Key Points
Nivolumab plus relatlimab was overall safe, tolerable, and manageable with no unexpected safety signals or treatment-related deaths.
The combination of nivolumab and relatlimab demonstrated antitumor activity in the subset of immune checkpoint–naive patients with HL.
Visual Abstract
Despite high response rates, anti–programmed death 1 (anti–PD-1) monotherapy eventually fails in most patients with relapsed/refractory (R/R) Hodgkin lymphoma (HL) and is ineffective in most other B-cell malignancies. The lymphocyte activation gene 3 (LAG-3) cell-surface receptor represents another immune checkpoint that can be targeted to induce remissions in these diseases; dual inhibition of PD-1 and LAG-3 is approved in advanced melanoma. We performed a multicenter phase 1/2a open-label study of the anti–LAG-3 antibody relatlimab (RELATIVITY-022) administered as monotherapy or in combination with nivolumab in patients with R/R B-cell malignancies. We treated 106 patients and no dose-limiting toxicities were observed during escalation. The recommended phase 2 dose was relatlimab 240 mg as monotherapy or nivolumab 240 mg plus relatlimab 160 mg, administered every 2 weeks. No unexpected safety signals were observed compared with anti–PD-1 monotherapy. In the HL expansion cohorts, objective response rate (ORR) was 62% and complete response rate (CRR) was 19% in anti–PD-1/anti–programmed death ligand 1 (anti–PD-[L]1)–naive patients (n = 21), with a median progression-free survival (PFS) of 19 months; ORR was 15% and CRR 0%, with median PFS of 6 months in anti–PD-(L)1-progressed patients (n = 20). In diffuse large B-cell lymphoma, ORR was 7% with no CRs (n = 15), and median PFS was 2 months. Nivolumab plus relatlimab appeared to be safe and tolerable. Responses in patients with anti–PD-(L)1–naive HL was encouraging, although the contribution of relatlimab to overall efficacy of the combination needs to be further evaluated. This trial was registered at www.ClinicalTrials.gov as #NCT02061761.
Introduction
Although most patients with classical Hodgkin lymphoma (HL) are cured by initial treatment, 10% to 30% of patients become treatment refractory after initial treatment or relapse.1 Programmed death-1 (PD-1) blockade has emerged as an effective treatment option, either as a single agent or in combination with chemotherapy.2-6 Nivolumab and pembrolizumab are anti–PD-1 monoclonal antibodies (mAbs) that have been approved by the US Food and Drug Administration for treatment of relapsed/refractory (R/R) HL.7,8 Although anti–PD-1 therapy is effective in primary mediastinal B-cell lymphoma,9,10 it has not been proven to be effective in R/R diffuse large B-cell lymphoma (DLBCL). Even when used as a single agent for R/R HL, a large proportion of patients eventually progress or relapse after therapy.11-13 Developing new and effective treatments for these patients therefore represents an important unmet clinical need.
The lymphocyte activation gene 3 (LAG-3) cell-surface receptor is another checkpoint molecule that plays a role in the regulation of T-cell activation and tolerance.14-16 LAG-3 and PD-1 are distinct immune checkpoints that are both often expressed on tumor-infiltrating lymphocytes.17 Preclinical and clinical data support the role of LAG-3 in immune evasion in classical HL,18-21 and a substantial proportion of tumors isolated from patients with HL have been found to overexpress programmed death ligand 1 (PD-L1) and LAG-3 in the tumor microenvironment.22 Therefore, LAG-3 may serve as another immune evasion pathway, and combining a PD-1 inhibitor with a LAG-3 inhibitor may enhance antitumor immunity and improve response rates.
Relatlimab is a first-in-class human immunoglobulin G4 LAG-3–blocking antibody that binds to LAG-3 and restores the effector function of T cells.23 Nivolumab plus relatlimab as a fixed-dose combination has been approved for the treatment of unresectable or metastatic melanoma in adults and pediatric patients aged >12 years.23
RELATIVITY-022 is phase 1/2a, open-label study of relatlimab administered as monotherapy or in combination with nivolumab in patients with advanced R/R B-cell malignancies. Here, we report the safety, tolerability, efficacy, pharmacokinetics, immunogenicity, and pharmacodynamics of nivolumab plus relatlimab in patients enrolled in the dose-expansion cohorts (parts B and D).
Methods
Study design
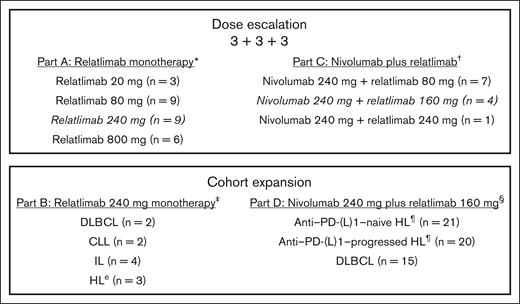
RELATIVITY-022 (ClinicalTrials.gov identifier: NCT02061761) was conducted in 9 centers in the United States and Canada. The study consisted of 4 parts, comprising 2 dose-escalation cohorts (3 + 3 + 3; parts A and C) and 2 dose-expansion cohorts (parts B and D) for relatlimab alone and in combination with nivolumab, respectively (Figure 1). Part B (relatlimab 240 mg monotherapy every 2 weeks) included patients with DLBCL, chronic lymphocytic leukemia, indolent lymphoma, and HL, whereas part D (nivolumab 240 mg plus relatlimab 160 mg every 2 weeks) only included patients with HL and DLBCL. All patients could receive up to 12 treatment cycles. RELATIVITY-022 was conducted in compliance with the Declaration of Helsinki, the International Council for Harmonisation guidelines for good clinical practice, and applicable national and local regulatory requirements. The study protocol was approved by the independent ethics committee or institutional review board at each participating site. All patients provided written informed consent before participation. The study overlapped with the period when the novel disease caused by severe acute respiratory syndrome coronavirus 2 was declared a public health emergency of international concern by the World Health Organization.24
RELATIVITY-022 study design. ∗Part A dose-escalation cohort includes patients with R/R CLL, HL, certain non-HLs, and MM. The 800-mg dose was stopped because of futility. †Part C dose-escalation cohort includes patients with R/R HL and DLBCL. ‡Part B cohort expansion included patients with R/R disease. §Patients with PD-(L)1–progressed HL had disease progression while on, or within 3 months of, treatment with an anti–PD-(L)1 as their most recent therapy. ¶Most patients had classic HL; 2 patients with nodular lymphocyte–predominant HL were included in part D (1 in PD-(L)1–progressed and 1 in PD-(L)1–naive). CLL, chronic lymphocytic leukemia; IL, indolent lymphoma; MM, multiple myeloma.
RELATIVITY-022 study design. ∗Part A dose-escalation cohort includes patients with R/R CLL, HL, certain non-HLs, and MM. The 800-mg dose was stopped because of futility. †Part C dose-escalation cohort includes patients with R/R HL and DLBCL. ‡Part B cohort expansion included patients with R/R disease. §Patients with PD-(L)1–progressed HL had disease progression while on, or within 3 months of, treatment with an anti–PD-(L)1 as their most recent therapy. ¶Most patients had classic HL; 2 patients with nodular lymphocyte–predominant HL were included in part D (1 in PD-(L)1–progressed and 1 in PD-(L)1–naive). CLL, chronic lymphocytic leukemia; IL, indolent lymphoma; MM, multiple myeloma.
Patients
Patients were aged ≥18 years and had histologically confirmed B-cell malignancies and experienced disease progression or were disease refractory to ≥1 standard therapy (eg, radiation, immunomodulatory therapy, immunotherapy [IO], cytotoxic chemotherapy, or select antibody-based therapy). Patients with HL in part D were further stratified into 2 groups: (1) those who had progressed or were refractory to ≥1 standard therapy and were naive to treatment with a PD-1/PD-L1 inhibitor (PD-(L)1–naive HL), and (2) those who experienced disease progression on or within 3 months of receiving a PD-(L)1 inhibitor as their most recent therapy (PD-(L)1–progressed HL). Patients with DLBCL who were PD-(L)1–naive or were previously treated with a PD-(L)1 inhibitor were eligible. Other eligibility criteria included passage of >100 days since autologous transplant, Eastern Cooperative Oncology Group performance status score of 0 or 1, adequate organ and hematologic function by clinical laboratory testing, and left ventricular ejection fraction of ≥50%. Previous exposure to immune-cell–modulating antibody-based regimens were prohibited as follows: for all patients in parts A through D, antibody regimens such as anti–CTLA-4, anti-KIR, and/or anti-OX40 antibodies were prohibited (prior anti-CD20, alemtuzumab, and anti-CD30 therapies were allowed). Additionally, anti–PD-(L)1 therapy was prohibited for patients in part A and the PD-(L)1–naive cohort of part D; the PD-(L)1–progressed HL portion of part D allowed only patients who had received PD-(L)1 as their most recent therapy, and who had progressed within 3 months of that therapy. Other key exclusion criteria included known or suspected central nervous system involvement (prior central nervous system involvement was permitted); known or suspected autoimmune disease; prior allogeneic transplant or autologous/allogeneic T-cell transfer; and positivity for HIV, hepatitis B, or hepatitis C. Patients with a history of a life-threatening toxicity related to prior IO were also excluded, except those that were unlikely to reoccur with standard countermeasures (eg, hormone replacement after endocrinopathy). Topical, ocular, intra-articular, intranasal, and inhalational corticosteroids were permitted, whereas immunosuppressive agents and systemic corticosteroids were allowed only for treating adverse events (AEs) and prophylaxis and were prohibited within 14 days before study initiation.
End points
The primary end point was safety, as measured by the rate of AEs, serious AEs, and AEs leading to treatment discontinuation, deaths, and laboratory abnormalities, assessed during treatment and for up to 135 days after the last treatment. Safety analyses were based on 30-day and 100-day safety windows. All patients who received at least 1 dose of study treatment were analyzed for safety. AEs were coded per the Medical Dictionary for Regulatory Activities version 25.1 and graded per the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.25 Coprimary end points in part D included objective response rate (ORR) based on investigator assessment (for patients with non-HL and HL, ORR was based on the International Working Group [IWG] 2007 response criteria)26 and duration of objective response (DOR). DOR was defined as the time between the date of first documented response (complete response [CR] or partial response [PR]) and the date of the first objectively documented progression as per IWG criteria relevant to each disease type in part D, or death due to any cause, whichever occurred first. For patients who neither progressed nor died, DOR was censored on the date of their last tumor assessment. Patients who started subsequent therapy without previously reported progression were censored at their last tumor assessment before starting subsequent therapy. Disease assessments were performed by positron emission tomography–computed tomography (PET-CT) and/or CT as appropriate according to the IWG criteria for malignant lymphoma (ie, PET-CT before treatment for patients with fluorodeoxyglucose-acid potentially curable lymphoma, such as HL or DLBCL, and in posttreatment assessment of HL or DLBCL),26 between days 50 and 56 of each 8-week treatment cycle, and at 30 days after treatment.
Secondary end points included pharmacokinetics (PKs) of relatlimab and nivolumab plus relatlimab (using the parameters outlined in supplemental Table 1) and immunogenicity. Select PK parameters were assessed from concentration–time data at select time points throughout treatment. Incidence of anti-drug antibodies (ADA) was assessed during treatment and for up to 135 days after each patient’s last treatment in posttreatment follow-up. A patient who was ADA+ at baseline was defined as a patient with positive seroconversion detected in the last sample before initiation of treatment. A patient considered ADA+ had at least 1 ADA+ sample relative to baseline after initiation of treatment.
Exploratory end points included pharmacodynamics of relatlimab in the peripheral blood, receptor occupancy of LAG-3 in the peripheral blood, overall survival, and progression-free survival (PFS) per IWG 2007 criteria.25 PFS was defined as the time from the first dose to the date of first objectively documented disease progression or death due to any cause, whichever occurred first. PD-L1 and LAG-3 analyses by immunohistochemistry were performed retrospectively when formalin-fixed paraffin-embedded blocks were available.
Patient samples
Serum for the PK analyses were collected from blood drawn during cycle 1 (before and after infusion on day 1, and before infusion on days 15, 29, and 42), cycles 2 and 3 (before and after infusion on day 1, and before infusion on day 15), and all subsequent odd-numbered treatment cycles (before infusion on day 1). The presence of ADA was evaluated using blood collected before infusion on days 1, 15, and 29 of cycle 1; day 1 of cycles 2 and 3; and day 1 of all subsequent odd-numbered treatment cycles. Blood was also drawn on days 60 and 135 after treatment for additional assessments. Blood for the pharmacodynamic analyses was drawn on days 1, 8, 16, 29, 43, and 50 through 56 of cycle 1; on day 29 of all even-numbered treatment cycles; and after confirmation of disease progression. Tumors were optionally biopsied on day 29 of cycle 1 and upon disease progression. The soluble factors analyzed in serum included cytokines (interleukin-2a, interleukin-8, and interleukin-10), chemokines (CXCL), and circulating receptors (LAG-3). Flow cytometry was used to quantify the proportion of activated CD8+ and CD4+ effector memory T cells in peripheral blood mononuclear cell preparations. Blood for the assessment of LAG-3 receptor occupancy was collected on days 1, 8, 15, and 43 of cycle 1, with a flow cytometry–based assay used to determine the percentage of relatlimab bound to CD3+CD8+ T cells.
Statistics
A 2-stage Fleming design, which allows an early stop for futility (and potentially for efficacy), was used to guide sample size for part D with a reasonable false-positive rate (<15%) and a false-negative rate (<10%) based on assumptions of true (target) and historic response rate for each cohort.27 Each disease cohort was handled independently and there was no multiplicity adjustment. For anti–PD-(L)1–progressed HL, the assumed target response rate will be 20% vs 2% of inefficacious rate because there is no standard of care available in this patient population. For anti–PD-(L)1–naive HL, the assumed target CR rate will be 45% vs 20% observed with anti–PD-1 monotherapy. For DLBCL, the assumed target ORR will be 45% vs 20%, which was obtained from another study of nivolumab for hematologic malignancies by averaging the observed rates of monotherapy (4/11 [36%]) and a combination treatment with ipilimumab (0/9 [0%]).2,28 The sample size and operational characteristics of using a Fleming 2-stage design are provided in supplemental Table 2, although not used for statistical hypothesis testing. Separate early stopping rules were applied based on prior PD-(L)1 exposure but not based on histology, and were determined by the 2-stage design characteristics. There was no stopping of a disease cohort for efficacy.
Safety data were summarized (1) overall by dose level and across all dose levels, and (2) by dose level and across all dose levels within each tumor type using descriptive statistics. The PK population included all patients who received ≥1 dose of study treatment and had evaluable serum concentration data. PK parameters were calculated using noncompartmental analyses (Phoenix WinNonlin, version 8.2; Certara, Princeton, NJ). Best overall response was summarized using frequency tables and 2-sided 95% confidence intervals (CIs), whereas DOR and PFS were estimated using the Kaplan-Meier method and, when appropriate, the median and 95% CIs (based on a log-log transformation method) were provided using Brookmeyer and Crowley methodology. Other exploratory end points were summarized using descriptive statistics.
Results
Patient characteristics and disposition
Between 12 March 2014 and 29 September 2021, a total of 136 patients were enrolled. There were 29 (21%) screen failures: patients no longer met eligibility criteria (n = 21), patient withdrew consent (n = 6), death (n = 1), and other unspecified reasons (n = 1). Additionally, 1 patient who entered the treatment period and was scheduled to receive treatment died before administration of the first dose. Therefore, 106 patients received at least 1 dose of the study treatment. In the dose-expansion cohorts, a total of 67 patients were treated (part B, n = 11; part D, n = 56; Figure 1). Baseline characteristics for patients in the dose-expansion cohorts can be found in Table 1 (baseline characteristics for those in the dose-escalation cohorts can be found in supplemental Table 3).
Baseline characteristics for patients enrolled in the expansion cohorts (parts B and D)
| . | Relatlimab monotherapy (240 mg; part B) . | Nivolumab plus relatlimab (240 mg/160 mg; part D) . | |||||
|---|---|---|---|---|---|---|---|
| CLL (n = 2) . | DLBCL (n = 2) . | IL (n = 4) . | HL∗ (n = 3) . | Anti–PD-(L)1–naive HL∗ (n = 21) . | Anti–PD-(L)1–progressed HL∗ (n = 20) . | DLBCL (n = 15) . | |
| Age, median (range), y | 67.5 (67-68) | 64.5 (39-90) | 62.0 (53-69) | 24.0 (20-47) | 35.0 (19-64) | 35.5 (25-74) | 73.0 (50-85) |
| Sex, n (%) | |||||||
| Male | 0 | 1 (50.0) | 4 (100.0) | 3 (100.0) | 15 (71.4) | 10 (50.0) | 10 (66.7) |
| Female | 2 (100.0) | 1 (50.0) | 0 | 0 | 6 (28.6) | 10 (50.0) | 5 (33.3) |
| Race, n (%) | |||||||
| White | 1 (50.0) | 2 (100.0) | 3 (75.0) | 3 (100.0) | 13 (61.9) | 16 (80.0) | 14 (93.3) |
| Black/African American | 1 (50.0) | 0 | 0 | 0 | 2 (9.5) | 1 (5.0) | 0 |
| Asian | 0 | 0 | 0 | 0 | 2 (9.5) | 2 (10.0) | 1 (6.7) |
| Native Hawaiian/other Pacific Islander | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 0 |
| Other | 0 | 0 | 1 (25.0) | 0 | 3 (14.3) | 1 (5.0) | 0 |
| Ethnicity, n (%) | |||||||
| Hispanic or Latino | 0 | 0 | 0 | 0 | 1 (4.8) | 1 (5.0) | 0 |
| Not Hispanic or Latino | 2 (100.0) | 2 (100.0) | 4 (100.0) | 3 (100.0) | 20 (95.2) | 19 (95.0) | 15 (100.0) |
| Country, n (%) | |||||||
| Canada | 0 | 0 | 0 | 0 | 1 (4.8) | 6 (30.0) | 0 |
| United States | 2 (100.0) | 2 (100.0) | 4 (100.0) | 3 (100.0) | 20 (95.2) | 14 (70.0) | 15 (100.0) |
| ECOG PS, n (%) | |||||||
| 0 | 2 (100.0) | 2 (100.0) | 3 (75.0) | 2 (66.7) | 17 (81.0) | 10 (50.0) | 4 (26.7) |
| 1 | 0 | 0 | 1 (25.0) | 1 (33.3) | 4 (19.0) | 10 (50.0) | 10 (66.7) |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (6.7) |
| Prior therapy regimens, n (%) | |||||||
| 1 | 0 | 1 (50.0) | 1 (25.0) | 0 | 5 (23.8) | 0 | 0 |
| 2 | 0 | 0 | 1 (25.0) | 0 | 6 (28.6) | 3 (15.0) | 3 (20.0) |
| 3 | 0 | 0 | 1 (25.0) | 1 (33.3) | 8 (38.1) | 1 (5.0) | 1 (6.7) |
| 4 | 1 (50.0) | 0 | 0 | 1 (33.3) | 1 (4.8) | 3 (15.0) | 7 (46.7) |
| ≥5 | 1 (50.0) | 1 (50.0) | 1 (25.0) | 1 (33.3) | 1 (4.8) | 13 (65.0) | 4 (26.7) |
| Previous anti–PD-(L)1 therapy, n (%) | |||||||
| 0 | 0 | 0 | 0 | 1 (33.3) | 21 (100.0) | 0 | 14 (93.3) |
| 1 | 0 | 1 (50.0) | 2 (50.0) | 0 | 0 | 4 (20.0) | 1 (6.7) |
| >1 | 2 (100.0) | 1 (50.0) | 2 (50.0) | 2 (66.7) | 0 | 16 (80.0) | 0 |
| Previous brentuximab therapy, n (%) | 0 | 0 | 0 | 2 (66.7) | 12 (57.1) | 15 (75.0) | 0 |
| Previous stem cell transplant, n (%) | 0 | 0 | 2 (50.0) | 2 (66.7) | 6 (28.6) | 13 (65.0) | 7 (46.7) |
| Sum of the product of diameters, median (range), mm2 | N/A | N/A | N/A | N/A | 1060.0 (0-5576) | 3 265.5 (551-26 758) | 1186.5 (155-8392) |
| . | Relatlimab monotherapy (240 mg; part B) . | Nivolumab plus relatlimab (240 mg/160 mg; part D) . | |||||
|---|---|---|---|---|---|---|---|
| CLL (n = 2) . | DLBCL (n = 2) . | IL (n = 4) . | HL∗ (n = 3) . | Anti–PD-(L)1–naive HL∗ (n = 21) . | Anti–PD-(L)1–progressed HL∗ (n = 20) . | DLBCL (n = 15) . | |
| Age, median (range), y | 67.5 (67-68) | 64.5 (39-90) | 62.0 (53-69) | 24.0 (20-47) | 35.0 (19-64) | 35.5 (25-74) | 73.0 (50-85) |
| Sex, n (%) | |||||||
| Male | 0 | 1 (50.0) | 4 (100.0) | 3 (100.0) | 15 (71.4) | 10 (50.0) | 10 (66.7) |
| Female | 2 (100.0) | 1 (50.0) | 0 | 0 | 6 (28.6) | 10 (50.0) | 5 (33.3) |
| Race, n (%) | |||||||
| White | 1 (50.0) | 2 (100.0) | 3 (75.0) | 3 (100.0) | 13 (61.9) | 16 (80.0) | 14 (93.3) |
| Black/African American | 1 (50.0) | 0 | 0 | 0 | 2 (9.5) | 1 (5.0) | 0 |
| Asian | 0 | 0 | 0 | 0 | 2 (9.5) | 2 (10.0) | 1 (6.7) |
| Native Hawaiian/other Pacific Islander | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 0 |
| Other | 0 | 0 | 1 (25.0) | 0 | 3 (14.3) | 1 (5.0) | 0 |
| Ethnicity, n (%) | |||||||
| Hispanic or Latino | 0 | 0 | 0 | 0 | 1 (4.8) | 1 (5.0) | 0 |
| Not Hispanic or Latino | 2 (100.0) | 2 (100.0) | 4 (100.0) | 3 (100.0) | 20 (95.2) | 19 (95.0) | 15 (100.0) |
| Country, n (%) | |||||||
| Canada | 0 | 0 | 0 | 0 | 1 (4.8) | 6 (30.0) | 0 |
| United States | 2 (100.0) | 2 (100.0) | 4 (100.0) | 3 (100.0) | 20 (95.2) | 14 (70.0) | 15 (100.0) |
| ECOG PS, n (%) | |||||||
| 0 | 2 (100.0) | 2 (100.0) | 3 (75.0) | 2 (66.7) | 17 (81.0) | 10 (50.0) | 4 (26.7) |
| 1 | 0 | 0 | 1 (25.0) | 1 (33.3) | 4 (19.0) | 10 (50.0) | 10 (66.7) |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (6.7) |
| Prior therapy regimens, n (%) | |||||||
| 1 | 0 | 1 (50.0) | 1 (25.0) | 0 | 5 (23.8) | 0 | 0 |
| 2 | 0 | 0 | 1 (25.0) | 0 | 6 (28.6) | 3 (15.0) | 3 (20.0) |
| 3 | 0 | 0 | 1 (25.0) | 1 (33.3) | 8 (38.1) | 1 (5.0) | 1 (6.7) |
| 4 | 1 (50.0) | 0 | 0 | 1 (33.3) | 1 (4.8) | 3 (15.0) | 7 (46.7) |
| ≥5 | 1 (50.0) | 1 (50.0) | 1 (25.0) | 1 (33.3) | 1 (4.8) | 13 (65.0) | 4 (26.7) |
| Previous anti–PD-(L)1 therapy, n (%) | |||||||
| 0 | 0 | 0 | 0 | 1 (33.3) | 21 (100.0) | 0 | 14 (93.3) |
| 1 | 0 | 1 (50.0) | 2 (50.0) | 0 | 0 | 4 (20.0) | 1 (6.7) |
| >1 | 2 (100.0) | 1 (50.0) | 2 (50.0) | 2 (66.7) | 0 | 16 (80.0) | 0 |
| Previous brentuximab therapy, n (%) | 0 | 0 | 0 | 2 (66.7) | 12 (57.1) | 15 (75.0) | 0 |
| Previous stem cell transplant, n (%) | 0 | 0 | 2 (50.0) | 2 (66.7) | 6 (28.6) | 13 (65.0) | 7 (46.7) |
| Sum of the product of diameters, median (range), mm2 | N/A | N/A | N/A | N/A | 1060.0 (0-5576) | 3 265.5 (551-26 758) | 1186.5 (155-8392) |
CLL, chronic lymphocytic leukemia; ECOG PS, Eastern Cooperative Oncology Group performance status; IL, indolent lymphoma; N/A, not available.
Most patients had classic HL; 2 patients with nodular lymphocyte–predominant HL were included in part D (1 in PD-(L)1–progressed and 1 in PD-(L)1–naive).
All patients in the dose-expansion cohorts were off treatment as of the data cutoff (Table 2; disposition for dose-escalation cohorts can be found in supplemental Table 4). Most patients in parts B and D discontinued treatment because of disease progression. In part D, the median duration of nivolumab plus relatlimab treatment was 24 weeks (range, 4-100) for patients in the anti–PD-(L)1–naive HL group, 25 weeks (range, 2-100) for patients in the anti–PD-(L)1–progressed HL group, and 8 weeks (range, 2-24) for patients in the DLBCL group (supplemental Table 5).
Patient disposition for patients enrolled in the expansion cohorts (parts B and D)
| . | Relatlimab monotherapy (240 mg; part B) . | Nivolumab plus relatlimab (240 mg/160 mg; part D) . | |||||
|---|---|---|---|---|---|---|---|
| CLL (n = 2) . | DLBCL (n = 2) . | IL (n = 4) . | HL∗ (n = 3) . | Anti–PD-(L)1–naive HL∗ (n = 21) . | Anti–PD-(L)1–progressed HL∗ (n = 20) . | DLBCL (n = 15) . | |
| Ongoing treatment, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Discontinued treatment, n (%) | 2 (100.0) | 2 (100.0) | 4 (100.0) | 3 (100.0) | 21 (100.0) | 20 (100.0) | 15 (100.0) |
| Disease progression | 1 (50.0) | 2 (100.0) | 4 (100.0) | 2 (66.7) | 2 (9.5) | 12 (60.0) | 14 (93.3) |
| Study drug toxicity | 0 | 0 | 0 | 0 | 3 (14.3) | 3 (15.0) | 0 |
| Patient request | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (6.7) |
| Withdrew consent | 0 | 0 | 0 | 0 | 1 (4.8) | 1 (5.0) | 0 |
| Maximum clinical benefit† | 0 | 0 | 0 | 0 | 5 (23.8)‡ | 1 (5.0)§ | 0 |
| No longer met study criteria | 0 | 0 | 0 | 0 | 0 | 1 (5.0) | 0 |
| Completed treatment per protocol | 0 | 0 | 0 | 0 | 4 (19.0) | 1 (5.0) | 0 |
| Other | 1 (50.0) | 0 | 0 | 1 (33.3) | 5 (23.8) | 1 (5.0) | 0 |
| Discontinued study, n (%) | 1 (50.0) | 2 (100.0) | 0 | 2 (66.7) | 4 (19.0) | 5 (25.0) | 14 (93.3) |
| Withdrew consent | 0 | 0 | 0 | 0 | 2 (9.5) | 1 (5.0) | 2 (13.3) |
| Lost to follow-up | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 |
| Death | 1 (50.0) | 2 (100.0) | 0 | 0 | 1 (4.8) | 3 (15.0) | 9 (60.0) |
| Other | 0 | 0 | 0 | 1 (33.3) | 1 (4.8) | 1 (5.0) | 3 (20.0) |
| . | Relatlimab monotherapy (240 mg; part B) . | Nivolumab plus relatlimab (240 mg/160 mg; part D) . | |||||
|---|---|---|---|---|---|---|---|
| CLL (n = 2) . | DLBCL (n = 2) . | IL (n = 4) . | HL∗ (n = 3) . | Anti–PD-(L)1–naive HL∗ (n = 21) . | Anti–PD-(L)1–progressed HL∗ (n = 20) . | DLBCL (n = 15) . | |
| Ongoing treatment, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Discontinued treatment, n (%) | 2 (100.0) | 2 (100.0) | 4 (100.0) | 3 (100.0) | 21 (100.0) | 20 (100.0) | 15 (100.0) |
| Disease progression | 1 (50.0) | 2 (100.0) | 4 (100.0) | 2 (66.7) | 2 (9.5) | 12 (60.0) | 14 (93.3) |
| Study drug toxicity | 0 | 0 | 0 | 0 | 3 (14.3) | 3 (15.0) | 0 |
| Patient request | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (6.7) |
| Withdrew consent | 0 | 0 | 0 | 0 | 1 (4.8) | 1 (5.0) | 0 |
| Maximum clinical benefit† | 0 | 0 | 0 | 0 | 5 (23.8)‡ | 1 (5.0)§ | 0 |
| No longer met study criteria | 0 | 0 | 0 | 0 | 0 | 1 (5.0) | 0 |
| Completed treatment per protocol | 0 | 0 | 0 | 0 | 4 (19.0) | 1 (5.0) | 0 |
| Other | 1 (50.0) | 0 | 0 | 1 (33.3) | 5 (23.8) | 1 (5.0) | 0 |
| Discontinued study, n (%) | 1 (50.0) | 2 (100.0) | 0 | 2 (66.7) | 4 (19.0) | 5 (25.0) | 14 (93.3) |
| Withdrew consent | 0 | 0 | 0 | 0 | 2 (9.5) | 1 (5.0) | 2 (13.3) |
| Lost to follow-up | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 |
| Death | 1 (50.0) | 2 (100.0) | 0 | 0 | 1 (4.8) | 3 (15.0) | 9 (60.0) |
| Other | 0 | 0 | 0 | 1 (33.3) | 1 (4.8) | 1 (5.0) | 3 (20.0) |
BOR, best overall response; SD, stable disease.
Most were classic HL; 2 patients with nodular lymphocyte–predominant HL were included in part D (1 in PD-(L)1–progressed and 1 in PD-(L)1–naive).
Maximum clinical benefit was per investigator assessment.
BOR of PR (3) and SD (2) was observed among 5 patients with anti–PD-(L)1–naive HL with maximum clinical benefit.
BOR of SD (1) was observed among 1 patient with anti–PD-(L)1–progressed HL with maximum clinical benefit.
Safety
No patient in the dose-escalation cohorts (parts A and C) experienced a dose-limiting toxicity (a safety summary for these patients can be found in supplemental Table 6). The maximum tolerated dose was not reached. Safety for patients in the dose-expansion cohorts (parts B and D) is shown in Table 3 (additional safety for these cohorts can be found in supplemental Table 7). Grade 3/4 treatment-related AEs (TRAEs) were reported in 2 patients (1 with chronic lymphocytic leukemia and 1 with indolent lymphoma) in part B, and in 7 patients (2 with anti–PD-(L)1–naive HL, 4 with anti–PD-(L)1–progressed HL, and 1 with DLBCL) in part D. Any-grade TRAEs (≥5%) were similar to that in solid tumors, including melanoma. No treatment-related deaths were reported.
Safety summary for patients treated in the expansion cohorts (parts B and D)
| . | Relatlimab monotherapy (240 mg; part B) . | Nivolumab plus relatlimab (240 mg/160 mg; part D) . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLL (n = 2) . | DLBCL (n = 2) . | IL (n = 4) . | HL∗ (n = 3) . | Total (n = 11) . | HL∗ anti–PD-(L)1– naive (n = 21) . | HL∗ anti–PD-(L)1–progressed (n = 20) . | DLBCL (n = 15) . | Total (n = 56) . | ||||||||||
| Grade, n (%) | Any | 3/4 | Any | 3/4 | Any | 3/4 | Any | 3/4 | Any | 3/4 | Any | 3/4 | Any | 3/4 | Any | 3/4 | Any | 3/4 |
| All-causality AEs | 2 (100.0) | 2 (100.0) | 2† (100.0) | 0 | 4 (100.0) | 2 (50.0) | 3 (100.0) | 1 (33.3) | 11 (100.0) | 5 (45.4) | 21 (100.0) | 12 (57.1) | 20 (100.0) | 8 (40.0) | 14‡ (93.3) | 4 (26.7) | 55 (98.2) | 24 (42.8) |
| TRAEs | 1 (50.0) | 1 (50.0) | 1 (50.0) | 0 | 3 (75.0) | 1 (25.0) | 1 (33.3) | 0 | 6 (54.5) | 2 (18.2) | 14 (66.7) | 2 (9.5) | 16 (80.0) | 4 (20.0) | 5 (33.3) | 1 (6.7) | 35 (62.5) | 7 (12.5) |
| TRAEs in ≥5% of patients | ||||||||||||||||||
| Amylase increased | 0 | 0 | 0 | 0 | 1 (25.0) | 0 | 0 | 0 | 1 (9.1) | 0 | 1 (4.8) | 0 | 2 (10.0) | 1 (5.0) | 0 | 0 | 3 (5.4) | 1 (1.8) |
| Anemia | 1 (50.0) | 1 (50.0) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (9.1) | 1 (9.1) | 1 (4.8) | 0 | 2 (10.0) | 0 | 0 | 0 | 3 (5.4) | 0 |
| Blood alkaline phosphatase increased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 2 (10.0) | 0 | 0 | 0 | 3 (5.4) | 0 |
| C-reactive protein increased | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 1 (9.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chills | 0 | 0 | 1 (50.0) | 0 | 0 | 0 | 0 | 0 | 1 (9.1) | 0 | 1 (4.8) | 0 | 0 | 0 | 0 | 0 | 1 (1.8) | 0 |
| Constipation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (5.0) | 0 | 1 (6.7) | 0 | 3 (5.4) | 0 |
| Diarrhea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (14.3) | 0 | 1 (5.0) | 0 | 0 | 0 | 4 (7.1) | 0 |
| Fatigue | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 1 (9.1) | 0 | 1 (4.8) | 0 | 4 (20.0) | 0 | 3 (20.0) | 0 | 8 (14.3) | 0 |
| Headache | 1 (50.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (9.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyperglycemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (9.5) | 0 | 1 (5.0) | 1 (5.0) | 0 | 0 | 3 (5.4) | 1 (1.8) |
| Hypothyroidism | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (19.0) | 0 | 1 (5.0) | 0 | 0 | 0 | 5 (8.9) | 0 |
| Infusion-related reaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (19.0) | 0 | 3 (15.0) | 0 | 0 | 0 | 7 (12.5) | 0 |
| Leukocytosis | 0 | 0 | 0 | 0 | 1 (25.0) | 0 | 0 | 0 | 1 (9.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lipase increased | 0 | 0 | 0 | 0 | 1 (25.0) | 1 (25.0) | 0 | 0 | 1 (9.1) | 1 (9.1) | 0 | 0 | 2 (10.0) | 1 (5.0) | 0 | 0 | 2 (3.6) | 1 (1.8) |
| Lymph node pain | 1 (50.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (9.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (5.0) | 0 | 1 (6.7) | 0 | 3 (5.4) | 0 |
| Pneumonitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (5.0) | 0 | 1 (6.7) | 1 (6.7) | 3 (5.4) | 1 (1.8) |
| Pyrexia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 4 (20.0) | 0 | 0 | 0 | 5 (8.9) | 0 |
| Rash | 0 | 0 | 0 | 0 | 1 (25.0) | 0 | 0 | 0 | 1 (9.1) | 0 | 2 (9.5) | 0 | 0 | 0 | 0 | 0 | 2 (3.6) | 0 |
| Rash maculopapular | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 2 (10.0) | 0 | 0 | 0 | 3 (5.4) | 0 |
| All-causality SAEs | 1 (50.0) | 1 (50.0) | 2 (100.0) | 0 | 1 (25.0) | 1 (25.0) | 1 (33.3) | 1 (33.3) | 5 (45.4) | 3 (27.3) | 5 (23.8) | 5 (23.8) | 9 (45.0) | 6 (30.0) | 10 (66.7) | 2 (13.3) | 24 (42.8) | 13 (23.2) |
| Treatment-related SAEs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (9.5) | 2 (9.5) | 5 (25.0) | 3 (15.0) | 1 (6.7) | 1 (6.7) | 8 (14.3) | 6 (10.7) |
| All-causality AEs leading to discontinuation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (19.0) | 1 (4.8) | 3 (15.0) | 2 (10.0) | 0 | 0 | 7 (12.5) | 3 (5.4) |
| TRAEs leading to discontinuation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (14.3) | 1 (4.8) | 3 (15.0) | 2 (10.0) | 0 | 0 | 6 (10.7) | 3 (5.4) |
| Immune-mediated TRAEs | ||||||||||||||||||
| Adrenal insufficiency | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8) | 1 (4.8) | 0 | 0 | 0 | 0 | 1 (1.8) | 1 (1.8) |
| Diabetes mellitus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5.0) | 1 (5.0) | 0 | 0 | 1 (1.8) | 1 (1.8) |
| Diarrhea/colitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 0 | 0 | 0 | 0 | 1 (1.8) | 0 |
| Hepatitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5.0) | 1 (5.0) | 0 | 0 | 1 (1.8) | 1 (1.8) |
| Hypersensitivity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyperthyroidism | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypophysitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypothyroidism | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (19.0) | 0 | 1 (5.0) | 0 | 0 | 0 | 5 (8.9) | 0 |
| Hypothyroidism/thyroiditis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (19.0) | 0 | 1 (5.0) | 0 | 0 | 0 | 5 (8.9) | 0 |
| Nephritis and renal dysfunction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pneumonitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (5.0) | 0 | 1 (6.7) | 1 (6.7) | 3 (5.4) | 1 (1.8) |
| Rash | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (20.0) | 0 | 0 | 0 | 4 (7.1) | 0 |
| Thyroiditis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| . | Relatlimab monotherapy (240 mg; part B) . | Nivolumab plus relatlimab (240 mg/160 mg; part D) . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLL (n = 2) . | DLBCL (n = 2) . | IL (n = 4) . | HL∗ (n = 3) . | Total (n = 11) . | HL∗ anti–PD-(L)1– naive (n = 21) . | HL∗ anti–PD-(L)1–progressed (n = 20) . | DLBCL (n = 15) . | Total (n = 56) . | ||||||||||
| Grade, n (%) | Any | 3/4 | Any | 3/4 | Any | 3/4 | Any | 3/4 | Any | 3/4 | Any | 3/4 | Any | 3/4 | Any | 3/4 | Any | 3/4 |
| All-causality AEs | 2 (100.0) | 2 (100.0) | 2† (100.0) | 0 | 4 (100.0) | 2 (50.0) | 3 (100.0) | 1 (33.3) | 11 (100.0) | 5 (45.4) | 21 (100.0) | 12 (57.1) | 20 (100.0) | 8 (40.0) | 14‡ (93.3) | 4 (26.7) | 55 (98.2) | 24 (42.8) |
| TRAEs | 1 (50.0) | 1 (50.0) | 1 (50.0) | 0 | 3 (75.0) | 1 (25.0) | 1 (33.3) | 0 | 6 (54.5) | 2 (18.2) | 14 (66.7) | 2 (9.5) | 16 (80.0) | 4 (20.0) | 5 (33.3) | 1 (6.7) | 35 (62.5) | 7 (12.5) |
| TRAEs in ≥5% of patients | ||||||||||||||||||
| Amylase increased | 0 | 0 | 0 | 0 | 1 (25.0) | 0 | 0 | 0 | 1 (9.1) | 0 | 1 (4.8) | 0 | 2 (10.0) | 1 (5.0) | 0 | 0 | 3 (5.4) | 1 (1.8) |
| Anemia | 1 (50.0) | 1 (50.0) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (9.1) | 1 (9.1) | 1 (4.8) | 0 | 2 (10.0) | 0 | 0 | 0 | 3 (5.4) | 0 |
| Blood alkaline phosphatase increased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 2 (10.0) | 0 | 0 | 0 | 3 (5.4) | 0 |
| C-reactive protein increased | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 1 (9.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chills | 0 | 0 | 1 (50.0) | 0 | 0 | 0 | 0 | 0 | 1 (9.1) | 0 | 1 (4.8) | 0 | 0 | 0 | 0 | 0 | 1 (1.8) | 0 |
| Constipation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (5.0) | 0 | 1 (6.7) | 0 | 3 (5.4) | 0 |
| Diarrhea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (14.3) | 0 | 1 (5.0) | 0 | 0 | 0 | 4 (7.1) | 0 |
| Fatigue | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 1 (9.1) | 0 | 1 (4.8) | 0 | 4 (20.0) | 0 | 3 (20.0) | 0 | 8 (14.3) | 0 |
| Headache | 1 (50.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (9.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyperglycemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (9.5) | 0 | 1 (5.0) | 1 (5.0) | 0 | 0 | 3 (5.4) | 1 (1.8) |
| Hypothyroidism | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (19.0) | 0 | 1 (5.0) | 0 | 0 | 0 | 5 (8.9) | 0 |
| Infusion-related reaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (19.0) | 0 | 3 (15.0) | 0 | 0 | 0 | 7 (12.5) | 0 |
| Leukocytosis | 0 | 0 | 0 | 0 | 1 (25.0) | 0 | 0 | 0 | 1 (9.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lipase increased | 0 | 0 | 0 | 0 | 1 (25.0) | 1 (25.0) | 0 | 0 | 1 (9.1) | 1 (9.1) | 0 | 0 | 2 (10.0) | 1 (5.0) | 0 | 0 | 2 (3.6) | 1 (1.8) |
| Lymph node pain | 1 (50.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (9.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (5.0) | 0 | 1 (6.7) | 0 | 3 (5.4) | 0 |
| Pneumonitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (5.0) | 0 | 1 (6.7) | 1 (6.7) | 3 (5.4) | 1 (1.8) |
| Pyrexia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 4 (20.0) | 0 | 0 | 0 | 5 (8.9) | 0 |
| Rash | 0 | 0 | 0 | 0 | 1 (25.0) | 0 | 0 | 0 | 1 (9.1) | 0 | 2 (9.5) | 0 | 0 | 0 | 0 | 0 | 2 (3.6) | 0 |
| Rash maculopapular | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 2 (10.0) | 0 | 0 | 0 | 3 (5.4) | 0 |
| All-causality SAEs | 1 (50.0) | 1 (50.0) | 2 (100.0) | 0 | 1 (25.0) | 1 (25.0) | 1 (33.3) | 1 (33.3) | 5 (45.4) | 3 (27.3) | 5 (23.8) | 5 (23.8) | 9 (45.0) | 6 (30.0) | 10 (66.7) | 2 (13.3) | 24 (42.8) | 13 (23.2) |
| Treatment-related SAEs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (9.5) | 2 (9.5) | 5 (25.0) | 3 (15.0) | 1 (6.7) | 1 (6.7) | 8 (14.3) | 6 (10.7) |
| All-causality AEs leading to discontinuation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (19.0) | 1 (4.8) | 3 (15.0) | 2 (10.0) | 0 | 0 | 7 (12.5) | 3 (5.4) |
| TRAEs leading to discontinuation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (14.3) | 1 (4.8) | 3 (15.0) | 2 (10.0) | 0 | 0 | 6 (10.7) | 3 (5.4) |
| Immune-mediated TRAEs | ||||||||||||||||||
| Adrenal insufficiency | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8) | 1 (4.8) | 0 | 0 | 0 | 0 | 1 (1.8) | 1 (1.8) |
| Diabetes mellitus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5.0) | 1 (5.0) | 0 | 0 | 1 (1.8) | 1 (1.8) |
| Diarrhea/colitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 0 | 0 | 0 | 0 | 1 (1.8) | 0 |
| Hepatitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5.0) | 1 (5.0) | 0 | 0 | 1 (1.8) | 1 (1.8) |
| Hypersensitivity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyperthyroidism | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypophysitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypothyroidism | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (19.0) | 0 | 1 (5.0) | 0 | 0 | 0 | 5 (8.9) | 0 |
| Hypothyroidism/thyroiditis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (19.0) | 0 | 1 (5.0) | 0 | 0 | 0 | 5 (8.9) | 0 |
| Nephritis and renal dysfunction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pneumonitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (5.0) | 0 | 1 (6.7) | 1 (6.7) | 3 (5.4) | 1 (1.8) |
| Rash | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (20.0) | 0 | 0 | 0 | 4 (7.1) | 0 |
| Thyroiditis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Includes AEs reported after first dose and within 100 days after last dose of study therapy. Relatlimab 240 mg was the RP2D.
RP2D, recommended phase 2 dose; SAE, serious AE.
Most patients had classic HL; 2 patients with nodular lymphocyte–predominant HL were included in part D (1 in PD-(L)1–progressed and 1 in PD-(L)1–naive).
Grade 5 events.
Includes 8 grade 5 events.
Efficacy (part D)
Median follow-up for efficacy in the 56 patients in part D was 37.7 months (range, 1.2-54.5) for the anti–PD-(L)1–naive HL group (n = 21), 39.1 months (range, 4.8-54.6) for the anti–PD-(L)1–progressed HL group (n = 20), and 4.7 months (range, 0.6-53.5) for the DLBCL group (n = 15; Table 4). In part D, the ORR for the anti–PD-(L)1–naive HL group was 62% (95% CI, 38.4-81.9); 4 patients (19%) achieved a CR, and 9 patients (43%) achieved a PR (Table 4). The ORR for the anti–PD-(L)1–progressed HL group was 15% (95% CI, 3.2-37.9; all 3 responders achieved a PR); and the ORR for the DLBCL group was 7% (95% CI, 0.2-31.9; only 1 patient achieved a PR). Median DOR in part D in the anti–PD-(L)1–naive HL group, the anti–PD-(L)1–progressed HL group, and the DLBCL group was 14.2 (95% CI, 2.6 to not reached [NR]) months, 6.4 (95% CI, 1.8 to NR) months, and not evaluable, respectively. Of the 13 responders (4 CR and 9 PR) in the anti–PD-(L)1–naive HL group, 4 subsequently progressed with DORs of 13.1, 14.2, 2.6, and 28.9 months. All 3 responders in the anti–PD-(L)1–progressed HL group progressed with DORs of 1.8, 6.4, and 7.8 months. The 1 responder in the DLBCL group was censored for DOR at 2.2 months because of study discontinuation. The DLBCL group was terminated early for futility, and neither of the coprimary end points were met.
Efficacy summary for patients enrolled in part D
| . | Anti–PD-(L)1–naive HL∗ (n = 21) . | Anti–PD-(L)1–progressed HL∗ (n = 20) . | DLBCL (n = 15) . |
|---|---|---|---|
| Follow-up, median (range), mo | 37.7 (1.2-54.5) | 39.1 (4.8-54.6) | 4.7 (0.6-53.5) |
| BOR, n (%) | |||
| CR | 4 (19) | 0 | 0 |
| PR | 9 (43) | 3 (15) | 1 (7) |
| SD | 6 (29) | 15 (75) | 2 (13) |
| PD | 0 | 1 (5) | 10 (67) |
| UTD† | 2 (10) | 1 (5) | 2 (13) |
| ORR, n (%), [95% CI] | 13 (62), [38.4-81.9] | 3 (15), [3.2-37.9] | 1 (7), [0.2-31.9] |
| DOR, median (95% CI), mo | 14.2 (2.6 to NR) | 6.4 (1.8 to NR) | NE |
| PFS, median (95% CI), mo | 19 (5.4 to NR) | 6 (3.7-10.0) | 2 (0.7-1.9) |
| OS | NE | NE | NE |
| . | Anti–PD-(L)1–naive HL∗ (n = 21) . | Anti–PD-(L)1–progressed HL∗ (n = 20) . | DLBCL (n = 15) . |
|---|---|---|---|
| Follow-up, median (range), mo | 37.7 (1.2-54.5) | 39.1 (4.8-54.6) | 4.7 (0.6-53.5) |
| BOR, n (%) | |||
| CR | 4 (19) | 0 | 0 |
| PR | 9 (43) | 3 (15) | 1 (7) |
| SD | 6 (29) | 15 (75) | 2 (13) |
| PD | 0 | 1 (5) | 10 (67) |
| UTD† | 2 (10) | 1 (5) | 2 (13) |
| ORR, n (%), [95% CI] | 13 (62), [38.4-81.9] | 3 (15), [3.2-37.9] | 1 (7), [0.2-31.9] |
| DOR, median (95% CI), mo | 14.2 (2.6 to NR) | 6.4 (1.8 to NR) | NE |
| PFS, median (95% CI), mo | 19 (5.4 to NR) | 6 (3.7-10.0) | 2 (0.7-1.9) |
| OS | NE | NE | NE |
Includes all response-evaluable patients. Responses were investigator-assessed. ORR per investigator was defined as CR + PR. PFS per investigator was defined as the time from first dose to the date of first objectively documented progression per the criteria relevant to each disease type or death due to any cause, whichever occurred first. OS was defined as the time between the date of the first dose and the date of death.
NE, not evaluable; OS, overall survival; PD, progressive disease; SD, stable disease; UTD, unable to determine.
Most patients had classic HL; 2 patients with nodular lymphocyte–predominant HL were included in part D (1 in PD-(L)1–progressed, with a BOR of SD, and 1 in PD-(L)1–naive, with a BOR of NE).
The reasons for response being UTD included death before disease assessment, NE, and other.
Among the anti–PD-(L)1–naive HL group, all patients had a reduction in tumor burden from baseline, with 13 of 20 (65%) patients having a ≥50% reduction in target lesion(s) from baseline (supplemental Figure 1). For the anti–PD-(L)1–progressed HL group, 17 of 19 (89%) patients had a reduction in tumor burden from baseline, with 3 of 19 (16%) patients having a ≥50% reduction in target lesion(s) from baseline. Among the DLBCL group, 1 of 10 (10%) evaluable patients had a reduction in tumor burden from baseline, which was a ≥50% reduction in target lesion(s) from baseline.
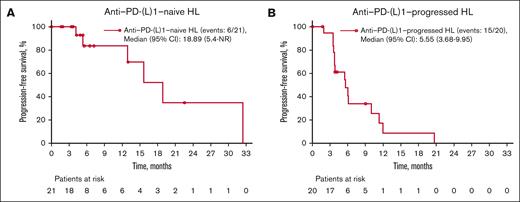
Median PFS was 19 months (95% CI, 5.4 to NR) for the anti–PD-(L)1–naive HL group, 6 months (95% CI, 3.7-10.0) for the anti–PD-(L)1–progressed HL group, and 2 months (95% CI, 0.7-1.9) for the DLBCL group (Figure 2; Table 4). Overall survival was not evaluable because of an insufficient number of events (1 event in the PD-(L)1–naive HL group and 3 events in the PD-(L)1–progressed HL group; Table 4).
Investigator-assessed PFS in part D patients. Patients with anti–PD-(L)1–naive HL (A) and anti–PD-(L)1–progressed HL (B). PFS for patients with DLBCL was not captured because of low patient numbers (<0), and also because these patients were heavily pretreated. Most patients had classic HL; 2 patients with nodular lymphocyte–predominant HL were included in part D (1 in PD-(L)1–progressed and 1 in PD-(L)1–naive).
Investigator-assessed PFS in part D patients. Patients with anti–PD-(L)1–naive HL (A) and anti–PD-(L)1–progressed HL (B). PFS for patients with DLBCL was not captured because of low patient numbers (<0), and also because these patients were heavily pretreated. Most patients had classic HL; 2 patients with nodular lymphocyte–predominant HL were included in part D (1 in PD-(L)1–progressed and 1 in PD-(L)1–naive).
PKs
The point estimates for the slope of linear regression model of log of PK parameters (maximum plasma concentration [Cmax] and area under plasma concentration–time curve in one dosing interval [TAU]) on log (dose) indicated a dose-proportional increase in Cmax of relatlimab and slightly more than a dose-proportional increase in the area under the curve (TAU) of relatlimab in the studied dose range of 20 to 800 mg at cycle 1, day 1 (supplemental Table 8). The mean serum concentration–time profiles for relatlimab monotherapy and in combination with nivolumab showed typical mAb characteristics with slow distribution and elimination (supplemental Figures 2A-B, 3A-B, 4A-B, and 5A-B).
Immunogenicity
An ADA assessment for relatlimab monotherapy cohorts (parts A and B) can be found in supplemental Table 9. The incidence of relatlimab ADA in all relatlimab 240 mg monotherapy patients was 37% (7/19 patients). In patients enrolled in the combination cohorts (parts C and D) with nivolumab 240 mg plus relatlimab 160 mg dose, the incidence of relatlimab ADA was 8% (4/52 patients) and the incidence of nivolumab ADA was 2% (1/46 patients; supplemental Table 10).
Biomarkers
LAG-3 receptor occupancy of CD3+CD8+ T cells reached 82% at the lowest relatlimab monotherapy dose (20 mg every 2 weeks) at cycle 1, day 15, and dropped <15% by cycle 1, day 43. A similar pattern was observed for dose of 80 mg; the median receptor occupancy reached >90% at dose of 240 mg and 100% at dose of 800 mg at cycle 1, day 15, and maintained at the same level until cycle 1, day 43, with the 2 highest relatlimab doses (240 mg and 800 mg every 2 weeks; supplemental Figure 6A). A similar percent LAG-3 receptor occupancy was observed at the 160 mg relatlimab dose in combination with nivolumab (240 mg) across the indications of HL and DLBCL at cycle 1, day 15 (median, 92.7%-100%) and reached 100% at cycle 1, day 43 for both indications and regardless of IO-naive or progression in HL (supplemental Figure 6B). The median level of free serum LAG-3 in the periphery that was not bound to drug decreased from baseline during treatment compared with baseline levels in all relatlimab monotherapy cohorts (except at the lowest dose of 20 mg; supplemental Figure 7A) and during treatment with nivolumab plus relatlimab (all doses) in part C (supplemental Figure 7B). A higher baseline for free serum LAG-3 in patients with anti–PD-(L)1–progressed HL was detected as compared with levels detected in naive patients (supplemental Figure 7C).
Increased levels of serum interferon gamma–induced CXCL9 were observed after treatment with relatlimab monotherapy at all doses as early as cycle 1, day 15 (supplemental Figure 8A). CXCL9 increased upon nivolumab plus relatlimab combination treatment consistently across all doses in part C, with relatlimab dose dependency (supplemental Figure 8B). The increase was also observed at cycle 1, day 15 in part D, which was sustained until cycle 4, day 29 (supplemental Figure 8C). Patients with anti–PD-(L)1-naive HL and DLBCL had early, peak, and sustained increase to late time point compared with patients with anti–PD-(L)1-progressed HL.
Discussion
RELATIVITY-022 was a phase 1/2a dose-escalation and dose-expansion study of the safety, tolerability, and efficacy of the anti–LAG-3 mAb relatlimab administered alone and in combination with the anti–PD-1 mAb nivolumab in patients with R/R B-cell malignancies. This report emphasizes the results of the dose-expansion cohorts for monotherapy (part B) and combination therapy (part D).
Nivolumab plus relatlimab demonstrated a tolerable and manageable safety profile in the dose-expansion cohorts. No treatment-related deaths were reported. TRAEs, such as adrenal insufficiency, were generally comparable with the nivolumab plus relatlimab combination therapy experience in solid tumors such as melanoma.29 Additionally, there was no apparent effect of treatment-emergent ADAs after treatment with nivolumab plus relatlimab on clinical safety (or efficacy). The relatlimab PKs were similar between monotherapy and combination therapy, indicating that there was no apparent PK interaction between nivolumab and relatlimab.
Nivolumab plus relatlimab demonstrated antitumor activity in R/R HL, particularly in patients who were anti–PD-(L)1–naive (ORR, 62%; CR, 19%; PR, 43%; median DOR, 14 months). In patients who had progressed or relapsed after anti–PD-(L)1 inhibitor therapy, efficacy was modest (ORR, 15%; CR, 0%; PR, 15%; median DOR, 6.4 months). Although the results do not provide robust support for a clinical benefit from the addition of relatlimab to PD-1 blockade, the study’s limitations (such as a small sample size, lack of a comparator group, and nonrandomized design) make comparing efficacy challenging. The preliminary efficacy results (ORR, 1 [7%]; CR, 0; PR, 1 [7%]) in DLBCL also do not provide support for a clinical benefit from the addition of relatlimab to PD-1 blockade in this disease; although, the aforementioned study limitations similarly make comparing efficacy challenging. Furthermore, because of study initiation early in the checkpoint inhibitor era, eligibility criteria were not limited to classic HL. Two patients were enrolled with a diagnosis of nodular lymphocyte–predominant HL, 1 each in part D HL naive and HL progressed, who, in retrospect, would not be expected to respond to checkpoint inhibitor therapy.
The slow overall recruitment rate was most likely due to a combination of some of the following factors: the sequential enrollment of multiple cohorts; a small patient pool available for part D due to fewer patients without prior PD-1 blockade in HL, because clinical practice and clinical trials evolved to begin using these treatments earlier in therapy and the low number of patients who were PD-1 refractory at the time of recruitment; stringent eligibility criteria; patient/physician preferences; and the onset of the COVID-19 pandemic.29 There was also a relatively low rate of prior brentuximab vedotin exposure and autologous stem cell transplant across subsets of the trial, with 24% of patients identified as having PD-1–naive R/R HL receiving nivolumab plus relatlimab therapy in second line without having been treated with autologous stem cell transplant or brentuximab vedotin. Potential reasons (which were not captured in the data) may have included lack of eligibility for those therapies, patient or investigator preference, and limited access to alternative treatments.
Lastly, reassessing responses using the Lugano 2014 criteria would have been valuable, however these criteria were not available when the study was designed.30 A study retrospectively comparing the Lugano 2014 criteria and the Cheson 2007 criteria by blinded independent review found increased duration of confirmed response in 12% and DOR in 24% of patients using the Lugano 2014 criteria.26,31 This difference was attributed to the assessment of PET positivity (blood pool vs liver), leading to earlier CRs and subsequently longer duration of confirmed response and DOR. Therefore, we believe that the results here would have been similar, with a potentially modestly higher rate of complete (metabolic) response, had it been possible to apply the Lugano 2014 criteria as well.
Regarding tissue biomarkers, there were too few samples with tissue evaluable for LAG-3 or PD-L1 immunohistochemistry, and too few responding patients in the checkpoint inhibitor progressed group, to correlate protein expression and clinical outcome. There remains an unmet medical need to identify a subpopulation that might benefit from combined checkpoint blockade.
Preliminary safety and efficacy results have been recently reported for favezelimab (an anti–LAG-3 antibody) and pembrolizumab in adults with anti–PD-(L)1–naive and anti–PD-(L)1–progressed HL (ClinicalTrials.gov identifier: NCT03598608).32,33 Again, the small cohort sizes and different eligibility criteria preclude direct comparison between the studies.
Noting the small sample size, the data do not support a clinical benefit from combined LAG-3 and PD-1 blockade in lymphomas such as DLBCL based on the preliminary efficacy results (ORR, 1 [7%]; CR, 0; PR, 1 [7%]). The lack of a meaningful apparent efficacy signal parallels data with single-agent anti–PD-1 therapies in this setting.34,35
Overall, the study provided safety, clinical pharmacology, pharmacodynamic, and preliminary efficacy data in several dose-expansion cohorts. The combination demonstrated a tolerable and manageable safety profile that is consistent with previously published data, but with limited evidence of clinical activity. Nivolumab plus relatlimab demonstrated evidence of antitumor activity only in a subset of immune checkpoint–naive patients with HL, but it remains unclear whether the addition of LAG-3 inhibition enhances activity over PD-1 inhibitor alone. Therefore, an important unmet need for patients with R/R disease remains, particularly for those who progress after PD-(L)1 blockade.
Acknowledgments
The authors thank the patients and investigators who participated in the RELATIVITY-022 trial. The authors acknowledge the following individuals from Bristol Myers Squibb: Mena Abaskharoun and Maurice Lobo for contributions to clinical data analysis and interpretation; Charlie Garnett-Benson for contributions to biomarker data analysis and interpretation; Diedereik Grootendorst for contributions to imaging response data analysis and interpretations; and Jenn Liu for contributions to biospecimen and imaging operations. The authors acknowledge personnel at Dako, an Agilent Technologies company, for collaborative development of the PD-L1 immunohistochemistry (IHC) 28-8 pharmDx assay, as well as personnel at LabCorp for collaborative development of the LAG-3 IHC assay, including analytic and clinical assay validations. Professional medical writing and editorial assistance were provided by Jessica R. Augello, Michele Salernitano, and Melissa Kirk of Ashfield MedComms, an Inizio company, and funded by Bristol Myers Squibb.
Authorship
Contribution: A.K.G., P.A., A.B.G., N.A., S.D., S.P.B., R.S., J.Q.W., S.W., and D.E.G. conceptualized and designed the study; and all authors were responsible for data acquisition, analysis, and interpretation, and had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict-of-interest disclosure: A.K.G. has received research funding from Merck, I-Mab Bio, IGM Bio, Takeda, Gilead, AstraZeneca, Agios, Janssen, Bristol Myers Squibb (BMS), Seagen, Teva, and Genmab; reports consultancy for/honoraria from Incyte, Kite, MorphoSys/Incyte, ADC Therapeutics, Acrotech, Merck, Karyopharm, Servier, BeiGene, Cellectar, Janssen, Seagen, Epizyme, I-Mab Bio, Gilead, Genentech, Lilly, Caribou, Fresenius Kabi, and Scitek; and has stock in Compliment Corporation. P.A. has received grants/contracts from Leukemia and Lymphoma Society and Kite/Gilead; reports consultancy for/honoraria from Merck, BMS, Pfizer, Affimed, Adaptive, Infinity, ADC Therapeutics, Celgene, MorphoSys, Daiichi Sankyo, Miltenyi, Tessa, Genmab, C4, Enterome, Regeneron, Epizyme, AstraZeneca, Genentech/Roche, Xencor, Foresight, and ATB Therapeutics; reports travel support from BMS and Genmab; and has participated on an advisory board for ADC and Merck. S.S.N. received research support from Kite/Gilead, BMS, Allogene, Precision Biosciences, Adicet Bio, Sana Biotechnology, and Cargo Therapeutics; served as advisory board member/consultant for Kite/Gilead, Merck, Sellas Life Sciences, Athenex, Allogene, Incyte, Adicet Bio, BMS, bluebird bio, Fosun Kite, Sana Biotechnology, Caribou, Astellas Pharma, MorphoSys, Janssen, Chimagen, ImmunoACT, Orna Therapeutics, Takeda, Synthekine, CARsgen, Appia Bio, and GlaxoSmithKline; has stock options in Longbow Immunotherapy Inc; and has intellectual property related to cell therapy. N.L.B. has received research funding from ADC Therapeutics, Autolus, BMS/Celgene, Forty-Seven, Gilead/Kite Pharma, Janssen, Merck, Millennium, Pharmacyclincs, Roche/Genentech, and Seagen; and has served on the advisory board at ADC Therapeutics, Foresight Diagnostics, Kite, Roche/Genentech, and Seattle Genetics. S.E.S. has received research support from Celgene/BMS, Merck, Janssen, Acerta Pharma, Profound Bio, Shrodinger, Genmab, Gilead Sciences, Incyte, Genentech, and BeiGene; and reports consultancy with/honoraria from ADC Therapeutics, BeiGene, Janssen, and AbbVie. J.K. has received research support from Roche, AstraZeneca, and Merck; and reports consulting for/honoraria from AbbVie, BMS, Gilead, Merck, Roche, Seattle Genetics, Amgen, AstraZeneca, Incyte, Janssen, Karyopharm, Merck, Novartis, and Pfizer. K.J.S. has received research support from BMS and Roche; and reports consultancy with/honoraria from Seagen, AbbVie, Janssen, and Merck. J.P.L. has received research support from the National Institutes of Health/National Cancer Institute, Leukemia and Lymphoma Society, Genentech Foundation, Lymphoma Research Foundation, Follicular Lymphoma Foundation, Epizyme, and Janssen; and reports consulting for/honoraria from AbbVie, Astellas, AstraZeneca, Bayer, BeiGene, BMS, Calithera, Constellation, Eisai, Lilly, Epizyme, Genmab, Grail, Incyte, Janssen, Karyopharm, Merck, Mustang Bio, Novartis, Pfizer, Roche/Genentech, Seattle Genetics, Second Genome, Sutro, Caribou Biosciences, and Treeline Biosciences. A.B.G., N.A., S.D., S.P.B, R.S., J.Q.W., and S.W. are employees of BMS. D.E.G. declares no competing financial interests.
Correspondence: Ajay K. Gopal, University of Washington/Fred Hutchinson Cancer Center, 825 Eastlake Ave E, Seattle, WA 98109; email: agopal@uw.edu; and Philippe Armand, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; email: philippe_armand@dfci.harvard.edu.
References
Author notes
A.K.G. and P.A. contributed equally to this study.
The Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
The full-text version of this article contains a data supplement.