Key Points
p-S6 is particularly overexpressed in Myc/Bcl-2 DE DLBCL, indicating poor prognosis of patients with DLBCL.
The inhibition of p-S6 in fibroblastic cells suppressed DLBCL cell proliferation and exhibited synergistic effects with chemotherapy.
Visual Abstract
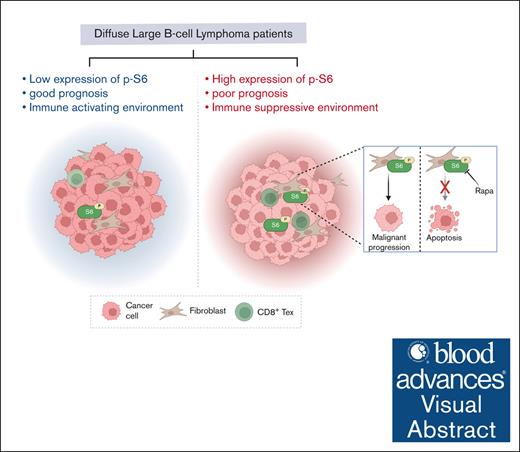
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous disease characterized by distinct morphological, genetic, and clinical features. DLBCL with Myc/Bcl-2 double expression (DE) has been positively correlated with poor prognosis and a low response rate to chemotherapy. However, molecular mechanisms underlying the malignant progression of DE DLBCL and the tumor microenvironment of DE DLBCL have not been fully elucidated. In this study, we assessed protein expression in samples from 18 patients with DLBCL using imaging mass cytometry with a panel of 26 metal-tagged antibodies, identifying 8 cell types within the tumor microenvironment. Although the ratio of immune cells did not significantly differ between DE and non-DE tissues, a strong interaction between fibroblasts and exhausted CD8+ T cells was specifically observed in DE tissues. By comparing protein expression ratios between DE and non-DE tissues, we found that p-S6 levels were significantly higher in B cells and fibroblasts of DE samples than non-DE samples. In our retrospective study, p-S6 independently predicted an inferior prognosis in patients with DLBCL (n = 71; hazard ratio, 5.758; 95% confidence interval, 1.297-25.558; P = .021). In addition, coculture with fibroblasts accelerated the in vitro and in vivo growth of DLBCL cells, and enrichment of the mammalian target of rapamycin/S6 pathways was identified using bulk RNA sequencing. Conversely, specific inhibition of p-S6 suppressed DLBCL cell proliferation and exhibited synergistic effects with chemotherapy in vivo. In summary, our findings elucidated the specific tumor microenvironment in DE DLBCL tissues and identified p-S6 as a promising therapeutic target to enhance the efficacy of chemotherapy.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common form of non-Hodgkin lymphoma, accounting for ∼30% to 40% of all adult cases. DLBCL is remarkably heterogeneous on both clinical and molecular levels, characterized by diverse biological features and varying responses to treatment.1,2 One of the key molecular distinctions in DLBCL lies in its classification into germinal center B-cell and activated B-cell (ABC) subtypes, each linked to different signaling pathways and clinical outcomes. More recently, an additional layer of complexity has emerged in the form of Myc/Bcl-2 protein double-expression (DE) DLBCL, which has been found to be associated with a particularly aggressive disease course.3,4 Beyond intrinsic tumor cell characteristics, the tumor microenvironment (TME) plays a critical role in shaping lymphoma behavior and treatment response. Within the TME, immune cells such as T cells and macrophages exert both pro- and antitumor influences. Recently, mounting evidence suggest that cross talk between lymphoma cells and fibroblasts can enhance tumor survival, promote immune evasion, and dampen therapy efficacy.5,6
Patients with DE DLBCL often have inferior clinical outcomes compared with those with non-DE DLBCL, probably owing to enhanced antiapoptotic signaling and activation of survival pathways. After standard chemotherapy treatment with R-CHOP (rituximab, cyclophosphamide [CTX], doxorubicin [DOX], Oncovin [vincristine [VCR]], and prednisone), patients with DE DLBCL typically face aggressive disease progression and poor prognosis.7 Approximately 40% of patients with DE DLBCL are resistant to standard chemotherapy and will eventually progress into relapsed diseases.8,9 Although clinical trials have shown promise with therapeutic regimens such as anti-CD20–based immunotherapy combined with Bcl-2 inhibition, it remains crucial to explore the TME features in DE DLBCL tissues and identify the molecular determinants of prognosis and malignancy.10,11 Recently, data suggest that cross talk between lymphoma cells and fibroblasts can enhance tumor survival, promote immune evasion, and dampen therapy efficacy.12 Fibroblasts may secrete soluble mediators that stimulate lymphoma cell proliferation or support the expansion of immunosuppressive macrophages.13 Meanwhile, T cells can shift toward an exhausted phenotype in regions enriched with fibroblasts, weakening the host antitumor response.14 These complex cellular interactions require comprehensive investigations to reveal microenvironmental features on a cellular level.
Given that protein expression directly influences cell fate, we aimed to examine the protein expression landscape in tissue samples from patients with DE and non-DE DLBCL, rather than focusing on the transcriptional level.15,16 Imaging mass cytometry (IMC) enables simultaneous in situ protein analysis using metal-tagged antibodies.17 These markers, including CD4, CD8, CD20, CD68, CD163, CD223, CD274, CD366, and ɑ-SMA, help identify various cell types such as B cells, T cells, macrophages, and fibroblasts.18,19 In this study, we deciphered the TME composition in DE DLBCL, highlighting the potential role of fibroblasts in promoting tumor growth. In addition, p-S6 was significantly overexpressed in B cells and fibroblasts of DE DLBCL tissues and was identified as an independent prognostic factor associated with poor prognosis in patients with DLBCL. Our in vitro and in vivo experiments underscored the significance of the mammalian target of rapamycin (mTOR)/S6 pathway through coculture of fibroblasts and DLBCL cells, suggesting the potential value of p-S6 as a therapeutic target in DLBCL.
Materials and methods
Patient samples
The study followed the Declaration of Helsinki principles and was approved by the medical ethics committee of the Hangzhou First People’s Hospital, Westlake University, for research purposes. Biopsy specimens of patients with DLBCL were obtained from Hangzhou First People’s Hospital, Westlake University, and the First Affiliated Hospital of Zhejiang University. Tissues were acquired by regular biopsy or endoscopic ultrasound fine needle. The expression of Myc/Bcl-2 in tissue sections of 71 patients with DLBCL was based on routine clinical pathological assessments.
Hematoxylin and eosin (H&E) stain was performed and examined with an optical microscope (Leica DMi8, Wetzlar, Germany) by a pathological expert to recognize tumor areas. Three regions of interest (ROIs; 1 mm2 per area) for each formalin-fixed paraffin-embedded (FFPE) sample were acquired and scanned by IMC.
Antibody panel, hyperion tissue scan, and data acquisition
FFPE tissues from patients with DLBCL by biopsies were cut into 4 μm sections followed by heating at 68°C for 1 hour. Dewaxing in xylene was performed twice for 10 minutes each. Sections were rehydrated sequentially in 95%, 85%, and 75% ethanol for 5 minutes each, followed by 100°C heat-mediated antigen retrieval for 30 minutes. After cooling naturally, sections were washed twice for 5 minutes each with phosphate-buffered saline with Tween (PBS-TB, PBS with 0.5% Tween-20; catalog no. 93773, Sigma-Aldrich) and 1% bovine serum albumin (catalog no. SRE0098, Sigma-Aldrich). Then, sections were blocked with SuperBlock (catalog no. 37515, Thermo Fisher Scientific) for 30 minutes. After another 3 PBS-TB washes, sections were incubated with an antibody cocktail at 4°C overnight. The antibodies were mostly labeled with metals using the Maxpar X8 Antibody Labeling Kit (Fluidigm). Briefly, an X8 polymer was linked to the reduced antibody, and a specific metal was loaded onto the polymer. The final antibody panel is presented in supplemental Table 1. After antibody incubation overnight at 4°C, 3 additional PBS-TB washes were performed. The sections were incubated with a 1.25 μM Intercalator-Ir solution (catalog no. 201192B, Fluidigm) in PBS-TB for 30 minutes at room temperature to label nuclei, followed by 2 PBS-TB washes and 1 final double-distilled water wash. An IMC (Fluidigm, Hyperion) was used to scan the prepared sections to generate the multiplexed images.20,21
Univariate and multivariate analysis
The R package “survival” (version 3.2-10) was used to conduct survival and Cox analyses. In the univariate analysis, all variables with P value <.1 were included in the multivariate analysis.
Analysis of DEGs from RNA sequencing data
Quality-trimmed FASTQ files of RNA sequencing data were aligned to the mouse reference genome GRCm39 using HISAT2 (version 2.2.1). We used featureCounts to generate raw gene expression counts for each sample. Subsequently, GFOLD (version 1.1.4) was used to conduct differential expression analysis, identifying differentially expressed genes (DEGs) based on non-0 GFOLD values. mTOR pathway–related genes were retrieved from the Molecular Signatures Database. Gene Ontology (GO) enrichment analysis for DEGs was performed using the R package clusterProfiler (version 4.12.2), with pathways exhibiting P value <.05 considered significantly enriched. Dotplot and centplot were plotted by the R package enrichplot (version 1.24.2).
Extraction of mouse primary splenic FRCs and transwell coculture
Freshly extracted spleen from C57BL/6J mice was washed twice with sterile PBS at 4°C and cut into pieces in a sterile penicillin bottle. Notably, 5 to 10 mL of digestion solution (containing 0.2 mg/mL collagenase P) was added into the bottle and allowed for digestion at 37°C for 1 hour. After digestion, the supernatant was collected and mixed with complete medium (Dulbecco modified Eagle medium containing 10% fetal bovine serum and 1% penicillin streptomycin, same hereafter) at a 1:1 ratio. After centrifuging at 300g for 5 minutes at 4°C, the supernatant was discarded, and the cell pellet was obtained. Cells were resuspended in complete medium and cultured at 37°C with 5% CO2. After replacement of fresh complete medium, mouse splenic reticular fibroblasts were collected using trypsin, and their phenotype was determined using flow cytometry as indicated by specific marker expression (PDPN+, CD31–, and CD45–).
The transwell coculture system was used to study the effect of fibroblastic reticular cells (FRCs) on DLBCL cell proliferation. The number of 1 × 105 DLBCL cells was placed in the upper insert (6.5-mm diameter with polycarbonate membrane filters containing 0.4-μm pores; Corning Inc, Corning, NY), and FRC cells were seeded at a 1:1 ratio onto the lower chambers of transwell coculture system. After coculture at 37°C incubator for indicated times, DLBCL cells from the upper insert of transwell coculture system were collected and placed in 15 mL centrifuge tubes. The cells were then centrifuged, resuspended, and counted using the Countstar cell counter (Shanghai Ruiyu Biotechnology Co, Ltd). Data collected over 3 days were analyzed using GraphPad Prism 8 for experimental results and graphing.
Statistical analysis
The results are expressed as the mean ± standard deviation of at least 3 independent experiments. Differences between means were analyzed using Student t test and were considered statistically significant when P value <.05. Statistical analyses and data visualization were performed using IBM SPSS version 22.0 (IBM SPSS, Inc, Chicago, IL) and GraphPad Prism version 6.01 (GraphPad Software Inc, San Diego, CA).
Results
IMC analysis of DLBCL tissue sections
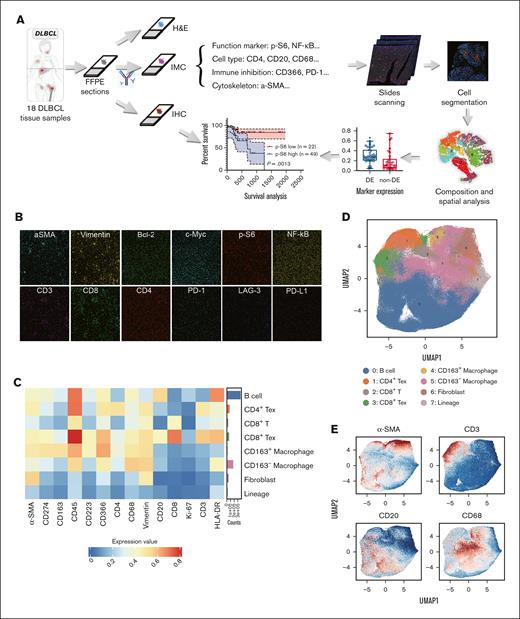
Tissue sections from 18 patients with DLBCL were obtained for the IMC assay. IMC data were collected from tissue sections adjacent to those used for immunohistochemistry (IHC) and H&E staining. H&E staining was used to identify tumoral areas (supplemental Figure 1), and at least 3 distinct ROIs of 1 mm2 in the tumoral areas of each section were selected for IMC analysis. A panel of 26 metal-tagged antibodies (supplemental Table 1) was used to examine a total of 824 243 cells. A schematic overview of this workflow is presented in Figure 1A.
IMC pipeline based on the 26-marker panel. (A) The workflow of IMC. Continuous tissue sections of 18 patients with DLBCL were analyzed by IMC, H&E stain, and IHC. Protein expression of functional markers analyzed by IHC was to confirm the results from IMC. Patient prognosis based on differential expression of functional markers was retrospectively studied later in this study. (B) Representative IMC images showed the distribution of various markers. Scale bar, 200 μm. (C) Heat map of cellular phenotypes. A heat map represents major cell types identified within the TME. (D) UMAP plots were based on the single-cell data extracted from IMC images; 8 clusters from samples collected from 18 patients with DLBCL were defined according to their marker expression. (E) Representative images showed the distribution of cell type markers in UMAP plots. UMAP, uniform manifold approximation and projection.
IMC pipeline based on the 26-marker panel. (A) The workflow of IMC. Continuous tissue sections of 18 patients with DLBCL were analyzed by IMC, H&E stain, and IHC. Protein expression of functional markers analyzed by IHC was to confirm the results from IMC. Patient prognosis based on differential expression of functional markers was retrospectively studied later in this study. (B) Representative IMC images showed the distribution of various markers. Scale bar, 200 μm. (C) Heat map of cellular phenotypes. A heat map represents major cell types identified within the TME. (D) UMAP plots were based on the single-cell data extracted from IMC images; 8 clusters from samples collected from 18 patients with DLBCL were defined according to their marker expression. (E) Representative images showed the distribution of cell type markers in UMAP plots. UMAP, uniform manifold approximation and projection.
Initial IMC images were visualized as pseudocolor representations, and representative images demonstrated the spatial distribution of various markers within a single ROI (Figure 1B). Eight major cell types were identified, including CD20+ B cells, exhausted CD4+/CD366+ T (CD4+ Tex) cells, CD8+ T cells, CD8+/CD274+/CD223+/CD366+ exhausted T (CD8+ Tex) cells, CD68+/CD163+ macrophages (CD163+ macrophages), CD68+/CD163– macrophages (CD163– macrophages), α-SMA+/CD20– fibroblasts, and lineage-negative cells, as depicted in Figure 1C-D.
Similar to findings reported in other studies, costaining of markers such as CD3 and CD20 (Figure 1E) was observed in some regions, which was not biologically anticipated.22 This phenomenon may be attributed to the limited spatial resolution of IMC technology, which is unable to accurately distinguish between neighboring cells that are extremely close to each other, either horizontally or vertically. Furthermore, this overlapping of markers may suggest close cellular interactions within the DLBCL microenvironment, particularly between exhausted T and B cells, potentially influencing tumor behavior and immune responses.23
TME and cell type interaction in DLBCL
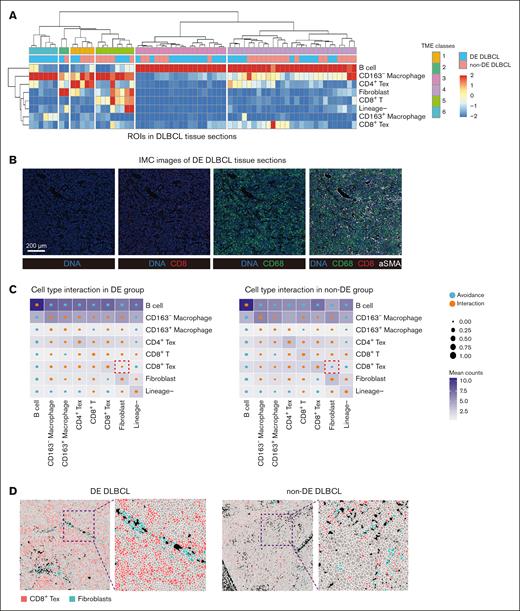
Eighteen DLBCL samples were categorized into 2 groups based on their Myc/Bcl-2 expression status: DE (DE, n = 10) and non-DE (n = 8). Comparing the proportions of various cell types between DE and non-DE samples, we observed no significant differences in immune cells, including B cells, CD8+ T cells, CD8+ Tex cells, and macrophages (supplemental Figure 2). To explore the composition of the microenvironment in DE DLBCL samples and understand how TME organization differs between DE and non-DE samples, we performed a broad classification based on the densities of major immune cell types and identified common TME archetypes in DLBCL.24 Through unsupervised hierarchical clustering, 6 common TME classes were defined across all samples (Figure 2A). We noted that B cells were largely enriched in classes 3 and 4, whereas fibroblasts were mostly found in classes 2 and 5, together with the distribution of M1 (CD163–) macrophages and exhausted T cells. In addition, a unique enrichment of M2 (CD163+) macrophages in class 6 was found in DE DLBCL samples, indicating an unfavorable immune environment in DE DLBCL. Notably, fibroblasts were clustered with macrophages and CD8+ T/CD8+ Tex cells in class 5, suggesting a potential role of fibroblasts in modulating immune responses.25 Representative IMC images were shown to elaborate the distribution of different cell types in DLBCL tissue sections (Figure 2B).
TME classes and cell type interaction differ in DE and non-DE DLBCL samples. (A) Heat map showing the classes of TME classes across samples. Subset frequency was normalized to total cells and row scaled by z score. (B) Representative images showed the expression of indicated markers in the scanning area. Scale bar, 200 μm. (C) Circles indicating patterns of cell-cell interactions/avoidance for DE (n = 10) and non-DE (n = 8). The circle size shows the percentage of ROIs with significant interactions/avoidance determined by the permutation test. Rows represent the cell type of interest (center cell), and columns represent other cell types surrounding the interest cell type (neighboring cell). Colors in the heat map indicate the Pearson correlation between cell types across all ROIs in DE and non-DE, respectively. (D) Representative images showed the distribution of CD8+ Tex cells and fibroblasts in DE and non-DE DLBCL tissues. FFPE, formalin-fixed paraffin-embedded.
TME classes and cell type interaction differ in DE and non-DE DLBCL samples. (A) Heat map showing the classes of TME classes across samples. Subset frequency was normalized to total cells and row scaled by z score. (B) Representative images showed the expression of indicated markers in the scanning area. Scale bar, 200 μm. (C) Circles indicating patterns of cell-cell interactions/avoidance for DE (n = 10) and non-DE (n = 8). The circle size shows the percentage of ROIs with significant interactions/avoidance determined by the permutation test. Rows represent the cell type of interest (center cell), and columns represent other cell types surrounding the interest cell type (neighboring cell). Colors in the heat map indicate the Pearson correlation between cell types across all ROIs in DE and non-DE, respectively. (D) Representative images showed the distribution of CD8+ Tex cells and fibroblasts in DE and non-DE DLBCL tissues. FFPE, formalin-fixed paraffin-embedded.
To explore cell type interactions, we performed regional correlation analysis to investigate the potential spatial co-occurrence patterns of different cells and identified statistically significant interaction or avoidance pairs within cellular neighborhoods across DE and non-DE samples (Figure 2C). We found that fibroblasts frequently interacted with CD8+ Tex cells in DE DLBCL samples, while showing avoidance of CD8+ Tex cells in non-DE DLBCL samples. Although the quantitative analysis showed no significant difference in cell type interactions between DE and non-DE samples, the IMC images indicated a closer interaction of CD8+ Tex cells and fibroblasts in DE DLBCL rather than non-DE DLBCL tissues (Figure 2D). These results suggested that fibroblasts probably facilitated a suppressed immune milieu in DE DLBCL tissues.
Differential marker expression reveals p-S6 as an independent prognosis marker
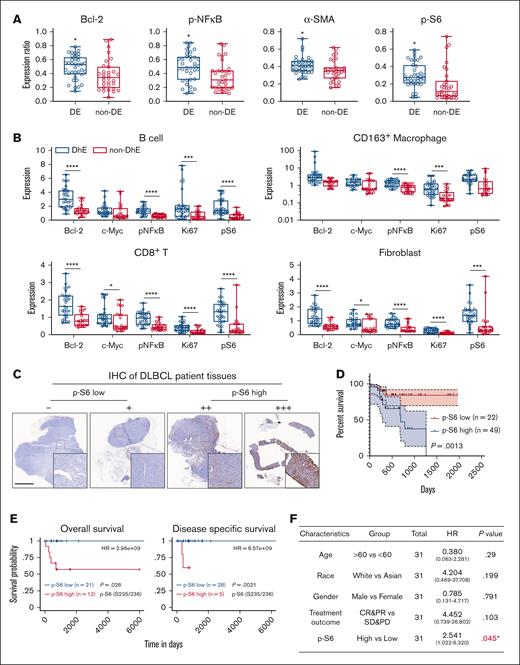
To investigate the levels of functional markers in DE and non-DE DLBCL samples, a comprehensive statistical analysis was conducted. The results showed an overabundant expression of Bcl-2, pNF-κB, α-SMA, and p-S6 in DE DLBCL samples compared with non-DE samples (Figure 3A). It was anticipated that Bcl-2 and pNF-κB would be significantly overexpressed in DE DLBCL, given that the activation of NF-κB is crucial in the pathogenesis of ABC-like DLBCL, a subtype associated with poor prognosis for patients with DLBCL.26,27 However, the roles of α-SMA and p-S6 in DLBCL prognosis or chemotherapy response remain rarely explored. The expression of α-SMA, a hallmark of mature fibroblasts, provides the structural foundation for tissues and organs, but recent studies have revealed that α-SMA+ cancer-associated fibroblasts contribute to the creation of an immunosuppressive microenvironment, indicating the immunosuppressive role of fibroblasts in addition to structural support.28 In contrast, aberrant activation of p-S6 has been less studied in DLBCL. Surprisingly, the levels of p-S6 were found to be significantly higher in B cells, macrophages, CD8+ T cells, and fibroblasts in DE DLBCL samples than non-DE samples (Figure 3B). This unexpected finding prompted us to investigate the potential link between p-S6 expression and DLBCL malignancy.
Differential protein expression in DE and non-DE DLBCL samples. (A) Bar plots showing the statistical analysis of indicated protein expression in DE and non-DE DLBCL samples. (B) The differential expression of indicated functional markers in various cell types between DE and non-DE DLBCL samples. (C) IHC was performed to validate the expression of p-S6 in 18 DLBCL specimens, and 4 representative images of p-S6 staining were shown to validate IHC staining scores. (D) Survival analysis of 71 patients with DLBCL in our respective study was performed using GraphPad Prism software (version 8). (E) Overall survival (OS) and disease-specific survival analysis of p-S6 (235/236). Protein arrays data were performed on TRGAted (https://nborcherding.shinyapps.io/TRGAted/). Grouping by “auto select best cutoff.” (F) Univariate analysis of The Cancer Genome Atlas database. HR >1 is poorly prognostic; HR <1 indicated improved OS and any characteristic crossing the line at 1 is not significant. CR, complete response; HR, hazard ratio; PD, progressive disease; PR, partial response; SD, stable disease. ∗p < .05; ∗∗∗p < .001; ∗∗∗∗p < .0001.
Differential protein expression in DE and non-DE DLBCL samples. (A) Bar plots showing the statistical analysis of indicated protein expression in DE and non-DE DLBCL samples. (B) The differential expression of indicated functional markers in various cell types between DE and non-DE DLBCL samples. (C) IHC was performed to validate the expression of p-S6 in 18 DLBCL specimens, and 4 representative images of p-S6 staining were shown to validate IHC staining scores. (D) Survival analysis of 71 patients with DLBCL in our respective study was performed using GraphPad Prism software (version 8). (E) Overall survival (OS) and disease-specific survival analysis of p-S6 (235/236). Protein arrays data were performed on TRGAted (https://nborcherding.shinyapps.io/TRGAted/). Grouping by “auto select best cutoff.” (F) Univariate analysis of The Cancer Genome Atlas database. HR >1 is poorly prognostic; HR <1 indicated improved OS and any characteristic crossing the line at 1 is not significant. CR, complete response; HR, hazard ratio; PD, progressive disease; PR, partial response; SD, stable disease. ∗p < .05; ∗∗∗p < .001; ∗∗∗∗p < .0001.
We first validated the expression of p-S6 in consecutive tissue sections used for IMC using IHC (supplemental Figure 3), and the results showed that p-S6 sporadically distributed across DE DLBCL tissues (Figure 3C), which were consistent with IMC images. To examine the clinical relevance of p-S6, baseline demographics of these 18 patients and another 53 patients are presented in Table 1 and supplemental Figure 4. We observed that, after 6 cycles of standard chemotherapy, patients with low levels of p-S6 exhibited higher response rates than those with elevated levels of p-S6 (supplemental Figure 5A). We also examined the expression of p-S6 by IHC in additional samples from53 patients DLBCL (supplemental Figure 3) and combined the prognosis data with 18 patients. Among the total of 71 patients, p-S6 displayed as an independent risk factor for DLBCL (hazard ratio, 5.758; 95% confidence interval, 1.297-25.558; P = .021) in the multivariate analysis (Table 2). Meanwhile, the univariate Cox analysis demonstrated p-S6 as a prognostic factor (P = .0013) in 71 patients with DLBCL (Figure 3D) and also in patients with DE DLBCL (supplemental Figure 6) estimated by probability of survival. Analysis using the TRGAted database showed that high p-S6 expression was significantly associated with shorter overall survival and disease-specific survival than patients with low p-S6 expression (Figure 3E; supplemental Figure 5B). In addition, we used the “median-based” method to validate that high p-S6 expression is associated with poor prognosis in patients with DLBCL (supplemental Figure 7). It should be noted that although DE is generally associated with a poorer prognosis in patients with DLBCL, this trend was not observed in our multivariate analysis, likely owing to the limited sample size of the study.
Baseline demographics of 18 patients with DLBCL undergoing IMC assay
| Specimen no. . | Sex . | Age . | ECOG grade . | Ann Arbor stage . | Secondary lesions . | Molecular subtype . | Ki-67 (%) . | Chemotherapy regimens . | Response assessment . | p-S6 expression by IHC . | OS∗ (mo) . | Protein expression of Myc/Bcl-2 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16-6625 | F | 57 | 0 | IV A | None | Non-GCB | 80-90 | R-CHOP + E-CHOP | CR | Weak | 50 | DE |
| 16-40368 | F | 56 | 0 | IV A | Spleen, marrow | Non-GCB | 70 | R-CHOP | CR | Moderate | 49 | DE |
| 16-41410 | M | 81 | 3 | IV B | None | GCB | 10-20 | R-GDP | PR | Weak | 7 | Non-DE |
| 17-16583 | M | 48 | 0 | I E | Stomach | Non-GCB | 90 | R-CHOP | CR | Strong | 56 | Non-DE |
| 17-28349 | M | 63 | 1 | IV B | Nasal cavity + gut | Unknown | 80 | R-CHOP + R-EPOCH | PR | Weak | 7† | DE |
| 17-36374 | M | 52 | 0 | II A | Tonsil | Non-GCB | 60-70 | R-CHOP + R-EPOCH | PR | Weak | 22 | DE |
| 17-39665 | F | 82 | 2 | I E | Stomach | Non-GCB | 90 | R-GemoX | CR | Weak | 53 | Non-DE |
| 19-11239 | F | 64 | 0 | IV A | Stomach | GCB | Unknown | CHOP + CTX/radiotherapy | PR | Strong | 51 | DE |
| 19-20798 | F | 40 | 1 | IV B | Lung + pelvic + mediastinum | GCB | 80-90 | R-CHOP | PD | Strong | 9† | DE |
| 19-20900 | F | 81 | 0 | I E | Stomach | GCB | 70 | None (surgical resection) | CR | Negative | 32 | Non-DE |
| 19-24750 | M | 37 | 0 | I E | Thymus gland | Non-GCB | 30 | None (surgical resection) | CR | Negative | 27 | Non-DE |
| 19-35522 | M | 78 | 1 | IV B | Marrow | GCB | 30-40 | R-CHOP | SD | Weak | 23 | DE |
| 19-37115 | F | 72 | 2 | IS B | Spleen | Non-GCB | 80-90 | R-CHOP | PR | Moderate | 42 | Non-DE |
| 19-37398 | F | 65 | 0 | III E A | Tonsil | GCB | 60 | R-CHOP | CR | Weak | 29 | Non-DE |
| 19-51710 | F | 65 | 0 | I A | None | Unknown | 90 | E-POCH | CR | Weak | 22 | DE |
| 19-66735 | M | 83 | 0 | 1E | Stomach | GCB | 60 | R-CHOP | CR | Weak | 29 | Non-DE |
| 20-10803 | M | 58 | 1 | Unknown | Parotid gland | Unknown | 80-90 | R-CHOP | SD | Moderate | 12 | DE |
| 20-20699 | F | 78 | 0 | IV B | Marrow | Non-GCB | 80 | R-CHOP | PR | Moderate | 1† | DE |
| Specimen no. . | Sex . | Age . | ECOG grade . | Ann Arbor stage . | Secondary lesions . | Molecular subtype . | Ki-67 (%) . | Chemotherapy regimens . | Response assessment . | p-S6 expression by IHC . | OS∗ (mo) . | Protein expression of Myc/Bcl-2 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16-6625 | F | 57 | 0 | IV A | None | Non-GCB | 80-90 | R-CHOP + E-CHOP | CR | Weak | 50 | DE |
| 16-40368 | F | 56 | 0 | IV A | Spleen, marrow | Non-GCB | 70 | R-CHOP | CR | Moderate | 49 | DE |
| 16-41410 | M | 81 | 3 | IV B | None | GCB | 10-20 | R-GDP | PR | Weak | 7 | Non-DE |
| 17-16583 | M | 48 | 0 | I E | Stomach | Non-GCB | 90 | R-CHOP | CR | Strong | 56 | Non-DE |
| 17-28349 | M | 63 | 1 | IV B | Nasal cavity + gut | Unknown | 80 | R-CHOP + R-EPOCH | PR | Weak | 7† | DE |
| 17-36374 | M | 52 | 0 | II A | Tonsil | Non-GCB | 60-70 | R-CHOP + R-EPOCH | PR | Weak | 22 | DE |
| 17-39665 | F | 82 | 2 | I E | Stomach | Non-GCB | 90 | R-GemoX | CR | Weak | 53 | Non-DE |
| 19-11239 | F | 64 | 0 | IV A | Stomach | GCB | Unknown | CHOP + CTX/radiotherapy | PR | Strong | 51 | DE |
| 19-20798 | F | 40 | 1 | IV B | Lung + pelvic + mediastinum | GCB | 80-90 | R-CHOP | PD | Strong | 9† | DE |
| 19-20900 | F | 81 | 0 | I E | Stomach | GCB | 70 | None (surgical resection) | CR | Negative | 32 | Non-DE |
| 19-24750 | M | 37 | 0 | I E | Thymus gland | Non-GCB | 30 | None (surgical resection) | CR | Negative | 27 | Non-DE |
| 19-35522 | M | 78 | 1 | IV B | Marrow | GCB | 30-40 | R-CHOP | SD | Weak | 23 | DE |
| 19-37115 | F | 72 | 2 | IS B | Spleen | Non-GCB | 80-90 | R-CHOP | PR | Moderate | 42 | Non-DE |
| 19-37398 | F | 65 | 0 | III E A | Tonsil | GCB | 60 | R-CHOP | CR | Weak | 29 | Non-DE |
| 19-51710 | F | 65 | 0 | I A | None | Unknown | 90 | E-POCH | CR | Weak | 22 | DE |
| 19-66735 | M | 83 | 0 | 1E | Stomach | GCB | 60 | R-CHOP | CR | Weak | 29 | Non-DE |
| 20-10803 | M | 58 | 1 | Unknown | Parotid gland | Unknown | 80-90 | R-CHOP | SD | Moderate | 12 | DE |
| 20-20699 | F | 78 | 0 | IV B | Marrow | Non-GCB | 80 | R-CHOP | PR | Moderate | 1† | DE |
CR, complete response; E-CHOP, cyclophosphamide, DOX hydrochloride (hydroxydaunorubicin), VCR sulfate (Oncovin), prednisone, and etoposide phosphate; ECOG, Eastern Cooperative Oncology Group; F, female; GCB, germinal center B-cell; Ki-67, rates of Ki-67 positive cells; M, male; non-DE, do not exhibit simultaneous high expression of both Myc and Bcl-2; OS, overall survival; PD, progressive disease; PR, partial response; R-EPOCH, rituximab, etoposide phosphate, prednisone, VCR sulfate (Oncovin), CTX, and DOX hydrochloride; R-GemoX, gemcitabine-oxaliplatin plus rituximab; SD, stable disease.
OS data was updated on 31 December 2021.
Patient had died from the disease.
Univariate and multivariate analysis of patients with DLBCL
| Characteristics . | Total (n) . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) . | P value . | Hazard ratio (95% CI) . | P value . | ||
| Gender | 71 | ||||
| Male | 45 | Reference | |||
| Female | 26 | 0.723 (0.263-1.985) | .529 | ||
| Age | 71 | 1.045 (1.001-1.092) | .046 | 1.054 (1.008-1.103) | .022 |
| MYC/BCL2 expression | 71 | ||||
| Non-DE | 29 | Reference | |||
| DE | 42 | 1.659 (0.620-4.435) | .313 | ||
| p-S6 expression | 71 | ||||
| Low | 22 | Reference | |||
| High | 49 | 4.913 (1.118-21.585) | .035 | 5.758 (1.297-25.558) | .021 |
| Hans | 68 | ||||
| Non-GCB | 45 | Reference | |||
| GCB | 23 | 1.255 (0.454-3.469) | .662 | ||
| Ann Arbor stage | 71 | ||||
| Ⅰ | 10 | Reference | |||
| Ⅱ | 12 | 75 715 781.210 (0.000 to Inf) | .998 | ||
| Ⅲ | 9 | 203 509 622.443 (0.000 to Inf) | .998 | ||
| Ⅳ | 40 | 406 929 403.918 (0.000 to Inf) | .998 | ||
| Characteristics . | Total (n) . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) . | P value . | Hazard ratio (95% CI) . | P value . | ||
| Gender | 71 | ||||
| Male | 45 | Reference | |||
| Female | 26 | 0.723 (0.263-1.985) | .529 | ||
| Age | 71 | 1.045 (1.001-1.092) | .046 | 1.054 (1.008-1.103) | .022 |
| MYC/BCL2 expression | 71 | ||||
| Non-DE | 29 | Reference | |||
| DE | 42 | 1.659 (0.620-4.435) | .313 | ||
| p-S6 expression | 71 | ||||
| Low | 22 | Reference | |||
| High | 49 | 4.913 (1.118-21.585) | .035 | 5.758 (1.297-25.558) | .021 |
| Hans | 68 | ||||
| Non-GCB | 45 | Reference | |||
| GCB | 23 | 1.255 (0.454-3.469) | .662 | ||
| Ann Arbor stage | 71 | ||||
| Ⅰ | 10 | Reference | |||
| Ⅱ | 12 | 75 715 781.210 (0.000 to Inf) | .998 | ||
| Ⅲ | 9 | 203 509 622.443 (0.000 to Inf) | .998 | ||
| Ⅳ | 40 | 406 929 403.918 (0.000 to Inf) | .998 | ||
Bold: classification of patients with DLBCL; CI, confidence interval; Inf, infinity
The prognostic role of p-S6 was further supported by The Cancer Genome Atlas database analysis (hazard ratio, 2.541; 95% confidence interval, 1.022-6.320; P = .045; Figure 3F). These findings strongly suggested that the overexpression of p-S6 correlated with poor prognosis in patients with DLBCL. The aberrant activation of p-S6 across multiple cell types, including B cells and fibroblasts, underscored its potential as a key driver of DLBCL progression. As such, p-S6 may serve as both a biomarker for predicting patient outcomes and a potential therapeutic target in DLBCL treatment strategies.
Fibroblasts promoted DLBCL cell growth via the activation of mTOR/S6 pathway
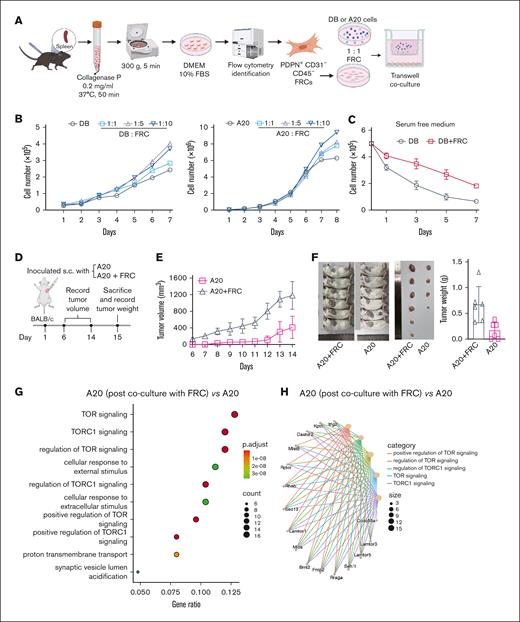
Fibroblasts localized inside lymph nodes, known as FRCs, are capable of maintaining a suppressive immune environment by impeding optimal T-cell migration and inhibiting the lytic function of CD8+ T cells,29 whereas this phenomenon is partially validated via IMC analysis in this study. However, the direct interaction between fibroblasts and DLBCL cells remains unclear. To explore the role of FRC in DLBCL, primary FRCs were isolated from mouse spleen via centrifugation and then cocultured with DLBCL cells in a transwell coculture system (Figure 4A). Upon coculturing FRCs with DLBCL cells at indicated ratios, we observed that FRCs significantly promoted the cell proliferation rate of both DB (human DLBCL cell line) and A20 (murine B-cell lymphoma) cells (Figure 4B). In a serum-free medium, DB cells in monoculture gradually died over time, whereas coculture with FRCs partially rescued these cells and delayed cell death (Figure 4C). In addition, FRCs promoted tumor growth in an in vivo model. A20 cells, with or without FRCs mixed at 1:1 ratio, were subcutaneously injected into BALB/c mice, and tumor volume was measured every day once tumors became palpable (Figure 4D). As illustrated in Figure 4E, A20 mixed with FRCs exhibited a marked increase in both tumor volume and tumor weight (Figure 4E-F). Notably, 2 mice injected solely with A20 cells did not develop tumors (Figure 4F).
Coculture with fibroblasts promoted cell proliferation and enhanced the mTOR/S6 pathway. (A) Primary fibroblasts were obtained from mice spleen via centrifugation. FRCs were cocultured with DLBCL cells in a transwell coculture system. (B) DB or A20 cells were cocultured with FRCs for indicated days at various ratios. The number of DB cells was recorded by cell counting. (C) In a serum-free medium, the number of DB cells, with or without the coculture with FRCs, was determined by cell counting. (D) A schematic illustration of animal models. (E) Tumor volume was measured every day once the tumors became palpable. (F) Mice were euthanized on day 15, and tumors were dissected and weighed. (G) A20 cells were cocultured with FRCs using the transwell coculture system for 24 hours before RNA sequencing (RNA-seq) sample preparation. GO enrichment for upregulated genes of “FRC cocultured with A20” vs A20. The yellow-green color scale corresponds to the enrichment significance of the genes. Dot size is proportional to the number of genes annotated to the corresponding categories. P values are calculated by Fisher exact test. (H) A20 cells were cocultured with FRCs using the transwell coculture system for 24 hours before RNA-seq sample preparation. Network of “FRC cocultured with A20” upregulated genes and enriched concepts. Different colors represent various categories, and the size of the dots corresponds to the number of genes linked to each category. DMEM, Dulbecco modified Eagle medium; FBS, fetal bovine serum.
Coculture with fibroblasts promoted cell proliferation and enhanced the mTOR/S6 pathway. (A) Primary fibroblasts were obtained from mice spleen via centrifugation. FRCs were cocultured with DLBCL cells in a transwell coculture system. (B) DB or A20 cells were cocultured with FRCs for indicated days at various ratios. The number of DB cells was recorded by cell counting. (C) In a serum-free medium, the number of DB cells, with or without the coculture with FRCs, was determined by cell counting. (D) A schematic illustration of animal models. (E) Tumor volume was measured every day once the tumors became palpable. (F) Mice were euthanized on day 15, and tumors were dissected and weighed. (G) A20 cells were cocultured with FRCs using the transwell coculture system for 24 hours before RNA sequencing (RNA-seq) sample preparation. GO enrichment for upregulated genes of “FRC cocultured with A20” vs A20. The yellow-green color scale corresponds to the enrichment significance of the genes. Dot size is proportional to the number of genes annotated to the corresponding categories. P values are calculated by Fisher exact test. (H) A20 cells were cocultured with FRCs using the transwell coculture system for 24 hours before RNA-seq sample preparation. Network of “FRC cocultured with A20” upregulated genes and enriched concepts. Different colors represent various categories, and the size of the dots corresponds to the number of genes linked to each category. DMEM, Dulbecco modified Eagle medium; FBS, fetal bovine serum.
Among the 353 mTOR pathway–related genes cataloged in the Molecular Signatures Database, our study identified 126 upregulated genes in the coculture of FRCs with A20 cells vs A20 cells alone. GO enrichment analysis revealed a significant enrichment of these genes in the biological processes “TOR signaling,” “TORC1 signaling,” and “regulation of TOR signaling” (P < .05; Figure 4G), indicating the activation of the mTOR pathway in the coculture condition. The comprehensive network illustrating the intricate relationships between these genes and the enriched biological concepts is depicted in Figure 4H.
Suppression of p-S6 delayed cell proliferation and sensitized chemotherapy
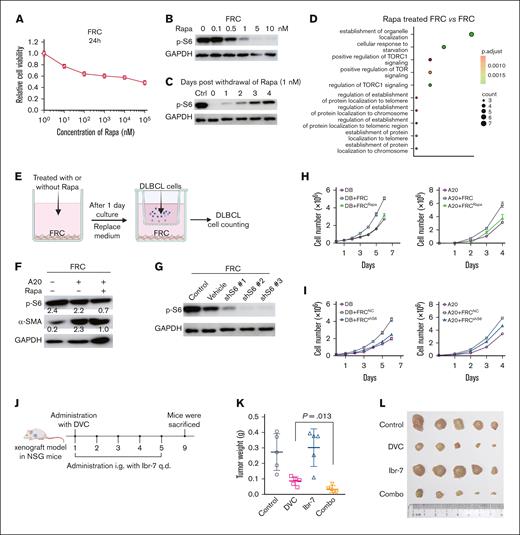
FRCs cocultured with A20 cells dramatically promoted the tumor growth of the A20 cells, which was probably attributed to the activation of the mTOR pathway. To investigate the impact of mTOR/S6 inhibition on the supportive role of FRCs in promoting DLBCL cell survival, we treated FRCs with rapamycin, a specific mTOR inhibitor. Treatment with rapamycin for 48 hours inhibited the viability of FRCs in a dose-dependent manner (Figure 5A). At a concentration of 1 nM, rapamycin sufficiently inhibited the activation of p-S6 (Figure 5B). To minimize potential cytotoxic effects, we used 1-nM rapamycin in subsequent experiments. After the withdrawal of 1-nM rapamycin treatment for 24 hours, p-S6 levels progressively recovered (Figure 5C). Gene expression analysis of rapamycin-treated FRCs revealed downregulation of 47 genes compared with untreated controls. Subsequent GO enrichment analysis indicated that these genes were significantly enriched in biological processes related to “establishment of organelle localization,” “cellular response to starvation,” and most notably “positive regulation of TORC1 signaling” (Figure 5D). The significant enrichment of genes associated with “positive regulation of TORC1 signaling” strongly suggests that mTOR signaling activity in FRCs is suppressed after rapamycin treatment.
Suppression of p-S6 inhibited DLBCL cell proliferation and sensitized chemotherapy. (A) Primary mice fibroblasts (FRCs) were treated with the indicated concentration of Rapa for 24 hours. Relative cell viability was determined by CCK-8 assay. (B) FRCs were treated with the indicated concentrations of Rapa for 24 hours. Cell lysates were collected and the expression of p-S6 was determined by western blotting. (C) FRCs were treated with 1 nM of Rapa for 24 hours, and were collected at 1 to 4 days after withdrawal of Rapa. The expression of p-S6 was determined by western blotting. (D) FRCs were treated with 1 nM of Rapa for 24 hours before RNA-seq sample preparation. GO enrichment for downregulated genes of “Rapa-treated FRC” vs FRC. The red-green color scale corresponds to the enrichment significance of the genes. Dot size is proportional to the number of genes annotated to the corresponding categories. P values are calculated by Fisher exact test. (E) A schematic diagram of FRCs treatment with Rapa and coculture with DLBCL cells. (F) FRC lysates were collected to determine the protein expression of p-S6 and α-SMA. (G) FRCs were treated with shS6 to knock down the level of p-S6. (H) FRCs were pretreated with Rapa to suppress the expression of p-S6 and then cocultured with DB or A20 cells. DB or A20 cell number was counted by Countstar. (I) FRCs were pretreated with shS6 to suppress the expression of p-S6 and then cocultured with DB or A20 cells. DB or A20 cell number was counted by Countstar. (J) A schematic diagram of in vivo antitumor study using DB xenograft mice. (K) Tumors were weighted after dissection on day 9. (L) Photos of tumors were taken accompanied by the scale. DVC, DOX+VCR+CTX; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Rapa, rapamycin.
Suppression of p-S6 inhibited DLBCL cell proliferation and sensitized chemotherapy. (A) Primary mice fibroblasts (FRCs) were treated with the indicated concentration of Rapa for 24 hours. Relative cell viability was determined by CCK-8 assay. (B) FRCs were treated with the indicated concentrations of Rapa for 24 hours. Cell lysates were collected and the expression of p-S6 was determined by western blotting. (C) FRCs were treated with 1 nM of Rapa for 24 hours, and were collected at 1 to 4 days after withdrawal of Rapa. The expression of p-S6 was determined by western blotting. (D) FRCs were treated with 1 nM of Rapa for 24 hours before RNA-seq sample preparation. GO enrichment for downregulated genes of “Rapa-treated FRC” vs FRC. The red-green color scale corresponds to the enrichment significance of the genes. Dot size is proportional to the number of genes annotated to the corresponding categories. P values are calculated by Fisher exact test. (E) A schematic diagram of FRCs treatment with Rapa and coculture with DLBCL cells. (F) FRC lysates were collected to determine the protein expression of p-S6 and α-SMA. (G) FRCs were treated with shS6 to knock down the level of p-S6. (H) FRCs were pretreated with Rapa to suppress the expression of p-S6 and then cocultured with DB or A20 cells. DB or A20 cell number was counted by Countstar. (I) FRCs were pretreated with shS6 to suppress the expression of p-S6 and then cocultured with DB or A20 cells. DB or A20 cell number was counted by Countstar. (J) A schematic diagram of in vivo antitumor study using DB xenograft mice. (K) Tumors were weighted after dissection on day 9. (L) Photos of tumors were taken accompanied by the scale. DVC, DOX+VCR+CTX; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Rapa, rapamycin.
We next used a transwell coculture system to study the effects of mTOR suppression on the supportive role of FRCs (Figure 5E). DB cell line was used in this study because it exhibited robust basal p-S6 expression (supplemental Figure 8). As shown in Figure 5F, FRCs cocultured with A20 cells exhibited enhanced levels of α-SMA, whereas treatment with rapamycin significantly reduced the activation of both p-S6 and α-SMA. Notably, rapamycin treatment markedly inhibited the growth of A20 and DB cells cocultured with FRCs (Figure 5G). A similar inhibitory effect on A20 and DB cell proliferation was observed with p-S6 knockdown via short hairpin RNA (Figure 5H-I).
The combination of DOX, VCR, and CTX represents the first-line chemotherapy regimen for DLBCL. We investigated whether p-S6 inhibition could potentiate the antitumor effects of this chemotherapy regimen. For this purpose, we used Ibr-7, a selective p-S6 inhibitor characterized in our previous study.30 When tumor volumes reached ∼200 mm3, mice were administered a single injection of DOX, VCR, and CTX, along with intragastric administration of Ibr-7 for 5 consecutive days (Figure 5J). Measurement of dissected tumor weights revealed that chemotherapy alone achieved a substantial tumor inhibition rate of 69%. Although Ibr-7 alone had minimal impact on tumor growth, its combination with chemotherapy further increased the tumor inhibition rate to 88% (Figure 5K-L). These results indicated that p-S6 suppression enhanced the efficacy of chemotherapy in DLBCL.
Discussion
DLBCL, especially with double high expression of Myc/Bcl-2 (DE DLBCL), is an aggressive and malignant disease that is at high risk of becoming relapsed and refractory, not only because the current standard treatments have achieved limited efficacy but also because the TME of DLBCL needs to be fully clarified. Herein, we capitalized on IMC to establish the protein expression pattern of DLBCL patient samples31,32. DE DLBCL displayed an abnormal interaction between fibroblasts and CD8+ Tex cells in comparison with non-DE DLBCL samples. Quantitative analysis of protein expression revealed an aberrant overexpression of p-S6 in B cells and fibroblasts, and the high level of p-S6 was positively correlated with the inferior prognosis of patients with DLBCL. Notably, fibroblasts promoted the tumor growth of DLBCL cells, whose gene expression was enriched in the mTOR pathway by GO enrichment analysis. In addition, the suppression of mTOR/S6 sabotaged the supportive activity of fibroblasts in the proliferation of DLBCL cells, thus enlightening a therapeutic role of p-S6 in DLBCL.
In DE DLBCL, a distinctive interaction between fibroblasts and CD8+ Tex cells emerges as a key feature of the TME.33 Unlike non-DE tissues, where fibroblast activity seems more passive, DE tissues exhibit active fibroblast involvement. This interaction may be mediated by direct cell-cell communication and the release of cytokines or growth factors, creating a microenvironment that sustains immune modulation.34 Fibroblasts, known for their role in wound healing and tissue remodeling, become activated in the TME, potentially adopting a cancer-associated fibroblast phenotype, which can alter immune cell function.35,36 These activated fibroblasts can suppress immune function via secreting immunosuppressive molecules such as transforming growth factor β and upregulate immune checkpoints (eg, programmed death-ligand 1 expression) in CD8+ Tex cells.37,38 This complex interplay suggests that fibroblasts in DE DLBCL could contribute to the immune evasion mechanisms used by malignant cells.
p-S6 is a downstream effector of the mTOR signaling pathway, which plays a pivotal role in the progression of DLBCL.39 Notably, p-S6 in B cells can also be activated by B-cell receptor signaling through the phosphoinositide 3-kinase (PI3K)/serine/threonine kinase (AKT)/mTOR pathway, which is known to be active in both germinal center B-cell and ABC subtypes of DLBCL. This activation underscores the convergence of multiple signaling pathways on the mTOR axis, driving tumor growth and survival across diverse molecular backgrounds.40,41 In DE DLBCL, the overexpression of MYC and Bcl-2 genes contributes to uncontrolled proliferation and resistance to apoptosis.42MYC is a well-known oncogene that promotes cell cycle progression and metabolism, whereas Bcl-2 inhibits apoptosis, allowing malignant cells to survive longer.10 The concomitant overexpression of these genes creates a synergistic effect that enhances tumor aggressiveness. The elevated p-S6 levels observed in our study suggested that the mTOR/S6 pathway is hyperactivated in DE DLBCL, further promoting oncogenic processes. In DLBCL, previous studies have reported that activation of the mTOR pathway is associated with poor prognosis and chemoresistance.43 Fibroblasts within the TME can influence cancer progression by secreting growth factors and cytokines that activate signaling pathways like mTOR in tumor cells.38 The strong interaction between fibroblasts and B cells in DE samples observed in our study probably facilitated the activation of the mTOR/S6 pathway, leading to increased p-S6 levels. This interaction could create a supportive niche for lymphoma cells, enhancing their growth and survival. In addition, we found that fibroblasts cocultured with BCL cells increased the expression of p-S6, along with a slightly enhanced expression of fibro-adipogenic progenitor (FAP) and vimentin, which indicates the activation of fibroblasts. Therefore, targeting the mTOR/S6 pathway presented a promising therapeutic strategy. Combining mTOR inhibitors with standard chemotherapy could potentially overcome chemoresistance in patients with DE DLBCL. Clinical trials are warranted to evaluate the efficacy of such combination therapies.
In conclusion, the significant increase of p-S6 levels in B cells and fibroblasts of DE DLBCL samples highlights the importance of the mTOR/S6 pathway in the pathogenesis of this lymphoma subtype. The role of p-S6 in promoting cell proliferation and survival pathways provides insight into the aggressive nature of DE DLBCL and offers a potential target for therapeutic intervention.
Acknowledgments
The authors thank Westlake Laboratory of Life Sciences and Biomedicine of Zhejiang Province, Westlake University, for their financial support on this project. This work was supported by Leading Talents of Health Commission Zhejiang Province, National Natural Science Foundation of China (82100200), Clinical Pharmacy of Hangzhou Medical Key Discipline (2021-21-16), and Zhejiang Provincial Medical and Health Technology Project (2021KY881).
Authorship
Contribution: B.Z. analyzed data and wrote the manuscript; H.T., X.T., and N.L. designed the experiments; Y.X., J.S., and Z.C. obtained patient information; J.W. and M.S. explored the online database; J.Z., Q.L. and Y.L. performed the immunohistochemistry; C.C. and J.F. obtained patient tissues; Y.Y. performed in vitro experiments; W.W. analyzed the tumoral area of formalin-fixed paraffin-embedded tissue sections; and S.Q. and Z.Z. performed the data analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hongyan Tong, Department of Hematology, Zhejiang Provincial Key Laboratory of Hematopoietic Malignancy, The First Affiliated Hospital, Cancer Center, Zhejiang University School of Medicine, #79 Qingchun Road, Hangzhou, China; email: tonghongyan@zju.edu.cn; Nengming Lin, Key Laboratory of Clinical Cancer Pharmacology and Toxicology Research of Zhejiang Province, Hangzhou First People's Hospital, Westlake University School of Medicine, #261 Huansha Road, Hangzhou 310006, China; email: lnm1013@zju.edu.cn; Xiangmin Tong, Department of Hematology, Hangzhou First People’s Hospital, Westlake University School of Medicine, #261 Huansha Road, Hangzhou, China; email: tongxiangmin@163.com; and Shenxian Qian, Department of Hematology, Hangzhou First People’s Hospital, Westlake University School of Medicine, #261 Huansha Road, Hangzhou 310006, China; email: sxqian@hotmail.com.
References
Author notes
B.Z., Y.X., M.S., J.S., and Y.L. contributed equally to this study.
Original data are available on request from the author, Bo Zhang (zhangbo@hospital.westlake.edu.cn).
The full-text version of this article contains a data supplement.