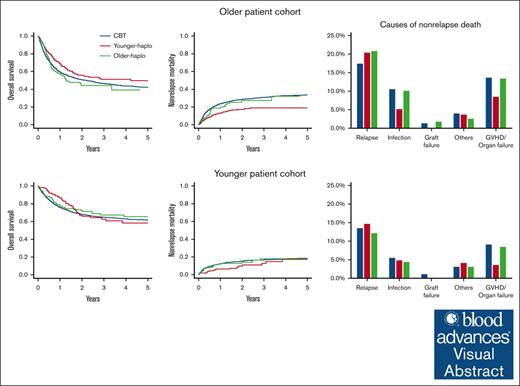
Key Points
HSCT from younger haplo donors provided better overall survival and lower nonrelapse mortality in older patients compared with CBT.
The finding highlights the importance of considering donor and patient age in transplantation strategies, with outcome varying by age group.
Visual Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) from HLA-matched donors is the gold standard. However, haploidentical stem cell transplantation using posttransplant cyclophosphamide (PTCY-haplo) and cord blood transplants (CBTs) are alternatives when HLA-matched donors are not available. Using Japanese registry data, we evaluated the impact of haploidentical donor age on posttransplant outcomes by comparing PTCY-haplo and CBT. We analyzed data for 5161 patients aged 16 to 70 years who received their first HSCT for acute leukemia, myelodysplastic syndrome, or chronic myeloid leukemia. Haploidentical donors were categorized as “younger” (aged <40 years) or “older” (aged ≥40 years), and the patients were divided into younger (aged <50 years) and older (aged ≥50 years) cohorts. In the older cohort, PTCY-haplo from younger donors had better overall survival (OS; 55.5% vs 50.8%, P = .006), lower nonrelapse mortality (NRM; 17.3% vs 28.6%, P < .001), and higher relapse rates (33.0% vs 24.9%, P = .017) than with CBT. PTCY-haplo from older donors had comparable OS (44.1% vs 50.8%, P = 1.00), NRM (27.3% vs 28.6%, P = 1.00), and relapse (29.2% vs 24.9%, P = .90) to that with CBT. In the younger cohort, PTCY-haplo from younger and older donors showed OS, NRM, and relapse comparable with CBT. In the older cohort, cumulative incidence of acute graft-versus-host disease (GVHD) was higher with CBT than with PTCY-haplo, regardless of donor age. However, in the younger cohort, acute GVHD was lower in PTCY-haplo from younger donors than with CBT. PTCY-haplo from younger donors to older patients offers better clinical outcomes than CBT.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially curative option for high-risk hematologic malignancies, with HSCT from a HLA-matched donor being the standard approach. However, for patients without an HLA-matched donor, haploidentical stem cell transplantation with posttransplant cyclophosphamide (PTCY-haplo) and cord blood transplantation (CBT) are well-established alternatives. These options provide life-saving treatment, along with rapid donor availability and flexibility in transplantation timing.
Several prospective and retrospective studies have compared PTCY-haplo with CBT as alternatives to HSCT.1-8 However, the findings were inconsistent, with some studies reporting comparable outcomes for PTCY-haplo and CBT,3-8 whereas others showed lower nonrelapse mortality (NRM) and improved overall survival (OS) with PTCY-haplo.1,2 These variations may be because of differences in disease types or transplantation methods across studies. Notably, donor age played a significant role in HSCT from haplo donors, as highlighted in the European Society for Blood and Marrow Transplantation guidelines.9 Additionally, multiple studies have reported lower NRM with HSCT from younger haplo donors.10-15
Haploidentical donor age may be a key factor when comparing PTCY-haplo and CBT; however, no study has specifically examined this comparison based on donor age differences. Therefore, this study aimed to determine the optimal alternative donor selection by analyzing posttransplant outcomes of PTYC-haplo, categorized by donor age, and CBT using data from the Japanese registry. Patients undergoing PTCY-haplo were classified into older and younger donor groups to assess the impact of donor age.
Patients and methods
Patient selection
Clinical data were collected by the Japanese Society for Transplantation and Cellular Therapy and the Japanese Data Center for Hematopoietic Cell Transplantation through the Transplant Registry Unified Management Program.16,17 The study included patients aged 16 to 70 years with acute myeloid leukemia (AML), acute lymphoblastic leukemia, myelodysplastic syndrome (MDS), or chronic myeloid leukemia (CML) who underwent initial PTCY-haplo using peripheral blood stem cells, or CBT, between January 2016 and December 2021. For eligible patients, data were collected on age, sex, HLA, donor source, disease status at HSCT, and survival status at the last follow-up. Haploidentical donors were defined as related donors mismatched at 2 to 3 HLA antigen levels for HLA-A, HLA-B, and HLA-DR. Patients who received in vivo T-cell depletion with antithymocyte globulin or alemtuzumab were excluded. CBT was defined as a single cord blood (CB) unit matched at a minimum of 4 antigen levels for HLA-A, HLA-B, and HLD-DR. This retrospective study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional review board at Jichi Medical University.
Definitions and end points
Donors were classified into younger and older groups, and patients were further categorized by age based on differences in OS.
The primary end point was OS, defined as the time from HSCT to death from any cause or the last follow-up for surviving patients. Several secondary end points were also analyzed. Relapse was defined as the recurrence of the underlying hematological disease, whereas NRM referred to death without relapse. Disease-free survival (DFS) was measured from HSCT to relapse, death, or last follow-up. Acute and chronic graft-versus-host disease (GVHD) were diagnosed and graded according to established criteria.18,19 GVHD-free, relapse-free survival (GRFS) was defined as the time from HSCT to the occurrence of any of the following events: grade 3/4 acute GVHD, chronic GVHD requiring systemic treatment, relapse, or death from any cause.20 The cause of death was determined based on the primary cause reported by the attending physicians.
Conditioning regimens were categorized as myeloablative conditioning (MAC) or reduced-intensity conditioning (RIC).21,22 High-risk diseases included acute leukemia beyond the second complete remission or in nonremission, as well as CML beyond the second chronic phase or in blast crisis. MDS with refractory anemia with 5% to 9% excess blasts, MDS with refractory anemia with 10% to 19% excess blasts, or MDS with excess blasts, based on the 2008 or 2016 World Health Organization classification,23,24 were also classified as high risk, whereas all other cases were considered low risk.
Statistical analysis
Patient characteristics were compared using the Fisher exact test for categorical variables and the Kruskal-Wallis test for continuous variables. OS was estimated using the Kaplan-Meier method with 95% confidence intervals (CIs), and comparisons were made using the log-rank test. The cumulative incidence method was used to assess GVHD, relapse, NRM, neutrophil engraftment, and platelet engraftment, accounting for competing risks. For GVHD, death or relapse without GVHD was considered a competing event. Relapse and NRM were treated as competing events for each other. The incidence of acute and chronic GVHD was analyzed in patients who survived without relapse for at least 60 and 100 days posttransplant, respectively. Multivariate analyses were conducted using a Cox proportional hazards model, with the hazard ratio for HCT adjusted for disease risk (high vs low), underlying disease (AML vs acute lymphoblastic leukemia, CML, or MDS), HCT-specific comorbidity index (≥3 vs <3), performance status (0-1 vs ≥2), conditioning intensity (MAC vs RIC), and donor–recipient sex mismatch (female to male vs others). Each cause of NRM was analyzed using the Fisher exact test. Receiver operating characteristic curve analysis was performed to determine the optimal patient age cutoff for predicting OS. To assess the impact of acute GVHD on DFS, a landmark analysis was conducted using day 43 after HSCT as the landmark, corresponding to the time when 75% of patients had developed acute GVHD.25 The analysis included only patients who had survived without relapse by that time. A 2-tailed P value < .05 was considered statistically significant, except for interaction terms, in which P < .1 was used. All statistical analyses were performed using EZR version 1.62, a graphical user interface for R.26
Results
Determining cutoff ages for patients and haplo donors
To establish the optimal patient age cutoff for the entire cohort, receiver operating characteristic curves were plotted for OS. The age of 51 years yielded the highest area under the curve (0.608), with a sensitivity of 0.707 and specificity of 0.456. Based on this, patients were classified into 2 groups: those aged ≥50 years (older cohort) and those aged <50 years (younger cohort).
When analyzing haplo-donor age as a continuous variable, no linear relationship with OS was found (P = .784). The distribution of donor ages across the older and younger patient cohorts is shown in supplemental Figure 1. In the older patient cohort, fewer donors were aged >40 years. In the younger cohort, a bimodal distribution was observed, with more donors in their 20s and 40s, and the median donor age was 39 years. Based on these findings, a donor age of 40 years was established as the clinical cutoff, with donors aged ≥40 years classified as older and those aged <40 years as younger. Previous studies have also suggested a cutoff between 30 and 40 years for haplo-donor age,14,27,28 so survival outcomes were also analyzed using a 30-year cutoff.
Patient characteristics
A total of 5116 eligible patients were identified: the older patient cohort (n = 3246; CBT, n = 2675, younger haplo, n = 451; older haplo, n = 120) and the younger patient cohort (n = 1870; CBT, n = 1532; younger haplo, n = 171; older haplo donor, n = 167; Table 1). The median follow-up was 443 days (range, 0-2397). Although most PTCY-haplo transplants were performed during the latter half of the study period (2019-2021), there was no significant difference in the follow-up period between CBT and younger haplo/older haplo within both cohorts (older cohort: CBT median follow-up period of 359 days, younger haplo median follow-up period of 409 days, and older haplo median follow-up period of 285 days; P = .088; younger cohort: CBT median follow-up period of 626 days, younger haplo median follow-up period of 504 days, and older haplo median follow-up period of 534 days; P = .55). The HCT-specific comorbidity index, performance status, and cytomegalovirus status did not significantly differ between the 3 groups. However, in the older patient cohort, AML was less common in the younger haplo group, high-risk disease was less frequent in the older haplo group, and the MAC regimen was more often used in the CBT group. In the younger patient cohort, younger patients were more prevalent in the younger haplo group, and high-risk disease and the MAC regimen were more common in the CBT group. In both patient cohorts, the infused CD34+ cell dose was higher in the younger haplo than the older haplo group. In the older patient cohort, the median infused CD34+ cell dose was 4.54 × 106/kg (interquartile range [IQR], 3.12 × 106/kg to 6.11 × 106/kg) for younger haplo and 3.02 × 106/kg (IQR, 2.43 × 106/kg to 4.86 × 106/kg) for older haplo (P < .001). In the younger patient cohort, the median infused CD34+ cell dose was 4.70 × 106/kg (IQR, 3.72 × 106/kg to 6.22 × 106/kg) for younger haplo and 4.03 × 106/kg (IQR, 2.87 × 106/kg to 5.03 × 106/kg) for older haplo (P = .002).
Patient characteristics
| Variables . | Older cohort (patient age ≥50 y) . | Younger cohort (patient age <50 y) . | ||||||
|---|---|---|---|---|---|---|---|---|
| CBT . | Younger haplo . | Older haplo . | P value . | CBT . | Younger haplo . | Older haplo . | P value . | |
| n = 2675 . | n = 451 . | n = 120 . | n = 1532 . | n = 171 . | n = 167 . | |||
| Age, y (range) | 61 (50-70) | 61 (50-70) | 59 (50-70) | .034 | 40 (16-49) | 34 (16-49) | 41 (16-49) | <.001 |
| Follow-up period, d (range) | 359 (0-2397) | 409 (8-2379) | 285 (3-1698) | .088 | 626 (0-2382) | 504 (7-2058) | 534 (7-2400) | .55 |
| HSCT y (%) | ||||||||
| 2016-2018 | 1266 (47.3) | 134 (29.7) | 30 (25.0) | <.001 | 735 (48.0) | 57 (33.3) | 66 (39.5) | <.001 |
| 2019-2021 | 1409 (52.7) | 317 (70.3) | 90 (75.0) | 797 (52.0) | 114 (66.7) | 101 (60.5) | ||
| Donor age, y (range) | 0 (0-0) | 30 (14- 39) | 48 (40- 65) | 0 (0-0) | 25 (13- 39) | 47 (40- 65) | ||
| Disease (%) | ||||||||
| AML | 1679 (62.8) | 241 (53.4) | 75 (62.5) | .001∗ | 914 (59.7) | 96 (56.1) | 85 (50.9) | .074∗ |
| ALL | 364 (13.6) | 70 (15.5) | 16 (13.3) | 428 (27.9) | 51 (29.8) | 61 (36.5) | ||
| MDS | 579 (21.6) | 135 (29.9) | 25 (20.8) | 141 (9.2) | 13 (7.6) | 18 (10.8) | ||
| CML | 53 (2.0) | 5 (1.1) | 4 (3.3) | 49 (3.2) | 11 (6.4) | 3 (1.8) | ||
| Disease risk (%) | ||||||||
| Low risk | 1296 (48.7) | 235 (53.0) | 72 (60.0) | .017 | 980 (64.1) | 116 (68.2) | 123 (74.1) | .026 |
| High risk | 1365 (51.3) | 208 (47.0) | 48 (40.0) | 549 (35.9) | 54 (31.8) | 43 (25.9) | ||
| HCT-CI (%) | ||||||||
| 1,2 | 2035 (76.5) | 346 (76.7) | 95 (79.8) | .703 | 1335 (87.7) | 152 (89.4) | 140 (84.8) | .435 |
| >2 | 625 (23.5) | 105 (23.3) | 24 (20.2) | 188 (12.3) | 18 (10.6) | 25 (15.2) | ||
| Performance status (%) | ||||||||
| 0-1 | 2424 (90.8) | 408 (90.5) | 110 (92.4) | .8 | 1430 (93.5) | 163 (95.3) | 157 (94.0) | .628 |
| 2-4 | 247 (9.2) | 43 (9.5) | 9 (7.6) | 100 (6.5) | 8 (4.7) | 10 (6.0) | ||
| Conditioning regimen (%) | ||||||||
| MAC | 1492 (55.8) | 146 (32.4) | 43 (35.8) | <.001 | 1335 (87.1) | 138 (80.7) | 133 (79.6) | .004 |
| RIC | 1183 (44.2) | 305 (67.6) | 77 (64.2) | 197 (12.9) | 33 (19.3) | 34 (20.4) | ||
| Sex mismatch (%) | ||||||||
| Others | 1864 (69.9) | 349 (77.4) | 78 (65.0) | .002 | 1145 (74.8) | 129 (75.4) | 108 (68.7) | .016 |
| Female to male | 803 (30.1) | 102 (22.6) | 42 (35.0) | 385 (25.2) | 42 (24.6) | 59 (35.3) | ||
| Source (%) | ||||||||
| CB | 2675 (100.0) | 0 (0.0) | 0 (0.0) | 1532 (100.0) | 0 (0.0) | 0 (0.0) | ||
| PBSC | 0 (0.0) | 451 (100) | 120 (00) | 0 (0.0) | 171 (100) | 167 (100) | ||
| Cytomegalovirus serostatus (%) | ||||||||
| (+) | 2286 (85.5) | 401 (88.9) | 105 (87.5) | .244 | 1165 (76.1) | 125 (73.1) | 134 (80.2) | .646 |
| (−) | 311 (11.6) | 36 (8.0) | 12 (10.0) | 337 (22.0) | 42 (24.6) | 30 (18.0) | ||
| Not reported | 78 (2.9) | 14 (3.1) | 3 (2.5) | 29 (1.9) | 4 (2.3) | 3 (1.8) | ||
| Variables . | Older cohort (patient age ≥50 y) . | Younger cohort (patient age <50 y) . | ||||||
|---|---|---|---|---|---|---|---|---|
| CBT . | Younger haplo . | Older haplo . | P value . | CBT . | Younger haplo . | Older haplo . | P value . | |
| n = 2675 . | n = 451 . | n = 120 . | n = 1532 . | n = 171 . | n = 167 . | |||
| Age, y (range) | 61 (50-70) | 61 (50-70) | 59 (50-70) | .034 | 40 (16-49) | 34 (16-49) | 41 (16-49) | <.001 |
| Follow-up period, d (range) | 359 (0-2397) | 409 (8-2379) | 285 (3-1698) | .088 | 626 (0-2382) | 504 (7-2058) | 534 (7-2400) | .55 |
| HSCT y (%) | ||||||||
| 2016-2018 | 1266 (47.3) | 134 (29.7) | 30 (25.0) | <.001 | 735 (48.0) | 57 (33.3) | 66 (39.5) | <.001 |
| 2019-2021 | 1409 (52.7) | 317 (70.3) | 90 (75.0) | 797 (52.0) | 114 (66.7) | 101 (60.5) | ||
| Donor age, y (range) | 0 (0-0) | 30 (14- 39) | 48 (40- 65) | 0 (0-0) | 25 (13- 39) | 47 (40- 65) | ||
| Disease (%) | ||||||||
| AML | 1679 (62.8) | 241 (53.4) | 75 (62.5) | .001∗ | 914 (59.7) | 96 (56.1) | 85 (50.9) | .074∗ |
| ALL | 364 (13.6) | 70 (15.5) | 16 (13.3) | 428 (27.9) | 51 (29.8) | 61 (36.5) | ||
| MDS | 579 (21.6) | 135 (29.9) | 25 (20.8) | 141 (9.2) | 13 (7.6) | 18 (10.8) | ||
| CML | 53 (2.0) | 5 (1.1) | 4 (3.3) | 49 (3.2) | 11 (6.4) | 3 (1.8) | ||
| Disease risk (%) | ||||||||
| Low risk | 1296 (48.7) | 235 (53.0) | 72 (60.0) | .017 | 980 (64.1) | 116 (68.2) | 123 (74.1) | .026 |
| High risk | 1365 (51.3) | 208 (47.0) | 48 (40.0) | 549 (35.9) | 54 (31.8) | 43 (25.9) | ||
| HCT-CI (%) | ||||||||
| 1,2 | 2035 (76.5) | 346 (76.7) | 95 (79.8) | .703 | 1335 (87.7) | 152 (89.4) | 140 (84.8) | .435 |
| >2 | 625 (23.5) | 105 (23.3) | 24 (20.2) | 188 (12.3) | 18 (10.6) | 25 (15.2) | ||
| Performance status (%) | ||||||||
| 0-1 | 2424 (90.8) | 408 (90.5) | 110 (92.4) | .8 | 1430 (93.5) | 163 (95.3) | 157 (94.0) | .628 |
| 2-4 | 247 (9.2) | 43 (9.5) | 9 (7.6) | 100 (6.5) | 8 (4.7) | 10 (6.0) | ||
| Conditioning regimen (%) | ||||||||
| MAC | 1492 (55.8) | 146 (32.4) | 43 (35.8) | <.001 | 1335 (87.1) | 138 (80.7) | 133 (79.6) | .004 |
| RIC | 1183 (44.2) | 305 (67.6) | 77 (64.2) | 197 (12.9) | 33 (19.3) | 34 (20.4) | ||
| Sex mismatch (%) | ||||||||
| Others | 1864 (69.9) | 349 (77.4) | 78 (65.0) | .002 | 1145 (74.8) | 129 (75.4) | 108 (68.7) | .016 |
| Female to male | 803 (30.1) | 102 (22.6) | 42 (35.0) | 385 (25.2) | 42 (24.6) | 59 (35.3) | ||
| Source (%) | ||||||||
| CB | 2675 (100.0) | 0 (0.0) | 0 (0.0) | 1532 (100.0) | 0 (0.0) | 0 (0.0) | ||
| PBSC | 0 (0.0) | 451 (100) | 120 (00) | 0 (0.0) | 171 (100) | 167 (100) | ||
| Cytomegalovirus serostatus (%) | ||||||||
| (+) | 2286 (85.5) | 401 (88.9) | 105 (87.5) | .244 | 1165 (76.1) | 125 (73.1) | 134 (80.2) | .646 |
| (−) | 311 (11.6) | 36 (8.0) | 12 (10.0) | 337 (22.0) | 42 (24.6) | 30 (18.0) | ||
| Not reported | 78 (2.9) | 14 (3.1) | 3 (2.5) | 29 (1.9) | 4 (2.3) | 3 (1.8) | ||
ALL, acute lymphoblastic leukemia; BM, bone marrow; HCT-CI, HCT-specific comorbidity index; PBSC, peripheral stem cell.
AML vs others.
Survival outcomes according to donor age in the younger and older patient cohorts
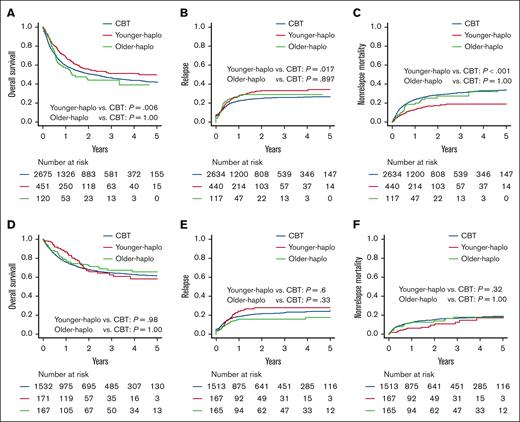
We assessed the survival outcomes of each donor group in both the older and younger patient cohorts. In the older patient cohort, the 2-year OS was significantly higher for younger haplo (55.5% [95% CI, 50.2-60.5]) than with CBT (50.8% [95% CI, 48.7-52.8]; P = .01), whereas the 2-year OS for older haplo (44.1% [95% CI, 33.6-54.0]) was similar to that with CBT (50.8% [95% CI, 48.7-52.8]; P = 1.00; Figure 1A). The 2-year relapse was also significantly higher for younger haplo (33.0% [95% CI, 28.3-37.7]) than with CBT (24.9% [95% CI, 23.2-26.6]; P = .017), whereas the relapse rate for older haplo (29.2% [95% CI, 20.9-38.0]) was comparable with CBT (24.9% [95% CI, 23.2-26.6]; P = .90; Figure 1B). In contrast, the 2-year NRM was significantly higher for younger haplo (17.3% [95% CI, 13.6-21.3]) than with CBT (28.6% [95% CI, 26.7-30.4]; P < .001), whereas the NRM for older haplo (27.3% [95% CI, 18.5-36.9]) was comparable with CBT (28.6% [95% CI, 26.7-30.4]; P = 1.00; Figure 1C).
Comparing OS, relapse, and NRM: CBT vs haplo transplants. OS (A,D), cumulative incidences of relapse (B,E), and NRM (C,F) for younger haplo, older haplo, and CBT in the older patient cohort in panels A-C and the younger patient cohort in panels D-F.
Comparing OS, relapse, and NRM: CBT vs haplo transplants. OS (A,D), cumulative incidences of relapse (B,E), and NRM (C,F) for younger haplo, older haplo, and CBT in the older patient cohort in panels A-C and the younger patient cohort in panels D-F.
In the younger patient cohort, the 2-year OS was similar for younger haplo (66.9% [95% CI, 57.5-74.7]) and older haplo (72.4% [95% CI, 64.2-79.0]) compared with CBT (68.1% [95% CI, 65.5-70.5]; P = 1.00 for both; Figure 1D). The 2-year relapse rate was also similar for younger haplo (28.0% [95% CI, 20.9-35.5]) and older haplo (15.9% [95% CI, 10.6-22.1]) compared with CBT (21.4% [95% CI, 19.3-23.6]; P = .60 for younger haplo, P = .33 for older haplo; Figure 1E). The 2-year NRM was similar for younger haplo (9.6% [95% CI, 5.2-15.6]) and older haplo (13.9% [95% CI, 8.9-20.1]) compared with CBT (15.3% [95% CI, 13.5-17.3]; P = .34 for younger haplo, P = 1.00 for older haplo; Figure 1F). These findings were confirmed by multivariate analyses (Table 2).
Multivariate analysis evaluating the impact of each HSCT type on acute and chronic GVHD, relapse, NRM, and OS in the older and younger patient cohorts
| . | Acute GVHD24 . | Acute GVHD34 . | Chronic GVHD . | Relapse . | NRM . | OS . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recipient age, ≥50 y . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . |
| Donor | ||||||||||||||||||
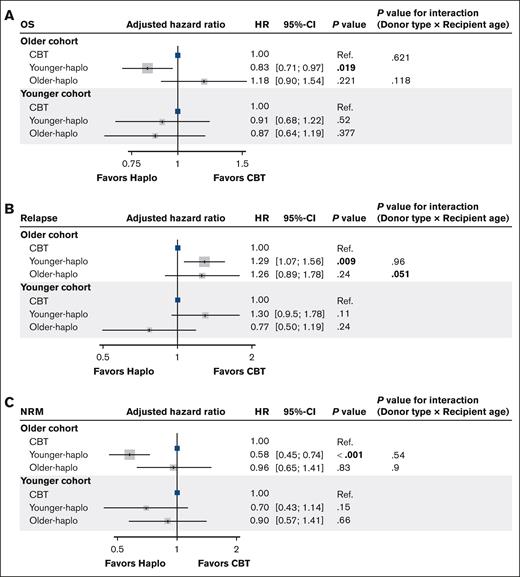
| Younger-H vs CBT | 0.52 | 0.41-0.64 | <.001 | 0.35 | 0.22-0.57 | <.001 | 1.15 | 0.93-1.43 | .200 | 1.29 | 1.07-1.56 | .009 | 0.58 | 0.45-0.74 | <.001 | 0.83 | 0.70-0.97 | .019 |
| Older-H vs CBT | 0.63 | 0.42-0.93 | .020 | 0.44 | 0.20-0.99 | .049 | 0.80 | 0.50-1.27 | .34 | 1.26 | 0.88-1.78 | .200 | 0.96 | 0.65-1.41 | .83 | 1.18 | 0.90-1.54 | .221 |
| Disease risk | ||||||||||||||||||
| High vs low | 1.00 | 0.89-1.14 | .950 | 1.34 | 1.07-1.66 | .009 | 1.05 | 0.89-1.24 | .540 | 2.57 | 2.21-2.99 | <.001 | 1.22 | 1.06-1.41 | .005 | 2.10 | 1.89-2.34 | <.001 |
| Disease | ||||||||||||||||||
| AML vs others | 0.87 | 0.77-0.99 | .030 | 0.94 | 0.75-1.17 | .590 | 0.98 | 0.83-1.15 | .780 | 1.19 | 1.03-1.38 | .020 | 1.04 | 0.90-1.20 | .6 | 1.22 | 1.10-1.36 | <.001 |
| HCT-CI | ||||||||||||||||||
| 3- vs 1-2 | 1.17 | 1.01-1.35 | .037 | 1.13 | 0.87-1.45 | .36 | 1.03 | 0.84-1.25 | .800 | 0.93 | 0.79-1.10 | .420 | 1.48 | 1.28-1.73 | <.001 | 1.31 | 1.17-1.47 | <.001 |
| PS24 | ||||||||||||||||||
| 2-4 vs 0,1 | 0.90 | 0.70-1.16 | .410 | 1.08 | 0.72-1.62 | .72 | 0.95 | 0.68-1.34 | .790 | 1.65 | 1.34-2.03 | <.001 | 1.31 | 1.04-1.64 | .02 | 2.00 | 1.72-2.32 | <.001 |
| RICMAC | ||||||||||||||||||
| RIC vs MAC | 0.78 | 0.68-0.88 | <.001 | 0.76 | 0.60-0.95 | .017 | 0.76 | 0.64-0.89 | <.001 | 1.21 | 1.05-1.40 | .008 | 0.77 | 0.66-0.88 | <.001 | 0.88 | 0.80-0.99 | .032 |
| Sex mismatch | ||||||||||||||||||
| F to M vs others | 0.84 | 0.73-0.97 | .015 | 0.77 | 0.60-0.99 | .042 | 1.17 | 0.99-1.40 | .073 | 1.11 | 0.95-1.29 | .190 | 1.05 | 0.90-1.21 | .56 | 1.15 | 1.03-1.28 | .013 |
| Recipient age, <50 y | ||||||||||||||||||
| Donor | ||||||||||||||||||
| Younger-H vs CBT | 0.52 | 0.37-0.75 | <.001 | 0.18 | 0.06-0.57 | .004 | 1.01 | 0.73-1.39 | .97 | 1.30 | 0.94-1.78 | .110 | 0.70 | 0.43-1.14 | .150 | 0.91 | 0.67-1.22 | .520 |
| Older-H vs CBT | 1.02 | 0.78-1.33 | .880 | 1.29 | 0.80-2.08 | .300 | 1.25 | 0.93-1.68 | .14 | 0.77 | 0.50-1.19 | .240 | 0.90 | 0.58-1.41 | .660 | 0.87 | 0.63-1.19 | .377 |
| Disease risk | ||||||||||||||||||
| High vs low | 1.09 | 0.92-1.29 | .290 | 1.29 | 0.93-1.78 | .130 | 0.88 | 0.71-1.09 | .24 | 3.36 | 2.74-4.13 | <.001 | 1.13 | 0.88-1.45 | .330 | 2.40 | 2.03-2.84 | <.001 |
| Disease | ||||||||||||||||||
| AML vs others | 1.05 | 0.89-1.23 | .570 | 0.78 | 0.57-1.07 | .120 | 1.08 | 0.89-1.30 | .45 | 0.99 | 0.80-1.21 | .900 | 0.89 | 0.70-1.14 | .360 | 1.02 | 0.86-1.21 | .818 |
| HCT-CI | ||||||||||||||||||
| 3- vs 1-2 | 0.79 | 0.61-1.02 | .076 | 0.89 | 0.55-1.43 | .630 | 1.08 | 0.82-1.42 | .6 | 0.9 | 0.66-1.22 | .500 | 1.79 | 1.33-2.42 | <.001 | 1.35 | 1.08-1.69 | .001 |
| PS24 | ||||||||||||||||||
| 2-4 vs 0,1 | 1.14 | 0.81-1.64 | .460 | 2.10 | 1.22-3.62 | .007 | 0.99 | 0.61-1.60 | .96 | 1.57 | 1.11-2.20 | .010 | 2.12 | 1.44-3.10 | <.001 | 2.41 | 1.87-3.11 | <.001 |
| RICMAC | ||||||||||||||||||
| RIC vs MAC | 0.92 | 0.73-1.18 | .520 | 0.96 | 0.61-1.50 | .850 | 1.04 | 0.80-1.36 | .75 | 1.01 | 0.75-1.34 | .970 | 0.75 | 0.52-1.09 | .130 | 0.87 | 0.68-1.11 | .227 |
| Sex mismatch | ||||||||||||||||||
| F to M vs others | 0.96 | 0.80-1.14 | .620 | 0.78 | 0.54-1.13 | .190 | 1.07 | 0.86-1.32 | .55 | 0.94 | 0.74-1.19 | .600 | 1.21 | 0.92-1.57 | .170 | 1.07 | 0.89-1.29 | .456 |
| . | Acute GVHD24 . | Acute GVHD34 . | Chronic GVHD . | Relapse . | NRM . | OS . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recipient age, ≥50 y . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . |
| Donor | ||||||||||||||||||
| Younger-H vs CBT | 0.52 | 0.41-0.64 | <.001 | 0.35 | 0.22-0.57 | <.001 | 1.15 | 0.93-1.43 | .200 | 1.29 | 1.07-1.56 | .009 | 0.58 | 0.45-0.74 | <.001 | 0.83 | 0.70-0.97 | .019 |
| Older-H vs CBT | 0.63 | 0.42-0.93 | .020 | 0.44 | 0.20-0.99 | .049 | 0.80 | 0.50-1.27 | .34 | 1.26 | 0.88-1.78 | .200 | 0.96 | 0.65-1.41 | .83 | 1.18 | 0.90-1.54 | .221 |
| Disease risk | ||||||||||||||||||
| High vs low | 1.00 | 0.89-1.14 | .950 | 1.34 | 1.07-1.66 | .009 | 1.05 | 0.89-1.24 | .540 | 2.57 | 2.21-2.99 | <.001 | 1.22 | 1.06-1.41 | .005 | 2.10 | 1.89-2.34 | <.001 |
| Disease | ||||||||||||||||||
| AML vs others | 0.87 | 0.77-0.99 | .030 | 0.94 | 0.75-1.17 | .590 | 0.98 | 0.83-1.15 | .780 | 1.19 | 1.03-1.38 | .020 | 1.04 | 0.90-1.20 | .6 | 1.22 | 1.10-1.36 | <.001 |
| HCT-CI | ||||||||||||||||||
| 3- vs 1-2 | 1.17 | 1.01-1.35 | .037 | 1.13 | 0.87-1.45 | .36 | 1.03 | 0.84-1.25 | .800 | 0.93 | 0.79-1.10 | .420 | 1.48 | 1.28-1.73 | <.001 | 1.31 | 1.17-1.47 | <.001 |
| PS24 | ||||||||||||||||||
| 2-4 vs 0,1 | 0.90 | 0.70-1.16 | .410 | 1.08 | 0.72-1.62 | .72 | 0.95 | 0.68-1.34 | .790 | 1.65 | 1.34-2.03 | <.001 | 1.31 | 1.04-1.64 | .02 | 2.00 | 1.72-2.32 | <.001 |
| RICMAC | ||||||||||||||||||
| RIC vs MAC | 0.78 | 0.68-0.88 | <.001 | 0.76 | 0.60-0.95 | .017 | 0.76 | 0.64-0.89 | <.001 | 1.21 | 1.05-1.40 | .008 | 0.77 | 0.66-0.88 | <.001 | 0.88 | 0.80-0.99 | .032 |
| Sex mismatch | ||||||||||||||||||
| F to M vs others | 0.84 | 0.73-0.97 | .015 | 0.77 | 0.60-0.99 | .042 | 1.17 | 0.99-1.40 | .073 | 1.11 | 0.95-1.29 | .190 | 1.05 | 0.90-1.21 | .56 | 1.15 | 1.03-1.28 | .013 |
| Recipient age, <50 y | ||||||||||||||||||
| Donor | ||||||||||||||||||
| Younger-H vs CBT | 0.52 | 0.37-0.75 | <.001 | 0.18 | 0.06-0.57 | .004 | 1.01 | 0.73-1.39 | .97 | 1.30 | 0.94-1.78 | .110 | 0.70 | 0.43-1.14 | .150 | 0.91 | 0.67-1.22 | .520 |
| Older-H vs CBT | 1.02 | 0.78-1.33 | .880 | 1.29 | 0.80-2.08 | .300 | 1.25 | 0.93-1.68 | .14 | 0.77 | 0.50-1.19 | .240 | 0.90 | 0.58-1.41 | .660 | 0.87 | 0.63-1.19 | .377 |
| Disease risk | ||||||||||||||||||
| High vs low | 1.09 | 0.92-1.29 | .290 | 1.29 | 0.93-1.78 | .130 | 0.88 | 0.71-1.09 | .24 | 3.36 | 2.74-4.13 | <.001 | 1.13 | 0.88-1.45 | .330 | 2.40 | 2.03-2.84 | <.001 |
| Disease | ||||||||||||||||||
| AML vs others | 1.05 | 0.89-1.23 | .570 | 0.78 | 0.57-1.07 | .120 | 1.08 | 0.89-1.30 | .45 | 0.99 | 0.80-1.21 | .900 | 0.89 | 0.70-1.14 | .360 | 1.02 | 0.86-1.21 | .818 |
| HCT-CI | ||||||||||||||||||
| 3- vs 1-2 | 0.79 | 0.61-1.02 | .076 | 0.89 | 0.55-1.43 | .630 | 1.08 | 0.82-1.42 | .6 | 0.9 | 0.66-1.22 | .500 | 1.79 | 1.33-2.42 | <.001 | 1.35 | 1.08-1.69 | .001 |
| PS24 | ||||||||||||||||||
| 2-4 vs 0,1 | 1.14 | 0.81-1.64 | .460 | 2.10 | 1.22-3.62 | .007 | 0.99 | 0.61-1.60 | .96 | 1.57 | 1.11-2.20 | .010 | 2.12 | 1.44-3.10 | <.001 | 2.41 | 1.87-3.11 | <.001 |
| RICMAC | ||||||||||||||||||
| RIC vs MAC | 0.92 | 0.73-1.18 | .520 | 0.96 | 0.61-1.50 | .850 | 1.04 | 0.80-1.36 | .75 | 1.01 | 0.75-1.34 | .970 | 0.75 | 0.52-1.09 | .130 | 0.87 | 0.68-1.11 | .227 |
| Sex mismatch | ||||||||||||||||||
| F to M vs others | 0.96 | 0.80-1.14 | .620 | 0.78 | 0.54-1.13 | .190 | 1.07 | 0.86-1.32 | .55 | 0.94 | 0.74-1.19 | .600 | 1.21 | 0.92-1.57 | .170 | 1.07 | 0.89-1.29 | .456 |
CBT, cord blood transplantation; F to M, female to male; GVHD24, grade 2 to 4 GVHD; GVHD34, grade 3 to 4 GVHD; HCT-CI, HCT-specific comorbidity index; Older-H, HSCT from older haplo donor; PS, performance status; Younger-H, HSCT from younger haplo donor.
A significant interaction was observed between older haplo (vs CBT) and patient age for relapse (P for interaction = 0.051), suggesting that the effect of haplo-donor age on relapse differed according to the patient’s age (Figure 2).
Adjusted hazard ratios for OS, relapse, and NRM: CBT vs haplo transplants. Forest plots for the adjusted HRs and 95% CIs for OS (A), relapse (B), and NRM (C) in the multivariate analysis. Ref., reference.
Adjusted hazard ratios for OS, relapse, and NRM: CBT vs haplo transplants. Forest plots for the adjusted HRs and 95% CIs for OS (A), relapse (B), and NRM (C) in the multivariate analysis. Ref., reference.
To address the short observation period in the haplo cohort, the data were reanalyzed with the observation period truncated at 1 year (supplemental Figure 2). The outcomes at 1 year after transplant for both the older and younger cohorts are shown in supplemental Figure 2. The 1-year OS, relapse, and NRM followed similar trends to those observed over the entire observation period.
We also assessed OS, relapse, and NRM based on the haplo-donor age cutoff at 30 years and analyzed these outcomes separately for the older and younger patient cohorts. The results are shown in supplemental Figure 3 and supplemental Table 1. Multivariate analysis revealed that OS and NRM were similar for both donor age cutoffs of 40 and 30 years. However, with the 30-year cutoff, relapse was significantly higher in the older cohort with older haplo and in the younger cohort with younger haplo, than with CBT.
Subgroup analysis of survival outcomes by conditioning regimen
A subgroup analysis was conducted based on conditioning regimens, dividing patients into those who received MAC and those who received RIC. In the older cohort, among those receiving MAC, no significant differences in OS, relapse, or NRM were found between younger haplo and CBT, unlike the overall results. For those receiving RIC, similar to the overall findings, relapse was lower, but NRM was higher in CBT than with younger haplo (supplemental Figure 4). In the younger cohort, regardless of the conditioning regimen (MAC or RIC), no significant differences in survival outcomes were observed between the haplo and CBT groups, consistent with the overall results (supplemental Figure 5).
Acute and chronic GVHD according to donor age in the younger and older patient cohorts
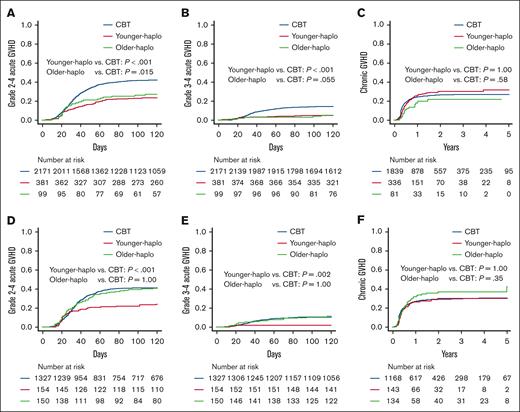
We assessed the occurrence of grade 2 to 4 and 3 to 4 acute GVHD and chronic GVHD in each donor group. In the older patient cohort, the cumulative incidence of grade 2 to 4 acute GVHD at day 100 was significantly lower for younger haplo (23.1% [95% CI, 19.0-27.5]) and older haplo (26.3% [95% CI, 18.0-35.3]) than with CBT (41.9% [95% CI, 39.8-43.9]; P < .001 and P = .015, respectively; Figure 3A). The cumulative incidence of grade 3 to 4 acute GVHD at day 100 was also significantly lower for younger haplo (5.0% [95% CI, 3.1-7.5]) and marginally lower for older haplo (4.1% [95% CI, 1.3-9.4]) than for CBT (14.1% [95% CI, 12.6-15.6]; P < .001 and P = .055, respectively; Figure 3B). The cumulative incidence of chronic GVHD at 2 years was similar for younger haplo (29.7% [95% CI, 24.7-34.9]) and older haplo (22.3% [95% CI, 13.4-32.5]) compared with CBT (25.9% [95% CI, 23.9-27.9]; P = 1.00 and P = .77, respectively; Figure 3C).
Comparing acute and chronic GVHD: CBT vs haplo transplants. Cumulative incidences of grade 2 to 4 acute GVHD (A,D), cumulative incidences of grade 3 to 4 acute GVHD (B,E), and cumulative incidences of chronic GVHD (C,F) for younger haplo, older haplo, and CBT in the older patient cohort in panels A-C and the younger patient cohort in panels D-F.
Comparing acute and chronic GVHD: CBT vs haplo transplants. Cumulative incidences of grade 2 to 4 acute GVHD (A,D), cumulative incidences of grade 3 to 4 acute GVHD (B,E), and cumulative incidences of chronic GVHD (C,F) for younger haplo, older haplo, and CBT in the older patient cohort in panels A-C and the younger patient cohort in panels D-F.
In the younger patient cohort, the cumulative incidence of grade 2 to 4 acute GVHD at day 100 was significantly lower for younger haplo (22.1% [95% CI, 15.9-28.9]) than for CBT (41.2% [95% CI, 38.5-43.8]; P < .001) but similar for older haplo (39.3% [95% CI, 31.5-47.1]) compared with CBT (P = 1.00; Figure 3D). The cumulative incidence of grade 3 to 4 acute GVHD at day 100 was significantly lower for younger haplo (1.9% [95% CI, 0.5-5.0]) than CBT (10.4% [95% CI, 8.8-12.1]; P = .002) but comparable for older haplo (10.7% [95% CI, 6.4-16.2]) compared with CBT (P = 1.00; Figure 3E). The cumulative incidence of chronic GVHD at 2 years was similar for younger haplo (31.0% [95% CI, 23.1-39.1]) and older haplo (36.7% [95% CI, 28.0-45.3]) compared with CBT (29.2% [95% CI, 26.6-31.9]; P = 1.00 and P = .38, respectively; Figure 3F). Similar findings were confirmed by multivariate analyses (Table 2).
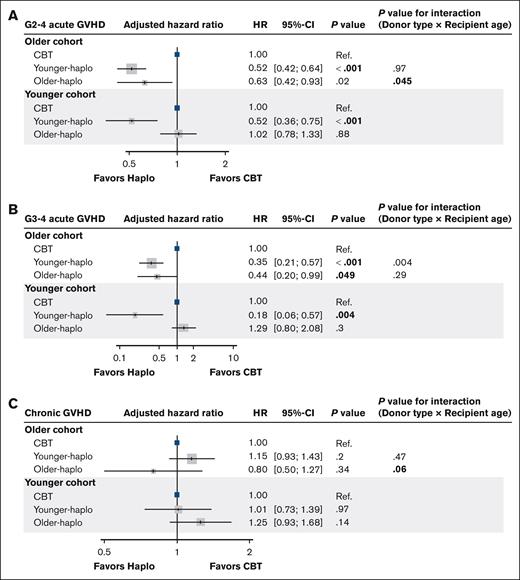
Significant interactions were found between older haplo (vs CBT) and patient age for grade 2 to 4 and chronic GVHD (P for interaction = 0.045 and 0.06, respectively; Figure 4A-C).
Adjusted hazard ratios for acute and chronic GVHD: CBT vs haplo transplants. Forest plots for the adjusted HRs and 95% CIs for grade 2 to 4 acute GVHD (A), grade 3 to 4 acute GVHD (B), and chronic GVHD (C) in the multivariate analysis. G, grade; Ref., reference.
Adjusted hazard ratios for acute and chronic GVHD: CBT vs haplo transplants. Forest plots for the adjusted HRs and 95% CIs for grade 2 to 4 acute GVHD (A), grade 3 to 4 acute GVHD (B), and chronic GVHD (C) in the multivariate analysis. G, grade; Ref., reference.
Additionally, as part of a subgroup analysis, we compared DFS between patients with and without grade 1 to 2 acute GVHD, separately for CBT, older haplo, and younger haplo. In the older patient cohort, 2-year DFS was higher for those with grade 1 to 2 acute GVHD compared with those without GVHD in CBT (57.3% [95% CI, 54.1-60.5] vs 42.4% [95% CI, 39.4-45.3], P < .001) but similar in both groups for older haplo (47.9% [95% CI, 30.9-63.1] vs 39.6% [95% CI, 26.3-52.5]; P = .60) and younger haplo cohorts (54.3% [95% CI, 45.8-62.0] vs 47.3% [95% CI, 40.2-54.0]; P = .20; supplemental Figure 6A-C). In the younger patient cohort, 2-year DFS was higher for those with grade 1 to 2 acute GVHD than those without GVHD in CBT (73.8% [95% CI, 70.2-77.1] vs 55.7% [95% CI, 51.7-59.6]; P < .001), and a similar trend was observed in the older haplo cohort (78.9% [95% CI, 67.3-86.8] vs 62.0% [95% CI, 48.4-73.0]; P = .053). However, in the younger haplo cohort, the rates were comparable (67.8% [95% CI, 53.9-78.3] vs 57.6% [95% CI, 45.5-68.0]; P = .16) (supplemental Figure 6D-F).
GRFS
In the older patient cohort, the 2-year GRFS was significantly higher for younger haplo (37.6% [95% CI, 32.1-43.1] vs 33.5% [95% CI , 31.3-35.7]; P < .001) and similar for older haplo (36.5% [95% CI , 26.2-46.8] vs 33.5% [95% CI , 31.3-35.7]; P = .56) compared with CBT (supplemental Figure 7A).
In the younger patient cohort, the 2-year GRFS was similar for younger haplo (54.5% [95% CI, 44.9-63.1] vs 47.3% [95% CI, 44.2-50.3]; P = .086) and older haplo (38.1% [95% CI , 28.8-47.4] vs 47.3% [95% CI , 44.2-50.3]; P = .693) compared with CBT (supplemental Figure 7B).
PTLD
In the older patient cohort, posttransplant lymphoproliferative disorder (PTLD) was observed in 41 patients in the CBT group, 2 patients in the younger haplo group, and 1 patient in the older haplo group, with no significant difference in incidence rates (younger haplo, 1.5%, vs CBT, 0.4%; P = .078; older haplo, 0.8%, vs CBT, 1.5%; P = 1.0).
In the younger patient cohort, PTLD occurred in 15 patients in the CBT group, with no cases in the younger haplo and older haplo groups and no difference in the incidence rates between these groups (younger haplo, 0.0%, vs CBT, 1.0%; P = .388; older haplo, 0.0%, vs CBT, 1.0%; P = .387).
Causes of death
In the older patient cohort, the causes of nonrelapse death were as follows: GVHD in 81 patients, organ failure in 259, thrombotic microangiopathy/veno-occlusive disease (TMA/VOD) in 80, infection in 314, graft failure in 36, bleeding in 51, secondary malignancies in 26, and other causes in 123 patients. Relapse-related death occurred in 585 patients. The CBT group had a higher incidence of fatal infections (10.4% vs 5.1%; P = .002), fatal TMA/VOD (2.7% vs 1.1%; P = .048), and graft failure (1.3% vs 0%; P = .007) than the younger haplo group (supplemental Figure 8). No significant differences were found between the CBT and older haplo groups in the proportion of each cause of nonrelapse death.
In the younger patient cohort, the causes of nonrelapse death were GVHD in 26 patients, organ failure in 98, infection in 98, graft failure in 16, bleeding in 17, secondary malignancies in 6, and other causes in 35. Relapse-related death occurred in 251 patients. The CBT group had fewer fatal TMA/VOD cases than the older haplo group (1.7% vs 4.8%; P = .014) but more organ failure than the younger haplo group (5.9% vs 1.2; P = .007; supplemental Figure 8).
Discussion
This study found that haplo-donor age influenced survival outcomes, with younger haplo donors providing better OS and lower NRM compared to CBT in the older patient cohort. However, these differences were not observed in younger patients, and survival outcomes between the older haplo and CBT groups were not significantly different, regardless of patient age.
To our knowledge, this is the first study to assess the impact of haplo-donor age in comparing PTCY-haplo and CBT. In a large prospective study, the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 1101) conducted a randomized controlled trial with 368 adult patients with leukemia and lymphoma, comparing PTCY-haplo bone marrow transplant with double-unit CBT following the RIC regimen. Although the 2-year progression-free survival was similar between the groups, PTCY-haplo showed lower NRM and higher OS than double-unit CBT.1 Conflicting results have been reported on the superiority of PTCY-haplo vs CBT, likely because of variations in disease type and status, conditioning regimen, GVHD prophylaxis, and the use of single or double CB units.3-8 This study offers new insights into the impact of haplo-donor age by patient age on survival outcomes for PTCY-haplo and CBT, which may aid in the selection of alternative donors.
Previous studies have shown that haplo-HSCT from older donors had lower relapse but higher NRM, with similar OS compared with younger donors.11-15 However, in this cohort, relapse, NRM, and OS were not significantly different between younger and older haplo groups. In the older patient cohort, only the younger haplo group experienced more relapse, lower NRM, and better OS than CBT. Notably, in this cohort, NRM due to infections, TMA/VOD, or graft failure was significantly higher in the CBT group than in the younger haplo group, which may have influenced these findings. The subgroup analysis also suggested that, even in older patients, MAC could negate survival outcome differences between younger haplo and CBT. Furthermore, the significantly higher relapse rate in the younger haplo group than the CBT group in the older cohort could be artifactual, because the higher NRM in the CBT group might have contributed to the increased relapse rate in the younger haplo group.
Haplo-donor age may have influenced the outcome through the number of infused CD34+ cells,29,30 which was significantly higher in younger donors. However, because the number of infused CD34+ cells was higher in younger donors across both the older and younger patient cohorts, this result cannot be attributed solely to the number of infused CD34+ cells. Thus, the observed differences in outcomes based on patient age highlight the need for further research to optimize donor selection and transplantation strategies considering both donor and patient characteristics.
Previous studies have indicated that acute GVHD was either comparable1,2,4 or more common5,7 in CBT compared with PTCY-haplo. Most studies reported similar rates of chronic GVHD.1,2,4-7 In our study, acute GVHD was more frequent in CBT in the older patient cohort, irrespective of the haplo-donor age group. In contrast, acute GVHD was higher in CBT in younger patients than in younger haplo but similar between older haplo and CBT, suggesting a significant interaction between donor type and patient age. CBT was associated with a stronger graft-versus-leukemia effect in patients with mild acute GVHD than those with PTCY-haplo, potentially because of better DFS.25 Our subgroup analysis showed that CBT improved DFS with mild acute GVHD in older patients, whereas PTCY-haplo did not, which aligns with previous reports.31 In contrast, PTCY-haplo (regardless of donor age) demonstrated a higher DFS rate in younger patients with mild acute GVHD, although no statistically significant difference was found because of the small sample size. This could explain the similar recurrence rates between PTCY-haplo and CBT in the younger patient cohort. Further research is required to investigate the differences in graft-versus-leukemia effects related to patient age in PTCY-haplo.
In this study, infection was the primary cause of nonrelapse death in both CBT and PTCY-haplo. Infection is a significant issue in CBT because of delayed engraftment and slow immune reconstitution. In fact, when CBT was compared with PTCY-haplo, there was a higher incidence of infection-related deaths in CBT.32 In this study, infection-related death was higher in the CBT group than in younger haplo only in the older patient group. There was no difference in infection-related deaths between CBT and older haplo in either older or younger patients. Additionally, acute GVHD was more common in CBT compared with haplo in older patients. However, there was no significant difference in GVHD-related deaths between CBT and PTCY-haplo, possibly because acute GVHD was more likely to respond to steroid treatment.33
With the increasing use of PTCY-haplo, the number of haplo-HSCTs has risen in the United States and Europe, whereas the number of CBT has decreased.34 In Japan, the number of haplo-HSCTs, primary using peripheral blood stem cells, has also increased, with ∼600 cases performed in 2020.35 However, single-unit umbilical CBT (UCBT) is still commonly performed, with ∼1500 cases in Japan in 2020. UCBT remains the most prevalent transplant type, outnumbering both related and unrelated donor transplants. In fact, Japan accounts for approximately half of the global UCBT cases performed each year.36 The JSCTC guideline notes that the outcome of transplants from alternative donors (CBT or haplo) are influenced by the transplant center’s experience, and donor selection should therefore be based on the center’s experience and the patient’s preferences.37
This study has several limitations. First, it was a retrospective analysis, and donor selection was influenced by physician judgment and institutional factors. Second, the median follow-up period of 443 days was too short to fully assess the long-term impact of donor types on prognosis, especially because PTCY-haplo has only recently been used in Japan. Third, the cutoff value for donor age was another limitation. Although previous studies suggested a cutoff of 30 years,13 this study showed similar results with both 30- and 40-year cutoffs. The optimal cutoff may vary with patient age and, as shown in this study, the available donor age range differs by patient age, making it challenging to define a single cutoff. Finally, in older patients, younger haplo may result in lower NRM than CBT, leading to more survivors and a relatively higher incidence of relapse. When examining DFS as a composite end point, older patients receiving haplo transplants showed a trend toward better DFS than those receiving CB. However, no significant difference in DFS was found between older patients with haplo transplants based on the presence or absence of GVHD, unlike in CBT (supplemental Figure 6). This suggests that GVHD (graft-versus-leukemia effect) may contribute to differences in relapse rates. Ultimately, assessing the true relapse rate is challenging when NRMs are significantly different.
Our study showed that HSCT from younger haplo donors resulted in better OS and lower NRM than CBT in older patients, although this benefit was less noticeable in younger patients. These findings highlight the importance of selecting donors based on both donor and patient age. Further research with a longer follow-up period is needed to confirm these results and refine transplantation strategies.
Acknowledgments
The authors thank all the physicians and staff at the hospital. The authors also thank all the members of the Transplant Registry Unified Management Committees at the Japanese Society for Transplantation and Cellular Therapy for their data management.
Authorship
Contribution: T. Nagayama designed the study, analyzed the data, performed the statistical analysis, and wrote the manuscript; S.-i.F. designed the study, advised on the methods, and revised the manuscript; S.N. advised on the methods and revised the manuscript; F.W. revised the manuscript; N.U., M. Tanaka, M.S.-Y., M.O., K.I., Y.H., S.O., N.D., H. Nakamae, T. Nishida, T.K., M.S., M. Tokunaga, F.I., and T.F. collected data and revised the manuscript; Y.K. collected data, advised on the methods, and revised the manuscript; Y.A. managed the unified registry database and revised the manuscript; and H. Nakasone advised on the methods, revised the manuscript, and was responsible for the project of JSTCT Donor/Source Working Group.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shin-ichiro Fujiwara, Division of Cell Transplantation and Transfusion, Jichi Medical University, 3311-1 Yakushiji, Shimotsuke 329-0498, Japan; email: sfujiwar@jichi.ac.jp.
References
Author notes
The data of this study are not publicly available because of ethical restrictions that exceed the scope of the recipient/doctor consent for research use in the registry.
The full-text version of this article contains a data supplement.