Key Points
Real-world efficacy among YAs receiving CD19 CAR T was comparable irrespective of CAR T construct.
CRS and ICANS were observed more frequently in YA recipients of brexu-cel vs tisa-cel.
Visual Abstract
Tisagenlecleucel (tisa-cel) and brexucabtagene autoleucel (brexu-cel) are approved CD19 chimeric antigen receptor T-cell therapy (CAR T) products for young adults (YA) with relapsed/refractory B-cell acute lymphoblastic leukemia. A distinct analysis of YAs receiving commercial CD19 CAR T has not been reported. Using retrospective data from the Pediatric Real-World CAR T Consortium and the Real-World Outcomes of CAR T in Adult ALL collaboration, we describe the efficacy and safety of tisa-cel and brexu-cel in 70 YAs (18-26 years; tisa-cel, n = 50; brexu-cel, n = 20). Cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS) were observed more frequently for brexu-cel vs tisa-cel (CRS, 85% vs 56%; ICANS, 40% vs 18%). Complete response rates were similar between products at 80% for brexu-cel and 88% for tisa-cel. Relapse-free survival (RFS) at 12 months was 46% for brexu-cel and 36% for tisa-cel. Durability of remission over 12 months was 61% for brexu-cel vs 41% for tisa-cel; 12-month overall survival (OS) for brexu-cel was 84% vs 68% for tisa-cel. In multivariate analysis, low disease burden was associated with improved OS, whereas inotuzumab before CAR T was associated with inferior outcomes. This study demonstrates comparable real-world efficacy among YAs receiving CD19 CAR T irrespective of CAR T construct; however, rates of toxicity seem higher with brexu-cel.
Introduction
The incidence of B-cell acute lymphoblastic leukemia (B-ALL) is increasing over time among adolescents and young adults (AYAs); however, these patients experience worse survival and toxicities than younger pediatric patients.1 Several factors contribute to inferior outcomes among AYAs with B-ALL, including distinct disease biology, less access to specialized care, inferior tolerance of intensive chemotherapy, and early treatment discontinuation.2-4 B-ALL in AYAs is enriched with adverse genetic risk factors, including more frequent Philadelphia chromosome (Ph)–like B-ALL characterized by multiple kinase-activating alterations, Ph+ B-ALL with BCR::ABL1 fusion, and KMT2A-rearranged ALL.5-7 Although intensive pediatric chemotherapy regimens such as that utilized in the prospective clinical trial CALGB10403 improved outcomes for AYAs relative to nonrandomized controls, psychosocial barriers can lead to poor adherence to complex regimens.8,9 AYAs also experience increased toxicities with key components of B-ALL therapy, including asparaginase.10,11 Studies demonstrate improved outcomes among AYAs treated at specialized centers with AYA expertise, yet less than half of AYAs receive treatment at these centers owing to challenges in accessing care.4,12
Chimeric antigen receptor T-cell therapy (CAR T)–targeting CD19 is a promising therapy for children and adults with relapsed/refractory (R/R) B-ALL. Based on the results of the pivotal ELIANA and ZUMA-3 studies, 2 CD19 CAR T products are US Food and Drug Administration (FDA)–approved for B-ALL. Tisagenlecleucel (tisa-cel) is approved for patients aged ≤25 years, and brexucabtagene autoleucel (brexu-cel) for adult patients ≥18 years.13,14 One key difference between these products is the costimulatory domain: tisa-cel includes a 41BB costimulatory domain, facilitating longer persistence in preclinical studies, whereas brexu-cel has a CD28 costimulatory domain.15 Long-term studies of tisa-cel in pediatric patients demonstrate durable remissions in patient subsets without consolidative allogeneic hematopoietic stem cell transplant (HCT).16 Although some studies of CD28 CAR T cells demonstrate improved outcomes in the setting of consolidative HCT, the identification of patients who will benefit from HCT after CAR T remains challenging.17-20 There are no clinical studies that directly compare 41BB with CD28 CAR T products for patients with R/R B-ALL. In addition, there are no published reports examining distinct outcomes among young adult (YA) CAR T recipients. There is an overlap in the approved age range in the United States for tisa-cel and brexu-cel, which presents a choice in therapy for YA patients and an opportunity to compare outcomes with these 2 CAR T products. Given that brexu-cel is not approved for adolescents, we focused our analysis on YAs (aged 18-26 years). We leverage the commercial experience using tisa-cel and brexu-cel to establish toxicity, response, and survival rates among YAs with B-ALL treated with standard-of-care CD19 CAR Ts and identify variables associated with improved outcomes.
Methods
Study design
We conducted a retrospective analysis of YAs who received CD19-directed CAR T and were registered with the Pediatric Real-World CAR Consortium (PRWCC) or the Real-World Outcomes of CAR T in Adult ALL (ROCCA). Patients between the ages of 18 and 26 years were included in the analysis. The PRWCC was launched in August 2019 to study CAR T outcomes in a real-world setting. It includes 40 sites across the United States and 185 patients. The patients derived from the PRWCC database all received tisa-cel; patients who received tisa-cel on a managed access program were included, whereas those on clinical trials were excluded.21 ROCCA was formed in 2022 after the FDA approval of brexu-cel for adult B-ALL. It includes 30 sites and 189 patients in the initial cohort.22,23 All patients derived from the ROCCA database received brexu-cel. Patients who received brexu-cel as part of a clinical trial or an expanded access protocol before FDA approval were excluded.
All institutions obtained institutional review board approval. Data were collected in REDCap databases housed at Stanford University in a fashion compliant with the Health Insurance Portability and Accountability Act of 1996. All YA patients who received tisa-cel were comparatively analyzed with patients who received brexu-cel.
Study end points
The primary outcomes were 12-month overall survival (OS), relapse-free survival (RFS), and duration of remission (DOR). Secondary outcomes included best overall response rates (ORRs) and toxicity. The best overall response was defined as complete response (CR) within 3 months after CAR infusion. CR was defined as ≤5% bone marrow (BM) blasts by morphology or minimal residual disease (MRD) negativity regardless of blood count recovery. CR with incomplete hematologic recovery was not reported on data collection forms. Central nervous system (CNS) disease was graded by standard cytology metrics per the Children’s Oncology Group classification: CNS 1, no cerebrospinal fluid (CSF) blasts; CNS 2, white blood cell count of <5/μL with CSF blasts; and CNS 3, white blood cell count of ≥5/μL with CSF blasts or signs of CNS involvement.5 MRD assessment was per institutional practices. Methodologies for MRD measurement included flow cytometry, quantitative reverse transcription polymerase chain reaction for BCR::ABL1 fusion, or next-generation sequencing/clonoSEQ (Adaptive Biotechnologies). Relapse was defined as any new evidence of medullary or extramedullary B-ALL. Cytokine release syndrome (CRS) grading was obtained according to criteria published by the American Society for Transplantation and Cellular Therapy (ASTCT).24 Immune effector cell–associated neurotoxicity syndrome (ICANS) grading was obtained according to institutional standard practice, using guidelines per ASTCT or CAR T cell–related encephalopathy syndrome.24,25
Statistical analysis
The infusion date of either tisa-cel or brexu-cel was considered time 0 for all analyses. Data cutoff was 6 March 2020 for patients receiving tisa-cel and 1 October 2023 for patients receiving brexu-cel. RFS was defined as the duration from time 0 to death from any cause or presence of disease in peripheral blood, BM, or extramedullary site. OS was defined as the duration from time 0 to death from any cause. DOR was defined as the time from day 28 after infusion (time of established remission) to disease relapse in patients who achieved remission. Censoring events for RFS and DOR included last follow-up and HCT in remission. Censoring events for OS included last follow-up. Disease status before CAR-T infusion was determined by the disease evaluation that was most proximal to infusion. Postbridging disease assessment was used for patients who received bridging chemotherapy and had results of postbridging assessment reported.
Descriptive analyses were conducted to compare patient characteristics between YA patients who received brexu-cel and patients who received tisa-cel. χ2 tests were conducted to test statistical differences between the 2 groups. Kaplan-Meier survival curves were generated to visualize the probability of survival. Univariate and multivariate Cox models were created to evaluate OS, RFS, and DOR. Univariate Cox models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for each group variable and covariates. Multivariate Cox models were generated, including the group variable and covariates. The tisa-cel group was used as a reference group. Covariates were selected based on variables that were thought to be prognostic based on clinician expertise and included CAR T product, age, ALL subtype, disease burden before infusion, and HCT, blinatumomab, or inotuzumab before CAR T. All analyses were conducted using SAS 9.4 (SAS Institute Inc, Cary, NC). A statistical significance level was set at .05 (2-sided).
Results
Patient characteristics
Of 374 patients across the PRWCC (n = 185) and ROCCA retrospective cohorts (n = 189), 70 were between ages 18 and 26 years with R/R B-ALL and met the inclusion criteria for this study (consort diagram shown in supplemental Figure 1). Twenty patients received brexu-cel at 10 study sites, and 50 patients received tisa-cel at 15 study sites. Baseline characteristics for these patients are presented in Table 1. The median age at infusion was 22.5 years in the brexu-cel group and 21 years in the tisa-cel group (range, 18-26 for both groups). Ph– (brexu-cel, n = 12, 60%; tisa-cel, n = 33, 66%) and Ph-like ALL (brexu-cel, n = 5, 25%; tisa-cel, n = 11, 22%) were the predominant ALL subtypes; 3 patients with Ph+ ALL received brexu-cel, and 5 received tisa-cel. There were no significant differences in demographic or baseline disease characteristics between the 2 groups.
Baseline patient and disease characteristics
| Characteristic . | Tisa-cel (n = 50) . | Brexu-cel (n = 20) . | P value . |
|---|---|---|---|
| Sex, n (%) | .35 | ||
| M | 29 (58) | 14 (70) | |
| F | 21 (42) | 6 (30) | |
| Age at CAR T-cell infusion, median (range), y | 21 (18-26) | 22.5 (18-26) | |
| Race/ethnicity, n (%) | .81 | ||
| Non-Hispanic White | 24 (48) | 9 (45) | |
| Hispanic | 19 (38) | 8 (40) | |
| Black | 2 (4) | 0 | |
| Asian/Pacific Islander | 0 | 2 (10) | |
| Other/not reported | 5 (10) | 1 (5) | |
| ALL subtype, n (%) | .8 | ||
| Ph– ALL∗ | 33 (66) | 12 (60) | |
| Ph+ ALL | 5 (10) | 3 (15) | |
| Ph-like ALL | 11 (22) | 5 (25) | |
| Unknown | 1 (2) | 0 | |
| Marrow disease before CAR T, n (%) | .1 | ||
| Undetectable MRD | 15 (30) | 3 (15) | |
| Detectable MRD with <5% blasts† | 12 (24) | 11 (55) | |
| Flow cytometry | 12 | 6 | |
| clonoSEQ | 0 | 3 | |
| qPCR | 0 | 1 | |
| NGS | 0 | 0 | |
| 5 to <50% blasts | 9 (18) | 2 (10) | |
| >50% blasts | 11 (22) | 3 (15) | |
| Unknown | 3 (6) | 1 (5) | |
| Extramedullary disease before CAR T, n (%) | .11 | ||
| Present | 5 (10) | 5 (25) | |
| Not present | 45 (90) | 3 (15) | |
| Not assessed/unknown | 0 | 12 (60) | |
| CNS disease status before CAR T, n (%) | .11 | ||
| CNS 1 | 41 (82) | 11 (55) | |
| CNS 2 | 1 (2) | 1 (5) | |
| CNS 3 | 2 (4) | 1 (5) | |
| Not assessed/unknown | 6 (12) | 7 (35) | |
| Therapy before CAR T | |||
| Total lines of therapy, median (range) | 3 (1-5) | 3 (1-9) | |
| Blinatumomab, n (%)‡ | 12 (24) | 9 (45) | .08 |
| Inotuzumab, n (%)‡ | 13 (26) | 6 (30) | .73 |
| Allogeneic HCT, n (%) | 20 (40) | 3 (15) | .04 |
| Bridging therapy between apheresis and lymphodepletion, n (%) | 26 (52) | 15 (75) | .08 |
| Lymphodepletion regimen, n (%) | .22 | ||
| Fludarabine/cyclophosphamide | 47 (94) | 17 (85) | |
| Other | 3 (6) | 3 (15) |
| Characteristic . | Tisa-cel (n = 50) . | Brexu-cel (n = 20) . | P value . |
|---|---|---|---|
| Sex, n (%) | .35 | ||
| M | 29 (58) | 14 (70) | |
| F | 21 (42) | 6 (30) | |
| Age at CAR T-cell infusion, median (range), y | 21 (18-26) | 22.5 (18-26) | |
| Race/ethnicity, n (%) | .81 | ||
| Non-Hispanic White | 24 (48) | 9 (45) | |
| Hispanic | 19 (38) | 8 (40) | |
| Black | 2 (4) | 0 | |
| Asian/Pacific Islander | 0 | 2 (10) | |
| Other/not reported | 5 (10) | 1 (5) | |
| ALL subtype, n (%) | .8 | ||
| Ph– ALL∗ | 33 (66) | 12 (60) | |
| Ph+ ALL | 5 (10) | 3 (15) | |
| Ph-like ALL | 11 (22) | 5 (25) | |
| Unknown | 1 (2) | 0 | |
| Marrow disease before CAR T, n (%) | .1 | ||
| Undetectable MRD | 15 (30) | 3 (15) | |
| Detectable MRD with <5% blasts† | 12 (24) | 11 (55) | |
| Flow cytometry | 12 | 6 | |
| clonoSEQ | 0 | 3 | |
| qPCR | 0 | 1 | |
| NGS | 0 | 0 | |
| 5 to <50% blasts | 9 (18) | 2 (10) | |
| >50% blasts | 11 (22) | 3 (15) | |
| Unknown | 3 (6) | 1 (5) | |
| Extramedullary disease before CAR T, n (%) | .11 | ||
| Present | 5 (10) | 5 (25) | |
| Not present | 45 (90) | 3 (15) | |
| Not assessed/unknown | 0 | 12 (60) | |
| CNS disease status before CAR T, n (%) | .11 | ||
| CNS 1 | 41 (82) | 11 (55) | |
| CNS 2 | 1 (2) | 1 (5) | |
| CNS 3 | 2 (4) | 1 (5) | |
| Not assessed/unknown | 6 (12) | 7 (35) | |
| Therapy before CAR T | |||
| Total lines of therapy, median (range) | 3 (1-5) | 3 (1-9) | |
| Blinatumomab, n (%)‡ | 12 (24) | 9 (45) | .08 |
| Inotuzumab, n (%)‡ | 13 (26) | 6 (30) | .73 |
| Allogeneic HCT, n (%) | 20 (40) | 3 (15) | .04 |
| Bridging therapy between apheresis and lymphodepletion, n (%) | 26 (52) | 15 (75) | .08 |
| Lymphodepletion regimen, n (%) | .22 | ||
| Fludarabine/cyclophosphamide | 47 (94) | 17 (85) | |
| Other | 3 (6) | 3 (15) |
F, female; M, male; NGS, next-generation sequencing; qPCR, quantitative polymerase chain reaction.
Ph– ALL tisa-cel group includes 1 mixed-phenotype acute leukemia.
MRD methodology unknown in 1 patient with detectable MRD.
One patient in brexu-cel group received both blinatumomab and inotuzumab before CAR T.
Median lines of therapy before CAR T was 3 in both groups (range: brexu-cel, 1-9; tisa-cel, 1-5). Fewer patients in the brexu-cel group underwent HCT before CAR T (n = 3 [15%]), whereas 20 tisa-cel patients (40%) had received HCT (P = .04). Nine patients (45%) in the brexu-cel group received blinatumomab compared with 12 patients (24%) in the tisa-cel group (P = .08). Inotuzumab was administered to 6 brexu-cel patients (30%) and 13 tisa-cel patients (26%) before CAR T (P = .73). Fifteen brexu-cel patients (75%) and 26 tisa-cel patients (52%) received bridging therapy between apheresis and lymphodepletion (P = .08). Bridging regimens consisted of systemic chemotherapy in most cases (brexu-cel, n = 10; tisa-cel, n = 24), with 2 patients in each group receiving inotuzumab and 1 brexu-cel patient receiving blinatumomab. Fludarabine/cyclophosphamide was the most commonly used lymphodepletion regimen (brexu-cel, n = 17; tisa-cel, n = 47). Median follow-up was 9.9 months for patients receiving brexu-cel (range, 2.2-15.1) and 12.7 months for patients receiving tisa-cel (range, 1.1-27.2).
Regarding the timing of pre-CAR-T infusion disease assessment, 11 of 15 brexu-cel patients who received bridging therapy had disease status reported after bridging. For the remaining 9 brexu-cel patients, disease status was only reported before apheresis. Among tisa-cel patients who received bridging therapy, 16 of 26 had disease evaluation after bridging. Disease status was reported before lymphodepletion for 31 tisa-cel patients; the exact timing of assessment was not reported for the remaining 3 patients.
Most patients were in morphologic remission at the time of BM assessment (brexu-cel, n = 14, 70%; tisa-cel, n = 27, 54%; P = .22). Three patients (15%) in the brexu-cel group had ≥50% BM blasts compared with 11 patients (22%) in the tisa-cel group (P = .51). Fifteen tisa-cel recipients had undetectable MRD (30%) compared with 3 patients (15%) treated with brexu-cel. Flow cytometry was the most common method to determine MRD status. Most patients either did not have non-CNS extramedullary disease or extramedullary disease status was not assessed at the time of infusion. Five patients had CNS disease at infusion; 2 received brexu-cel and 3 received tisa-cel.
Toxicity
CRS of any grade was more common in patients receiving brexu-cel, with 85% experiencing any grade CRS vs 56% of patients in the tisa-cel group (P = .01). Severe CRS (grade ≥3) was observed in 10% of brexu-cel patients (n = 2) compared with 18% of tisa-cel recipients (n = 9; P = .41; Table 2). Neurotoxicity was more common in the brexu-cel group, with 40% of brexu-cel patients (n = 8) experiencing any grade ICANS compared with 18% of tisa-cel patients (n = 9; P = .05). Brexu-cel patients experienced more severe neurotoxicity: 35% of patients (n = 7) experienced grade ≥3 ICANS vs 4% of tisa-cel patients (n = 2; P < .001). No grade 5 CRS or ICANS was reported in either group. More patients in the brexu-cel group received tocilizumab for treatment of toxicities (n = 14 [70%]) than tisa-cel recipients (n = 12 [24%]; P = .04). Steroids were more commonly used in the brexu-cel group (n = 12 [48%]) than in tisa-cel recipients (n = 5 [10%]; P = .09).
Toxicity frequency including CRS, ICANS, neutropenia, and infections
| . | Tisa-cel (n = 50) . | Brexu-cel (n = 20) . | P value . |
|---|---|---|---|
| CRS any grade, n (%) | 28 (56) | 17 (85) | .01 |
| Maximum CRS grade, n (%) | |||
| 1 | 9 | 9 | .46 |
| 2 | 10 | 6 | |
| ≥3 | 9 | 2 | .41 |
| 3 | 6 | 2 | |
| 4 | 3 | 0 | |
| 5 | 0 | 0 | |
| ICANS any grade, n (%) | 9 (18) | 8 (40) | .05 |
| Maximum ICANS grade, n (%) | |||
| 1 | 5 | 1 | |
| 2 | 2 | 0 | |
| ≥3 | 2 | 7 | <.001 |
| 3 | 2 | 6 | |
| 4 | 0 | 1 | |
| 5 | 0 | 0 | |
| Patients receiving tocilizumab, n (%) | 12 (24) | 14 (70) | .04 |
| No. of tocilizumab doses, median (range) | 1 (1-4) | 1 (1-3) | |
| Patients receiving steroids, n (%) | 5 (10) | 12 (48) | .09 |
| Steroid exposure, median (range) | 0 days (0-16) | 13 mg (0-840) | |
| Peak serum ferritin, median (range), ng/mL | 3470 (150-238 520) | 1806 (11-28 587) | |
| Peak C-reactive protein, median (range), mg/dL | 11.4 (0-306) | 26 (0.4-285) | |
| Neutropenia after infusion | |||
| Grade 4 neutropenia, n (%) | 29 (58) | 16 (80) | .08 |
| Duration of grade 4 neutropenia, median (range), d | 13.5 (1-75) | 14 (3-39) | |
| Infections within 30 days of CAR T, n (%) | |||
| Any infection | 12 (24) | 5 (25) | .99 |
| Fungal infection | 2 (4) | 1 (5) | .88 |
| Hospital length of stay, median (range), d | 13.5 (0-53) | 14 (0-55) |
| . | Tisa-cel (n = 50) . | Brexu-cel (n = 20) . | P value . |
|---|---|---|---|
| CRS any grade, n (%) | 28 (56) | 17 (85) | .01 |
| Maximum CRS grade, n (%) | |||
| 1 | 9 | 9 | .46 |
| 2 | 10 | 6 | |
| ≥3 | 9 | 2 | .41 |
| 3 | 6 | 2 | |
| 4 | 3 | 0 | |
| 5 | 0 | 0 | |
| ICANS any grade, n (%) | 9 (18) | 8 (40) | .05 |
| Maximum ICANS grade, n (%) | |||
| 1 | 5 | 1 | |
| 2 | 2 | 0 | |
| ≥3 | 2 | 7 | <.001 |
| 3 | 2 | 6 | |
| 4 | 0 | 1 | |
| 5 | 0 | 0 | |
| Patients receiving tocilizumab, n (%) | 12 (24) | 14 (70) | .04 |
| No. of tocilizumab doses, median (range) | 1 (1-4) | 1 (1-3) | |
| Patients receiving steroids, n (%) | 5 (10) | 12 (48) | .09 |
| Steroid exposure, median (range) | 0 days (0-16) | 13 mg (0-840) | |
| Peak serum ferritin, median (range), ng/mL | 3470 (150-238 520) | 1806 (11-28 587) | |
| Peak C-reactive protein, median (range), mg/dL | 11.4 (0-306) | 26 (0.4-285) | |
| Neutropenia after infusion | |||
| Grade 4 neutropenia, n (%) | 29 (58) | 16 (80) | .08 |
| Duration of grade 4 neutropenia, median (range), d | 13.5 (1-75) | 14 (3-39) | |
| Infections within 30 days of CAR T, n (%) | |||
| Any infection | 12 (24) | 5 (25) | .99 |
| Fungal infection | 2 (4) | 1 (5) | .88 |
| Hospital length of stay, median (range), d | 13.5 (0-53) | 14 (0-55) |
Grade 4 neutropenia (absolute neutropenil count, <0.5 x 103/μL) was more common within 30 days of infusion of brexu-cel: 80% of brexu-cel patients (n = 16) vs 58% of tisa-cel patients (n = 29; P = .08). The median duration of grade 4 neutropenia was similar at 14 days (range, 3-39) with brexu-cel and 13.5 days (range, 1-75) with tisa-cel. Frequency of infection of any severity by day 30 was similar between groups, at 25% (n = 5) in brexu-cel patients and 24% (n = 12) in tisa-cel patients (P = .99). Fungal infections by day 30 were uncommon (brexu-cel, n = 1; tisa-cel, n = 2).
Response and survival
The best ORR was 80% for patients receiving brexu-cel and 88% for patients receiving tisa-cel (P = .39). In both groups, most patients attaining CR achieved undetectable MRD remission (brexu-cel, 70%; tisa-cel, 86%), with only 2 patients in each group reported to be in CR with detectable MRD by flow cytometry. Including only patients with detectable MRD by flow and those with morphologic disease (≥5% BM blasts) at infusion, 87% of patients receiving brexu-cel and 91% of those receiving tisa-cel achieved CR (P = .68).
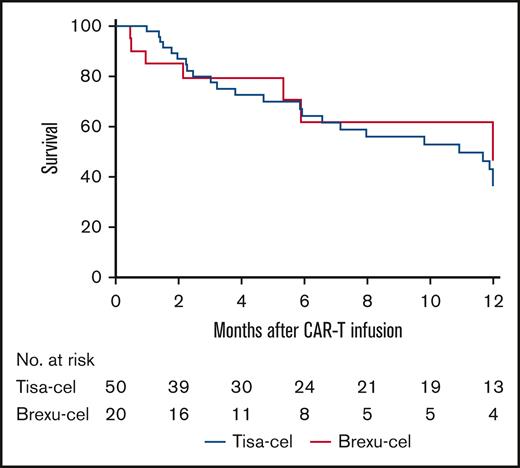
With a median follow-up period of 9.9 months for brexu-cel and 12.7 months for tisa-cel, 24 patients ultimately relapsed after infusion: 6 (30%) in the brexu-cel group vs 18 (36%) in the tisa-cel group (P = .63). Excluding 2 brexu-cel patients with recurrent disease before day 29, median time to relapse for brexu-cel recipients was 5.4 months (range, 1-6) compared with 3.9 months in the tisa-cel group (range, 1-13). Twelve-month RFS was 46% (95% CI, 31-81) for patients receiving brexu-cel and 36% (95% CI, 26-52) for patients receiving tisa-cel (Figure 1; HR, 1.12; 95% CI, 0.50-2.54; P = .79; RFS not censored for HCT shown in supplemental Figure 2A). Median RFS was 12 months (95% CI, 5.3 to not available [NA]) in the brexu-cel group and 11 months (95% CI, 5.9 to NA) in the tisa-cel group (HR, 0.91; 95% CI, 0.39-2.11). Most relapses were CD19+ in both groups. One CD19– relapse (17%) was reported in the brexu-cel group in a patient who did not achieve CR at day 28, whereas 6 CD19– relapses (33%) were reported in the tisa-cel group.
RFS at 12 months among YA patients receiving tisa-cel compared with those receiving brexu-cel. RFS was 36% for YA patients receiving tisa-cel (95% CI, 26-52) and 46% for those receiving brexu-cel 46% (95% CI, 31-81); HR, 1.12; 95% CI, 0.50-2.54; P = .79. RFS was censored for allogeneic stem cell transplant in remission and last follow-up.
RFS at 12 months among YA patients receiving tisa-cel compared with those receiving brexu-cel. RFS was 36% for YA patients receiving tisa-cel (95% CI, 26-52) and 46% for those receiving brexu-cel 46% (95% CI, 31-81); HR, 1.12; 95% CI, 0.50-2.54; P = .79. RFS was censored for allogeneic stem cell transplant in remission and last follow-up.
Eighteen patients died, including 3 (15%) in the brexu-cel group and 15 (30%) in the tisa-cel group. Causes of death included relapsed disease (brexu-cel, n = 1 at day 25; tisa-cel, n = 12 with median time to death 4.3 months [range, 1.4-23.9]) and infection (brexu-cel, n = 2 at 1 month and 2.2 months; tisa-cel, n = 2 at 2.3 months and 11.1 months); 1 patient in the tisa-cel group died at 11 months after CAR T owing to acute respiratory distress syndrome after HCT. No deaths were directly attributed to CRS or ICANS.
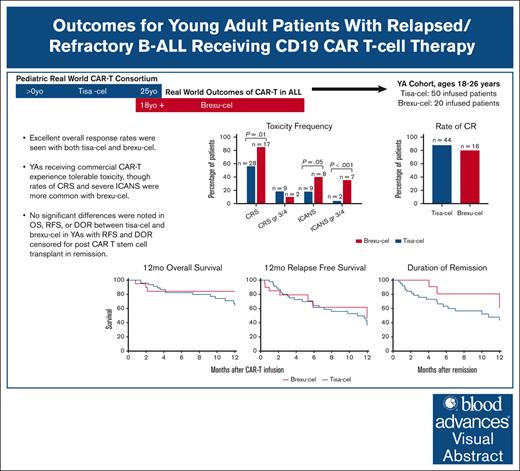
Twelve-month OS was 84% (95% CI, 81-100) in the brexu-cel group vs 68% (95% CI, 56-85) in the tisa-cel group (Figure 2; HR, 1.8; 95% CI, 0.62-5.19; P = .35). The median survival was not reached in the brexu-cel group (95% CI, 18.2 to NA) and was 23.5 months in the tisa-cel group (HR, 1.7; 95% CI, 0.57-4.80; P = .42).
OS at 12 months among YA patients receiving tisa-cel compared with those receiving brexu-cel. OS was 68% for YA patients receiving tisa-cel (95% CI, 56-85) and 84% for those receiving brexu-cel (95% CI, 81-100); HR, 1.8; 95% CI, 0.62-5.19; P = .35. OS was censored for the last follow-up.
OS at 12 months among YA patients receiving tisa-cel compared with those receiving brexu-cel. OS was 68% for YA patients receiving tisa-cel (95% CI, 56-85) and 84% for those receiving brexu-cel (95% CI, 81-100); HR, 1.8; 95% CI, 0.62-5.19; P = .35. OS was censored for the last follow-up.
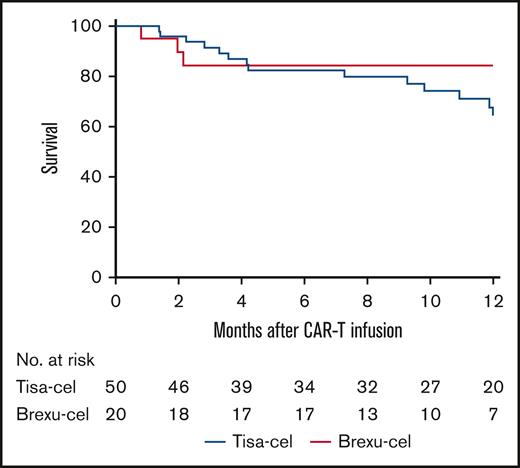
Eleven patients underwent post-CAR T HCT in remission (brexu-cel, n = 7 [35%]; tisa-cel, n = 4 [8%]). With DOR censored for the patients who received HCT in remission, 61% of brexu-cel patients (95% CI, 41-93) who achieved CR maintained remission at 12 months after infusion compared with 41% of tisa-cel patients (95% CI, 27-61) (Figure 3; HR, 2.55; 95% CI, 1.01-6.42; P = .12; DOR not censored for HCT shown in supplemental Figure 2B). The median DOR was not reached among brexu-cel patients and was 10.8 months among tisa-cel patients (95% CI, 5 to NA).
DOR at 12 months among YA patients achieving remission who received tisa-cel compared with those who received brexu-cel. DOR was 41% for YA patients receiving tisa-cel (95% CI, 27-61) and 61% for those receiving brexu-cel (95% CI, 41-93); HR, 2.55; 95% CI, 1.01-6.42; P = .12. DOR was censored for allogeneic stem cell transplant in remission and last follow-up. Time 0 was defined as day 28 after CAR T infusion, which was the first time point of established remission.
DOR at 12 months among YA patients achieving remission who received tisa-cel compared with those who received brexu-cel. DOR was 41% for YA patients receiving tisa-cel (95% CI, 27-61) and 61% for those receiving brexu-cel (95% CI, 41-93); HR, 2.55; 95% CI, 1.01-6.42; P = .12. DOR was censored for allogeneic stem cell transplant in remission and last follow-up. Time 0 was defined as day 28 after CAR T infusion, which was the first time point of established remission.
There was no reported use of tyrosine kinase inhibitors as post-CAR maintenance therapy in the brexu-cel group, whereas 1 patient with Ph+ B-ALL received tyrosine kinase inhibitors in the tisa-cel group.
Predictors of survival
Multivariate analysis was performed to identify factors associated with OS and RFS (Table 3). Inotuzumab was associated with inferior OS and RFS with HRs of 6.32 (95% CI, 1.48-27.0; P = .01) and 3.65 (95% CI, 1.41-9.46; P = .008), respectively. Lower disease burden at infusion was associated with improved OS compared to high disease burden (HR, 0.23; 95% CI, 0.06-0.86; P = .03) and trended toward improved RFS although this did not reach statistical significance (HR, 0.47; 95% CI, 0.20-1.10; P = .08). CAR T product was not found to be significantly associated with OS (HR, 0.71; 95% CI, 0.18-2.75; P = .62), RFS (HR, 1.01; 95% CI, 0.4-2.52; P = .98), or DOR (HR, 0.85; 95% CI, 0.26-2.74; P = .78). Leukemia subtype and pre-CAR T HCT had no significant impact on efficacy outcomes in multivariate analysis.
Multivariate analysis
| Characteristic . | OS . | RFS . | DOR . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| CAR T product: brexu-cel vs tisa-cel | 0.71 | 0.18-2.75 | .62 | 1.01 | 0.40-2.52 | .98 | 0.85 | 0.26-2.74 | .78 |
| Age, >21 vs ≤21 years | 1.08 | 0.36-3.24 | .90 | 1.59 | 0.68-3.72 | .29 | 1.36 | 0.46-4.01 | .57 |
| ALL subtype, Ph– vs Ph+ or Ph-like | 1.48 | 0.46-4.81 | .51 | 0.79 | 0.34-1.80 | .57 | 0.50 | 0.19-1.32 | .16 |
| Marrow disease before CAR T | |||||||||
| MRD− or MRD+ vs ≥5% blasts | 0.23 | 0.06-0.86 | .03 | 0.47 | 0.20-1.10 | .08 | 0.79 | 0.29-2.15 | .64 |
| Therapy before CAR T | |||||||||
| Allogeneic HCT, yes vs no | 0.54 | 0.20-2.33 | .38 | 0.47 | 0.17-1.30 | .15 | 0.43 | 0.12-1.53 | .20 |
| Blinatumomab, yes vs no | 0.23 | 0.05-1.17 | .08 | 0.33 | 0.11-1.02 | .05 | 0.42 | 0.10-1.75 | .23 |
| Inotuzumab, yes vs no | 6.32 | 1.48-27.0 | .01 | 3.65 | 1.41-9.46 | .008 | 2.17 | 0.66-7.18 | .21 |
| Characteristic . | OS . | RFS . | DOR . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| CAR T product: brexu-cel vs tisa-cel | 0.71 | 0.18-2.75 | .62 | 1.01 | 0.40-2.52 | .98 | 0.85 | 0.26-2.74 | .78 |
| Age, >21 vs ≤21 years | 1.08 | 0.36-3.24 | .90 | 1.59 | 0.68-3.72 | .29 | 1.36 | 0.46-4.01 | .57 |
| ALL subtype, Ph– vs Ph+ or Ph-like | 1.48 | 0.46-4.81 | .51 | 0.79 | 0.34-1.80 | .57 | 0.50 | 0.19-1.32 | .16 |
| Marrow disease before CAR T | |||||||||
| MRD− or MRD+ vs ≥5% blasts | 0.23 | 0.06-0.86 | .03 | 0.47 | 0.20-1.10 | .08 | 0.79 | 0.29-2.15 | .64 |
| Therapy before CAR T | |||||||||
| Allogeneic HCT, yes vs no | 0.54 | 0.20-2.33 | .38 | 0.47 | 0.17-1.30 | .15 | 0.43 | 0.12-1.53 | .20 |
| Blinatumomab, yes vs no | 0.23 | 0.05-1.17 | .08 | 0.33 | 0.11-1.02 | .05 | 0.42 | 0.10-1.75 | .23 |
| Inotuzumab, yes vs no | 6.32 | 1.48-27.0 | .01 | 3.65 | 1.41-9.46 | .008 | 2.17 | 0.66-7.18 | .21 |
Discussion
CAR T has revolutionized the treatment landscape for children and adults with R/R B-ALL.13,26-28 Although YAs (aged 18-25 years) are eligible to receive either tisa-cel or brexu-cel, to our knowledge this is the first multi-institutional analysis examining real-world clinical outcomes of CD19 CAR T among YAs. This work leverages data from 2 large consortia: ROCCA for adults and PRWCC for pediatrics.21,23,29 Median age reported in the ZUMA-3 and ELIANA studies was 40 and 11 years, respectively, limiting the ability to assess outcomes among YAs enrolled in the registrational studies.14,30 Our data confirm tolerability, high response rates, and encouraging survival among YAs, irrespective of the CAR T construct used.
The patient cohort analyzed here differs from the registrational studies in several important ways. Most patients in this study were in morphologic remission at the time of CAR T infusion, whereas ZUMA-3 and ELIANA required morphologic disease with >5% blasts.31 This reflects real-world practice to use CAR T in the context of a lower disease burden. Although limited by a small number of patients, we observed an ORR of >80% in those with morphologic disease before CAR T infusion, similar to registrational studies. Although baseline patient and disease characteristics were similar between recipients of tisa-cel and brexu-cel, there were more patients with previous HCT in the tisa-cel cohort, reflecting the evolving treatment paradigm of R/R B-ALL.
This analysis includes 39% of Hispanic patients (n = 27), mirroring the ethnic distribution of ALL in the United States. Given that Ph-like ALL is more common among Hispanic patients, future studies evaluating outcomes in this patient population are needed.6,17 Outcomes for adults with B-ALL treated in the frontline setting are worse than childhood ALL in part because of a higher incidence of high-risk ALL subsets among adults.32 Despite the enrichment of Ph-like disease among Hispanic and YA patients, ALL subtype was not associated with efficacy end points in our analysis. In a recent study examining outcomes of HCT after CAR T, the presence of Ph-like genotype was associated with inferior RFS.22
The rates of any grade CRS were lower than observed in registrational studies, likely reflecting less toxicity in patients with lower disease burden and improvements in toxicity management. Although there was a higher rate of CRS among brexu-cel patients, rates of severe CRS were similar between constructs. Any grade ICANS was observed in 24% of patients, which is favorable compared with the 40% and 60% reported in the ELIANA and ZUMA-3 studies, respectively.13,14 Despite overall lower rates of ICANS, brexu-cel recipients had higher grade ICANS with 35% experiencing grade ≥3 neurotoxicity than 4% of tisa-cel recipients. Toxicities in patients treated with tisa-cel were graded using various scoring systems, including ASTCT, CAR T cell–related encephalopathy syndrome, and institutional standards, and may therefore underestimate rates of severe ICANS.25 Nonetheless, the ROCCA consortia reported high rates of severe ICANS warranting further analysis, given that these patients may benefit from novel approaches for toxicity prevention.22,23 Notably, other key end points such as duration of grade 4 neutropenia, infection rates, and hospitalization length were similar between the 2 groups despite differences in immune-mediated toxicities. Because of the relatively small number with severe immune-mediated toxicities, we did not examine variables associated with toxicity. Further studies in this age group examining the association among toxicities, CAR T-cell expansion and persistence, and correlation with efficacy end points are needed. Moreover, survivorship is a particularly pressing issue for YAs because this age range represents a key stage of physical and psychosocial development.33 Studies examining patient-reported outcomes and long-term sequela of immune-mediated toxicities are warranted for this patient population.
41BB CAR T constructs such as tisa-cel are associated with longer persistence than CD28 costimulatory domains.15 We did not observe any differences in efficacy between tisa-cel and brexu-cel, underscoring that persistence alone may not explain the durability of response, although we do not have data on CAR T persistence or duration of B-cell aplasia (BCA) in this study. Loss of BCA, which has been used as a surrogate measure of loss of CD19 CAR T persistence, has been associated with a high risk of relapse in recipients of 41BB products.34,35 However, the significance of BCA and CAR T persistence remains unclear for patients treated with CD28 CAR constructs. In an updated analysis of ZUMA-3, CAR T cells were undetectable among all 4 patients in ongoing remission, suggesting that CAR T persistence may not be required for durable responses.27 However, in another study using a CD28 CAR, consolidative HCT was associated with significantly improved outcomes with no observed durable remissions in patients who did not proceed to transplant.17 In our study, 11 patients underwent consolidative transplants, most of whom were brexu-cel recipients. Duration of response favored brexu-cel; however, that benefit was not observed when censoring for HCT. The role of HCT in those who achieve undetectable MRD CR with CAR T remains unanswered and is of particular importance for YAs who are typically fit for HCT. Efforts such as the ongoing CAR CURE study (CinicalTrials.gov identifier: NCT05621291) are eagerly anticipated to determine who would benefit most from transplant after CAR T.
Differences in efficacy may emerge with longer follow-up times, particularly in the brexu-cel cohort that had a shorter median follow-up. We observed nonsignificant trends toward improvements in efficacy among brexu-cel recipients, which could become significant with longer follow-up. Given the 4-year gap between FDA approvals for tisa-cel and brexu-cel, data sets from PRWCC and ROCCA are not contemporaneous, highlighting a key limitation of our analysis. Most patients in the tisa-cel group from the PRWCC were treated between August 2017 and March 2020 compared with brexu-cel patients treated between October 2021 and August 2023. This may explain why patients in the PRWCC were more likely to have undergone HCT before CAR T. In our analysis, patients were treated in a variety of settings, which may have led to differences in practice for both pre-CAR treatment and use of consolidative HCT.
The treatment landscape of ALL has evolved with the integration of immunotherapy such as blinatumomab in upfront regimens, raising questions regarding optimal sequencing of therapy.36 In our report, previous use of blinatumomab trended toward improvement in RFS, which differs from the ZUMA-3 data.27 In the pediatric setting, data demonstrate that although pre-CAR T blinatumomab does not affect post-CAR T outcomes, nonresponse to blinatumomab is associated with inferior RFS and event-free survival.37 However, the depth of response to blinatumomab given before CAR-T infusion is unclear in this data set. Inotuzumab exposure before CAR T was associated with inferior OS and RFS in our multivariate analysis in line with recently reported results from ROCCA.22 Inotuzumab use before CAR T was similar among tisa-cel (26%) and brexu-cel recipients (30%) with 2 patients in each cohort receiving inotuzumab as a bridging therapy. Analysis from ROCCA demonstrated that the timing of inotuzumab (bridging vs apheresis) did not affect outcomes although one could postulate that it may affect apheresis yield.22 In this analysis, we did not capture response to inotuzumab, and therefore, more granular data are needed to determine whether inotuzumab-exposed patients have more adverse disease biology.22 In a study of mostly pediatric patients (median age of 13 years), inotuzumab before CAR T did not negatively affect efficacy outcomes.38 Further studies are needed to guide optimal sequence of therapy and bridging strategies.
The limitations of this study include its retrospective nature, a relatively small patient population with morphologic disease, short follow-up period, and differences in reporting periods between the tisa-cel and brexu-cel cohorts. We focused the analysis on patients who were eligible to receive either CAR T product, which limits the evaluation of outcomes in the full AYA population (ages 15-39 years). The recent approval of obecabtagene autoleucel for R/R B-ALL based on findings from the phase 1b/2 FELIX trial underscores importance of further studies examining the preferred CAR T product among AYAs.39
MRD testing in terms of both modality and timing was not uniform across sites. Although most patients in this study received bridging therapy, data on response to bridging were lacking in a subset of patients. Disease assessment was captured at the most proximal time point before CAR-T infusion, and thus, disease burden may have changed in some patients who had bridging therapy, limiting examination of optimal sequencing strategies and the impact of disease burden immediately preceding infusion.
Importantly, CAR T is provided at mostly tertiary care centers, limiting access to this novel therapy for the YA patient and ethnic minority populations who experience substantial barriers to care.4,12 Given the efficacy of commercial CD19 CAR T in this age group, further studies are needed to examine accessibility and barriers to CAR T among eligible YAs with B-ALL. Despite these limitations, we demonstrate that commercial CD19 CAR T is effective and safe among YAs irrespective of the product used.
Acknowledgments
The authors acknowledge the following individuals for their roles in supporting this study: for legal contracting, Joshua Murphy, Daniel Hernandez, and Kristine Martens-Ackeret, and for administrative support, Anika Lagto and Daisy Torres. The authors acknowledge the Stanford REDCap platform developed and operated by the Stanford Medicine Research information technology team. The REDCap platform services at Stanford are subsidized by Stanford School of Medicine Research Office and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH).
This work was partially supported by a grant from the Auxiliaries Foundation.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authorship
Contribution: H.L., R.F., L.M., L.M.S., G.W.R., and I.A. designed this study and were involved in data interpretation and editing; S.K. performed statistical analysis; K.N. and K.C.S. provided project administration and management support; L.M.S. and L.M. contributed equally to the study design and supervision; H.L. and R.F wrote the manuscript; and all authors were involved in data collection, manuscript revision, and final manuscript approval and are accountable for all aspects of this work.
Conflict-of-interest disclosure: L.M.S. has served on advisory committees for Novartis and Cargo Therapeutics. I.A. has served as a consultant for Kite, Sobi, Jazz, Pfizer, Amgen, and Takeda; received honoraria from Takeda; served on advisory committees for Amgen, Pfizer, Jazz, Kite, Takeda, Syndax, Sobi, and Wugen; and received research support from AbbVie and MacroGenics. J.R. has served as a consultant for Novartis. K.J.C. has served as a consultant for Novartis; served as a board member for Turn Bio and PromiCell; and received research funding from Novartis, Cellectis, and Atara Biotherapeutics. C.L.P. has served on an advisory committee for Novartis. S.H.C.B.’s spouse is employed by Takeda. S.C. has served as a consultant for Pfizer and Jazz. M.H. has served on advisory committees for Sobi and Novartis. P.S. has served on an advisory committee for Sobi. M.Q. has served as a consultant for Novartis and Mesoblast. R.D.C. has served on advisory committees for Autolus and PeproMene Bio; served as a consultant and received honoraria for Autolus, Amgen, Jazz, Kite, and Pfizer; and received research funding from Amgen, Kite, Incyte, Merck, Pfizer, Servier, and Vanda, and R.D.C.’s spouse was employed by and owned stock in Seagen. V.K.K. has received honoraria from Pfizer, Novartis, and Kite, and received research funding from Incyte. P.S. received honoraria from Autolus and Bristol Myers Squibb (BMS), and served on a speaker’s bureau for Sanofi. M.S. served as a consultant for Jazz and Novartis. A.L. served as a consultant for Pfizer and CTI BioPharma. G.Y. served on a speaker’s bureau for Jazz, Kite, BMS, Incyte, Sobi, GlaxoSmithKline, and Servier. V.B. served on advisory committees for AstraZeneca, Allogene, BeiGene, and CRISPR, and received research funding from Citius and Incyte. E.C. served as a consultant for AbbVie. A.S.A. served on advisory committees for Jazz, Taiho, and Novartis; received honoraria from Kite, Pfizer, Jazz, Beam, Nkarta, Novartis, Kura, and Amgen; and received research funding from Servier, ImmunoGen, GlycoMimetics, Pfizer, Incyte, Seattle Genetics, and MacroGenics. B.S. received research funding from Incyte, Jazz, Kite, and Servier; received honoraria from Janssen, Spectrum/Acrotech, BeiGene, and Gilead Sciences; served as a consultant for PeproMene, Lilly, Autolus, Deciphera, Century Therapeutics, Jazz, Kite, Precision Biosciences, Amgen, Pfizer, Novartis, BMS, Adaptive Biotechnologies, AstraZeneca, and Takeda; and served on an advisory committee for PeproMene Bio. L.M. served as a consultant for Amgen, Pfizer, Kite, Autolus, and Astellas; received honoraria from Kite; received research funding from BMS, Adaptive, Kite, Astellas, Orca Bio, and Jasper; and served on an advisory committee for Adaptive. R.F. received research funding from Kite and Novartis; and served on an advisory committee for Kite. The remaining authors declare no competing financial interests.
Correspondence: Hannah Lust, Division of Hematology, Oncology, Neuro-Oncology, and Stem Cell Transplantation, Ann & Robert H. Lurie Children’s Hospital, 225 E Chicago Ave, Box 30, Chicago, IL 60611; email: hlust@luriechildrens.org.
References
Author notes
Original data are available on request from the corresponding author, Hannah Lust (hlust@luriechildrens.org).
The full-text version of this article contains a data supplement.