Key Points
Tocilizumab was associated with decreased pre-engraftment syndrome, delayed myeloid recovery, and a nonsignificant reduction in aGVHD.
The use of tocilizumab had no survival benefit, thereby supporting alternative approaches to mitigate the aGVHD burden after dCBT.
Visual Abstract
Double-unit cord blood transplantation (dCBT) has been associated with high rates of progression-free survival (PFS) in adults with hematologic malignancies but also with relatively high rates of acute graft-versus-host disease (aGVHD). We conducted a single-arm, phase 2 clinical trial that investigated the addition of tocilizumab, an interleukin-6 receptor blocker, to cyclosporine-A (CSA) and mycophenolate mofetil (MMF) for aGVHD prophylaxis after intermediate-intensity dCBT. A total of 45 patients (median age, 47 years; range, 27-60 years; 80% acute leukemia; median hematopoietic cell transplant-comorbidity index, 2) were enrolled from March 2018 to March 2021. Transplant outcomes were compared with 39 previous CSA and MMF dCBT controls with similar inclusion criteria. Tocilizumab recipients had less pre-engraftment syndrome (38%; 95% confidence interval [CI], 24-52 vs 72%; 95% CI, 54-84; P < .001) but inferior day 45 neutrophil engraftment (93%; median, 25.5 days vs 97%; median, 22 days; P = .009]. The primary end point of day 100 grade 2 to 4 aGVHD was no different between groups (71%; 95% CI, 55-82 with tocilizumab vs 82%; 95% CI, 65-91; P = .11). However, there was a trend toward a lower day 100 incidence of stage 1 to 4 lower gastrointestinal aGVHD with tocilizumab (16%; 95% CI, 7-28 vs 33%; 95% CI, 19-48; P = .059). There were no significant differences in the 3-year incidences of relapse, transplant-related mortality, PFS, or overall survival between the groups. Tocilizumab recipients exhibited a distinct pattern of gut microbiome disruption. In summary, tocilizumab-based GVHD prophylaxis delayed neutrophil recovery without a significant reduction in aGVHD and had no survival benefit after dCBT. Investigation of alternative strategies to prevent severe aGVHD after dCBT is warranted. This trial was registered at www.clinicaltrials.gov as #NCT03434730.
Introduction
Cord blood (CB) is a valuable alternative stem cell source for patients in need of hematopoietic stem cell transplantation (HSCT) without readily available, HLA-matched, adult donors.1-3 Double-unit CB transplantation (dCBT) is standard therapy for adults with high-risk hematologic malignancies, and, in many series, dCBT has been associated with similar survival outcomes as HLA-matched donor HSCTs and enhanced protection against relapse.4-7 However, dCBT has also been associated with relatively high rates of acute graft-versus-host disease (aGVHD). Although there may be a survival advantage associated with mild aGVHD,8,9 severe aGVHD can mediate posttransplant morbidity and transplant-related mortality (TRM).4,10 Anti-thymocyte globulin (ATG) has been associated with delayed immune reconstitution and higher TRM after CBT11-15 and, therefore, is now considered contraindicated in adult patients. Consequently, investigating alternative nonlymphodepleting strategies to mitigate the aGVHD burden after dCBT is warranted.
Interleukin 6 (IL-6), a proinflammatory cytokine, has been implicated in the pathogenesis of aGVHD.16,17 Moreover, attenuation of aGVHD via IL-6 signal inhibition has been demonstrated in murine models through increased production of regulatory T-cells or direct reduction of IL-6-induced inflammation in target organs, such as the gastrointestinal (GI) tract.16,17 In humans, high serum IL-6 levels early after transplant have been associated with aGVHD, fever, sepsis, and engraftment syndrome.18-25 Accordingly, IL-6 receptor (IL-6R) blockade with tocilizumab (a humanized anti-IL-6R antibody that binds soluble- and membrane-bound IL-6R) has been investigated for the prevention and treatment of aGVHD. In the first phase 1/2 trial, a single dose of tocilizumab (8 mg/kg) on day −1 was combined with calcineurin-inhibitor/methotrexate prophylaxis in HLA-matched adult-donor HSCT recipients. This ameliorated grades 2 to 4 and 3 to 4 aGVHD and GVHD-related TRM when compared with historic controls.26 A subsequent phase 2 trial corroborated these findings and showed aGVHD mitigation, especially in the lower GI tract.27 Additional small series have suggested that tocilizumab may be effective aGVHD therapy, especially GI disease.28-32 Despite these promising results, a more recent phase 3 placebo-controlled trial did not meet its primary end point because of the smaller than expected effect size in aGVHD reduction in the tocilizumab arm and no survival benefit.33
Based on the promising preclinical and early clinical data and before availability of the phase 3 clinical trial results, we conducted a single-arm, phase 2 clinical trial that investigated adding tocilizumab to standard cyclosporine-A (CSA)/mycophenolate mofetil (MMF) GVHD prophylaxis for adults undergoing dCBT. Our hypothesis was that tocilizumab would attenuate the incidence and severity of aGVHD after dCBT without compromising patient safety.
Methods
Patients
This phase 2 trial was conducted in accordance with the Declaration of Helsinki and approved by the Memorial Sloan Kettering Cancer Center’s institutional review/privacy board. Patients were enrolled between March 2018 and March 2021. The primary end point was the incidence of grade 2 to 4 aGVHD at day 100. A Simon 2-stage minimax design was initially implemented for this trial with a maximum sample size of 27 (16 + 11) patients. Type 1 and type 2 errors were both set at 0.10. The study was going to be considered promising for further investigation if the day 100 grade 2 to 4 aGVHD rate was 40% or less and unpromising if the rate was 65% or more. Although the primary end point was not met in the first stage of the trial, we observed improvements in key secondary end points. Therefore, after receiving institutional review board approval, the study was amended to expand the sample size to a total of 45 patients to obtain better end point estimates. The primary end point remained the same after the sample size expansion.
The trial enrolled adult patients aged 18 to 65 years with no suitable matched related or unrelated donors in the required timeframe. Eligible diagnoses included acute myelogenous leukemia (AML) in morphologic complete remission (CR) (<5% blasts) or relapsed/refractory with ≤10% blasts, acute lymphoblastic leukemia (ALL) and mixed phenotype acute leukemia (MPAL) in morphologic CR, myelodysplastic syndrome (MDS)/myeloproliferative neoplasm with ≤10% blasts, aggressive lymphomas in metabolic CR, and indolent lymphomas with at least stable disease. Adequate performance status and organ function (Karnofsky Performance Scale ≥ 70; glomerular filtration rate ≥ 60 mL/min/1.73m2; bilirubin <1.5 mg/dL; alanine aminotransferase ≤ 3 × upper limit of normal; diffusion capacity of the lungs for carbon monoxide adjusted for hemoglobin ≥ 50% predicted; left ventricle ejection fraction > 50%; albumin ≥ 3.0 g/dL, and age-adjusted hematopoietic cell transplantation-comorbidity index [aaHCT-CI]34 ≤ 7) were required. The exclusion criteria included myelofibrosis, previous allograft, HIV or human T-lymphotropic virus 1 positivity, and active infection. During the study period, all consecutive patients who met the eligibility criteria were enrolled in the trial, except for 1 owing to patient/physician preference.
We compared the outcomes of trial subjects with 39 dCBT recipients with identical eligibility criteria who previously received dCBT at our center between 2014 and 2017.4 A subanalysis of the stool microbiome in trial and control patients was also performed.
CB graft selection
All patients received a dCB graft, and each unit had a total nucleated cell (TNC) of ≥ 1.5 × 107 per kg, a CD34+ cell dose of ≥ 1 × 105 per kg, and ≥ 3/8 HLA-A, -B, -C, -DRB1 allele donor-recipient HLA match. Unit selection prioritized quality and cryopreserved CD34+ cell dose over 8-allele HLA match, especially in more recent years.35-37 The presence of recipient donor-specific HLA antibodies was considered but was not a contraindication for unit selection.38
Conditioning regimen, GVHD prophylaxis, and supportive care
All patients received intermediate-intensity conditioning consisting of cyclophosphamide 50 mg/kg (day −6), fludarabine 150 mg/m2 (30 mg/m2/day on days −6 to −2), thiotepa 10 mg/kg (5 mg/kg/day on days −5 and −4), and total body irradiation 400 cGy (200 cGy/day on days −2 and −1).4 GVHD prophylaxis consisted of IV CSA and MMF (15 mg/kg every 8 hours [capped at 1.5 g per dose]) starting on day −3 as previously described. Trial patients also received 1 dose of tocilizumab at 8 mg/kg (capped at 800 mg) on day −1.
All patients received antimicrobial prophylaxis with a fluoroquinolone. In addition, per institutional practice, patients received IV vancomycin prophylaxis starting on day −2 (except between August 2019 and November 2020 when omission of vancomycin was evaluated per institution-wide policy). All patients received acyclovir prophylaxis. Letermovir at a dose of 240 mg daily was started on day +7 in cytomegalovirus (CMV) seropositive patients since 2018 and was continued for a minimum of 180 days (insurance permitting), as previously described.39 Micafungin was given from admission and switched to a mold-active azole after day 7 for at least 75 days, or longer in the presence of aGVHD. All patients received pneumocystis jirovecii pneumonia prophylaxis from 3 to 4 weeks after the transplant (timing and type based on toxoplasma serology). Patients received granulocyte colony-stimulating factor at a dose of 5 μg/kg/day from day +7 until neutrophil recovery. Patients with a first neutropenic fever between days 7 and 21, in addition to receiving broader antibiotic coverage as appropriate, were started on IV methylprednisolone at a dose of 1 mg/kg daily for 3 days for pre-engraftment syndrome (PES).40 The corticosteroid course could be extended for persistent signs or symptoms of PES. CMV, Epstein-Barr virus (EBV), human herpesvirus 6 (HHV-6), and adenovirus viral loads in peripheral blood were monitored using polymerase chain reaction after transplant according to institutional guidelines. Treatment for CMV reactivation was considered for any detected viral load before institution of letermovir prophylaxis, and for levels > 300 copies in letermovir recipients. Treatment for EBV, HHV-6, and adenovirus reactivation was based on viral load kinetics and levels and on concern for organ involvement.
Study definitions
HCT-CI41 and aaHCT-CI34 were used to classify patient comorbidities. Neutrophil recovery was defined as the first of 3 consecutive days with an absolute neutrophil count of ≥ 0.5 × 109 per L. Graft failure was defined as lack of donor-derived neutrophil recovery by day 45. PES was defined as fever ≥38°C with negative infectious workup and/or skin rash not attributable to a drug reaction before engraftment.40 Diagnosis of aGVHD was predominantly clinical but a biopsy was pursued when clinically indicated; aGVHD staging and grading were done according to the International Bone Marrow Transplant Registry criteria.42 For chronic GVHD (cGVHD), the National Institutes of Health consensus criteria were used.43 The diagnosis and grading of aGVHD and cGVHD were adjudicated by independent patient review by the programmatic GVHD grading panel without the involvement of the investigators of this protocol. TRM was death from any cause other than disease relapse. Cause of death attribution was done by the Copelan algorithm.44 Progression-free survival (PFS) was defined from date of transplant until progression, relapse, or death or until last follow-up. Overall survival (OS) was from date of transplant until death or last follow-up.
Statistical methods
The patient and graft characteristics are presented using descriptive statistics. Such characteristics were compared across groups using the Wilcoxon rank sum test for continuous variables and the Pearson χ2 and Fisher exact tests for categorical variables. Cumulative incidence functions were used to estimate engraftment, PES, GVHD, relapse, and TRM. The competing risks for each outcome were death for engraftment and PES, death or relapse for GVHD, death in the absence of relapse for relapse, and relapse for TRM. Gray’s test was used to compare the cumulative incidence across patient and treatment characteristics. OS and PFS were calculated using the Kaplan-Meier methodology and compared using a log-rank test. Multivariable models for the incidence of neutrophil recovery, platelet recovery, grades 2 to 4 and 3 to 4 aGVHD, TRM, and relapse were estimated using cause-specific hazards for competing risks regression. Multivariable models for OS and PFS were calculated using Cox proportional hazards models. For all multivariable Cox and competing risks regression models, baseline covariates of clinical interest were specified a priori. A 2-sided P value of < .05 was considered significant. R statistical software version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses.
Microbiome
Patients were offered participation in a separate study of the stool microbiome with serial stool sample collection throughout transplantation for sequencing of the genomic 16S ribosomal-RNA gene V5-V5 variable region as previously described.45 Alpha diversity, a measure of taxa diversity within individual samples, was calculated using the Inverse Simpson method and compared over time between the trial and historic patients. Given our previous report on the association between tocilizumab and the mitigation of enterococcal microbiome domination after transplant,46 a mixed-effects model was used to estimate the contribution of tocilizumab to relative enterococcal and, for comparison, Erysipelatoclostridium abundance.45 Mixed-effects models were adjusted for day after transplant and exposure to anti-anaerobic antibiotic agents47 with a random-effects term for individual patients and a random slope for the posttransplant day. To accommodate zero values in log-transformation of relative abundances, an epsilon of 10-7 was added to the relative abundances for each genus and the central log ratio was obtained. Finally, a principal coordinates visualization was constructed at the genus level; samples were assigned to 4 clusters using the k-means method, and the contribution of tocilizumab vs no tocilizumab samples to each cluster was compared using the Kruskal-Wallis test.
Results
Patient and graft characteristics
Between March 2018 and March 2021, 45 patients (Toci group; median age, 47 years; range, 27-60) were enrolled (Table 1). More than half (n = 24, 53%) had non-European ancestry. The median aaHCT-CI was 2 (range, 0-6). The most common diagnosis was acute leukemia (n = 36, 80%; 21 AML, 12 ALL, and 3 MPAL). The remaining patients had MDS (n = 4), CML (n = 2), or non-Hodgkin lymphoma (NHL; n = 3). The disease characteristics are presented in Table 2. The CB units (n = 90) had a median TNC dose of 2.81 × 107 (range, 1.49× 107-7.53× 107) per kg and a median CD34+ dose of 2.26 × 105 (range, 1.02× 105-7.71× 105) per kg. The median unit-recipient 8-allele HLA match was 4/8 (range, 3/8 to 6/8). The median survivor follow-up was 4.6 (range, 1.4-5.7) years.
Comparison of patient and graft characteristics between trial patients and historic controls
| Characteristic . | Toci (n = 45) . | No Toci (n = 39) . | P value . |
|---|---|---|---|
| Age, median (range), y | 47 (27-60) | 47 (24-63) | .6 |
| Sex (male), n (%) | 21 (47%) | 19 (49%) | .9 |
| Weight (kg), median (range) | 79 (54-131) | 74 (49-114) | .4 |
| Ancestry, n (%) | .9 | ||
| European | 21 (47) | 18 (46) | |
| Non-European | 24 (53) | 21 (54) | |
| CMV serostatus, n (%) | .8 | ||
| Positive | 28 (62) | 24 (62) | |
| Negative | 14 (31) | 14 (36) | |
| Equivocal | 3 (7) | 1 (3) | |
| HCT-CI, n (%) | .11 | ||
| 0 | 17 (38) | 6 (15) | |
| 1 | 5 (11) | 9 (23) | |
| 2 | 8 (18) | 8 (21) | |
| ≥3 | 15 (33) | 16 (41) | |
| HCT-CI, median (range) | 2 (0-6) | 2 (0-6) | |
| aaHCT-CI, n (%) | .2 | ||
| 0 | 5 (11) | 2 (5) | |
| 1 | 14 (31) | 5 (13) | |
| 2 | 7 (16) | 11 (28) | |
| 3 | 6 (13) | 9 (23) | |
| ≥4 | 13 (29) | 12 (31) | |
| aaHCT-CI, median (range) | 2 (0-6) | 3 (0-7) | |
| Diagnosis, n (%) | .12 | ||
| AML | 21 (47) | 29 (74) | |
| ALL | 12 (27) | 4 (10) | |
| MPAL | 3 (7) | 1 (3) | |
| MDS | 4 (9) | 1 (3) | |
| CML | 2 (4) | 3 (8) | |
| NHL | 3 (7) | 1 (3) | |
| Cryopreserved TNC (per kg × 107 per unit), median (range)∗ | 2.81 (1.49-7.53) | 3.02 (1.54-8.07) | .4 |
| Cryopreserved CD34+ (per kg × 105 per unit), median (range)∗ | 2.26 (1.02-7.71) | 2.27 (1.11-8.34) | .8 |
| 8-allele HLA match∗, n (%) | .5 | ||
| 3 | 10 (11) | 11 (14) | |
| 4 | 36 (40) | 25 (32) | |
| 5 | 34 (38) | 29 (37) | |
| 6 | 20 (22) | 11 (14) | |
| 7 | 0 (0) | 2 (3) |
| Characteristic . | Toci (n = 45) . | No Toci (n = 39) . | P value . |
|---|---|---|---|
| Age, median (range), y | 47 (27-60) | 47 (24-63) | .6 |
| Sex (male), n (%) | 21 (47%) | 19 (49%) | .9 |
| Weight (kg), median (range) | 79 (54-131) | 74 (49-114) | .4 |
| Ancestry, n (%) | .9 | ||
| European | 21 (47) | 18 (46) | |
| Non-European | 24 (53) | 21 (54) | |
| CMV serostatus, n (%) | .8 | ||
| Positive | 28 (62) | 24 (62) | |
| Negative | 14 (31) | 14 (36) | |
| Equivocal | 3 (7) | 1 (3) | |
| HCT-CI, n (%) | .11 | ||
| 0 | 17 (38) | 6 (15) | |
| 1 | 5 (11) | 9 (23) | |
| 2 | 8 (18) | 8 (21) | |
| ≥3 | 15 (33) | 16 (41) | |
| HCT-CI, median (range) | 2 (0-6) | 2 (0-6) | |
| aaHCT-CI, n (%) | .2 | ||
| 0 | 5 (11) | 2 (5) | |
| 1 | 14 (31) | 5 (13) | |
| 2 | 7 (16) | 11 (28) | |
| 3 | 6 (13) | 9 (23) | |
| ≥4 | 13 (29) | 12 (31) | |
| aaHCT-CI, median (range) | 2 (0-6) | 3 (0-7) | |
| Diagnosis, n (%) | .12 | ||
| AML | 21 (47) | 29 (74) | |
| ALL | 12 (27) | 4 (10) | |
| MPAL | 3 (7) | 1 (3) | |
| MDS | 4 (9) | 1 (3) | |
| CML | 2 (4) | 3 (8) | |
| NHL | 3 (7) | 1 (3) | |
| Cryopreserved TNC (per kg × 107 per unit), median (range)∗ | 2.81 (1.49-7.53) | 3.02 (1.54-8.07) | .4 |
| Cryopreserved CD34+ (per kg × 105 per unit), median (range)∗ | 2.26 (1.02-7.71) | 2.27 (1.11-8.34) | .8 |
| 8-allele HLA match∗, n (%) | .5 | ||
| 3 | 10 (11) | 11 (14) | |
| 4 | 36 (40) | 25 (32) | |
| 5 | 34 (38) | 29 (37) | |
| 6 | 20 (22) | 11 (14) | |
| 7 | 0 (0) | 2 (3) |
Total infused units per group: Toci (n = 90), No Toci (n = 78).
Comparison of disease characteristics in clinical trial patients and historic controls
| Characteristic . | Toci (n = 45) . | No Toci (n = 39) . | P value . |
|---|---|---|---|
| Diagnosis, n (%) | .12 | ||
| AML | 21 (47%) | 29 (74%) | |
| ALL | 12 (27%) | 4 (10%) | |
| MPAL | 3 (7%) | 1 (3%) | |
| MDS | 4 (9%) | 1 (3%) | |
| CML | 2 (4%) | 3 (8%) | |
| NHL | 3 (7%) | 1 (3%) | |
| Diagnosis and disease status, n (%)∗ | |||
| AML/ALL/MPAL | n = 36 | n = 34 | .1 |
| CR1 | 34 (94) | 27 (80) | |
| CR2 | 2 (6) | 6 (18) | |
| Relapsed/refractory | 0 | 1 (3) | |
| MDS | |||
| <5% blasts | 2 (50) | 1 (100) | |
| >5% blasts | 2 (50) | 0 | |
| CML | |||
| Chronic phase CR | 2 (100) | 2 (67) | |
| Accelerated phase | 0 | 1 (33) | |
| NHL | |||
| CR | 2 | 1 | |
| PR | 1 | 0 | |
| Acute leukemia MRD status | n = 36 | n = 34 | .2 |
| CR MRD positive | 7 | 12 | |
| CR MRD negative | 24 | 19 | |
| CR MRD unknown | 5 | 2 | |
| Relapsed/refractory | 0 | 1 | |
| ELN2010 classification for AML | n = 21 | n = 29 | .8 |
| Favorable | 3 | 3 | |
| Intermediate-1 | 7 | 11 | |
| Intermediate-2 | 8 | 8 | |
| Adverse | 3 | 7 | |
| MDS genetic risk | n = 4 | n = 1 | |
| Standard risk | 1 | 1 | |
| Adverse risk | 3 | 0 |
| Characteristic . | Toci (n = 45) . | No Toci (n = 39) . | P value . |
|---|---|---|---|
| Diagnosis, n (%) | .12 | ||
| AML | 21 (47%) | 29 (74%) | |
| ALL | 12 (27%) | 4 (10%) | |
| MPAL | 3 (7%) | 1 (3%) | |
| MDS | 4 (9%) | 1 (3%) | |
| CML | 2 (4%) | 3 (8%) | |
| NHL | 3 (7%) | 1 (3%) | |
| Diagnosis and disease status, n (%)∗ | |||
| AML/ALL/MPAL | n = 36 | n = 34 | .1 |
| CR1 | 34 (94) | 27 (80) | |
| CR2 | 2 (6) | 6 (18) | |
| Relapsed/refractory | 0 | 1 (3) | |
| MDS | |||
| <5% blasts | 2 (50) | 1 (100) | |
| >5% blasts | 2 (50) | 0 | |
| CML | |||
| Chronic phase CR | 2 (100) | 2 (67) | |
| Accelerated phase | 0 | 1 (33) | |
| NHL | |||
| CR | 2 | 1 | |
| PR | 1 | 0 | |
| Acute leukemia MRD status | n = 36 | n = 34 | .2 |
| CR MRD positive | 7 | 12 | |
| CR MRD negative | 24 | 19 | |
| CR MRD unknown | 5 | 2 | |
| Relapsed/refractory | 0 | 1 | |
| ELN2010 classification for AML | n = 21 | n = 29 | .8 |
| Favorable | 3 | 3 | |
| Intermediate-1 | 7 | 11 | |
| Intermediate-2 | 8 | 8 | |
| Adverse | 3 | 7 | |
| MDS genetic risk | n = 4 | n = 1 | |
| Standard risk | 1 | 1 | |
| Adverse risk | 3 | 0 |
ELN2010, European LeukemiaNet 2010 genetic risk.
P values are omitted for MDS, CML, NHL, and MDS genetic risk because of the small sample size.
A total of 39 patients who met identical inclusion criteria and who previously received dCBT at our center between 2014 and 2017 without tocilizumab4 served as the comparison (No Toci group). The patient and graft characteristics were similar between the 2 groups (Tables 1 and 2), although there were relatively more AML and fewer ALL and MDS cases in the No Toci historic controls. The median survivor follow-up in the No Toci group was 7 years (range, 2.7-9.2).
Hematopoietic engraftment
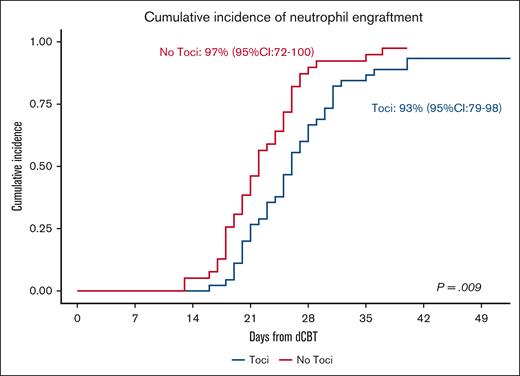
Of the 45 Toci patients, 1 patient died from graft failure in the setting of low post-thaw graft viability and disseminated adenovirus. Two more patients died with incomplete count recovery at 26 and 33 days after transplant because of organ failure and infection, respectively. The remaining 42 patients engrafted at a median of 25.5 (range, 16-40) days for a day 45 cumulative incidence of neutrophil recovery of 93% (95% confidence interval [CI], 79-98) (Figure 1). All 42 patients engrafted platelets (median, 35 days; range, 17-57; day 60 cumulative incidence, 93%; 95% CI, 78-98).
Neutrophil recovery in Toci and No Toci patients. The cumulative incidence of neutrophil recovery was 93% (95% CI, 79-98) in the Toci patient group (median day, 25.5) vs 97% (95% CI, 72-100) in the No Toci patient group (median, 22 days); P = .009.
Neutrophil recovery in Toci and No Toci patients. The cumulative incidence of neutrophil recovery was 93% (95% CI, 79-98) in the Toci patient group (median day, 25.5) vs 97% (95% CI, 72-100) in the No Toci patient group (median, 22 days); P = .009.
Among the 39 No Toci controls, 1 had graft failure in the setting of low graft viability and HLA sensitization. The remaining 38 patients engrafted neutrophils at a median of 22 (range, 13-37) days for a day 45 cumulative incidence of 97% (95% CI, 72-100; P = .009; Figure 1). A total of 37 patients achieved platelet recovery (median, 40 days; range, 24-60) for a day 60 incidence of 95% (95% CI, 77-99; P = .4).
In multivariable analyses controlling for CD34+ cell dose, tocilizumab was associated with delayed neutrophil engraftment (hazard ratio [HR], 0.6; 95% CI, 0.38-0.95; P = .028) but a trend toward faster platelet recovery (HR, 1.55; 95% CI, 0.98-2.46; P = .06; Table 3).
Multivariable analysis of dCBT outcomes
| Variables∗ . | Number . | Multivariate analysis . | |
|---|---|---|---|
| HR (95% CI) . | P value . | ||
| Neutrophil recovery | |||
| Patient group | .028 | ||
| No Toci | 39 | - | |
| Toci | 45 | 0.60 (0.38-0.95) | |
| CD34+ cell dose per kg (continuous) | 84 | 1.21 (1.04-1.41) | .014 |
| Platelet recovery | |||
| Patient group | .060 | ||
| No Toci | 39 | - | |
| Toci | 45 | 1.55 (0.98-2.46) | |
| CD34+ cell dose per kg (continuous) | 84 | 1.34 (1.12-1.61) | .001 |
| Grade 2 to 4 aGVHD | |||
| Patient group | .16 | ||
| No Toci | 39 | - | |
| Toci | 45 | 0.70 (0.43-1.15) | |
| HLA match | .57 | ||
| 3-5 | 68 | - | |
| 6-7 | 16 | 1.19 (0.65-2.20) | |
| Grade 3-4 aGVHD | |||
| Patient group | .15 | ||
| No Toci | 39 | - | |
| Toci | 45 | 0.45 (0.15-1.34) | |
| HLA match | .67 | ||
| 3-5 | 68 | - | |
| 6-7 | 16 | 1.32 (0.37-4.74) | |
| TRM | |||
| Patient group | .46 | ||
| No Toci | 39 | - | |
| Toci | 45 | 1.48 (0.52-4.16) | |
| Age (continuous) | 84 | 1.03 (0.97-1.08) | .31 |
| CD34+ cell dose per kg (continuous) | 84 | 0.88 (0.56-1.37) | .57 |
| Relapse | |||
| Patient group | .11 | ||
| No Toci | 39 | - | |
| Toci | 45 | 2.56 (0.77-8.55) | |
| Diagnosis | .38 | ||
| Acute leukemia | 70 | - | |
| (AML/ALL/MPAL) | |||
| MDS/CML | 10 | 2.79 (0.73-10.6) | |
| NHL | 4 | 1.32 (0.16-10.8) | |
| HLA match | .86 | ||
| 3-5 | 68 | - | |
| 6-7 | 16 | 0.87 (0.19-4.09) | |
| PFS | |||
| Patient group | .17 | ||
| No Toci | 39 | - | |
| Toci | 45 | 1.68 (0.79-3.56) | |
| Age | 84 | 0.99 (0.96-1.03) | .65 |
| CD34+ cell dose per kg | 84 | 0.83 (0.60-1.15) | .24 |
| OS | |||
| Patient group | .17 | ||
| No Toci | 39 | - | |
| Toci | 45 | 1.86 (0.74-4.68) | |
| Age | 84 | 1.01 (0.97-1.06) | .66 |
| CD34+ cell dose/kg | 84 | 0.88 (0.60-1.30) | .51 |
| Variables∗ . | Number . | Multivariate analysis . | |
|---|---|---|---|
| HR (95% CI) . | P value . | ||
| Neutrophil recovery | |||
| Patient group | .028 | ||
| No Toci | 39 | - | |
| Toci | 45 | 0.60 (0.38-0.95) | |
| CD34+ cell dose per kg (continuous) | 84 | 1.21 (1.04-1.41) | .014 |
| Platelet recovery | |||
| Patient group | .060 | ||
| No Toci | 39 | - | |
| Toci | 45 | 1.55 (0.98-2.46) | |
| CD34+ cell dose per kg (continuous) | 84 | 1.34 (1.12-1.61) | .001 |
| Grade 2 to 4 aGVHD | |||
| Patient group | .16 | ||
| No Toci | 39 | - | |
| Toci | 45 | 0.70 (0.43-1.15) | |
| HLA match | .57 | ||
| 3-5 | 68 | - | |
| 6-7 | 16 | 1.19 (0.65-2.20) | |
| Grade 3-4 aGVHD | |||
| Patient group | .15 | ||
| No Toci | 39 | - | |
| Toci | 45 | 0.45 (0.15-1.34) | |
| HLA match | .67 | ||
| 3-5 | 68 | - | |
| 6-7 | 16 | 1.32 (0.37-4.74) | |
| TRM | |||
| Patient group | .46 | ||
| No Toci | 39 | - | |
| Toci | 45 | 1.48 (0.52-4.16) | |
| Age (continuous) | 84 | 1.03 (0.97-1.08) | .31 |
| CD34+ cell dose per kg (continuous) | 84 | 0.88 (0.56-1.37) | .57 |
| Relapse | |||
| Patient group | .11 | ||
| No Toci | 39 | - | |
| Toci | 45 | 2.56 (0.77-8.55) | |
| Diagnosis | .38 | ||
| Acute leukemia | 70 | - | |
| (AML/ALL/MPAL) | |||
| MDS/CML | 10 | 2.79 (0.73-10.6) | |
| NHL | 4 | 1.32 (0.16-10.8) | |
| HLA match | .86 | ||
| 3-5 | 68 | - | |
| 6-7 | 16 | 0.87 (0.19-4.09) | |
| PFS | |||
| Patient group | .17 | ||
| No Toci | 39 | - | |
| Toci | 45 | 1.68 (0.79-3.56) | |
| Age | 84 | 0.99 (0.96-1.03) | .65 |
| CD34+ cell dose per kg | 84 | 0.83 (0.60-1.15) | .24 |
| OS | |||
| Patient group | .17 | ||
| No Toci | 39 | - | |
| Toci | 45 | 1.86 (0.74-4.68) | |
| Age | 84 | 1.01 (0.97-1.06) | .66 |
| CD34+ cell dose/kg | 84 | 0.88 (0.60-1.30) | .51 |
HLA match and CD34+ cell dose refer to engrafted unit.
Pre-engraftment syndrome and acute and chronic GVHD
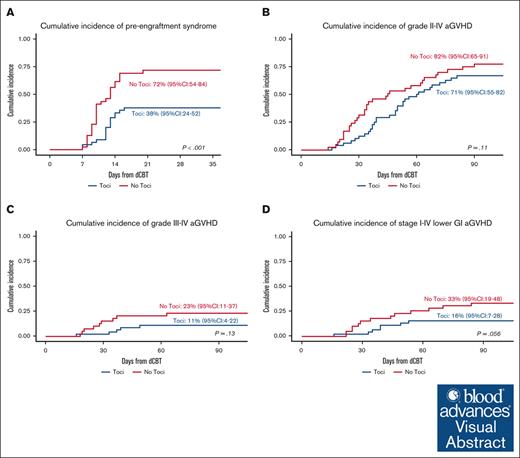
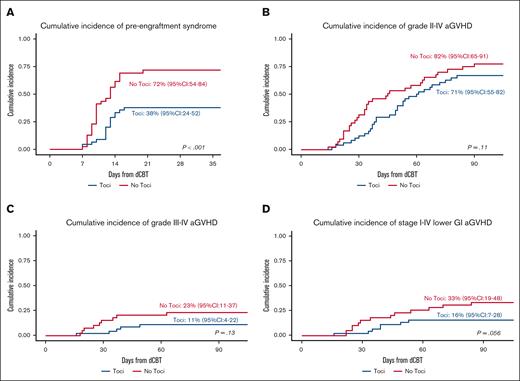
Seventeen of the 45 trial patients developed PES at a median of 12 (range, 7-16) days for a day 21 cumulative incidence of 38% (95% CI, 24-52). Of the 17 Toci patients with PES, only 8 (47%) had fever ≥38°C, and 10 (59%) received IV corticosteroids with only 2 of those requiring >3 days of treatment. In comparison, 28 of the 39 No Toci patients developed PES (median onset, 10 days; range, 7-20) for a day 21 cumulative incidence of 72% (95% CI, 54-84; P < .001; Figure 2A). All of the 28 No Toci patients who developed PES had fever ≥ 38°C, and all 28 (100%) received IV corticosteroids with 9 of those requiring >3 days of treatment. Only 2 Toci and 1 No Toci patients required supplemental oxygen for hypoxia attributed to PES. No patients in either cohort required intensive care unit level of care for PES.
PES and aGVHD in the Toci and No Toci patient groups. (A) The cumulative incidence of PES was of 38% (95% CI, 24-52) in the Toci group vs 72% (95% CI, 54-84) in the No Toci group (P < .001). (B) The cumulative incidence of grade 2 to 4 aGVHD was 71% (95% CI, 55-82) in the Toci group vs 82% (95% CI, 65-91) in the No Toci group (P = .11). (C) The cumulative incidence of grade 3 to 4 aGVHD was 11% (95% CI, 4-22) in the Toci group vs 23% (95% CI, 11-37) in the No Toci group (P = .13). (D) The cumulative incidence of stage 1 to 4 lower GI GVHD by day 100 was 16% (95% CI, 7-28) in the Toci group vs 33% (95% CI, 19-48) in the No Toci group (P = .059).
PES and aGVHD in the Toci and No Toci patient groups. (A) The cumulative incidence of PES was of 38% (95% CI, 24-52) in the Toci group vs 72% (95% CI, 54-84) in the No Toci group (P < .001). (B) The cumulative incidence of grade 2 to 4 aGVHD was 71% (95% CI, 55-82) in the Toci group vs 82% (95% CI, 65-91) in the No Toci group (P = .11). (C) The cumulative incidence of grade 3 to 4 aGVHD was 11% (95% CI, 4-22) in the Toci group vs 23% (95% CI, 11-37) in the No Toci group (P = .13). (D) The cumulative incidence of stage 1 to 4 lower GI GVHD by day 100 was 16% (95% CI, 7-28) in the Toci group vs 33% (95% CI, 19-48) in the No Toci group (P = .059).
The day 100 grades 2 to 4 aGVHD incidence (trial primary end point of the trial) and 3 to 4 aGVHD incidence among Toci patients were 71% (95% CI, 55-82) and 11% (95% CI, 4-22), respectively (Figure 2B-C). The median onset of grade 2 to 4 aGVHD was 49 (range, 16-81) days. The organ distribution of aGVHD stage in the 35 patients who developed aGVHD by day 100 is shown in Table 4. The upper GI tract was the most common organ involved (n = 29, 83%). Seven patients had lower GI involvement (stage 1, n = 3; stage 2, n = 1; stage 3, n = 1; stage 4, n = 2). A total of 14 patients (40%) had skin involvement (stage 1, n = 5; stage 2, n = 7; stage 3, n = 2). Liver involvement was uncommon and limited to patients who also had high stage involvement of the skin or lower GI tract. The day 100 cumulative incidence of stage 1 to 4 lower GI aGVHD was 16% (95% CI, 7-28; Figure 2D).
Toci patients who developed aGVHD by day 100 (n = 35)
| Organ stage . | Skin . | Upper GI . | Lower GI . | Liver . |
|---|---|---|---|---|
| 0 | 21 (60%) | 6 (17%) | 28 (80%)∗ | 32 (91%) |
| 1 | 5 (14%) | 29 (83%) | 3 (9%) | 0 |
| 2 | 7 (20%) | - | 1 (3%) | 1 (3%) |
| 3 | 2 (6%) | - | 1 (3%) | 1 (3%) |
| 4 | 0 | - | 2 (6%) | 1 (3%) |
| Organ stage . | Skin . | Upper GI . | Lower GI . | Liver . |
|---|---|---|---|---|
| 0 | 21 (60%) | 6 (17%) | 28 (80%)∗ | 32 (91%) |
| 1 | 5 (14%) | 29 (83%) | 3 (9%) | 0 |
| 2 | 7 (20%) | - | 1 (3%) | 1 (3%) |
| 3 | 2 (6%) | - | 1 (3%) | 1 (3%) |
| 4 | 0 | - | 2 (6%) | 1 (3%) |
A total of 7 patients had stage 0+ lower GI GVHD (<500 mL/day).
The day 100 grade 2 to 4 and 3 to 4 aGVHD incidence among the No Toci patients was 82% (95% CI, 65-91; P = .11) and 23% (95% CI, 11-37; P = .13), respectively (Figure 2B-C). The median onset of grade 2 to 4 aGVHD was 34 days (range, 14-90). The day 100 organ distribution of aGVHD stage among the 34 No Toci patients is shown in Table 5. Similar to the Toci group, the upper GI tract was the organ most commonly involved. However, there was a higher proportion of lower GI involvement (n = 13, 39%) primarily because of the higher frequency of stage 1 lower GI aGVHD (n = 10). There was a trend toward a higher incidence of day 100 stage 1 to 4 lower GI aGVHD of 33% (95% CI, 19-48; P = .059; Figure 2D) when compared with the Toci recipients.
No Toci patients who developed aGVHD by day 100 (n = 34)
| Organ stage . | Skin . | Upper GI . | Lower GI . | Liver . |
|---|---|---|---|---|
| 0 | 18 (53%) | 6 (18%) | 21 (62%) | 34 (100%) |
| 1 | 6 (18%) | 28 (82%) | 10 (30%) | 0 |
| 2 | 4 (12%) | - | 0 | 0 |
| 3 | 6 (18%) | - | 2 (6%) | 0 |
| 4 | 0 | - | 1 (3%) | 0 |
| Organ stage . | Skin . | Upper GI . | Lower GI . | Liver . |
|---|---|---|---|---|
| 0 | 18 (53%) | 6 (18%) | 21 (62%) | 34 (100%) |
| 1 | 6 (18%) | 28 (82%) | 10 (30%) | 0 |
| 2 | 4 (12%) | - | 0 | 0 |
| 3 | 6 (18%) | - | 2 (6%) | 0 |
| 4 | 0 | - | 1 (3%) | 0 |
Between day 100 and day 180, 2 patients in the Toci group with no previous aGVHD developed late aGVHD (1 grade 2 [upper GI], 1 grade 3 [stage 3 skin]), whereas 2 patients with persistent or recurrent aGVHD progressed from stage 2 to 3 skin involvement. In the No Toci group, 3 patients with no previous aGVHD developed late disease (all grade 2), whereas 1 with persistent or recurrent aGVHD progressed to grade 4 lower GI disease.
In multivariate analyses, the incidence of grade 2 to 4 and grade 3 to 4 aGVHD was not different between the 2 groups (Table 3). The incidence of cGVHD was very low in both groups with only 2 Toci patients and 3 No Toci patients developing cGVHD during the follow-up period.
TRM, relapse, PFS and OS
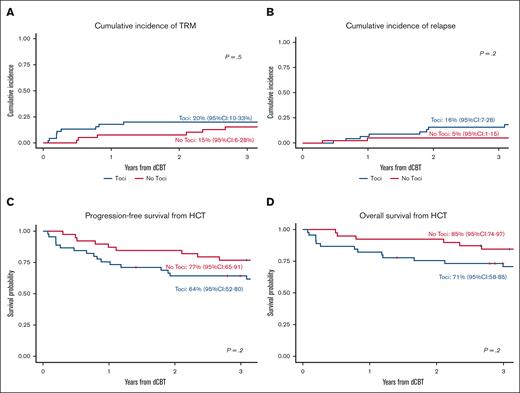
Among the Toci patients, the cumulative incidence of TRM at 1 and 3 years was 18% (95% CI, 8-30) and 20% (95% CI, 10-33), respectively (Figure 3A). Causes of TRM at 3 years included infection (n = 3), aGVHD (n = 3), organ failure (n = 2), and graft failure (n = 1). The 1- and 3-year relapse incidences were 7% (95% CI, 2-17) and 16% (95% CI, 7-28), respectively (Figure 3B). Of the 7 patients who relapsed by 3 years after transplant, 2 had KMT2A-rearranged AML, 3 had TP53-mutated and/or complex karyotype MDS, 1 had B-ALL, and 1 had MPAL. The 3-year PFS and OS were 64% (95% CI, 52-80) (Figure 3C) and 71% (95% CI, 58-85) (Figure 3D), respectively.
Survival outcomes in the Toci and No Toci groups. There were no significant differences in TRM (A), relapse (B), PFS (C), or OS (D) between the Toci and No Toci patients.
Survival outcomes in the Toci and No Toci groups. There were no significant differences in TRM (A), relapse (B), PFS (C), or OS (D) between the Toci and No Toci patients.
In the No Toci group, the 1- and 3-year TRM incidences were 8% (95% CI, 2-19) and 15% (95% CI, 6-28; P = .5), respectively. The causes of TRM were aGVHD (n = 4), organ failure (n = 1), and unknown (n = 1). The 3-year relapse incidence was 5% (95% CI, 1-15; P = .2). The 3-year PFS and OS estimates were 77% (95% CI, 65-91) and 85% (95% CI, 74-97), respectively. In multivariable analyses adjusting for clinically relevant covariates, there were no significant differences in TRM, relapse, PFS, or OS between groups (Table 3).
Immune reconstitution and infections
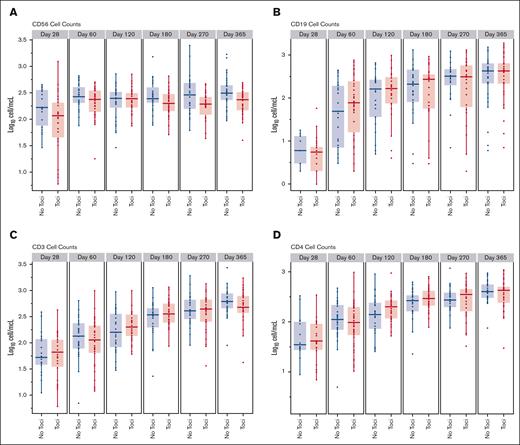
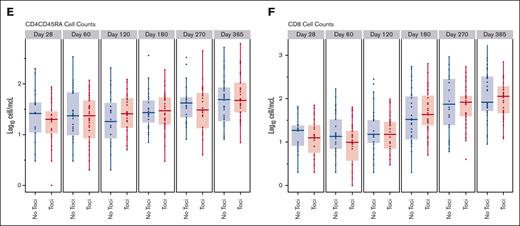
The recovery of natural killer cells, B cells and T cells, and the CD4+, CD8+, and CD4+CD45RA+ T cell subsets in the Toci and No Toci patient groups is shown in Figure 4. Tocilizumab-treated patients had comparable recovery of lymphocyte subsets as the No Toci group. A comparison of infections in the first 100 days between groups (Table 6) suggested that there was a higher incidence of gram-positive bacteremias and total bacterial infections in the Toci group, however, this was confounded by the omission of vancomycin prophylaxis in more than half of the trial patients. As expected, CMV seropositive Toci patients who received letermovir prophylaxis had no CMV infections in the first 100 days. The incidence of other DNA viruses was not different between the 2 groups.
Comparison of immune reconstitution between the Toci and No Toci groups. Tocilizumab did not adversely impact immune subset recovery.
Comparison of immune reconstitution between the Toci and No Toci groups. Tocilizumab did not adversely impact immune subset recovery.
Comparison of infectious complications between clinical trial patients and historic controls
| . | Toci n = 45 . | No Toci n = 39 . | P value . |
|---|---|---|---|
| Total bacterial infections,∗n | 65 | 39 | |
| Incidence rate ratio | 1.52 (95% CI, 0.93-2.48) | P = .092 | |
| Bacteremias | |||
| Gram-positive bacteremia, n | 24 | 8 | |
| Incidence rate ratio | 2.74 (95% CI, 1.31-5.73) | P = .007 | |
| Gram-negative bacteremia, n | 13 | 11 | |
| Incidence rate ratio | 1.08 (95% CI, 0.4-2.91) | P = .88 | |
| CMV,†n | 0 | 19 | |
| Cumulative incidence (day 90) | - | 79% (95% CI, 55-91) | |
| HHV-6,‡n | 43 | 39 | |
| Cumulative incidence (day 90) | 96% (95% CI, 81-99) | 100% (95% CI, 100-100) | |
| Other viral infections,§n (%) | |||
| Adenovirus | 4 (8.9) | 3 (7.7) | |
| EBV | 2 (4.4) | 0 | |
| Respiratory virus, n (%) | 6 (13) | 5 (13) | |
| Fungal infections,§n (%) | |||
| Possible | 1 (2) | 1 (3) | |
| Probable | 0 | 0 | |
| Proven | 0 | 1 (3) | |
| . | Toci n = 45 . | No Toci n = 39 . | P value . |
|---|---|---|---|
| Total bacterial infections,∗n | 65 | 39 | |
| Incidence rate ratio | 1.52 (95% CI, 0.93-2.48) | P = .092 | |
| Bacteremias | |||
| Gram-positive bacteremia, n | 24 | 8 | |
| Incidence rate ratio | 2.74 (95% CI, 1.31-5.73) | P = .007 | |
| Gram-negative bacteremia, n | 13 | 11 | |
| Incidence rate ratio | 1.08 (95% CI, 0.4-2.91) | P = .88 | |
| CMV,†n | 0 | 19 | |
| Cumulative incidence (day 90) | - | 79% (95% CI, 55-91) | |
| HHV-6,‡n | 43 | 39 | |
| Cumulative incidence (day 90) | 96% (95% CI, 81-99) | 100% (95% CI, 100-100) | |
| Other viral infections,§n (%) | |||
| Adenovirus | 4 (8.9) | 3 (7.7) | |
| EBV | 2 (4.4) | 0 | |
| Respiratory virus, n (%) | 6 (13) | 5 (13) | |
| Fungal infections,§n (%) | |||
| Possible | 1 (2) | 1 (3) | |
| Probable | 0 | 0 | |
| Proven | 0 | 1 (3) | |
Bacterial infections (total bacterial infections, gram-positive bacteremias, gram-negative bacteremias) were compared using Poisson regression to account for multiple infections per patient with an offset term to account for days at risk.
CMV infection cumulative incidence was calculated among CMV seropositive patients only with death and second transplant as competing events.
HHV-6 reactivation is presented using cumulative incidence with death and second transplant as competing events. Only 2 patients in each group required HHV-6 directed therapy.
Other viral infections (EBV, adenovirus, respiratory viruses) and fungal infections are presented descriptively because of small numbers. Only 1 adenovirus infection in each group required antiviral therapy. One patient with EBV received pre-emptive rituximab before day 100.
Microbiome
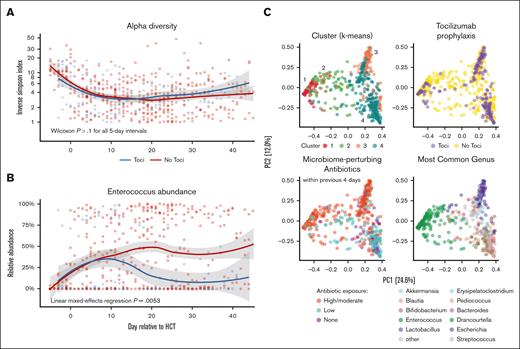
Stool samples were available for 29 of the 45 (64%) Toci patients and for 32 of the 39 (82%) No Toci patients (median, 9 samples per patient between days −14 and +45). The alpha diversity, calculated using the Inverse Simpson method, was plotted longitudinally (Figure 5A). No significant difference in the mean per-patient alpha diversity was identified at any time interval between groups (P > .1 in all 5-day bins).
Tocilizumab-based GVHD prophylaxis is associated with lower Enterococcus abundance. (A) The alpha diversity, as measured by the Inverse Simpson index, is shown over time relative to transplant day. Both the Toci and No Toci groups exhibited a similar decrease in diversity over the first 2 weeks following infusion. No difference was observed in diversity between groups when compared using the Wilcoxon test for 5-day intervals. (B) The relative abundance of Enterococcus over time was markedly different between the Toci and No Toci cohorts (multivariable linear mixed-effects regression P = .0056). (B) Principal component plots are shown; 4 clusters were identified when using k-means clustering (top left). Toci exposure (top-right), antibacterial exposure within previous 4 days (bottom left), and most prevalent genus in each sample (bottom right) are shown. The Toci vs No Toci contribution to the clusters was significantly different (Kruskal-Wallis; P = 1.3e-14). Antibiotics to which patients were exposed in the 4 days before each sample collection were classified by their extent of anticipated perturbation of human intestinal microbiome communities as previously described.47
Tocilizumab-based GVHD prophylaxis is associated with lower Enterococcus abundance. (A) The alpha diversity, as measured by the Inverse Simpson index, is shown over time relative to transplant day. Both the Toci and No Toci groups exhibited a similar decrease in diversity over the first 2 weeks following infusion. No difference was observed in diversity between groups when compared using the Wilcoxon test for 5-day intervals. (B) The relative abundance of Enterococcus over time was markedly different between the Toci and No Toci cohorts (multivariable linear mixed-effects regression P = .0056). (B) Principal component plots are shown; 4 clusters were identified when using k-means clustering (top left). Toci exposure (top-right), antibacterial exposure within previous 4 days (bottom left), and most prevalent genus in each sample (bottom right) are shown. The Toci vs No Toci contribution to the clusters was significantly different (Kruskal-Wallis; P = 1.3e-14). Antibiotics to which patients were exposed in the 4 days before each sample collection were classified by their extent of anticipated perturbation of human intestinal microbiome communities as previously described.47
Analysis of the relative abundance of Enterococcus over time (Figure 5B) revealed a marked divergence in the patterns of enterococcal abundance between the 2 groups. To account for differences in the baseline characteristics and antibiotic exposure, a mixed-effects linear regression model adjusting for recent (within 4 days) exposure to high-risk anti-anaerobic antibiotics and time after transplantation was performed. Tocilizumab exposure was associated with markedly decreased enterococcal abundance (abundance loge-scaled; β = −2.79; P = .0053).
Finally, to analyze taxonomic composition variation across samples, a principal coordinates ordination was constructed. This revealed 4 generally distinct clusters of samples (defined by k-means clustering and labeled with black numerals in Figure 5C). Samples from Toci patients were enriched in clusters 3 and 4 but relatively sparse in clusters 1 and 2 (Kruskal-Wallis; P = 1.3e-14). Notably, Enterococcus was prominently one of the most common taxa for samples in cluster 1 (Toci 15% vs No Toci 22%) and cluster 2 (Toci 3% vs No Toci 22%), whereas samples in cluster 3 were enriched for other gram-positive genera (eg, Streptococcus, Pediococcus, and Lactobacillus; Toci 17% vs No Toci 22%) and samples in cluster 4 for strict anaerobes (eg, Blautia, Erysipelatoclostridium; Toci 65% vs No Toci 33%). Using the mixed-effects model applied previously to Enterococcus, we confirmed that the abundance of both Erysipelatoclostridium and Blautia, predominant in cluster 4, were not associated with tocilizumab exposure (P > .1). In time-dependent Cox regression models, enterococcal abundance was not associated with PES, OS or GVHD-related mortality across this cohort (P > .1).
Discussion
Herein, we report the results of a novel phase 2 trial that investigated, for the first time to our knowledge, the addition of tocilizumab to standard CSA/MMF GVHD prophylaxis in adults with hematologic malignancies who underwent dCBT. When compared with identical dCBT historic controls from our center, we observed a significant reduction in the incidence of PES. Although the reduction in the incidence of grade 2 to 4 and 3 to 4 aGVHD was in tocilizumab recipients was nonsignificant, there was a strong trend toward a reduction in the incidence of lower GI tract aGVHD. Neutrophil recovery, however, was delayed by a median of 3.5 days. There were no significant differences in TRM, relapse, PFS, or OS.27,33
Our findings are consistent with the phase 3 adult-donor HSCT trial,33 which showed only a modest reduction in grade 2 to 4 aGVHD with tocilizumab. However, the effect was mostly noted in the incidence of grade 2 aGVHD in that trial, whereas the relative reduction was more pronounced for grade 3 to 4 aGVHD in our dCBT recipients. Differences in the patient demographics, conditioning, the use of HLA-disparate grafts, and combining tocilizumab with CSA and MMF in our trial rather than a calcineurin inhibitor and methotrexate may have accounted for this difference. Based on recent experimental data, the only modest reduction in aGVHD incidence in the phase 3 trial and our trial may in fact be explained by the IL-6 pathway potentially being dispensable for GI protection in aGVHD.48 Nevertheless, we observed a trend toward a reduction in lower GI aGVHD involvement, albeit primarily stage 1, corroborating the findings by Drobyski et al 27,46 and the case series of patients with advanced GI aGVHD successfully treated with tocilizumab.30,32 Notably, the onset of grade 2 to 4 aGVHD in our trial was ∼2 weeks later when compared with the dCBT controls. This delayed onset may be associated with the significant decline of tocilizumab levels and reversal of IL-6R blockade beyond the first month after transplant.26,33 Although a second dose of tocilizumab may therefore have further mitigated aGVHD, this was not considered given that the safety and efficacy of a single dose of tocilizumab26,27 specifically in CBT recipients had not been established when the trial was designed.
PES, a complication that resembles cytokine release syndrome (CRS), is seen in up to 75% of CBT recipients.49,50 PES has been attributed to graft-versus-graft and graft-versus-recipient immune reactions in the presence of multiple HLA-mismatches by some investigators. Serum IL-6 concentrations are increased in PES after CBT.51,52 Tocilizumab has been successfully used to treat CRS in recipients of chimeric antigen receptor T cells or haploidentical HSCT,53,54 as well as in severe PES in a small trial of CBT recipients.52 In our trial, as expected, dCBT patients who received tocilizumab were approximately half as likely to develop PES than historic dCBT controls. Moreover, tocilizumab recipients who were diagnosed with PES were less likely to have a fever (ie, presentation with rash only) and only 59% of patients with PES required IV corticosteroid treatment. In contrast, all dCBT control patients who developed PES were febrile and received IV corticosteroids. Therefore, tocilizumab was associated with a significant reduction in both the incidence and severity of PES. Nevertheless, serious complications of PES were very infrequent in both cohorts because of the prompt initiation of PES treatment per institutional practice.40
Experimental inhibition of IL-6R signaling has not been shown to impair graft-versus-leukemia effects in murine transplantation models.17,48 In addition, tocilizumab-based GVHD prophylaxis was not associated with increased relapse risk after adult-donor HSCT.27,33 Although the incidence of relapse appeared higher in our trial of dCBT patients when compared with the historic control group, this difference was not significant and may have been confounded by differences in patient characteristics or disease risk between the 2 cohorts. Nevertheless, it is interesting to postulate that tocilizumab may have somewhat blunted the graft-versus-leukemia effects after dCBT by mitigating early alloreactivity, graft-versus-graft interactions, and PES55 and by its modest reduction of aGVHD.8,9 Importantly, the overall low risk for relapse observed in both cohorts, including in high-risk patients, supports robust graft-versus-leukemia effects associated with CB as a graft source.4,5
IL-6 is a proliferation and maturation factor for stem and progenitor cells, thereby influencing hematopoietic recovery after myelosuppression.56-59 In our trial patients, we observed a median 3.5 day delay in neutrophil recovery, and a similar delay of 2 to 3 days was shown in adult-donor HSCTs.33 Bacteremias caused by gram-positive bacteria were more common in tocilizumab dCBT recipients. This finding could be explained by the institutional change in bacterial prophylaxis during the trial and/or the slower neutrophil recovery. In contrast with neutrophil engraftment, platelet recovery was a median of 5 days earlier, which approached significance in the multivariate analysis. We hypothesize that the lower incidence and delayed aGVHD onset in tocilizumab recipients may have contributed to this observation. The effective mitigation of CMV infections by letermovir in trial patients (thereby avoiding the need for CMV therapy) may have also been beneficial. Immune reconstitution was not adversely affected by tocilizumab.
We have previously reported that Enterococcus domination is the most frequent pattern of microbiome disruption in fecal samples from patients who were undergoing HSCT in general.60 Notably, we have also previously observed a lower burden of Enterococcus domination in a different cohort of tocilizumab-treated HSCT patients who received peripheral blood allografts.46 Herein, we confirmed our previous observation of decreased enterococcal abundance in dCBT recipients exposed to tocilizumab prophylaxis. We initially hypothesized that tocilizumab recipients would have a lower burden of exposure to broad-spectrum antibiotics because tocilizumab decreases the frequency of febrile episodes. However, a strong association was still observed after adjusting for this variable. Although a causal relationship between tocilizumab administration and decreased enterococcal abundance cannot be definitively established based on these data, our observations further support the hypothesis that tocilizumab may directly modulate the gut microbiome composition through decreasing intestinal inflammation and restoring elements of gut homeostasis. This is notable given that Enterococcus domination is a harbinger of bloodstream infections, lower-GI GVHD, and mortality in HSCT recipients.60-62
In summary, tocilizumab has a modest effect in reducing the GVHD burden after dCBT, but any potential benefits are offset by delayed neutrophil recovery with no survival improvement. Taken together with the negative results of the phase 3 randomized clinical trial in adult HSCT recipients, our findings do not support further investigation of IL-6 blockade in GVHD prevention after dCBT. Recently, dCBT with optimized practices37,63-67 is associated with high survival in adults with hematologic malignancies despite aGVHD.4 Nonetheless, investigating novel, nonlymphodepleting agents to reduce GVHD burden after dCBT is warranted.
Acknowledgments
This work was supported, in part, by the National Cancer Institute of the National Institutes of Health (NIH/NCI) under grant P01 CA23766 and the NIH/NCI Cancer Center Support grant P30 CA008748, and (J.U.P.) by the National Heart, Lung, and Blood Institute under award K08HL143189. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authorship
Contribution: I.P. and J.N.B. designed the clinical trial (NCT03434730); I.P., J.A.F., J.U.P., and J.N.B. assembled and analyzed the data and wrote the manuscript; I.P., S.B., J.A.F., and S.D. performed the statistical analysis; S.E., K.C., S.C., and S.Q. maintained the patient database and procured data for the study; I.P., A.S., C.C., P.D., S.A.G., B.G., A.M.H., A.J., E.B.P., M.-A.P., D.M.P., B.C.S., R.T., J.W.Y., J.U.P., and J.N.B. provided patient care; and all authors interpreted the data, reviewed and edited the manuscript, and have approved the submitted version of the manuscript.
Conflict-of-interest disclosure: I.P. reports receiving research funding from Merck; honoraria from PRECISIONheor; and serving on a data and safety monitoring board (DSMB) for ExCellThera. A.S. reports serving as a consultant on the scientific advisory board of ExCellThera. S.A.G. reports serving as a consultant for and receiving honoraria and research funding from Celgene and Novartis; serving as a consultant for and receiving research funding from Amgen, Actinuum, and Miltenyi Biotech; serving as a consultant for and receiving honoraria from Jazz Pharmaceuticals and Omeros; receiving research funding from Takeda; and serving as a consultant for Kite Pharma. B.G. reports receiving research funding from Actinium Pharmaceuticals and serving on the DSMB for Synthetic Biologics, Inc. M.A.P. reports receiving honoraria from Adicet, Allogene, AlloVir, Caribou Biosciences, Celgene, Bristol Myers Squibb, Equilium, Exevir, ImmPACT Bio, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Sanofi, Syncopation, VectivBio AG, and Vor Biopharma; serving on DSMBs for Cidara Therapeutics and Sellas Life Sciences; serving on on the scientific advisory board of NexImmune; reports ownership interests in NexImmune, Omeros, and OrcaBio; and reports institutional research support for clinical trials from Allogene, Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis. D.M.P. reports serving as a consultant for Kadmon/Sanofi Corporation, CareDx, Incyte, and Ceramedix, and receiving research funding from Incyte. B.C.S. reports receiving consulting fees from Hansa Biopharma and Gamida Cell. R.T. reports serving as a Blood and Marrow Transplant Clinical Trials Network medical monitor for Angiocrine Bioscience and Omeros. J.W.Y. reports owning equity in Merck, Pfizer, and Amgen. J.U.P. reports receiving research funding, intellectual property fees, and travel reimbursement from Seres Therapeutics and consulting fees from DaVolterra, CSL Behring, Crestone Inc, and from MaaT Pharma. He reports serving on an advisory board of and holding equity in Postbiotics Plus Research and Prodigy Biosciences. He further reports filing intellectual property applications related to the microbiome (reference numbers #62/843,849, #62/977,908, and #15/756,845). Memorial Sloan Kettering Cancer Center has financial interests relative to Seres Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Ioannis Politikos, Adult Bone Marrow Transplantation Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 545 E 73rd St, Box 61, New York, NY 10021; email: politiki@mskcc.org.
References
Author notes
S.B. and J.A.F. contributed equally to this work.
Original data are available upon reasonable request from the corresponding author, Ioannis Politikos (politiki@mskcc.org).