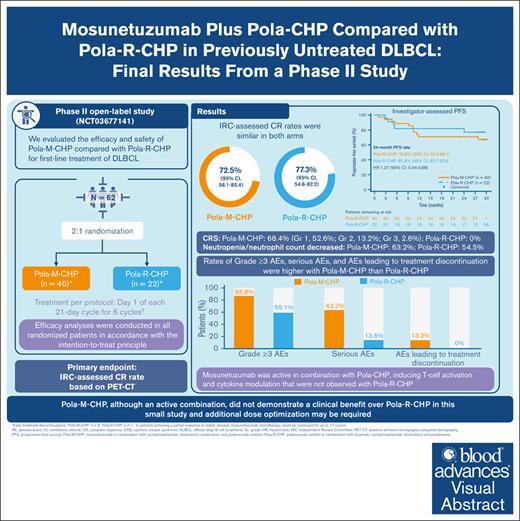
Key Points
First-line Pola-M-CHP and Pola-R-CHP demonstrate similar response rates in previously untreated diffuse large B-cell lymphoma.
Pola-M-CHP did not demonstrate a clinical benefit over Pola-R-CHP in this small study and additional dose optimization may be required.
Visual Abstract
This phase 2 study evaluated mosunetuzumab plus cyclophosphamide, doxorubicin, prednisone, and polatuzumab vedotin (Pola-M-CHP) vs Pola-rituximab (R)-CHP for first-line treatment of diffuse large B-cell lymphoma. Patients were randomized 2:1 to receive 6 cycles of Pola-M-CHP or Pola-R-CHP on day 1 of each 21-day cycle. Mosunetuzumab was administered intravenously via step-up dosing during cycle 1 and at 30 mg on day 1 of subsequent cycles. The primary end point was independent review committee–assessed complete response (CR) rate by positron emission tomography–computed tomography. Overall, 62 patients were enrolled and received Pola-M-CHP (n = 40) or Pola-R-CHP (n = 22). CR rates were similar in both arms (72.5% with Pola-M-CHP vs 77.3% with Pola-R-CHP); the 24-month investigator-assessed progression-free survival rate was 70.8% (95% confidence interval [CI], 55.6-86.1) with Pola-M-CHP vs 81.8% (95% CI, 65.7-97.9) with Pola-R-CHP. The most common adverse event (AE) was cytokine release syndrome (68.4%; mostly grade 1 [52.6%], and primarily confined to cycle 1) with Pola-M-CHP and neutropenia/neutrophil count decreased (54.5%) with Pola-R-CHP. Neutropenia/neutrophil count decreased was the most frequently observed grade ≥3 AE in both arms (Pola-M-CHP, 36.8%; Pola-R-CHP, 22.7%). Rates of grade ≥3 AEs (86.8% vs 59.1%), serious AEs (63.2% vs 13.6%), and AEs leading to treatment discontinuation (13.2% vs 0%) were higher with Pola-M-CHP than Pola-R-CHP, respectively. Pharmacodynamic changes were supportive of mosunetuzumab’s mechanism of action and its addition to the Pola-CHP combination. Pola-M-CHP, although an active combination, did not demonstrate a clinical benefit over Pola-R-CHP in this small study. This trial was registered at www.clinicaltrials.gov as #NCT03677141.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of aggressive non-Hodgkin lymphoma (NHL) and accounts for approximately 30% of all NHL cases.1 Although most patients with newly diagnosed DLBCL are cured with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), which was the standard of care for >2 decades, up to 40% of patients who receive R-CHOP have a disease that is refractory or experience relapse.1-4 Recent efforts to improve the standard of care led to the replacement of vincristine in R-CHOP with polatuzumab vedotin, a CD79b-directed antibody-drug conjugate.5,6 In the phase 3 POLARIX study (NCT03274492), polatuzumab vedotin in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone (Pola-R-CHP) demonstrated a significant progression-free survival (PFS) benefit compared with R-CHOP (stratified hazard ratio, 0.73; 95% confidence interval [CI], 0.57-0.95; P = .02) in the first-line treatment of DLBCL.7 The estimated 2-year PFS rate was 76.7% in the Pola-R-CHP arm compared with 70.2% in the R-CHOP arm.7 These findings led to the approval of Pola-R-CHP for patients with previously untreated DLBCL.8,9
However, there remains a need for novel combination regimens to further improve outcomes in patients with previously untreated DLBCL. Mosunetuzumab, a CD20 × CD3 T-cell engaging bispecific antibody that redirects T cells to eliminate malignant B cells,10 has recently been evaluated in combination with CHOP.11 In a phase 2 study of 40 patients with newly diagnosed DLBCL (GO40515; NCT03677141), mosunetuzumab (M)-CHOP demonstrated promising activity with high response rates (overall response rate [ORR], 87.5%; complete response [CR] rate, 85.0%) and a manageable safety profile.11 The response rates observed with M-CHOP suggest that patients with previously untreated DLBCL may benefit from combination regimens that incorporate enhanced immune effector molecules such as mosunetuzumab.11 Replacing rituximab with the CD20 × CD3 bispecific antibody mosunetuzumab has the potential to further improve patient outcomes through a distinct mechanism of action, whereby T cells are directly recruited to CD20-expressing lymphoma cells.10 The nonoverlapping mechanisms of action of mosunetuzumab and Pola-CHP, including results from the phase 2 study of M-CHOP and the phase 3 POLARIX study,5,7,10,11 provide a rationale for investigating this combination for the first-line treatment of DLBCL. Here, we report the safety and efficacy of mosunetuzumab in combination with Pola-CHP (Pola-M-CHP) compared with Pola-R-CHP in patients with previously untreated DLBCL.
Methods
Study design
This phase 1b/2 open-label, multicenter, randomized, controlled study (NCT03677141) included dose-escalation cohorts in patients with relapsed/refractory NHL and a dose-expansion cohort in patients with newly diagnosed DLBCL. Details of the dose-escalation and safety run-in phase of the study have been published previously.11 Here, we focus on the randomized, phase 2, dose-expansion cohort investigating Pola-M-CHP vs Pola-R-CHP in patients with previously untreated DLBCL.
The protocol was approved by institutional review boards or ethics committees. The trial was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practice, and applicable laws and regulations.
Patients
Patients aged ≥18 years with previously untreated, histologically confirmed DLBCL with an International Prognostic Index (IPI) score of 2 to 5 and an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 2 were included with additional criteria as previously published.11 Patients who had transformed lymphoma or who received prior therapy for B-cell NHL or prior allogeneic stem cell transplant were excluded. All patients provided a written informed consent and complied with the study protocol and procedures.
Randomization and masking
Eligible patients were randomly assigned in a 2:1 ratio to receive Pola-M-CHP or Pola-R-CHP. Randomization in this open-label study was performed by an interactive voice or web-based response system. Stratification factors included IPI score (2 vs 3-5) and bulky disease (present or absent, defined as at least one ≥7.5 cm lesion at baseline).
Treatments
Treatment per protocol was on day (D) 1 of each 21-day cycle for 6 cycles. Patients received oral prednisone 100 mg daily on days 1 to 5, polatuzumab vedotin 1.8 mg/kg IV on D1 (or D2 if rituximab infusion was prolonged due to infusion-related adverse events [AEs]), doxorubicin 50 mg/m2 IV, cyclophosphamide 750 mg/m2 IV, and rituximab 375 mg/m2 IV (in the Pola-R-CHP arm). In the Pola-M-CHP arm, mosunetuzumab was administered IV in step-up doses in cycle (C) 1 on D1 (1 mg), D8 (2 mg), and D15 (30 mg) to mitigate the risk of cytokine release syndrome (CRS). Thereafter, mosunetuzumab 30 mg was administered on D1 of each subsequent cycle. Patients in the Pola-M-CHP and Pola-R-CHP arms were required to receive prophylactic granulocyte colony-stimulating factor during the 6 cycles of treatment.
Patients with a partial response (PR) or stable disease at the end of C6 could continue mosunetuzumab monotherapy for up to an additional 11 cycles (total of 17 cycles) or until disease progression or unacceptable toxicity.
Assessments
Interim and primary response assessments (PRAs) were obtained after C4 and C6, respectively. Response was assessed based on positron emission tomography–computed tomography (PET-CT) scans using the Lugano 2014 criteria.12
Study end points
The primary efficacy end point was PET-CT CR rate at PRA (6-8 weeks after C6D1 or last dose of study medication if study treatment was discontinued before C6), as determined by a blinded independent review committee (IRC), using Lugano 2014 criteria.12 Patients with missing or no response assessments were classified as noncomplete responders. Secondary efficacy end points were investigator assessed using Lugano 2014 criteria and included CR rate at the PRA based on CT only, ORR (CR or PR at C6) based on PET-CT, best ORR based on PET-CT, duration of response (DoR) among patients with a best overall response of CR or PR, PFS, PFS at 1 year, and event-free survival (EFS). Safety and tolerability were assessed by analyzing the occurrence and severity of AEs, with severity determined according to National Cancer Institute Common Terminology Criteria for AEs version 5.0. CRS events were graded according to American Society for Transplantation and Cellular Therapy CRS consensus grading criteria.13 The relationship between exploratory biomarkers (cytokines and T-cell activation) was also assessed.
Biomarker sample collection and analysis
Peripheral whole blood and plasma were collected for central flow cytometry analysis of selected T-cell markers and cytokines, respectively, at predefined time points. For flow cytometry, whole blood samples were collected from all patients in the randomized arms 0 to 4 hours before initial dosing (C1D1 predose) and 2 hours (±30 minutes) after infusion in C1 to C6. Samples were collected in Becton Dickinson (BD) Vacutainer tubes containing sodium heparin and shipped at ambient temperature on the day of collection to ICON laboratories for central assessment. Samples were acquired on BD FACSCanto II flow cytometers (BD Biosciences) using FACSDiva software (BD Biosciences, version 6.1.3). T-cell markers were measured with a validated custom panel at ICON. T-cell activation was measured in both CD4 and CD8 T-cell populations using the early activation marker CD69 surface expression. Data were analyzed as a percentage of the parent population over time.
Plasma collection for the evaluation of cytokines included additional time points in C1. For the Pola-M-CHP arm, samples were collected in C1 before the initial dosing (C1D1 prechemotherapy) and before the initial mosunetuzumab dose (C1D1 premosunetuzumab). Subsequent plasma collections were after mosunetuzumab administration at ±30 minutes after the end of infusion (C1D1 end of infusion), 2 hours after the end of infusion (C1D1 2 hours), and then 24 hours after the end of infusion (C1D1 24 hours). Additional samples were collected before dosing (predose) and 2 hours after dose (2 hours). A similar collection strategy was performed for the Pola-R-CHP arm except for the C1D1 premosunetuzumab assessment. Cytokines were measured in human plasma collected in K2EDTA. Interferon gamma (IFN-γ) was measured centrally at Rules Based Medicine (Austin, TX) using a Quanterix Simoa assay and interleukin-6 (IL-6) was measured at Frontage Laboratories (Exton, PA) using an enzyme-linked immunosorbent assay method. Time-course assessments were analyzed and plotted relative to the C1 intensive collection time course for the Pola-M-CHP arm and the extended time course across both arms.
Statistical analysis
Efficacy analyses were conducted in all randomized patients in accordance with the intention-to-treat (ITT) principle. The safety population included all patients who received any amount of the study drug. Sample size determination and statistical analyses were performed as previously described.11 All comparisons between treatment arms were exploratory. P values were not corrected for multiple hypothesis testing.
Results
Patient characteristics
A total of 62 patients with previously untreated DLBCL were enrolled; 40 and 22 patients were randomized to receive Pola-M-CHP and Pola-R-CHP, respectively. Two patients randomized to the Pola-M-CHP arm did not receive any study treatment (screen failure, n = 1; enrolled in error, n = 1) but were included in the ITT population.
In the Pola-M-CHP and Pola-R-CHP arms, respectively, median age was 66.5 years (range, 39-81; 55.0% >65 years) and 63.0 years (range, 30-79; 27.3% >65 years), 9 patients (22.5%) and 6 patients (27.3%) had bulky disease ≥7.5 cm, 16 patients (40.0%) and 8 patients (36.4%) had an IPI score of 2, 24 patients (60.0%) and 14 patients (63.6%) had an IPI score of ≥3, and most patients had ECOG PS of 0 to 1 (94.7% and 95.2%) (Table 1).
Baseline patient and disease characteristics in all patients (clinical cutoff date 12 October 2023; ITT population)
| . | Pola-M-CHP, n = 40 . | Pola-R-CHP, n = 22 . |
|---|---|---|
| Median age (range), y | 66.5 (39-81) | 63.0 (30-79) |
| 18-65 | 18 (45.0) | 16 (72.7) |
| >65 | 22 (55.0) | 6 (27.3) |
| Male | 26 (65.0) | 14 (63.6) |
| ECOG PS | n = 38 | n = 21 |
| 0 | 16 (42.1) | 13 (61.9) |
| 1 | 20 (52.6) | 7 (33.3) |
| 2 | 2 (5.3) | 1 (4.8) |
| Ann Arbor stage | ||
| I-II | 5 (12.5) | 2 (9.1) |
| III-IV | 35 (87.5) | 20 (90.9) |
| IPI score | ||
| 2 | 16 (40.0) | 8 (36.4) |
| 3 | 14 (35.0) | 12 (54.5) |
| 4 | 10 (25.0) | 2 (9.1) |
| Cell of origin | ||
| ABC | 5 (12.5) | 0 |
| GCB | 16 (40.0) | 11 (50.0) |
| Non-GCB | 18 (45.0) | 7 (31.8) |
| Unknown | 1 (2.5) | 4 (18.2) |
| Additional characterization by local laboratory | ||
| MYC, BCL2 and/or BCL6 rearrangements | 5 (12.5) | 4 (18.2) |
| Double expressor (MYC and BCL2 overexpression without translocation) | 6 (15.0) | 1 (4.5) |
| None of above | 17 (42.5) | 15 (68.2) |
| Not determined/available | 12 (30.0) | 2 (9.1) |
| Bulky disease (≥7.5 cm) | 9 (22.5) | 6 (27.3) |
| Extranodal involvement | 25 (62.5) | 17 (77.3) |
| . | Pola-M-CHP, n = 40 . | Pola-R-CHP, n = 22 . |
|---|---|---|
| Median age (range), y | 66.5 (39-81) | 63.0 (30-79) |
| 18-65 | 18 (45.0) | 16 (72.7) |
| >65 | 22 (55.0) | 6 (27.3) |
| Male | 26 (65.0) | 14 (63.6) |
| ECOG PS | n = 38 | n = 21 |
| 0 | 16 (42.1) | 13 (61.9) |
| 1 | 20 (52.6) | 7 (33.3) |
| 2 | 2 (5.3) | 1 (4.8) |
| Ann Arbor stage | ||
| I-II | 5 (12.5) | 2 (9.1) |
| III-IV | 35 (87.5) | 20 (90.9) |
| IPI score | ||
| 2 | 16 (40.0) | 8 (36.4) |
| 3 | 14 (35.0) | 12 (54.5) |
| 4 | 10 (25.0) | 2 (9.1) |
| Cell of origin | ||
| ABC | 5 (12.5) | 0 |
| GCB | 16 (40.0) | 11 (50.0) |
| Non-GCB | 18 (45.0) | 7 (31.8) |
| Unknown | 1 (2.5) | 4 (18.2) |
| Additional characterization by local laboratory | ||
| MYC, BCL2 and/or BCL6 rearrangements | 5 (12.5) | 4 (18.2) |
| Double expressor (MYC and BCL2 overexpression without translocation) | 6 (15.0) | 1 (4.5) |
| None of above | 17 (42.5) | 15 (68.2) |
| Not determined/available | 12 (30.0) | 2 (9.1) |
| Bulky disease (≥7.5 cm) | 9 (22.5) | 6 (27.3) |
| Extranodal involvement | 25 (62.5) | 17 (77.3) |
Data presented as n (%) of patients unless otherwise stated.
ABC, activated B-cell like; GCB, germinal center B-cell like.
Treatment exposure, reasons for incomplete study treatment, and duration of follow-up
The median number of chemotherapy treatment cycles in both treatment arms was 6 (Pola-M-CHP, range 1-6; Pola-R-CHP, range 4-6; supplemental Table 1). The proportion of patients (safety evaluable: Pola-M-CHP, n = 38; Pola-R-CHP, n = 22) who received >90% dose intensity in the Pola-M-CHP arm was 92.1% (mosunetuzumab, cyclophosphamide, doxorubicin) and 97.4% (polatuzumab vedotin); 100% (rituximab and doxorubicin) and 95.5% of patients (cyclophosphamide and polatuzumab vedotin) in the Pola-R-CHP arm received >90% dose intensity. Two patients received consolidation with 6 additional cycles of mosunetuzumab monotherapy. One of the 2 patients achieved a PR and continued with an additional 4 cycles of mosunetuzumab monotherapy with subsequent progressive disease (PD). The other patient achieved a CR and proceeded with an additional cycle of mosunetuzumab monotherapy with subsequent PD.
Thirty safety-evaluable patients (78.9%) in the Pola-M-CHP arm completed 6 cycles of study treatment, whereas 8 patients received fewer treatment cycles. Investigators’ reasons for early treatment discontinuations in the patients who received <6 cycles were death (n = 2, due to unknown cause), lack of efficacy (n = 1), AE (n = 1, grade 4 respiratory failure with pulmonary hemorrhage), data entry error (n = 1, patient completed treatment but treatment exposure data were missing), physician decision (n = 2), and other (n = 1, patient was unable to travel to complete study treatment). Of the 8 patients in the Pola-M-CHP arm who discontinued treatment early, 1 patient received 5 cycles of treatment, 1 received 1 cycle, and 2 patients each received 2, 3, or 4 cycles of treatment. In the Pola-R-CHP arm, 21 patients (95.5%) received 6 cycles of treatment and 1 patient discontinued treatment early due to PD.
At the clinical cutoff date (12 October 2023), the median follow-up was 29.0 months (range, 0-34) in the Pola-M-CHP arm and 28.6 months (range, 14-30) in the Pola-R-CHP arm.
Efficacy
In the ITT population, the primary end point of PET-CT CR rate by IRC at the end-of-treatment assessment was 72.5% (29 of 40 patients; 95% CI, 56.1-85.4) in the Pola-M-CHP arm and 77.3% (17 of 22 patients; 95% CI, 54.6-92.2) in the Pola-R-CHP arm (Table 2); exploratory analysis of the CR rate in patients who received any study treatment showed 76.3% of patients (29 of 38) in the Pola-M-CHP arm and 77.3% of patients (17 of 22) in the Pola-R-CHP arm had a CR. Investigator-assessed best ORR and accompanying CR rate by either PET-CT or CT only, respectively, were 85.0% and 77.5% in the Pola-M-CHP arm and 95.5% and 81.8% in the Pola-R-CHP arm in the ITT population (Table 2). At the time of PRA, investigator-assessed ORR and CR rate by PET-CT, respectively, were 80.0% and 75.0% in the Pola-M-CHP arm and 77.3% and 68.2% in the Pola-R-CHP arm (Table 2). Investigator-assessed PD occurred in 1 (2.5%) and 5 patients (22.7%) in the Pola-M-CHP and Pola-R-CHP arms, respectively. Investigator-assessed CR rates in the Pola-M-CHP arm were similar across subgroups (supplemental Figure 1). There was concordance between CR rates according to IRC and investigator assessment (Pola-M-CHP arm, 87.5%; Pola-R-CHP arm, 90.9%).
Efficacy summary in all patients with previously untreated DLBCL (ITT population)
| . | Pola-M-CHP, n = 40 . | Pola-R-CHP, n = 22 . |
|---|---|---|
| IRC-assessed objective response | ||
| CR rate∗, n (%) | 29 (72.5) | 17 (77.3) |
| 95% CI | 56.1-85.4 | 54.6-92.2 |
| IRC-assessed ORR∗, n (%) | 30 (75.0) | 19 (86.4) |
| 95% CI | 58.8-87.3 | 65.1-97.1 |
| Response assessment at PRA∗(investigator assessed) | ||
| ORR, n (%) | 32 (80.0) | 17 (77.3) |
| 95% CI | 64.4-91.0 | 54.6-92.2 |
| CR rate, n (%) | 30 (75.0) | 15 (68.2) |
| 95% CI | 58.8-87.3 | 45.1-86.1 |
| Partial response, n (%) | 2 (5.0) | 2 (9.1) |
| Progressive disease, n (%) | 1 (2.5) | 5 (22.7) |
| Stable disease, n (%) | 0 | 0 |
| Missing or not done, n (%) | 7 (17.5) | 0 |
| Best overall response (investigator assessed)† | ||
| ORR, n (%) | 34 (85.0) | 21 (95.5) |
| 95% CI | 70.2-94.3 | 77.2-99.9 |
| CR rate, n (%) | 31 (77.5) | 18 (81.8) |
| 95% CI | 61.6-89.2 | 59.7-94.8 |
| Investigator-assessed DoR† | ||
| Median (95% CI), mo | NE (16.2-NE) | NE (NE-NE) |
| Event-free rate (95% CI), % | ||
| 12 months | 75.0 (60.0-90.0) | 85.7 (70.8-100) |
| 24 months | 71.4 (55.6-87.3) | 80.7 (63.6-97.7) |
| Investigator-assessed PFS† | ||
| Median (95% CI), mo | NE (NE-NE) | NE (NE-NE) |
| Event-free rate (95% CI), % | ||
| 12 months | 70.8 (55.6-86.1) | 81.8 (65.7-97.9) |
| 24 months | 70.8 (55.6-86.1) | 81.8 (65.7-97.9) |
| PFS HR (95% CI) | 1.27 (0.44-3.68) | |
| P value | .66 | |
| Investigator-assessed EFS† | ||
| Median EFS (95% CI), mo | NE (27.8-NE) | NE (NE-NE) |
| Event-free rate (95% CI), % | ||
| 12 months | 69.1 (53.8-84.3) | 81.8 (65.7-97.9) |
| 24 months | 69.1 (53.8-84.3) | 81.8 (65.7-97.9) |
| EFS HR (95% CI) | 1.46 (0.51-4.17) | |
| P value | .48 | |
| Overall survival | ||
| Median OS (95% CI), mo | NE (NE-NE) | NE (NE-NE) |
| Event-free rate (95% CI), % | ||
| 12 months | 86.3 (75.2-97.5) | 100.0 (100.0-100.0) |
| 24 months | 86.3 (75.2-97.5) | 86.4 (72.0-100.0) |
| OS HR (95% CI) | 1.02 (0.24-4.31) | |
| P value | .97 | |
| . | Pola-M-CHP, n = 40 . | Pola-R-CHP, n = 22 . |
|---|---|---|
| IRC-assessed objective response | ||
| CR rate∗, n (%) | 29 (72.5) | 17 (77.3) |
| 95% CI | 56.1-85.4 | 54.6-92.2 |
| IRC-assessed ORR∗, n (%) | 30 (75.0) | 19 (86.4) |
| 95% CI | 58.8-87.3 | 65.1-97.1 |
| Response assessment at PRA∗(investigator assessed) | ||
| ORR, n (%) | 32 (80.0) | 17 (77.3) |
| 95% CI | 64.4-91.0 | 54.6-92.2 |
| CR rate, n (%) | 30 (75.0) | 15 (68.2) |
| 95% CI | 58.8-87.3 | 45.1-86.1 |
| Partial response, n (%) | 2 (5.0) | 2 (9.1) |
| Progressive disease, n (%) | 1 (2.5) | 5 (22.7) |
| Stable disease, n (%) | 0 | 0 |
| Missing or not done, n (%) | 7 (17.5) | 0 |
| Best overall response (investigator assessed)† | ||
| ORR, n (%) | 34 (85.0) | 21 (95.5) |
| 95% CI | 70.2-94.3 | 77.2-99.9 |
| CR rate, n (%) | 31 (77.5) | 18 (81.8) |
| 95% CI | 61.6-89.2 | 59.7-94.8 |
| Investigator-assessed DoR† | ||
| Median (95% CI), mo | NE (16.2-NE) | NE (NE-NE) |
| Event-free rate (95% CI), % | ||
| 12 months | 75.0 (60.0-90.0) | 85.7 (70.8-100) |
| 24 months | 71.4 (55.6-87.3) | 80.7 (63.6-97.7) |
| Investigator-assessed PFS† | ||
| Median (95% CI), mo | NE (NE-NE) | NE (NE-NE) |
| Event-free rate (95% CI), % | ||
| 12 months | 70.8 (55.6-86.1) | 81.8 (65.7-97.9) |
| 24 months | 70.8 (55.6-86.1) | 81.8 (65.7-97.9) |
| PFS HR (95% CI) | 1.27 (0.44-3.68) | |
| P value | .66 | |
| Investigator-assessed EFS† | ||
| Median EFS (95% CI), mo | NE (27.8-NE) | NE (NE-NE) |
| Event-free rate (95% CI), % | ||
| 12 months | 69.1 (53.8-84.3) | 81.8 (65.7-97.9) |
| 24 months | 69.1 (53.8-84.3) | 81.8 (65.7-97.9) |
| EFS HR (95% CI) | 1.46 (0.51-4.17) | |
| P value | .48 | |
| Overall survival | ||
| Median OS (95% CI), mo | NE (NE-NE) | NE (NE-NE) |
| Event-free rate (95% CI), % | ||
| 12 months | 86.3 (75.2-97.5) | 100.0 (100.0-100.0) |
| 24 months | 86.3 (75.2-97.5) | 86.4 (72.0-100.0) |
| OS HR (95% CI) | 1.02 (0.24-4.31) | |
| P value | .97 | |
All patients who were assessed for a response had PET assessment.
HR, hazard ratio; NE, not estimable.
Based on PET-CT.
Based on PET-CT or CT only.
In the Pola-M-CHP arm, 7 patients (17.5%) lacked investigator-assessed response data at the time of PRA due to early treatment discontinuation (reasons: death due to unknown cause, n = 2; grade 5 AE [coma deemed unrelated to study treatment, concurrent with cytomegalovirus {CMV} reactivation], n = 1; withdrawal of consent, n = 1; physician decision, n = 1; and improper enrollment [no study treatment received], n = 2); an additional patient for whom an indicator lesion was not identified by IRC was classified as a case of missing data. Of the 33 patients in the Pola-M-CHP arm who had PRA data available, 30 (90.9%) had a CR (as assessed by the investigators); this is an estimate of the CR rate in patients who received treatment and remained in the study until the PRA. None of the patients in the Pola-R-CHP arm had missing response data at the time of PRA.
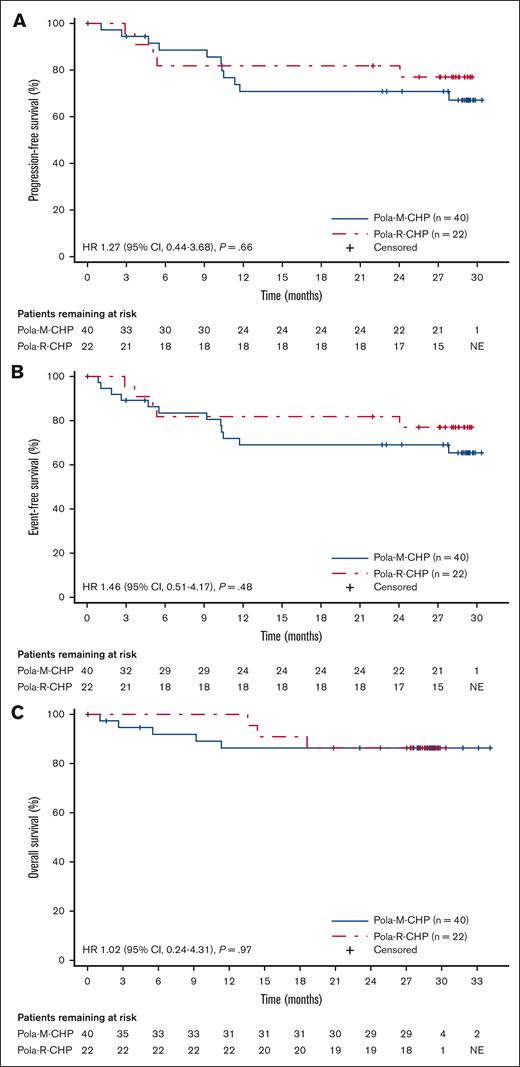
Median DoR (investigator assessed) was not estimable in either treatment arm (Table 2). Median values for (investigator-assessed) PFS (Figure 1A), EFS (Figure 1B), and overall survival (Figure 1C) were not estimable in either treatment arm; the stratified hazard ratios were 1.27 (95% CI, 0.44-3.68; P = .66), 1.46 (95% CI, 0.51-4.17; P = .48), and 1.02 (95% CI, 0.24-4.31; P = .97), respectively, for Pola-M-CHP compared with Pola-R-CHP (Table 2). The estimated 24-month PFS rate was 70.8% (95% CI, 55.6-86.1) in the Pola-M-CHP arm and 81.8% (95% CI, 65.7-97.9) in the Pola-R-CHP arm (Table 2).
PFS, EFS, and OS in the ITT population. Kaplan-Meier plot of (A) investigator-assessed PFS, (B) investigator-assessed EFS, and (C) OS in all randomized patients who received Pola-M-CHP vs Pola-R-CHP.
PFS, EFS, and OS in the ITT population. Kaplan-Meier plot of (A) investigator-assessed PFS, (B) investigator-assessed EFS, and (C) OS in all randomized patients who received Pola-M-CHP vs Pola-R-CHP.
Safety and tolerability
AEs with ≥10% incidence are summarized in Table 3. The most common AEs (reported in ≥40% of patients) were CRS (68.4%), neutropenia/neutrophil count decreased (63.2%), and nausea (44.7%) in the Pola-M-CHP arm and neutropenia/neutrophil count decreased (54.5%), fatigue (45.5%), and nausea and alopecia (both 40.9%) in the Pola-R-CHP arm. Grade ≥3 events occurred in 86.8% (33 of 38) and 59.1% of patients (13 of 22) in the Pola-M-CHP and Pola-R-CHP arms, respectively (Table 4). The most common grade ≥3 AE in both arms was neutropenia/neutrophil count decreased (Pola-M-CHP, 63.2%; Pola-R-CHP, 40.9%). Grade 5 (fatal) AEs occurred in 3 patients (7.9%; death at home due to unknown cause, n = 2; coma concurrent with CMV reactivation, n = 1) in the Pola-M-CHP arm; none were considered by the investigator as related to study treatment (Table 4). However, the possibility that the deaths were treatment related cannot be ruled out. Grade 5 AEs were not observed in the Pola-R-CHP arm.
Most common treatment-emergent AEs with at least 10% incidence and SAEs (at least 5% incidence; safety-evaluable population)
| . | Pola-M-CHP, n = 38 . | Pola-R-CHP, n = 22 . | ||
|---|---|---|---|---|
| Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | |
| Most common AEs (≥10% of patients) based on preferred term | ||||
| CRS | 26 (68.4) | 1 (2.6) | 0 | 0 |
| Nausea | 17 (44.7) | 0 | 9 (40.9) | 0 |
| Neutropenia/neutrophil count decreased | 23 (63.2) | 25 (63.2) | 12 (54.5) | 9 (40.9) |
| Diarrhea | 13 (34.2) | 3 (7.9) | 4 (18.2) | 0 |
| Fatigue | 12 (31.6) | 1 (2.6) | 10 (45.5) | 0 |
| Decreased appetite | 12 (31.6) | 1 (2.6) | 1 (4.5) | 1 (4.5) |
| Anemia | 10 (26.3) | 5 (13.2) | 5 (22.7) | 2 (9.1) |
| Alanine aminotransferase increased | 10 (26.3) | 2 (5.3) | 4 (18.2) | 1 (4.5) |
| Vomiting | 9 (23.7) | 0 | 5 (22.7) | 0 |
| Infusion-related reaction | 9 (23.7) | 0 | 2 (9.1) | 0 |
| Alopecia | 7 (18.4) | 0 | 9 (40.9) | 0 |
| Abdominal pain | 7 (18.4) | 1 (2.6) | 1 (4.5) | 0 |
| Constipation | 6 (15.8) | 0 | 4 (18.2) | 0 |
| Dyspepsia | 6 (15.8) | 0 | 0 | 0 |
| Thrombocytopenia | 6 (15.8) | 3 (7.9) | 1 (4.5) | 0 |
| Rash | 6 (15.8) | 0 | 4 (18.2) | 0 |
| Increased white blood cell count | 6 (15.8) | 4 (10.5) | 3 (13.6) | 2 (9.1) |
| Febrile neutropenia | 5 (13.2) | 5 (13.2) | 2 (9.1) | 2 (9.1) |
| Peripheral sensory neuropathy | 5 (13.2) | 0 | 4 (18.2) | 0 |
| Peripheral edema | 5 (13.2) | 0 | 0 | 0 |
| Pyrexia | 5 (13.2) | 0 | 0 | 0 |
| Back pain | 5 (13.2) | 0 | 3 (13.6) | 0 |
| Infection | 4 (10.5) | 3 (7.9) | 1 (4.5) | 1 (4.5) |
| Pruritus | 4 (10.5) | 0 | 1 (4.5) | 0 |
| Hypokalemia | 4 (10.5) | 0 | 1 (4.5) | 1 (4.5) |
| Hypomagnesemia | 4 (10.5) | 0 | 0 | 0 |
| Hypophosphatemia | 4 (10.5) | 0 | 1 (4.5) | 0 |
| Dyspnea | 4 (10.5) | 1 (2.6) | 3 (13.6) | 0 |
| Decreased weight | 4 (10.5) | 1 (2.6) | 1 (4.5) | 0 |
| Increased aspartate aminotransferase | 4 (10.5) | 3 (7.9) | 2 (9.1) | 1 (4.5) |
| Decreased platelet count | 4 (10.5) | 4 (10.5) | 3 (13.6) | 0 |
| Peripheral neuropathy | 3 (7.9) | 0 | 4 (18.2) | 0 |
| Headache | 2 (5.3) | 0 | 7 (31.8) | 0 |
| Insomnia | 2 (5.3) | 0 | 6 (27.3) | 0 |
| Most common SAEs (≥5% of patients) based on preferred term | ||||
| Febrile neutropenia | 5 (13.2) | 5 (13.2) | 1 (4.5) | 1 (4.5) |
| CRS | 5 (13.2) | 1 (2.6) | 0 | 0 |
| Infection not otherwise specified | 3 (7.9) | 3 (7.9) | 1 (4.5) | 1 (4.5) |
| Diarrhea | 3 (7.9) | 2 (5.3) | 0 | 0 |
| Anemia | 2 (5.3) | 2 (5.3) | 0 | 0 |
| Herpes zoster | 2 (5.3) | 1 (2.6) | 0 | 0 |
| Death | 2 (5.3) | 2 (5.3) | 0 | 0 |
| . | Pola-M-CHP, n = 38 . | Pola-R-CHP, n = 22 . | ||
|---|---|---|---|---|
| Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | |
| Most common AEs (≥10% of patients) based on preferred term | ||||
| CRS | 26 (68.4) | 1 (2.6) | 0 | 0 |
| Nausea | 17 (44.7) | 0 | 9 (40.9) | 0 |
| Neutropenia/neutrophil count decreased | 23 (63.2) | 25 (63.2) | 12 (54.5) | 9 (40.9) |
| Diarrhea | 13 (34.2) | 3 (7.9) | 4 (18.2) | 0 |
| Fatigue | 12 (31.6) | 1 (2.6) | 10 (45.5) | 0 |
| Decreased appetite | 12 (31.6) | 1 (2.6) | 1 (4.5) | 1 (4.5) |
| Anemia | 10 (26.3) | 5 (13.2) | 5 (22.7) | 2 (9.1) |
| Alanine aminotransferase increased | 10 (26.3) | 2 (5.3) | 4 (18.2) | 1 (4.5) |
| Vomiting | 9 (23.7) | 0 | 5 (22.7) | 0 |
| Infusion-related reaction | 9 (23.7) | 0 | 2 (9.1) | 0 |
| Alopecia | 7 (18.4) | 0 | 9 (40.9) | 0 |
| Abdominal pain | 7 (18.4) | 1 (2.6) | 1 (4.5) | 0 |
| Constipation | 6 (15.8) | 0 | 4 (18.2) | 0 |
| Dyspepsia | 6 (15.8) | 0 | 0 | 0 |
| Thrombocytopenia | 6 (15.8) | 3 (7.9) | 1 (4.5) | 0 |
| Rash | 6 (15.8) | 0 | 4 (18.2) | 0 |
| Increased white blood cell count | 6 (15.8) | 4 (10.5) | 3 (13.6) | 2 (9.1) |
| Febrile neutropenia | 5 (13.2) | 5 (13.2) | 2 (9.1) | 2 (9.1) |
| Peripheral sensory neuropathy | 5 (13.2) | 0 | 4 (18.2) | 0 |
| Peripheral edema | 5 (13.2) | 0 | 0 | 0 |
| Pyrexia | 5 (13.2) | 0 | 0 | 0 |
| Back pain | 5 (13.2) | 0 | 3 (13.6) | 0 |
| Infection | 4 (10.5) | 3 (7.9) | 1 (4.5) | 1 (4.5) |
| Pruritus | 4 (10.5) | 0 | 1 (4.5) | 0 |
| Hypokalemia | 4 (10.5) | 0 | 1 (4.5) | 1 (4.5) |
| Hypomagnesemia | 4 (10.5) | 0 | 0 | 0 |
| Hypophosphatemia | 4 (10.5) | 0 | 1 (4.5) | 0 |
| Dyspnea | 4 (10.5) | 1 (2.6) | 3 (13.6) | 0 |
| Decreased weight | 4 (10.5) | 1 (2.6) | 1 (4.5) | 0 |
| Increased aspartate aminotransferase | 4 (10.5) | 3 (7.9) | 2 (9.1) | 1 (4.5) |
| Decreased platelet count | 4 (10.5) | 4 (10.5) | 3 (13.6) | 0 |
| Peripheral neuropathy | 3 (7.9) | 0 | 4 (18.2) | 0 |
| Headache | 2 (5.3) | 0 | 7 (31.8) | 0 |
| Insomnia | 2 (5.3) | 0 | 6 (27.3) | 0 |
| Most common SAEs (≥5% of patients) based on preferred term | ||||
| Febrile neutropenia | 5 (13.2) | 5 (13.2) | 1 (4.5) | 1 (4.5) |
| CRS | 5 (13.2) | 1 (2.6) | 0 | 0 |
| Infection not otherwise specified | 3 (7.9) | 3 (7.9) | 1 (4.5) | 1 (4.5) |
| Diarrhea | 3 (7.9) | 2 (5.3) | 0 | 0 |
| Anemia | 2 (5.3) | 2 (5.3) | 0 | 0 |
| Herpes zoster | 2 (5.3) | 1 (2.6) | 0 | 0 |
| Death | 2 (5.3) | 2 (5.3) | 0 | 0 |
Data presented as n (%).
AE summary (safety-evaluable population)
| . | Pola-M-CHP, n = 38 . | Pola-R-CHP, n = 22 . |
|---|---|---|
| Patients with at least 1 AE | 38 (100) | 22 (100) |
| Mosunetuzumab/rituximab-related | 37 (97.4) | 16 (72.7) |
| Serious AE | 24 (63.2) | 3 (13.6) |
| Mosunetuzumab/rituximab-related | 20 (52.6) | 1 (4.5) |
| Any grade ≥3 AE | 33 (86.8) | 13 (59.1) |
| Any grade 5 AE (fatal) | 3 (7.9)∗ | 0 |
| Mosunetuzumab-related | 0 | NA |
| Infections concurrent to grade ≥3 neutropenia by laboratory abnormalities: | ||
| Infection | 2 (5.3) | 0 |
| Pneumonia | 0 | 1 (4.5) |
| Any AE leading to discontinuation of any study treatment | 5 (13.2) | 0 |
| Mosunetuzumab related | 2 (5.3) | NA |
| Any AE leading to mosunetuzumab/rituximab dose modification/interruption | 19 (50.0) | 3 (13.6) |
| . | Pola-M-CHP, n = 38 . | Pola-R-CHP, n = 22 . |
|---|---|---|
| Patients with at least 1 AE | 38 (100) | 22 (100) |
| Mosunetuzumab/rituximab-related | 37 (97.4) | 16 (72.7) |
| Serious AE | 24 (63.2) | 3 (13.6) |
| Mosunetuzumab/rituximab-related | 20 (52.6) | 1 (4.5) |
| Any grade ≥3 AE | 33 (86.8) | 13 (59.1) |
| Any grade 5 AE (fatal) | 3 (7.9)∗ | 0 |
| Mosunetuzumab-related | 0 | NA |
| Infections concurrent to grade ≥3 neutropenia by laboratory abnormalities: | ||
| Infection | 2 (5.3) | 0 |
| Pneumonia | 0 | 1 (4.5) |
| Any AE leading to discontinuation of any study treatment | 5 (13.2) | 0 |
| Mosunetuzumab related | 2 (5.3) | NA |
| Any AE leading to mosunetuzumab/rituximab dose modification/interruption | 19 (50.0) | 3 (13.6) |
Data presented as n (%).
NA, not applicable.
Death (due to unknown cause, n = 2; coma concurrent with cytomegalovirus reactivation, n = 1).
Serious AEs (SAEs) were reported in 63.2% (24 of 38) and 13.6% of patients (3 of 22) in the Pola-M-CHP and Pola-R-CHP arms, respectively. The most common SAEs (≥5% incidence) are summarized in Table 3. In the Pola-R-CHP arm, no single, specific SAE term was reported in >1 patient. Mosunetuzumab-related AEs leading to treatment discontinuation occurred in 2 patients (5.3%) in the Pola-M-CHP arm (grade 2 CRS with hypoxia, n = 1; grade 4 respiratory failure with pulmonary hemorrhage, n = 1). In the latter patient, CT images suggested a potential atypical/viral infection, but test results from an extensive microbiologic panel were negative, and pneumonitis was not reported. No treatment-related AEs led to treatment discontinuation in the Pola-R-CHP arm.
CRS events were reported in 26 patients (68.4%) in the Pola-M-CHP arm and were predominantly low grade (grade 1, 52.6%; grade 2, 13.2%; grade 3, 2.6%;); all events were resolved. Most CRS events were confined to C1 with the highest incidence occurring after the first dose of mosunetuzumab (65.8%; Figure 2); few events occurred after the first step-up dose. Median time to first CRS onset was 1 day and median duration of CRS was 2 days. Among patients who developed CRS, 6 patients (23.1%) received tocilizumab and 4 patients (15.4%) received corticosteroids; no patient required intensive care unit admission (supplemental Table 2).
CRS by treatment cycle and grade. Proportion of patients who experienced CRS events by grade and cycle in the Pola-M-CHP arm (safety-evaluable population). C, cycle.
CRS by treatment cycle and grade. Proportion of patients who experienced CRS events by grade and cycle in the Pola-M-CHP arm (safety-evaluable population). C, cycle.
Mean neutrophil count nadirs during each cycle were comparable between the 2 arms (supplemental Figure 2). Rates of low absolute neutrophil counts according to hematology laboratory tests were similar in both arms (Pola-M-CHP vs Pola-R-CHP: C1D1-7, 5.7% vs 0%; C1D8-14, 48.1% vs 42.1%; C1D15-21, 11.5% vs 15.8%; C2, 23.5% vs 27.3%; C3+, 14.7% vs 22.7%; supplemental Table 3).
Neurologic AEs potentially consistent with immune effector cell–associated neurotoxicity syndrome (ICANS) occurred in 3 patients (7.9%; grade 1 petit mal epilepsy, grade 3 syncope, grade 5 coma concurrent with CMV reactivation) in the Pola-M-CHP arm. After medical review, none of these AEs were classified as suspected ICANS. Peripheral neuropathy (all grade 1/2) was reported in 3 (7.9%) and 4 patients (18.2%) in the Pola-M-CHP and Pola-R-CHP arms, respectively.
Biomarkers
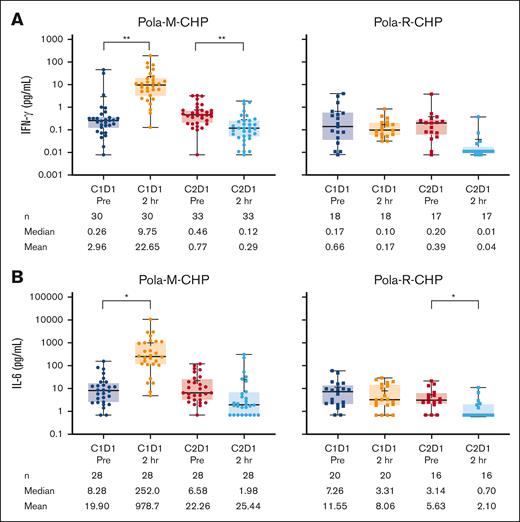
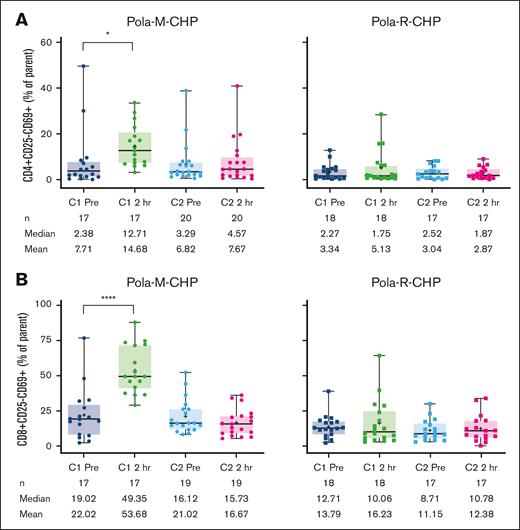
Pharmacodynamic changes including T-cell activation and cytokine modulation occurred after administration of Pola-M-CHP, consistent with the mechanism of action of mosunetuzumab. Levels of IFN-γ predose (n = 30; mean 2.96 pg/mL [median 0.26 pg/mL]) increased significantly after the initial mosunetuzumab dose (n = 30, mean 22.65 pg/mL [median 9.75 pg/mL]; Figure 3A; supplemental Figure 3A). A similar pattern was observed for IL-6 (predose, n = 28, mean 19.90 pg/mL [median 8.28 pg/mL]; C1D1 2-hour dose, n = 28, mean 978.7 pg/mL [median 252.0 pg/mL]; Figure 3B; supplemental Figure 3B). In contrast, no modulation in these cytokine levels was observed with the Pola-R-CHP regimen. Transient increases in activated T cells, as evidenced by increased surface expression of CD69+ on both CD4 and CD8 cells, were observed after Pola-M-CHP administration compared with minimal changes after Pola-R-CHP administration (Figure 4A-B).
Cytokine levels during treatment. Levels of (A) IFN-γ and (B) IL-6 during treatment with Pola-M-CHP and Pola-R-CHP. Paired t tests were performed on paired samples collected predose and 2 hours postdose during cycles 1 and 2. Boxes represent the 25th percentile, median, and 75th percentile, with the shaded regions showing the interquartile range. Plus signs represent mean values. Whiskers extend to the minimum and maximum values. ∗P = .01-.05; ∗∗P = .001-.01. 2 hr, 2 hours postdose; Pre, predose.
Cytokine levels during treatment. Levels of (A) IFN-γ and (B) IL-6 during treatment with Pola-M-CHP and Pola-R-CHP. Paired t tests were performed on paired samples collected predose and 2 hours postdose during cycles 1 and 2. Boxes represent the 25th percentile, median, and 75th percentile, with the shaded regions showing the interquartile range. Plus signs represent mean values. Whiskers extend to the minimum and maximum values. ∗P = .01-.05; ∗∗P = .001-.01. 2 hr, 2 hours postdose; Pre, predose.
T-cell activation markers during treatment. (A) CD4+ and (B) CD8+ in patients treated with Pola-M-CHP vs Pola-R-CHP. Paired t tests were performed on paired samples collected predose and 2 hours postdose during cycles 1 and 2. Boxes represent the 25th percentile, median, and 75th percentile, with the shaded regions showing the interquartile range. Plus signs represent mean values. Whiskers extend to the minimum and maximum values. ∗P = .01-.05; ∗∗∗∗P < .0001.
T-cell activation markers during treatment. (A) CD4+ and (B) CD8+ in patients treated with Pola-M-CHP vs Pola-R-CHP. Paired t tests were performed on paired samples collected predose and 2 hours postdose during cycles 1 and 2. Boxes represent the 25th percentile, median, and 75th percentile, with the shaded regions showing the interquartile range. Plus signs represent mean values. Whiskers extend to the minimum and maximum values. ∗P = .01-.05; ∗∗∗∗P < .0001.
Discussion
This is, to our knowledge, the first clinical trial to compare Pola-M-CHP with Pola-R-CHP in patients with newly diagnosed DLBCL. In this phase 2 study, response rates (IRC-assessed CR rate and ORR: Pola-M-CHP, 72.5% [95% CI, 56.1-85.4] and 75.0% [95% CI, 58.8-87.3]; Pola-R-CHP, 77.3% [95% CI, 54.6-92.2] and 86.4% [95% CI, 65.1-97.1], respectively) were similar in both treatment arms and in line with results from the recent POLARIX study of patients with previously untreated DLBCL.7 An important caveat is the high rate of patients without any investigator-assessed PET-CT scan response assessments in the Pola-M-CHP arm (17.5%, n = 7, including 2 patients who did not receive study treatment and 2 patients who died at home due to unknown causes before completing any response assessments) compared with the Pola-R-CHP arm where all patients had at least 1 postbaseline investigator-assessed response. These missing response assessment data may have contributed to an overall lower response rate reported in the Pola-M-CHP arm. Considering that caveat and the relatively small sample size, the IRC-assessed CR rate observed with Pola-M-CHP (72.5%) was comparable with that observed with Pola-R-CHP in POLARIX (78.0%).7 Similarly, the investigator-assessed CR rate observed with Pola-M-CHP (75.0%) was comparable with that previously reported for M-CHOP (85.0%),11 but relatively higher than R-CHOP in GOYA (59.1%).14 IRC- and investigator-assessed ORR (75.0% and 80.0%, respectively) were comparable with those reported for Pola-R-CHP (IRC assessed, 85.5%) in POLARIX,7 R-CHOP (investigator assessed, 77.6%) in GOYA,14 and M-CHOP (investigator assessed, 87.5%).11 However, it is important to note that the GOYA study used response assessments with or without PET according to modified Cheson 2007 criteria.15 The frequency of patients with investigator-assessed PD at the end of treatment was higher in the Pola-R-CHP arm (22.7%; 95% CI, 7.82-45.37) than in the Pola-M-CHP arm (2.5%; 95% CI, 0.06-13.16) despite the numerically greater anti-CD20 antibody therapy exposure observed in the Pola-R-CHP arm, suggesting a possible difference in activity against DLBCL between mosunetuzumab and rituximab. DoR, EFS, PFS, and overall survival were all not estimable; however, the 24-month PFS rates in the Pola-M-CHP and Pola-R-CHP arms (70.8% and 81.8%, respectively) were generally comparable with those reported in POLARIX (76.7%),7 taking into account the respective CIs.
The overall safety profile of Pola-M-CHP was comparable with previous experience with mosunetuzumab in relapsed/refractory B-cell NHL and M-CHOP in newly diagnosed DLBCL.11,16,17 Although CRS events were observed at a higher frequency than previously described in patients receiving mosunetuzumab monotherapy in B-cell NHL (27.4%)17 (possibly due to reasons described by Olszewski et al11), most were low grade in severity and confined to C1, with the exception of 1 patient with a grade 3 CRS event.
SAEs were more common in the Pola-M-CHP arm than in the Pola-R-CHP arm (63.2% vs 13.6%, respectively); however, the relatively small patient numbers in this randomized phase 2 trial do not allow for statistical power to evaluate differences between arms. Differences in demographic and baseline characteristics in the Pola-R-CHP vs Pola-M-CHP arm, respectively, such as enrollment of a younger population (patients aged <65 years, 72.7% vs 45.0%) and more patients with ECOG PS of 0 (61.9% vs 42.1%) might have contributed to this. Furthermore, the SAE rate observed in the Pola-R-CHP arm was much lower than anticipated when compared with the SAE rate observed with Pola-R-CHP in POLARIX (13.6% vs 34.0%),7 which may be due in part to the relatively small sample size in the current study. The numerically greater rate of serious febrile neutropenia in the Pola-M-CHP arm (13.2% vs 4.5% with Pola-R-CHP) was similar to that observed with Pola-R-CHP in POLARIX (13.8%).7
The frequency of neutropenia/neutrophil count decreased reported as an AE was numerically higher in the Pola-M-CHP arm than the Pola-R-CHP arm (63.2% vs 54.5%). However, the proportion of patients who had low absolute neutrophil counts according to hematology laboratory tests collected during the study (not dependent on investigator-initiated reporting as AEs) was similar between the 2, as were the means and CIs of neutrophil count nadirs during each cycle. Furthermore, the frequent protocol-mandated surveillance of hematology laboratory testing on days 1, 8, and 15 of the first 2 cycles of treatment during this study might have contributed to the higher rate of neutropenia than reported in other recent studies, such as POLARIX and GOYA, which did not require frequent hematology laboratory assessments.7,14
Three fatal AEs were reported in the Pola-M-CHP arm. No autopsy data were available and additional information was lacking for these events. Two patients died at home without a known cause or other informative data (one during C2 and the other during C4); the deaths occurred 5 days after the last study treatment in both cases. The third patient died after the completion of study treatment during C6. The patient experienced coma (26 days after treatment) in the setting of CMV reactivation. None of the fatal AEs were assessed by the investigator as related to study treatment; however, the possibility that the deaths were treatment related cannot be ruled out.
Neurologic AEs potentially consistent with ICANS were infrequent. Although peripheral neuropathy is consistent with the mechanism of action of polatuzumab vedotin,18,19 the incidence of peripheral neuropathy in this study was low in both treatment arms (Pola-M-CHP, 7.9%; Pola-R-CHP, 18.2%) compared with Pola-R-CHP (52.9%) and R-CHOP (53.9%) in POLARIX.7
Biomarker analyses demonstrated that a distinct pattern of cytokine release and T-cell activation is observed in patients treated with Pola-M-CHP compared with Pola-R-CHP, indicative of immune synapse formation. Maximal secretion of both IFN-γ and IL-6 occurred early (by the end of infusion) after the initial dose of mosunetuzumab, despite chemotherapy immediately before mosunetuzumab administration. T-cell activation (based on surface expression of the early activation marker CD69 on CD4 and CD8 T-cell subsets) was also increased after the first dose of mosunetuzumab in the Pola-M-CHP arm but was absent in the Pola-R-CHP arm. Although T-cell activation was observed in both CD4 and CD8 T cells, peak activation occurred in the CD8 subset 2 hours after the first mosunetuzumab dose.
Limitations of this study include the small sample size, open-label study design, and the high proportion of patients with no response assessment at the end of treatment in the Pola-M-CHP arm (mainly due to early treatment discontinuations, which may have led to a relative underestimation of response rates). Furthermore, the limited scope of IRC assessments that were performed (at screening, interim response, and primary response time points) restricted the determination of IRC-assessed PFS.
In summary, although patients who received Pola-M-CHP for first-line treatment of DLBCL showed better response rates than seen in past studies of R-CHOP in similar patient populations, Pola-M-CHP showed no clear efficacy advantage in this randomized phase 2 study. The frequency of PD at the end of treatment was lower with Pola-M-CHP compared with Pola-R-CHP, but this did not translate into increased PFS. The rate of grade ≥3 AEs was numerically higher in the Pola-M-CHP arm than the Pola-R-CHP arm (86.8% vs 59.1%), with SAEs reported in 63.2% and 13.6% of patients in the Pola-M-CHP and Pola-R-CHP arms, respectively. Factors such as differences in patient demographics and different rates of early treatment discontinuations between the treatment arms may have contributed to lower response rates and higher frequencies of SAEs and grade 3/4 AEs in the Pola-M-CHP arm. Biomarker studies confirmed that T-cell activation soon after mosunetuzumab administration following combination chemotherapy occurred similarly to observations with monotherapy. In conclusion, although an active combination, Pola-M-CHP did not demonstrate a clinical benefit over Pola-R-CHP in this small study and additional dose optimization may be required.
Acknowledgments
Third-party medical writing assistance, under the direction of the authors, was provided by Dikeledi Matlebjane of Ashfield MedComms, an Inizio company, and was funded by F. Hoffmann-La Roche Ltd. Statistical support in finalizing this manuscript was provided by Amy V. Kapp (employee of Genentech, Inc).
NCT03677141 is sponsored by Genentech, Inc. The sponsor was involved in the design and conduct of the study, collection, management, analysis, and interpretation of the data.
Authorship
Contribution: J. Westin and M.C.W. contributed to the study design; J. Westin, T.J.P., A.M., E.G.-B., S.G., and J.M.B.-B. provided study materials or patients; J. Westin, E.G.-B., J.S., E. Purev, and D.L.Y. collected and assembled the data; J. Westin, T.J.P., A.M., M.S.H., S.G., M.C.W., J. Wang, R.B., J.S., E. Purev, and D.L.Y. performed data analysis and interpretation; and J. Westin, T.J.P., A.M., M.S.H., E.G.-B., C.T., M.B.-O., R.G., S.G., M.C.W., J. Wang, R.B., J.S., E. Penuel, E. Purev, D.L.Y., and J.M.B.-B. carried out manuscript writing, provided final approval of the manuscript, and are accountable for all aspects of this work.
Conflict-of-interest disclosure: J. Westin reports a consulting role with Roche/Genentech, Kite, Bristol-Myers Squibb, Novartis, MorphoSys/Incyte, AstraZeneca, Merck, ADC Therapeutics, Monte Rosa, and Iksuda; and research funding from Roche/Genentech, Kite, Bristol-Myers Squibb, Novartis, MorphoSys/Incyte, AstraZeneca, and ADC Therapeutics. T.J.P. reports consultancy role with AbbVie, AstraZeneca, and ADC Therapeutics; research funding from AbbVie, Bayer, Bristol-Myers Squibb, and Genentech; and advisory board membership with Epizyme. A.M. reports a consulting role with Gilead, AstraZeneca, Seagen, Incyte/MorphoSys, BeiGene, Roche-Genentech, and ADC Therapeutics; honoraria from Gilead, AstraZeneca, Seagen, Incyte/MorphoSys, Kyowa Kirin, BeiGene, and Roche-Genentech; and research funding from Incyte, Takeda, Gilead, Bristol-Myers Squibb, Innate Pharma, Seagen, and ONO Pharmaceuticals. M.S.H. reports consultancy role with AbbVie, AstraZeneca, BeiGene, Eli Lilly, Janssen, Kite, Novartis, Pharmacyclics, and TG Therapeutics. E.G.-B. reports consultancy or advisory role with Janssen, AbbVie, Kiowa, EUSA Pharma, Incyte, Eli Lilly, BeiGene, and Novartis. C.T. reports consultancy or advisory role with F. Hoffmann-La Roche Ltd, Incyte, Kite/Gilead Sciences, Novartis, Bristol-Myers Squibb, and Takeda. M.B.-O. reports a consulting role for Incyte and Kite; research funding from Roche, Kite, Sociedad Española de Hematología y Hemoterapia, and Asociación Madrileña de Hematología y Hemoterapia; honoraria and speaker’s bureau fees from Bristol-Myers Squibb, Kite, Novartis, Roche, Incyte, and AbbVie; and membership on an entity’s Board of Directors or advisory committees with Sociedad Española de Hematología y Hemoterapia, and Asociación Madrileña de Hematología y Hemoterapia. R.G. reports a consulting role with Celgene, Novartis, Roche, Bristol-Myers Squibb, Takeda, AbbVie, AstraZeneca, Janssen, Merck Sharp & Dohme, Merck, Gilead, Daiichi Sankyo, and Sanofi; honoraria fees from Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, Bristol-Myers Squibb, Merck Sharp & Dohme, Sandoz, AbbVie, Gilead, Daiichi Sankyo, and Sanofi; meeting attendance and/or travel support from Roche, Amgen, Janssen, AstraZeneca, Novartis, Merck Sharp & Dohme, Celgene, Gilead, Bristol-Myers Squibb, AbbVie, and Daiichi Sankyo; and advisory board participation with Roche, Janssen, AstraZeneca, Novartis, Merck Sharp & Dohme, Celgene, Gilead, Bristol-Myers Squibb, AbbVie, Daiichi Sankyo, Takeda, Merck, and Sanofi. S.G. reports consultancy, honoraria, and speaker’s bureau fees from AbbVie, Amgen, AstraZeneca, Gilead, Janssen, Novartis, Roche, Pfizer, and Sobi; and honoraria and speaker’s bureau fees from Angelini, Servier, Swixx, and Zentiva. M.C.W. reports meeting attendance and/or travel support from Roche/Genentech; employment with Genentech; patents planned, issued, or pending for Genentech; and stock and stock options in Roche. J. Wang reports employment with Genentech and stocks in Roche. R.B. reports employment with Roche. J.S., E. Purev, and D.L.Y. report employment with Genentech and equity holding in Roche. E. Penuel reports employment with Genentech. J.M.B.-B. reports a consultancy role with Daiichi and research funding from Fundesalud (grants of European funds), and Daiichi.
Correspondence: Jason Westin, Lymphoma & Myeloma, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 0429, Houston, TX 77030; email: jwestin@mdanderson.org.
References
Author notes
All authors had full access to the data in the study. Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available at https://vivli.org/members/ourmembers/. For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
The full-text version of this article contains a data supplement.