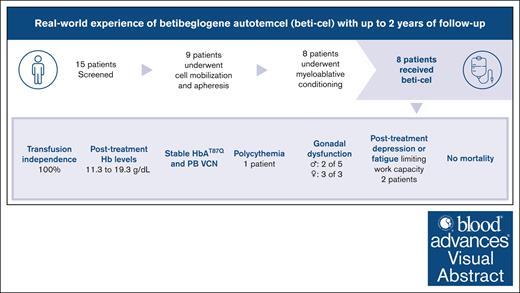
Key Points
In a real-world setting in Germany, beti-cel therapy was safe and effective in patients with transfusion-dependent non-β0/β0 thalassemia.
All patients achieved and maintained transfusion independence, and safety up to 24 months was consistent with the clinical trial data.
Visual Abstract
Gene addition and editing strategies for transfusion-dependent β-thalassemia have gained momentum as potentially curative treatment options, with studies showcasing their efficacy and safety. We report, to our knowledge, the first real-world application of betibeglogene autotemcel (beti-cel; Zynteglo) during its period of active license in Europe from January 2020 to March 2022 for patients aged ≥12 years without a β0/β0 genotype and without a HLA-matched sibling donor, before beti-cel marketing authorization was withdrawn by its holder because of nonsafety reasons. Among 15 screened patients, 4 opted out for fertility and safety concerns, 2 were excluded because of marked hepatic siderosis, and 1 had apheresis collection failure. Eight patients received beti-cel after busulfan myeloablative conditioning, all achieving transfusion independence within 8 to 59 days, with posttreatment hemoglobin levels ranging from 11.3 to 19.3 g/dL. No deaths occurred, but acute toxicity mirrored busulfan’s known effects. Posttreatment platelet management faced challenges because of HLA-antibodies in 3 patients. Monitoring up to month 24 revealed pituitary-gonadal endocrine dysfunction in all 3 female and in 2 of 5 male patients. Additionally, we observed unexpected posttreatment sequelae: 1 patient developed polycythemia that could not be explained by known genetic or acquired mechanisms, 1 patient developed posttreatment depression and anxiety prohibiting her from returning to work, and 1 patient developed fatigue severely compromising both quality of life and work capacity. This real-world experience corroborates beti-cel’s efficacy and safety and provides information on adverse events observed during real-world use of the therapy.
Introduction
β-thalassemia is an autosomal recessive, single-gene disorder that is caused by >350 different mutations of the β-globin gene.1 These mutations lead to reduced (β⁺) or absent (β0) β-globin chain production and α-globin chain aggregation in erythroid precursors as well as ineffective erythropoiesis, microcytic anemia, hypochromic anemia, and hemolysis. Patients with homozygous or a compound heterozygous genotype require lifelong red blood cell (RBC) transfusions; this is referred to as transfusion-dependent thalassemia (TDT), also known as thalassemia major. Regular packed RBC (pRBC) transfusions can correct for anemia but result in iron overload requiring iron chelation therapy.2 Despite advances in thalassemia care, patient life expectancy remains lower than the general population, and disease management remains a substantial burden for patients and their caregivers.3-5
Allogeneic hematopoietic stem cell transplantation (HSCT) has been available as a curative treatment option with favorable outcomes for patients with TDT, including young patients with limited iron overload and a HLA-matched sibling donor (MSD) or HLA-matched unrelated donor.6-8 However, MSD availability has been limited in communities with small families and in groups not well represented in international donor registries.9 The use of mismatched family donors has been associated with substantial mortality and morbidity due to acute and chronic graft-versus-host disease, graft rejection, and infertility.8,10,11 Although these factors have limited the feasibility of HSCT from mismatched donors, improvements in the conditioning regimen tailored for HSCT for TDT from haploidentical donors has resulted in promising outcomes with this treatment modality.12,13
Recent clinical studies have shown that gene therapy, either through gene addition of functional copies of a modified β-globin gene (HBB) by lentiviral vector (LVV) or gene editing of BCL11A enhancer to induce fetal hemoglobin (Hb) expression by CRISPR/CRISPR–associated protein 9, results in transfusion independence (TI) in most patients with TDT aged ≥12 years irrespective of genotype,14,15 including patients as young as 4 years of age with lentiviral-based gene therapy.14
Betibeglogene autotemcel (beti-cel; Zynteglo) was first licensed in Europe for the treatment of patients with TDT aged ≥12 years and a non-β0/β0 genotype. Beti-cel represents a cellular product produced by ex vivo transduction of autologous hematopoietic stem cells (HSCs) with the BB305 LVV,16 encoding HBB with a substitution at codon 87 (βA-T87Q). This modification preserves the function and oxygen affinity of the β-globin chain, thus allowing one to distinguish βA-T87Q from adult Hb (HbA) by high-performance liquid chromatography.17,18
The European Commission granted conditional marketing authorization for beti-cel for patients with non-β0/β0 TDT in May 2019 based on the data from 3 clinical trials (HGB-204, ClinicalTrials.gov identifier: NCT01745120; HGB-205, ClinicalTrials.gov identifier: NCT02151526; and HGB-207, ClinicalTrials.gov identifier: NCT02906202).19,20 Marketing was temporarily suspended by the marketing authorization holder from February to July 2021 after reports of myeloid neoplasms arising in 2 patients with sickle cell disease treated with BB305 vector.21-23 The European Medicines Agency’s safety committee concluded that the vector was unlikely the cause of the myeloid neoplasms and that the risk-to-benefit of beti-cel remained favorable. Marketing authorization in the European Union for this product was subsequently withdrawn at the request of the marketing authorization holder in March 2022 because of commercial reasons. Beti-cel was subsequently approved in the United States for the treatment of adult and pediatric patients with β-thalassemia who require regular RBC transfusions.
Here, we report data on 8 patients with non-β0/β0 TDT who received beti-cel at University Hospital Heidelberg after the approval of beti-cel in the European Union. Our findings demonstrate overall consistent safety and effectiveness of gene addition therapy in the real world as compared with the beti-cel clinical program. We highlight the manifestation of busulfan-induced endocrine dysfunction, likely resulting in infertility. Furthermore, we document a case of polycythemia of unknown etiology, 1 patient with paradoxical depression/anxiety, and a case of fatigue leading to diminished work capacity and quality of life (QoL).
Methods
Based on the criteria established by the European Medicines Agency for the marketing authorization of beti-cel, the medical service of the German statutory health insurance system devised operational criteria requiring that patients to be treated with beti-cel met the parameters set by the conditional license (supplemental Table 1).
HSCs for beti-cel manufacturing were collected by mobilization with granulocyte-colony stimulating factor (G-CSF) and plerixafor (supplemental Table 2) and apheresis in adherence with the beti-cel summary of product characteristics (SmPC; supplemental Table 3).19,20 HSCs were sent for central manufacturing of beti-cel.16 A backup reserve of HSCs (minimum 2 × 106 CD34+ cells per kg) was retained at the clinical site as a contingency for engraftment failure.
Collection of peripheral blood CD34+ cells was performed at University Hospital Heidelberg in cooperation with the Institute of Clinical Transfusion Medicine and Cell Therapy, Heidelberg, using Spectra Optia devices (Terumo). Apheresis was performed via central venous access. Up to 4 total blood volumes were processed over a maximum period of 5 hours. Acid-citrate-dextrose was used at a blood-to-anticoagulant ratio of 10:1 to 15:1. In the case of a low body weight of <40 kg, the intermediate density layer set had to be prefilled with an erythrocyte concentrate.
Patients were assessed for HLA antibodies to assess for platelet transfusion refractoriness. All patients underwent myeloablative conditioning with busulfan over 4 consecutive days (16 doses at 0.8 mg/kg IV in 6-hour intervals, totaling 12.8 mg/kg; therapeutic drug monitoring was not used). After a minimum 48-hour washout period, patients received freshly thawed beti-cel IV over ∼5 minutes.
Neutrophil engraftment was defined as an absolute neutrophil count of at least 500 cells per μL for 3 consecutive days. Platelet engraftment was defined as a platelet count exceeding 20 platelets per nL with an interval of ≥7 days since the last platelet transfusion.
Assessments included data that were generated as part of routine clinical care for patients receiving autologous HSCT. Overall survival, TI, kinetics of hematopoietic engraftment, and iron elimination requirements were evaluated. TI was defined as not receiving any β-thalassemia–related pRBC transfusions for ≥12 months after beti-cel infusion without postinfusion supportive pRBC transfusions. Liver iron concentration (LIC) was measured by FerriScan. Additional assessments included peripheral blood βA-T87Q-globin levels as monitored by high-performance liquid chromatography and vector copy number by quantitative polymerase chain reaction. Persistent oligoclonality was defined as relative frequency of ≥10% for the same integration site at 2 consecutive time points or relative frequency of ≥5% for the same ≥2 integration sites at 2 consecutive time points.
Continuous variables were presented with median, minimum, and maximum values, whereas categorical variables show the proportion of treated patients in each category. Wilcoxon and Mann-Whitney U tests were used for statistical significance, although the power of this analysis is limited because of the small number of patients.
Adverse events and serious adverse events were graded in line with Common Terminology Criteria for Adverse Events, version 5.0.
All patients were enrolled in the German Society of Pediatric Oncology and Hematology Rare Anemia Registry. Baseline data, including demographics and information regarding thalassemia manifestations and treatment, were retrospectively collected. This study was approved by the ethics committee of the Medical Faculty Heidelberg University (Ref S-217/2020).
Results
Patient characteristics
A total of 15 patients were evaluated for beti-cel eligibility between March and November 2020 (supplemental Figure 1). Of these patients, 4 withdrew because of patient choice, and 2 were considered ineligible because of severe hepatic siderosis and an LIC of 42.1 and 43 mg/g dry weight (dw), respectively. One patient had an apheresis collection failure (supplemental Table 1). As of 28 July 2023, the median duration of follow-up for 8 treated patients was 541.5 days (range, 365-734).
Eight patients, 3 male and 5 female, aged 13 to 26 years received beti-cel between February 2021 and May 2022 (Table 1). Of these patients, 4 had a β⁺/β⁺ genotype and 4 had a β⁺/β0 genotype. All patients had been receiving regular RBC transfusions for a median span of 15.8 years (range, 6-27) with an annual transfusion requirement between 114 and 260 mL/kg per year. All patients had an LIC of <8 mg/g dw, and none displayed evidence of cardiac iron overload. Two patients had undergone splenectomy before treatment with beti-cel. Adequate iron chelation had been administered to all patients for a minimum of 3 years before apheresis, with a median of 12 years (range, 3-22). No patients had a treatment history of prior HSCT or use of hydroxyurea or luspatercept.
Characteristics of 8 patients treated with beti-cel
| Patient . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . |
|---|---|---|---|---|---|---|---|---|
| Age at the time of beti-cel infusion, y | 15 | 29 | 18 | 17 | 14 | 22 | 26 | 13 |
| Sex | Male | Male | Male | Female | Female | Male | Female | Male |
| Country of origin | Ukraine | Turkey | Syria | Afghanistan | Lebanon | Syria | Thailand | Lebanon |
| Functional genotype | β°/β⁺ | β°/β⁺ | β⁺/β⁺ | β⁺/β⁺ | β⁺/β⁺ | β°/β⁺ | β°/β+E | β⁺/β⁺ |
| Molecular genotype (HGVS nomenclature) | HBB:c.92+1G>A/HBB:c.92+6T>C | HBB:c.92+1G>A/HBB:c.92+6T>C | HBB:c.93–21G>A/HBB:c.93–21G>A | HBB:c.92+5G>C/HBB:c.92+5G>C | HBB:c.93–21G>A/HBB:c.92+6T>C | HBB:c.92+1G>A/HBB:c.92+6T>C | HBB:c.27_28insAGAA/HBB:c.79G>A | HBB:c.92+6T>C/HBB:c.93–21G>A |
| Age at initiation of regular RBC transfusions, y | 9.8 | 2 | 1.5 | 0.6 | 1.5 | 1 | 11 | 3 |
| Average no. of RBC transfusions per year over last 2 years | 17.3 | 20.8 | 17.3 | 17.3 | 13.0 | 26.0 | 17.3 | 12.0 |
| Deferasirox, mg/kg per day | 28.2 | 22.0 | 15.0 | 23.0 | 12.5 | 21.1 | 14.9 | |
| Deferiprone, mg/kg per day | 56.9 | 76.0 | ||||||
| Splenectomy | No | Yes | Yes | No | No | No | No | No |
| LIC, mg/g dw | 2 | 0.7 | 6.9 | 3.5 | 7.9 | 7 | 2.3 | 1.9 |
| Cardiac T2∗, ms | 35 | 38 | 37 | 25 | 44 | 37 | 41 | >20 |
| Serum ferritin, μg/L | 1059 | 282 | 1338 | 967 | 2342 | 1315 | 392 | 1268 |
| Spleen volume, mL | 801 | Post splenectomy | Post splenectomy | 534 | 547 | 691 | 784 | 623 |
| Patient . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . |
|---|---|---|---|---|---|---|---|---|
| Age at the time of beti-cel infusion, y | 15 | 29 | 18 | 17 | 14 | 22 | 26 | 13 |
| Sex | Male | Male | Male | Female | Female | Male | Female | Male |
| Country of origin | Ukraine | Turkey | Syria | Afghanistan | Lebanon | Syria | Thailand | Lebanon |
| Functional genotype | β°/β⁺ | β°/β⁺ | β⁺/β⁺ | β⁺/β⁺ | β⁺/β⁺ | β°/β⁺ | β°/β+E | β⁺/β⁺ |
| Molecular genotype (HGVS nomenclature) | HBB:c.92+1G>A/HBB:c.92+6T>C | HBB:c.92+1G>A/HBB:c.92+6T>C | HBB:c.93–21G>A/HBB:c.93–21G>A | HBB:c.92+5G>C/HBB:c.92+5G>C | HBB:c.93–21G>A/HBB:c.92+6T>C | HBB:c.92+1G>A/HBB:c.92+6T>C | HBB:c.27_28insAGAA/HBB:c.79G>A | HBB:c.92+6T>C/HBB:c.93–21G>A |
| Age at initiation of regular RBC transfusions, y | 9.8 | 2 | 1.5 | 0.6 | 1.5 | 1 | 11 | 3 |
| Average no. of RBC transfusions per year over last 2 years | 17.3 | 20.8 | 17.3 | 17.3 | 13.0 | 26.0 | 17.3 | 12.0 |
| Deferasirox, mg/kg per day | 28.2 | 22.0 | 15.0 | 23.0 | 12.5 | 21.1 | 14.9 | |
| Deferiprone, mg/kg per day | 56.9 | 76.0 | ||||||
| Splenectomy | No | Yes | Yes | No | No | No | No | No |
| LIC, mg/g dw | 2 | 0.7 | 6.9 | 3.5 | 7.9 | 7 | 2.3 | 1.9 |
| Cardiac T2∗, ms | 35 | 38 | 37 | 25 | 44 | 37 | 41 | >20 |
| Serum ferritin, μg/L | 1059 | 282 | 1338 | 967 | 2342 | 1315 | 392 | 1268 |
| Spleen volume, mL | 801 | Post splenectomy | Post splenectomy | 534 | 547 | 691 | 784 | 623 |
HGVS, Human Genome Variation Society.
Treatment timeline
The time from obtaining informed consent to beti-cel infusion encompassed a median duration of 531 days (range, 338-585; supplemental Figure 2). The temporary suspension of marketing of beti-cel between February and July 2021 prolonged the interval between screening and infusion for 7 of 8 patients. As a result, the median time from apheresis to beti-cel infusion was 113 days (range, 88-298). Patients were discharged from in-patient care a median 31.5 days (range, 24-67) after beti-cel infusion.
Apheresis and manufacturing of beti-cel
Of 8 patients, 7 (87.5%) required 1 cycle of mobilization and apheresis. The median number of CD34+ cells collected for manufacturing was 30.56 × 106 cells per kg (range, 17.9 × 10⁶ to 43.79 × 10⁶; supplemental Table 4). One patient required a second collection cycle because of CD34+ cell loss during the manufacturing process.
Apheresis was not successful in 1 patient despite effective HSC mobilization by G-CSF and plerixafor. Although this patient had a peak peripheral blood CD34+ cell count of 288.7 cells per μL, the cell collection did not provide sufficient CD34+ cells because of high numbers of normoblasts (maximum of 56 per nL) and elevated total nucleated blood cell count (maximum of 145 per nL) accompanied by thrombocytosis (maximum of 1460 per nL).25 The patient, a male aged 28 years with a β+/β+ genotype (HBB:c.-80T>A/HBB:c.-80T>A), had undergone splenectomy 141 days before apheresis because of symptomatic splenomegaly (spleen volume measured by magnetic resonance imaging, 1091 mL; postsplenectomy spleen weight, 1465 g) and risk of splenic rupture during HSC mobilization.26
Conditioning and beti-cel infusion
All 8 patients underwent busulfan conditioning in accordance with the Zynteglo SmPC.27 Dosing was done in accordance with the busulfan SmPC without therapeutic drug monitoring. Supportive medications included clonazepam, heparin, ursodeoxycholic acid, liposomal amphotericin B, aciclovir, fluconazole, and cotrimoxazole. No patient received defibrotide. Beti-cel was administered through injection via a central line after a 48- to 60-hour busulfan washout.
HLA antibodies
Before beti-cel infusion, 7 of 8 patients underwent screening for HLA antibodies. Two had HLA antibodies identified before their first platelet transfusion and received a total of 4 (patient 6) and 7 (patient 5) HLA-matched platelet transfusions. One patient had HLA antibodies detected after refractoriness to platelet transfusions (patient 7). Despite the administration of 46 HLA-matched platelet transfusions between day +8 and day +61, the patient remained thrombocytopenic. Subsequently, eltrombopag treatment was initiated from day +43 to day +59, rendering the patient platelet-transfusion–free by day +61. However, the patient's platelet count remained low at 91 per nL at day +365.
Engraftment
The median time to neutrophil engraftment was 26 days (range, 21-44; supplemental Figure 3). Two patients received G-CSF before neutrophil engraftment (patient 4, from day +21 to +26; patient 7, from day +38 to +50). The median time to platelet engraftment was 44.5 days (range, 20-98). The median requirement for platelet transfusions was 7.5 days (range, 2-46) and for a median duration of 26.5 days (range, 14-63) after infusion of beti-cel.
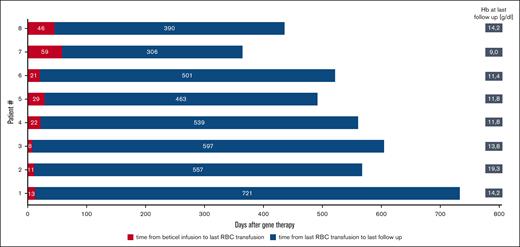
TI
All 8 patients achieved and maintained TI at last follow-up (Figure 1). Patients received a median of 7 pRBCs, equivalent to a median of 29.9 mL/kg (range, 18.7-72.7) after beti-cel infusion. The median time to last transfusion was 21.5 days (range, 8-59) after beti-cel infusion. The median Hb level at the last follow-up was 11.8 g/dL (range, 11.4-19.3). One patient received erythropoietin treatment from day +43 to day +54 because of delayed erythrocyte engraftment.
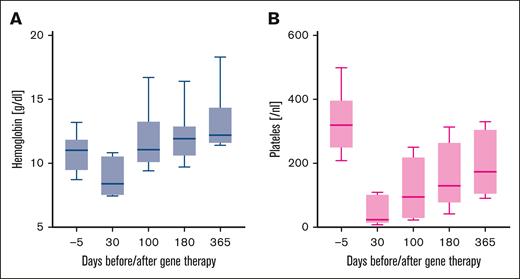
Hb levels and platelet counts improved over time during the first year of treatment (Figure 2).
Kinetics of Hb levels and platelet counts in 8 TDT patients after beti-cel infusion.
Kinetics of Hb levels and platelet counts in 8 TDT patients after beti-cel infusion.
Pharmacodynamic assessments
The median percentage of LVV-positive CD34+ cells was 86.5% (range, 76-98). The vector copy number in peripheral blood mononuclear cells remained stable over time (supplemental Figure 4).
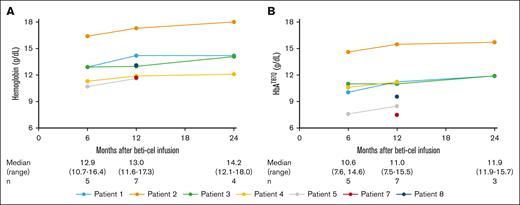
Peripheral blood Hb and HbAT87Q levels were stable up to month 24 (Figure 3). Stable and durable HbAT87Q accounted for normal median Hb levels across all visits (12.90, 13.00, and 14.15 g/dL at months 6, 12, and 24, respectively).
Iron management after beti-cel infusion
The median LIC was 5.4 mg/g dw (range, 1.1-14.8) and median ferritin serum concentration was 1794 μg/L (range, 247-2149) when assessed at a median 149 days (range, 119-263) after beti-cel infusion. Patients with iron overload after beti-cel infusion were treated with phlebotomies if the total Hb level exceeded 10 g/dL. Four patients started phlebotomy (10 mL/kg bodyweight) on days +184 to +371 at intervals of 2 to 4 weeks and as determined by their Hb recovery. Two patients with Hb of <10 g/dL and 1 patient with insufficient venous access restarted iron chelation therapy (deferasirox on days +90, +99, and +155). Chelation and/or phlebotomies were continued until LIC was <3 mg/g dw or serum ferritin was <500 μg/L.
Reconstitution of erythropoiesis after beti-cel infusion
Five patients had bone marrow aspirates assessed before beti-cel infusion, and all had a myeloid-to-erythroid ratio of <1 (supplemental Table 5). Of 7 patients with postinfusion evaluations, 1 had an elevated ratio (patient 5) and 5 had ratios below the normal range (patients 3, 4, 6, 7, and 8), suggesting hyperplastic erythropoiesis at a median time of 100 days (range, 99-190) after beti-cel infusion.
QoL
There were no meaningful short-term changes in the overall QoL as assessed by TranQol scores before treatment (median, 60 [range, 26-73]) and after treatment (median, 53 [range, 34-78]), as well as in domain analyses (supplemental Table 6). Of 8 patients, 6 were able to attend school or work a year after receiving beti-cel.
One patient (patient 6), at ∼3 months after gene therapy, developed a fatigue syndrome characterized by a perception that his mind is eager for a new life but his body is incapacitated and exhausted, with depleted energy reserves. Consequently, his scores for physical (43/100, lowest in group) and emotional (38/100, second lowest in group) health were low. No clinical causes for his fatigue were identified. Outpatient rehabilitation measures have been initiated, and the patient is currently receiving multidisciplinary supportive care.
Another patient (patient 7) manifested depressive symptoms with panic attacks at ∼15 months after therapy, experiencing shortness of breath and palpitations. Diagnostic assessment did not reveal any organic causes for these symptoms. The patient harbored concerns about the risk of disease recurrence, leukemia, or other fatal complications. Although the patient was working full time before treatment, they did not return to work because of feeling psychologically battered, lethargic, and depressed, with a corresponding drop in their school/career functioning score from 75% to 0% after gene therapy. The patient was undergoing outpatient psychotherapy as of last follow-up.
Safety
Adverse effects after conditioning were consistent with myeloablative conditioning with busulfan (Table 2; supplemental Table 7). Nonhematologic grade 4 toxicities were not observed, and no patients developed veno-occlusive liver disease (VOD) or seizures.
Nonhematologic adverse events grade 2, 3, and 4
| . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|
| Mucositis | 3 | 3 | 0 |
| Alopecia | 8 | 0 | 0 |
| Infection | 1∗ | 1† | 0 |
| Fever in neutropenia (n = 11)‡ | 4 | 0 | 0 |
| Erythrodermia | 0 | 2 | 0 |
| Pancreatitis | 0 | 1 | 0 |
| . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|
| Mucositis | 3 | 3 | 0 |
| Alopecia | 8 | 0 | 0 |
| Infection | 1∗ | 1† | 0 |
| Fever in neutropenia (n = 11)‡ | 4 | 0 | 0 |
| Erythrodermia | 0 | 2 | 0 |
| Pancreatitis | 0 | 1 | 0 |
Nonhematologic adverse events grade 2, 3, and 4 (according to Common Terminology Criteria for Adverse Events version 5.0 nomenclature) in 8 patients after beti-cel infusion.
Facial skin infection.
Dental caries with abscess formation.
Three patients developed febrile neutropenia twice.
Two patients experienced busulfan-induced erythroderma (grade 3). Another 2 patients developed abscesses after discharge from in-patient care on days 85 and 57 after beti-cel infusion, respectively. In 1 case, a minor superficial abscess emerged on the skin covering the malar bone (grade 2), which responded to oral antibiotics. The second patient suffered from a dental abscess (grade 3) necessitating tooth extraction and a course of oral antibiotics.
One patient was readmitted for clinical monitoring of a severe acute respiratory syndrome coronavirus 2 infection on day 39 (after hospital discharge by day +35). No antiviral therapy was necessitated. No other patients had to be readmitted to inpatient care after beti-cel infusion.
None of these adverse events were considered related to beti-cel. There were no patient deaths, and no patient met the criteria for persistent oligoclonality.
Polycythemia
One patient (patient 2) developed asymptomatic polycythemia with a peak Hb of 19.3 g/dL and a hematocrit of 0.546 L/L on day 365. Erythropoietin concentrations were found within the normal range, indicating insufficient suppression of erythropoietin synthesis despite high Hb levels. Further diagnostic assessment, including sequence analysis of JAK2 and CALR, did not reveal any cause for the polycythemia (Table 3). However, this patient had undergone splenectomy, which may potentially have a modulating effect on the Hb level.28 At the latest follow-up, 746 days after beti-cel infusion, the patient continued to be asymptomatic despite persisting polycythemia and had not received any treatment for polycythemia.
Overview of the diagnostic assessment of polycythemia in patient 2
| Erythropoietin concentration . |
|---|
| Renal function assessment, including urine, blood, and ultrasound of urinary tract |
| Spirometry |
| Cardiac function assessment, including echocardiography and ECG |
| Ultrasound of abdominal and pelvic organs for tumor detection |
| Polysomnography |
| Blood gas analyses, including estimation of Hb oxygen affinity and HbCO levels |
| Sequence analyses of α-globin (HBA) genes, pyruvate kinase (PKLR), and bisphosphoglycerate mutase (BPGM) |
| Integration site analysis of the therapeutic gene |
| Mutational analysis of JAK2 and CALR |
| Erythropoietin concentration . |
|---|
| Renal function assessment, including urine, blood, and ultrasound of urinary tract |
| Spirometry |
| Cardiac function assessment, including echocardiography and ECG |
| Ultrasound of abdominal and pelvic organs for tumor detection |
| Polysomnography |
| Blood gas analyses, including estimation of Hb oxygen affinity and HbCO levels |
| Sequence analyses of α-globin (HBA) genes, pyruvate kinase (PKLR), and bisphosphoglycerate mutase (BPGM) |
| Integration site analysis of the therapeutic gene |
| Mutational analysis of JAK2 and CALR |
All of these investigations revealed normal results.
ECG, electrocardiogram; HbCO, carboxyhemoglobin.
Assessment of the endocrine pituitary-gonadal axis
Of 8 patients, 6 opted for germ cell cryopreservation (ovarian tissue [n = 3]; sperm conservation [n = 3]), before undergoing busulfan conditioning. None had children before receiving beti-cel. All 3 female patients (patients 4, 5, and 7) showed an endocrine profile of hypergonadotropic hypogonadism, including low levels of anti–Müllerian hormone after conditioning (Table 4). Two with pretreatment data available showed normal (patient 4) and low (patient 7) anti–Müllerian hormone serum concentrations before treatment.
Endocrine profile of the pituitary-gonadal axis in female patients before and after busulfan conditioning and beti-cel infusion
| . | Before busulfan conditioning and beti-cel infusion . | After busulfan conditioning and beti-cel infusion . | ||||
|---|---|---|---|---|---|---|
| Patient 4 . | Patient 5 . | Patient 7 . | Patient 4 . | Patient 5 . | Patient 7 . | |
| LH, U/L (normal, 0.60-13.72) | 5.1 | 4.47 | 10.99 | 15.6 | 39.6 | 6.18 |
| FSH, U/L (normal, 0.97-11.60) | 3.3 | 6.47 | 13.87 | n.a. | 95.8 | 42.58 |
| Estradiol, pg/mL (normal, 40-250) | n.a. | n.a. | 12.6∗ | 18.6 | <11.8 | <11.8∗ |
| AMH, ng/mL (normal, 1.2-9.5) | 3.31 | n.a. | 0.03 | <0.02 | 0.01 | 0.01 |
| . | Before busulfan conditioning and beti-cel infusion . | After busulfan conditioning and beti-cel infusion . | ||||
|---|---|---|---|---|---|---|
| Patient 4 . | Patient 5 . | Patient 7 . | Patient 4 . | Patient 5 . | Patient 7 . | |
| LH, U/L (normal, 0.60-13.72) | 5.1 | 4.47 | 10.99 | 15.6 | 39.6 | 6.18 |
| FSH, U/L (normal, 0.97-11.60) | 3.3 | 6.47 | 13.87 | n.a. | 95.8 | 42.58 |
| Estradiol, pg/mL (normal, 40-250) | n.a. | n.a. | 12.6∗ | 18.6 | <11.8 | <11.8∗ |
| AMH, ng/mL (normal, 1.2-9.5) | 3.31 | n.a. | 0.03 | <0.02 | 0.01 | 0.01 |
AMH, anti–Müllerian hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone; n.a., not available.
Receiving hormonal contraception.
One patient (patient 7) who had regular menses of normal intensity and was on oral contraceptives before gene therapy developed hypomenorrhea after treatment under the same oral contraceptive dose. Two patients (patients 4 and 5) experienced secondary amenorrhea after treatment; 1 patient (patient 5) received hormone replacement therapy, and the other (patient 4) was undergoing gynecological assessment for planned hormone replacement therapy.
The endocrine profile of the male patients was more variable (Table 5). Of 5 patients, 1 showed low testosterone concentrations, and 2 of 4 patients with available measurements had low inhibin B concentrations after therapy. These data indicate impaired hormonal function and spermatogenesis after single-agent busulfan conditioning.
Endocrine profile of the pituitary-gonadal axis in male patients before and after busulfan conditioning and beti-cel infusion
| . | Before busulfan conditioning and beti-cel infusion . | After busulfan conditioning and beti-cel infusion . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 . | Patient 2 . | Patient 3 . | Patient 6 . | Patient 8 . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 6 . | Patient 8 . | |
| LH, U/L (normal, 0.79-11.75) | 1.4 | 7.86 | n.a. | 4.24 | 0.47 | 6 | 8.94 | 6.87 | 6.36 | 0.52 |
| FSH, U/L (normal, 1.08-12.85) | 2.2 | 4.99 | n.a. | 12.8 | 0.81 | n.a. | 9.38 | 9.94 | 12.05 | 3.08 |
| Testosterone, ng/mL (normal, 2-7) | n.a. | n.a. | n.a. | 6.83 | n.a. | 4.4 | 3.44 | 5.54 | 0.98 | 5 |
| Inhibin B, ng/L (normal, >120) | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 20 | 487 | 10 | 122 |
| . | Before busulfan conditioning and beti-cel infusion . | After busulfan conditioning and beti-cel infusion . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 . | Patient 2 . | Patient 3 . | Patient 6 . | Patient 8 . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 6 . | Patient 8 . | |
| LH, U/L (normal, 0.79-11.75) | 1.4 | 7.86 | n.a. | 4.24 | 0.47 | 6 | 8.94 | 6.87 | 6.36 | 0.52 |
| FSH, U/L (normal, 1.08-12.85) | 2.2 | 4.99 | n.a. | 12.8 | 0.81 | n.a. | 9.38 | 9.94 | 12.05 | 3.08 |
| Testosterone, ng/mL (normal, 2-7) | n.a. | n.a. | n.a. | 6.83 | n.a. | 4.4 | 3.44 | 5.54 | 0.98 | 5 |
| Inhibin B, ng/L (normal, >120) | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 20 | 487 | 10 | 122 |
Discussion
This real-world, single-center experience study in Germany demonstrates that in a real-world setting, beti-cel safety and efficacy was consistent with what was observed in the clinical setting. Of patients who carried at least 1 β⁺ allele, ∼90% achieved TI in the pivotal trials of beti-cel,19,20 a rate comparable with those who received allogeneic HSCT from an MSD.7,29 In this report, all patients achieved TI and had an unsupported Hb level of >11 g/dL, confirming the efficacy of beti-cel.
Although engraftment times were similar to those reported in beti-cel clinical studies,19,20 they were slower than what has been reported in the literature for patients with TDT who received allogeneic HSCT. These patients had a median time to neutrophil engraftment of 13 days (range, 10-25) and platelet engraftment of 23 days (range, 12-46).30 Consistent with the findings of the beti-cel clinical studies, we observed that 4 of 8 patients reported here had platelet counts of <100 per nL 100 days after beti-cel infusion,20,31 and 6 of 8 patients had normal platelet counts by day 365. The duration of hospitalization for beti-cel was similar to what we have observed at our center in patients undergoing allogeneic HSCT (median, 37.5 days [range, 29-55]) and is also consistent with what has been reported in the literature.32
Of 8 patients in our series, 5 were aged >16 years, an age group that experience higher risk of graft failure and treatment-related mortality after allogeneic HSCT because of hemosiderosis-associated organ dysfunction.7,8 In this respect, the safety profile of beti-cel after myeloablative busulfan conditioning should not be overinterpreted because of severe hemosiderosis (>15 mg/g dw) being an exclusion criterion. Posttreatment bone marrow aspirates continued to show erythroid hyperplasia with abnormal myeloid-to-erythroid ratios. In the clinical trial setting, improvement in ineffective erythropoiesis was observed with longer term follow-up of up to 3 years in patients who achieved TI.20 Therefore, additional follow-up will be necessary to monitor these patients for TDT-related complications, including increased iron resorption, osteoporosis, and space-occupying extramedullary hematopoiesis. Although the percentage of LVV-positive cells in the drug product for this cohort was numerically greater than was observed in the clinical trial setting, which ranged from 34% to 90% among patients who stopped transfusions, some ineffective erythropoiesis in the bone marrow is expected, because not all cells express βA-T87Q. Of 8 patients, 7 received iron management with deferasirox or phlebotomy after beti-cel infusion.
The duration between obtaining informed consent and administering the drug product was much longer than typically observed for allogeneic HSCT at our center (median, 18 days [range, 8-42]). This increased time to treatment can be attributed to health insurance approval (average, 101 days), suspension of beti-cel marketing (129 days), and the beti-cel manufacturing time (∼90 days).
None of the 15 screened patients had an MSD or HLA-matched unrelated donor available, and all had guaranteed insurance coverage for beti-cel despite the costs exceeding those of allogeneic HSCT. It was notable that 73% (11 of 15) of patients consented to be part of the first series of patients to be treated in the real-world setting. Although 53% (8 of 15) of the screened patients received beti-cel, 27% (4 of 15) declined gene therapy because of the expected side effects of busulfan conditioning, emphasizing the continued need for minimizing the toxicity of myeloablative conditioning.
Acute adverse events observed due to the conditioning regimen were comparable with those expected for myeloablative conditioning with busulfan and consistent with the safety data reported to date from the beti-cel clinical trial program.20,31 Because the patients reported here had an extended history of RBC transfusions, including long periods of regular transfusions in their countries of origin with non–leukocyte-depleted blood products, we anticipated a high likelihood of alloimmunization. As a result, we conducted HLA-alloantibody testing prior to conditioning and observed HLA-antibodies in 4 of 7 patients. Therefore, we used HLA-matched platelet concentrates for transfusion, although this did not prevent refractoriness to platelet transfusions in all patients.
Busulfan-based conditioning regimens are frequently used in patients undergoing allogeneic HSCT for nonmalignant conditions.33-35 In contrast to the clinical trials that assessed the safety and effectiveness of lentiviral gene therapy, we used the standard busulfan dosage as recommended by the manufacturer, forgoing therapeutic drug monitoring. Patients received VOD prophylaxis with ursodiol, and no VOD was observed.
Fertility preservation was a key consideration for younger patients before treatment, and some patients declined gene therapy because of potential loss of fertility associated with busulfan monotherapy. Prior HSCT studies have reported endocrine dysfunction as a long-term adverse event of busulfan combination therapy.36,37 Beti-cel clinical studies using busulfan monotherapy have not reported data on endocrine function. Here, we report that 5 patients (3 female and 2 male) showed gonadal dysfunction, although some patients may already have had diminished gonadal function before treatment because of hemosiderosis.38-40 Therefore, preservation of fertility by alternative nongonadotoxic conditioning methods remains an important clinical consideration for patients interested in gene therapies.41,42
The case of polycythemia reported here was not observed in the 63 patients treated in the pivotal beti-cel studies.43 Although we were not able to identify the cause of the polycythemia, it was associated with normal erythropoietin serum concentrations, indicating dysfunction of oxygen sensing or potential abnormal oxygen delivery by the Hb tetramer now including the therapeutic β-globin chain. We excluded increased oxygen affinity, and specifically an increase of carboxyhemoglobin, and did not find genetic evidence for dysfunction of the hypoxia-inducible transcription factor 2A–erythropoietin axis. Previously, it had been shown that oxygen affinity of HbA and HbAT87Q was similar.18 The cause for this unexpected effect thus remains unknown. Treating physicians should be aware of this, because excessively increased Hb levels can result in hyperviscosity complications.
QoL remains a key consideration when treating patients with chronically debilitating disorders. In this cohort followed-up for up to 2 years, TranQol scores aligned well with previously published findings.24,44-46 Although most patients may experience subjective improvements in QoL, 2 patients were unable to engage in daily activities or pursue their hobbies because of previously absent chronic fatigue and symptoms of depression after beti-cel treatment. Although this may appear to be counterintuitive, this was also observed in adolescent patients who have been cured from malignant and nonmalignant diseases by allogeneic HCST.47-49 These seemingly paradoxical effects on QoL, particularly in young adults, may act as an additional reason to initiate gene therapy for TDT earlier in life.
Conclusions
This real-world study of beti-cel in patients with TDT aged ≥12 years without a β0/β0 genotype provides efficacy results consistent with what was reported in the beti-cel clinical trial program. Our experience underscores challenges with busulfan conditioning and its impact on gonadal function, pinpointing the need for less toxic conditioning regimens. Because more patients are treated in the real world in the United States, it will be important to understand and assess newer challenges and events we encountered, such as polycythemia, impact on QoL, and frequency of HLA antibodies resulting in clinically relevant platelet refractoriness. Although beti-cel is not currently approved in the European Union, these data provide important follow-up information for patients in Germany previously treated with beti-cel, as well as those considering treatment in the United States in which beti-cel is approved.
Acknowledgments
The authors thank the patients and their families. Editorial and medical writing support was provided by Nelson Jen of bluebird bio, Inc. Language editing was supported by ChatGPT on an early draft of the manuscript. The Rare Anemia Registry of the German Society of Pediatric Oncology and Hematology is supported by the Dietmar Hopp Stiftung.
Authorship
Contribution: A.M., M.-L.R., H.T., J.K., and A.K. wrote and revised the manuscript; J.K. and A.K. designed and conceptualized the study; S.L., S.H., L.K., J.K., and M.D. provided clinical information before treatment with beti-cel; G.T. analyzed and interpreted the vector copy number, integration site analysis, and high-performance liquid chromatography data; D.J. documented clinical information before and after beti-cel infusion; J.G. performed conditioning and beti-cel infusion; and all authors reviewed and provided input to the manuscript.
Conflict-of-interest disclosure: A.K. received research support and royalties from bluebird bio, Inc. H.T. and G.T. are employees of, and hold stock/stock options in, bluebird bio. J.K. is a consultant for bluebird bio, Vertex, Novartis, and Pfizer, and receives research support from Pfizer, Novo Nordisk, Novartis, and Deutsche Kinderkrebsstiftung. The remaining authors declare no competing financial interests.
Correspondence: Andreas Kulozik, Department of Pediatric Oncology, Hematology and Immunology, Heidelberg University Hospital, Im Neuenheimer Feld 430, D-69120 Heidelberg, Germany; email: andreas.kulozik@med.uni-heidelberg.de; and Joachim Kunz, Department of Pediatric Oncology, Hematology and Immunology, Heidelberg University Hospital, Im Neuenheimer Feld 430, D-69120 Heidelberg, Germany; email: joachim.kunz@med.uni-heidelberg.de.
References
Author notes
A.M. and M.-L.R. contributed equally to this study.
Data are available on reasonable request from the corresponding authors, Andreas Kulozik (andreas.kulozik@med.uni-heidelberg.de) and Joachim Kunz (joachim.kunz@med.uni-heidelberg.de).
The full-text version of this article contains a data supplement.