Key Points
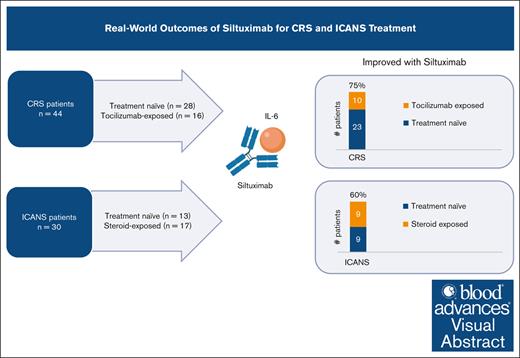
In this retrospective analysis, siltuximab effectively treated CRS and ICANS with an objective response rate of 75% and 60%, respectively.
Siltuximab was effective in patients with CRS previously exposed to tocilizumab, with an objective response rate of 63%.
Visual Abstract
Chimeric antigen receptor T-cell (CAR-T) therapies are effective in many hematologic malignancies; however, adverse events including cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS) can affect a significant number of patients. Those who develop refractory CRS or ICANS have few treatment options. Siltuximab, a monoclonal antibody binding circulating interleukin-6, has been proposed to have clinical activity in both CRS and ICANS. We conducted a multicenter retrospective analysis of siltuximab treatment for CRS and ICANS after CAR-T therapy in a real-world cohort from 6 academic centers. Fifty-four patients were evaluated. Sixteen patients had CRS previously treated with tocilizumab and 17 patients had ICANS previously treated with steroids. Of the patients with CRS at the time of siltuximab, 75% had improvement in CRS grade. Of the patients with ICANS at the time of siltuximab, 60% had improvement in ICANS grade. To our knowledge, this is the largest cohort of patients treated with siltuximab for CRS and/or ICANS after CAR-T therapies. Siltuximab appeared to be effective for both CRS and ICANS, including previously treated toxicities. These data support the use of siltuximab in CRS and ICANS as well as provide rationale for future prospective studies.
Introduction
Chimeric antigen receptor T-cell (CAR-T) therapy has been well studied in the treatment of hematologic malignancies such as acute lymphoblastic leukemia, multiple myeloma, and non-Hodgkin lymphoma.1-5 Although CAR-T therapy can lead to durable remissions in these diseases, adverse events including cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS) are frequent occurrences and increase morbidity in treated patients.1,4,6 Clinical trials data demonstrate axicabtagene ciloleucel, and brexucabtagene autoleucel, approved for the treatment of various non-Hodgkin lymphomas, were associated with high rates of CRS (91%-93%) and ICANS (63%-64%).7,8 Studies leading to the approvals of lisocabtagene maraleucel and tisagenlecleucel also note that development of CRS and ICANS was common, albeit slightly less frequent (CRS, 42%-58%; ICANS, 21%-30%).4,5 Similarly, trials studying idecabtagene vicleucel and ciltacabtagene autoleucel in multiple myeloma also demonstrate development of CRS (84%-95%) and ICANS (18%-21%) as common side effects.9,10
Tocilizumab, a humanized monoclonal antibody to interleukin-6 (IL-6) receptor, is the standard of care for managing CRS.11 The efficacy of tocilizumab was studied retrospectively through analysis of pooled clinical trials data of axicabtagene ciloleucel and tisagenlecleucel. Results demonstrated 69% of patients with CRS after tisagenlecleucel had resolution of symptoms within 14 days with no >2 doses of tocilizumab, whereas only 53% of patients with CRS after axicabtagene ciloleucel responded.11 These results demonstrate that a subset of patients does not respond to tocilizumab therapy; furthermore, studies have demonstrated that it is not only ineffective in the treatment of ICANS but may precipitate higher grade ICANS.12-14 Corticosteroids remain the standard for treatment of ICANS because short courses are associated with improvement in neurological symptoms without impairing CAR-T efficacy.15 Beyond first-line treatments for CRS and ICANS, current guidelines are unclear regarding the treatment of toxicities refractory to these drugs.
Siltuximab, a chimeric immunoglobulin monoclonal antibody against IL-6 has been used effectively in the management of CRS refractory to tocilizumab.16,17 In addition, siltuximab has also been proposed to have clinical activity in untreated CRS and ICANS.18,19 In this study, we assess the efficacy of siltuximab when used in the treatment of CRS and ICANS in a real-world cohort.
Methods
Study design
We conducted a retrospective review of adult patients with hematologic malignancies treated with CAR-T therapy who received siltuximab for treatment of CRS and/or ICANS. This study included patients from 6 academic medical centers in the United States between 2018 and 2023 and was conducted with approval from institutional review boards at each site including The Ohio State University.
CRS and ICANS assessment and management
CRS and ICANS grades at the time of siltuximab administration were noted through retrospective chart review according to the American Society for Transplantation and Cellular Therapy consensus grading.20 Management of CRS and ICANS were at the discretion of treating physicians and in accordance with National Comprehensive Cancer Network guidelines.21 In June 2021, an emergency use authorization issued by the US Food and Drug Administration for the use of tocilizumab in the treatment of severe COVID-19 resulted in a nationwide shortage. Because of the decreased availability of tocilizumab during this time, a higher number of patients likely received siltuximab as first-line treatment for CRS and/or ICANS. In patients with concurrent CRS and ICANS, these toxicities were evaluated independently of each other. Management of CRS and ICANS was per institutional guidelines.
Definitions
Improvement in CRS and ICANS was defined as a reduction in grade of CRS or ICANS after siltuximab administration. Tocilizumab-exposed CRS was defined as CRS that did not improve after ≥1 doses of tocilizumab. Steroid-exposed ICANS was defined as ICANS that did not improve after initiation of steroid therapy. Treatment-naïve CRS or ICANS was defined as CRS or ICANS that was untreated before siltuximab administration.
Statistical analysis
Coprimary end points of this study were: (1) the percentage patients who had improvement in CRS after receiving siltuximab and (2) the percentage who had improvement in ICANS after receiving siltuximab. Secondary end points included time to improvement of CRS or ICANS and overall survival (OS) after CAR-T therapies. Patient characteristics were summarized using the median and range for continuous variables and frequency and percentage for the categorical variables. OS was calculated from the date of CAR-T infusion to the date of death, censoring those alive at the date of last contact. OS rates were estimated using the Kaplan-Meier methodology and compared between patient groups using the log-rank test. Stata 18 was used for all the analysis.
Results
Patient, disease, and product characteristics
Fifty-four patients met eligibility criteria and were evaluated. The median age at diagnosis was 60 years (range, 23-81). Fifty percent of patients were female and 91% were White. The hematologic malignancies represented in this study were diffuse large B-cell lymphoma (52%), mantle cell lymphoma (22%), multiple myeloma (17%), follicular lymphoma (5%), and acute lymphoblastic leukemia (4%). Axicabtagene ciloleucel was the most used CAR-T product (42%) followed by brexucabtagene autoleucel (24%), idecabtagene vicleucel (17%), lisocabtagene maraleucel (15%), and tisagenlecleucel (2%). No patient received corticosteroid prophylaxis before CAR-T therapy (Table 1).
Patient, disease, and CAR-T product characteristics
| Characteristics . | N = 54 . | % . |
|---|---|---|
| Median age, y (range) | 60 (23-81) | |
| Sex | ||
| Female | 27 | 50 |
| Male | 27 | 50 |
| Race | ||
| Caucasian | 49 | 91 |
| African American | 1 | 2 |
| Other | 4 | 7 |
| Diagnosis | ||
| DLBCL | 28 | 52 |
| FL | 3 | 5 |
| MCL | 12 | 22 |
| ALL | 2 | 4 |
| Multiple myeloma | 9 | 17 |
| Cell product | ||
| Axicabtagene ciloleucel | 23 | 42 |
| Brexucabtagene autoleucel | 9 | 24 |
| Idecabtagene vicleucel | 13 | 17 |
| Lisocabtagene maraleucel | 8 | 15 |
| Tisagenlecleucel | 1 | 2 |
| Prophylaxis before CAR-T therapies | ||
| Corticosteroids | 0 | 0 |
| Characteristics . | N = 54 . | % . |
|---|---|---|
| Median age, y (range) | 60 (23-81) | |
| Sex | ||
| Female | 27 | 50 |
| Male | 27 | 50 |
| Race | ||
| Caucasian | 49 | 91 |
| African American | 1 | 2 |
| Other | 4 | 7 |
| Diagnosis | ||
| DLBCL | 28 | 52 |
| FL | 3 | 5 |
| MCL | 12 | 22 |
| ALL | 2 | 4 |
| Multiple myeloma | 9 | 17 |
| Cell product | ||
| Axicabtagene ciloleucel | 23 | 42 |
| Brexucabtagene autoleucel | 9 | 24 |
| Idecabtagene vicleucel | 13 | 17 |
| Lisocabtagene maraleucel | 8 | 15 |
| Tisagenlecleucel | 1 | 2 |
| Prophylaxis before CAR-T therapies | ||
| Corticosteroids | 0 | 0 |
ALL, acute lymphoblastic leukemia; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma.
CRS and ICANS management before siltuximab use
Fifty-three patients (98%) developed CRS, with a median time to onset of 2 days (range, 0-9). Tocilizumab was administered in 29 (54%) patients with CRS, with the majority (62%) receiving >1 dose. Of the patients treated with tocilizumab first, 9 patients (36%, n = 25) had resolution of CRS, 3 patients (12%, n = 25) had improvement in CRS grade without resolution, and 13 patients (52%, n = 25) had no improvement in CRS grade.
ICANS occurred before siltuximab administration in 31 patients (57%), with median time to onset of 6 days (range, 0-13). Corticosteroids were administered as initial treatment in 18 (58%) patients. Of 18 patients, 1 patient (6%, n = 16) had resolution of ICANS and 17 patients had persistent ICANS and were evaluated as “steroid-exposed” before siltuximab. Five patients (16%, n = 31) had grade 1 ICANS and were observed until treatment was indicated. Eight patients (26%, n = 31) were treated with siltuximab and corticosteroids as their initial therapy for ICANS.
Ten patients received additional treatments beyond tocilizumab and corticosteroids before siltuximab administration: anakinra (1 patients), lumbar puncture with intrathecal chemotherapy (3 patients), lumbar puncture with intrathecal steroids (2 patients), ruxolitinib (2 patient), and etanercept (1 patient).
Treatment-naïve CRS and ICANS outcomes with siltuximab administration
Twenty-eight patients with CRS were treatment naïve at the time of siltuximab administration, 21% (6/28 patients) had grade 1 CRS and 79% (22/28 patients) had grade 2 CRS (Table 2). Nineteen patients were treatment naïve with CRS with no ICANS, and 9 patients had concurrent ICANS. In this treatment-naïve group, 23 patients had improvement in CRS grade after receiving siltuximab: 20 patients (71%, n = 28) had improved CRS grade after 1 dose of siltuximab and the remaining 3 patients improved after 2 doses of siltuximab. Median time to CRS resolution from the date of first siltuximab administration was 1 day (range, 0-7), with 65% of patients resolving within 24 hours and 91% resolving within 72 hours (Table 3). Four patients with treatment-naïve CRS did later develop ICANS, median time to ICANS was 3.5 days (range, 1-7); of note, 3 of these 4 patients received tocilizumab after siltuximab administration.
CRS and ICANS grades at siltuximab administration
| . | Treatment naïve∗ . | Patients with prior treatment† . | ||
|---|---|---|---|---|
| n . | % . | n . | % . | |
| CRS grade | ||||
| 1 | 6 | 21 | 4 | 25 |
| 2 | 22 | 79 | 7 | 44 |
| 3 | 0 | 0 | 3 | 19 |
| 4 | 0 | 0 | 2 | 12 |
| ICANS grade | ||||
| 1 | 5 | 38 | 0 | 0 |
| 2 | 6 | 46 | 1 | 6 |
| 3 | 2 | 15 | 8 | 47 |
| 4 | 0 | 0 | 8 | 47 |
| . | Treatment naïve∗ . | Patients with prior treatment† . | ||
|---|---|---|---|---|
| n . | % . | n . | % . | |
| CRS grade | ||||
| 1 | 6 | 21 | 4 | 25 |
| 2 | 22 | 79 | 7 | 44 |
| 3 | 0 | 0 | 3 | 19 |
| 4 | 0 | 0 | 2 | 12 |
| ICANS grade | ||||
| 1 | 5 | 38 | 0 | 0 |
| 2 | 6 | 46 | 1 | 6 |
| 3 | 2 | 15 | 8 | 47 |
| 4 | 0 | 0 | 8 | 47 |
Patients who did not receive tocilizumab for CRS or steroids for ICANS before treatment with siltuximab.
Patients who received tocilizumab and/or steroids before treatment with siltuximab.
Treatment of CRS and ICANS with siltuximab
| . | At siltuximab administration . | Improvement after siltuximab, % . | Median days to resolution (range) . |
|---|---|---|---|
| CRS | |||
| Treatment-naïve | 28 | 82 (23/28) | 1 (0-7) |
| Tocilizumab-exposed∗ | 16 | 63 (10/16) | 2 (0-4) |
| Total | 44 | 75 (33/44) | |
| ICANS | |||
| Treatment-naïve† | 13 | 69 (9/13) | 3 (1-13) |
| Steroid-exposed‡ | 17 | 53 (9/17) | 5 (2-40) |
| Total | 30 | 60 (18/30) |
| . | At siltuximab administration . | Improvement after siltuximab, % . | Median days to resolution (range) . |
|---|---|---|---|
| CRS | |||
| Treatment-naïve | 28 | 82 (23/28) | 1 (0-7) |
| Tocilizumab-exposed∗ | 16 | 63 (10/16) | 2 (0-4) |
| Total | 44 | 75 (33/44) | |
| ICANS | |||
| Treatment-naïve† | 13 | 69 (9/13) | 3 (1-13) |
| Steroid-exposed‡ | 17 | 53 (9/17) | 5 (2-40) |
| Total | 30 | 60 (18/30) |
Patients who received ≥1 doses of tocilizumab before siltuximab.
Includes patients who received steroids at the same time as siltuximab or after siltuximab.
Patients who received steroids for treatment of ICANS before siltuximab.
Thirteen patients developed ICANS and were treatment naïve at the time of siltuximab administration. Siltuximab was administered alone (3 patients) or in combination with corticosteroids (10 patients). Of note, within the group of 10 patients who received siltuximab with corticosteroids, 70% received both medications on the same day whereas 30% received steroids 2 to 3 days after siltuximab. The majority of patients (90%) received a total dose of dexamethasone 24 to 40 mg daily, whereas 1 patient received methylprednisolone 1 g daily. In this treatment-naïve ICANS group, toxicity grade at the time of siltuximab administration was grade 1 in 38% patients (5/13), grade 2 in 46% (6/13), and grade 3 in 15% (2/13; Table 2). The rate of ICANS grade improvement was 69% (9/13 patients), with median time to resolution from the date of first siltuximab administration of 3 days (range, 1-13). ICANS resolved within 24 hours in 29%, within 72 hours in 57%, and within 5 days in 86% of patients.
Previously treated CRS and ICANS outcomes with siltuximab administration
Sixteen patients with CRS received siltuximab after tocilizumab administration; median number of doses of tocilizumab received in this group was 2 (range, 1-4). Median time between first dose of tocilizumab and siltuximab was 3 days (range, 1-15). Improvement in CRS grade was observed in 10 patients (63%), 9 of whom improved with 1 dose of siltuximab and 1 patient improved after 2 doses. Median number of days to resolution of CRS after siltuximab was 2 (range, 0-4), with 60% of patients experiencing resolution within 24 hours and 91% within 72 hours.
Of 17 patients with steroid-exposed ICANS, 53% (9/17) improved after receiving siltuximab (Table 3) with median time to resolution from the date of first siltuximab administration of 5 days (range, 2-40). Thirty-three percent of patients had resolution of symptoms within 72 hours and 50% within 5 days of siltuximab. Median time between first dose of steroids and siltuximab was 3 days (range, 1-25). Before siltuximab administration, the majority of patients (71%) received a total dose of dexamethasone ≥80 mg daily (highest dose of steroids reported was 1 g methylprednisolone twice daily). Overall, patients who were steroid exposed received higher doses of steroids than those who received steroids in combination with siltuximab for frontline treatment.
Full cohort outcomes
In a full cohort analysis, CRS grade at the time of siltuximab administration was grade 1/2 in 89%, and grade 3/4 in 11%. ICANS grade at the time of siltuximab administration was grade 1/2 in 40%, and grade 3/4 in 60%. Twenty-four patients only had CRS at the time of siltuximab administration, 10 patients only had ICANS, and 20 patients had concurrent CRS and ICANS. Overall, 75% (33/44 patients) of patients with CRS at the time of siltuximab administration had improvement in CRS grade after siltuximab treatment. The median time to CRS resolution after siltuximab was 1 day (range, 0-7). Overall, 60% (18/30 patients) of patients who had ICANS at the time of siltuximab administration had improvement in ICANS grade after siltuximab treatment. The median time to ICANS resolution after siltuximab was 4.5 days (range, 1-40).
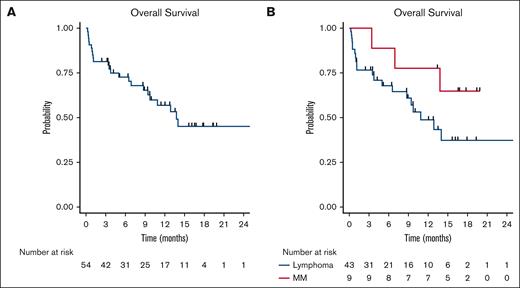
The median time to discharge after siltuximab administration was 14 days (range, 7 to 90). CAR-T therapy objective response rate was 70% and complete response rate was 46%. Twenty-three patients had died at the time of data collection; 4 deaths were deemed secondary to CAR-T toxicity and 14 deaths were secondary to disease progression. The median OS was 13.8 months (95% confidence interval, 9.4 to not reached) and was not statistically different based on indication of CAR-T therapy, log-rank P = .14 (Figure 1).
Cohort survival outcomes. (A) OS for full cohort. (B) OS for patients with lymphoma (blue) compared with those with myeloma (red).
Cohort survival outcomes. (A) OS for full cohort. (B) OS for patients with lymphoma (blue) compared with those with myeloma (red).
Discussion
Although efficacious in several hematologic malignancies, CAR-T therapy is often complicated by the development of CRS and ICANS. These toxicities can play a significant role in the course of a patient’s recovery after CAR-T therapy, and in some cases may lead to protracted hospital admissions, increased risk of infection, and death.22,23 The standard-of-care management of CRS and ICANS with the anti–IL-6 receptor monoclonal antibody tocilizumab and corticosteroids were established based on noncomparative efficacy analyses from the initial pivotal studies using CAR-T therapies.1,4 Although these treatments have demonstrated efficacy in the management of CRS and ICANS, there remains a subset of patients who experience toxicities refractory to first-line treatments.11,24,25 In this study, we demonstrate a potential role for siltuximab in the management of CRS and ICANS.
In this cohort of patients with CRS and/or ICANS treated with siltuximab, we demonstrated a CRS response rate of 75% and an ICANS response rate of 60%. This cohort is a heterogenous cohort of patients with respect to toxicity, grade of toxicity when siltuximab was administered, number of prior treatments, as well as possible combination therapy with corticosteroids. The cohort heterogeneity should be considered; however, in the absence of controlled clinical trial data, this study represents the largest analysis of potential efficacy of siltuximab in management of CRS and ICANS. Additionally, in a real-world clinical setting, patients with CRS and/or ICANS are often treated with multiple therapies concurrently. In this study, many patients received corticosteroids both sequentially as well as concurrently. Although this could potentially affect the response with siltuximab administration, the data presented in this study do represent real-world management of CRS and/or ICANS.
IL-6 secreted by monocyte-lineage cells is a key driver in the development of CRS. Although the pathogenesis of ICANS is not fully understood, CSF infiltration by cytokines and leukocytes likely plays a role.25-28 Furthermore, 1 of the most significant risk factors for development of ICANS is the presence preceding CRS. Theoretically, siltuximab not only treats CRS by directly binding IL-6 in the serum but also mitigates a rebound increase in IL-6 concentrations seen after tocilizumab administration and may reduce the risk of subsequent development of ICANS.29
In this study, we found that 4 of 19 patients (21%) treated with siltuximab for treatment-naïve CRS did develop ICANS; 75% of these patients had resolution of ICANS and median time to resolution was 9 days (range, 8-12). This supports the possibility that development of ICANS is not necessarily an IL-6–restricted process.30 This supports further investigation into mechanism of ICANS development in the setting if IL-6 blockade. Additionally, given the retrospective nature of this analysis, it is not possible to determine whether the rate of ICANS may have been higher without siltuximab, whether the ICANS grade may have been higher without siltuximab, or if resolution of ICANS could have taken longer.
There are limitations to this study. The retrospective nature of the study allows for possible recall bias, missing information, observer bias, and presence of unmeasured confounders. Treatment with corticosteroids was not standardized among patients in this cohort, possibly confounding the effect of siltuximab on CRS and ICANS symptoms. Similarly, duration of time between tocilizumab, steroids, siltuximab, and other toxicity-directed treatments before or after siltuximab was not standardized across patients and dependent on individual provider judgement. Data on toxicity-directed treatments after siltuximab were not collected.
To our knowledge, this is the largest cohort of patients treated with siltuximab for CAR-T therapy–related toxicities. These data demonstrate that siltuximab has a meaningful role in the treatment of CRS and ICANS and supports the need for further investigation of siltuximab through dose-optimization studies and prospective clinical trials.
Acknowledgments
This work was supported by the Lymphoma Research Foundation Career Development Award 1177432 (T.V.). C.L. was supported by National Institutes of Health/National Heart, Lung, and Blood Institute grant T32 HL007057-46.
Authorship
Contribution: A.B. and T.V. designed the research project, collected and analyzed data, and wrote the manuscript; Q.Z. analyzed data; and M. Geer, C.L., J.W., J.M., M. Ghosh, D.F., A.G., J.T., N.A., E.B., N.D., M.d.L., N.E., and P.C. were involved in data collection and editing of abstracts and manuscripts.
Conflict-of-interest disclosure: C.L. reports consultancy for Rigel and an advisory board role with Autolus. N.A. reports an advisory board role with Bristol Myers Squibb and Kite/Gilead, and consultancy for, and institutional research support from, Kite/Gilead. E.B. reports an advisory board role with Novartis and Kyverna Therapeutics. M.d.L. reports an advisory board role with AbbVie, Novartis, Bristol Myers Squibb, and Pfizer, and research funding from Miltenyi Biotec. N.E. reports consultancy for Merck, Novartis, ADC Therapeutics, and Lilly; an advisory board role with Merck, Novartis, and ADC Therapeutics; research funding from BeiGene; and speakers' bureau participation for Novartis and Incyte. P.C. reports research support from AbbVie, ADC Therapeutics, and Genmab; an advisory board role with Bristol Myers Squibb, Novartis, Sobi, and Synthekine; consultancy for Arvinas and Recordati; and equity in Abcon. T.V. reports an advisory board role with Genmab/AbbVie; consultancy for Novartis, Recordati, and Genmab; and research funding from Kite, Viracta, Incyte/MorphoSys, Genmab/AbbVie, and Recordati. The remaining authors declare no competing financial interests.
Correspondence: Timothy Voorhees, Division of Hematology, Internal Medicine, The Ohio State University, 2121 Kenny Rd, Columbus, OH 43210; email: timothy.voorhees@osumc.edu.
References
Author notes
Data are available on request from the corresponding author, Timothy Voorhees (timothy.voorhees@osumc.edu).