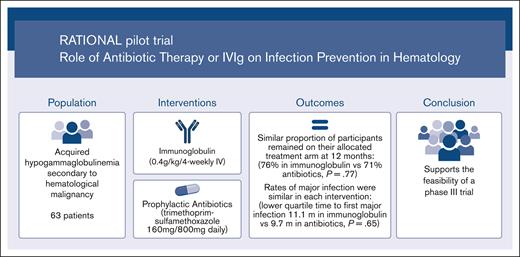
Feasibility trial comparing immunoglobulin and prophylactic antibiotics in patients with hypogammaglobulinemia due to blood cancers.
A similar proportion of participants remained on antibiotics at 12 months as those on immunoglobulins, with similar rates of major infection.
Visual Abstract
Immunoglobulin replacement and prophylactic antibiotics are commonly used to prevent infections in patients with secondary hypogammaglobulinemia due to hematological malignancies but have never been directly compared. In this randomized controlled feasibility trial conducted in 7 hospitals in Australia and New Zealand, we enrolled patients with secondary hypogammaglobulinemia with either a history of recurrent/severe infection or an immunoglobulin G level <4 g/L. Participants were randomized in a 1:2 ratio to immunoglobulin (0.4 g/kg per 4 weeks IV) or daily antibiotics (trimethoprim-sulfamethoxazole 160 mg/800 mg or, if contraindicated, 100 mg doxycycline) for 12 months. Participants allocated to antibiotics were allowed to crossover after grade ≥3 infections. The primary outcome was proportion of patients alive on the assigned treatment 12 months after randomization. Between August 2017 and April 2019, 63 patients were randomized: 42 to antibiotics and 21 to immunoglobulin. Proportion of participants alive on allocated treatment at 12 months was 76% in the immunoglobulin and 71% in the antibiotic arm (Fisher exact test P=.77; odds ratio, 0.78; 95% CI, 0.22-2.52). The lower quartile for time to first major infection (median, not reached) was 11.1 months for the immunoglobulin and 9.7 months for the antibiotic arm (log-rank test, P=.65). Three participants in the immunoglobulin and 2 in the antibiotic arm had grade ≥3 treatment-related adverse events. A similar proportion of participants remained on antibiotic prophylaxis at 12 months to those on immunoglobulin, with similar rates of major infections. Our findings support the feasibility of progressing to a phase 3 trial. Trial registration #ACTRN12616001723471.
Introduction
Acquired hypogammaglobulinemia is common in patients with hematological malignancies, especially multiple myeloma (MM), chronic lymphocytic leukemia (CLL), and non-Hodgkin lymphoma (NHL), and is associated with a reduced ability to mount antibody responses to a range of infectious agents and vaccines. This leads to an increased risk of infection and contributes to morbidity, mortality, and health care resource use.1-3
Immunoglobulin (Ig) replacement, either IV or subcutaneous, is often used to reduce the number and severity of infections in patients with acquired hypogammaglobulinemia secondary to hematological malignancies. However, the evidence supporting the use of Ig replacement therapy is limited. A recent systematic review and meta-analysis suggested that although prophylactic Ig reduced the risk of clinically documented infections, this was based on results from 5 trials enrolling 267 patients published more than 20 years ago.4 As most trials predate modern B-cell– and plasma cell–targeted therapies and supportive care, the applicability of these findings to current clinical practice is uncertain.4
Some international guidelines recommend a trial of prophylactic antibiotics before commencing Ig replacement5; however, the evidence supporting the use of antibiotic prophylaxis for patients with secondary hypogammaglobulinemia is limited. Prophylactic antibiotics reduced febrile episodes and deaths within the first 12 weeks of antimyeloma therapy in a randomized trial6 and reduced the risk of infection within the first 3 months of myeloma therapy in a meta-analysis.7 A more recent systematic review did not find any overall benefit of prophylactic antibiotics for preventing infections in patients with secondary hypogammaglobulinemia,4 but the included trials did not report on the concomitant use of Ig therapy and did not enroll patients with secondary hypogammaglobulinemia due to CLL or NHL.4 In Australia and New Zealand, a trial of prophylactic antibiotics is not required before commencing Ig replacement, and a recent practice survey showed that antibiotic use is not a standard of care for this indication.8
Because of the increasing demand, high cost, and limited global supply of Ig products, there is a need for further evidence to support rational Ig use and evaluate alternatives. We designed the Role of Antibiotic Therapy or IV Ig on Infections in Hematology (RATIONAL) trial to determine the feasibility of delivering prophylactic antibiotics as an alternative to Ig replacement for patients with acquired hypogammaglobulinemia secondary to a hematological malignancy.
Methods
The RATIONAL trial is an investigator-initiated, open-label, phase 2, parallel-randomized controlled feasibility trial conducted in Australia and New Zealand. The trial protocol and statistical analysis plan are provided in the supplemental Appendix. The trial was registered at the Australian and New Zealand Clinical Trials Registry (ACTRN12616001723471) and was approved by the relevant human research ethics committees at the participating sites. We obtained informed consent from all trial participants.
Trial sites and patients
The trial was conducted at 7 hospitals in Australia and New Zealand. We included patients aged ≥18 years with acquired hypogammaglobulinemia secondary to a hematological malignancy who met the Australian National Blood Authority’s Criteria for the Clinical Use of Ig, which is a total IgG level below the local lower limit of the reference range (excluding paraprotein) and a history of recurrent or severe bacterial infections or IgG < 4 g/L (excluding paraprotein). Other eligibility criteria included that patients had a life expectancy of >12 months and were able to attend monthly IV Ig infusions or self-administered subcutaneous Ig.
Patients were excluded if they had received an allogeneic stem cell transplant; had a known objection to receiving Ig products, known severe IgA deficiency, history of allergy to Ig preparations or all of the trial antibiotic options, or history of splenectomy; were already receiving daily antibiotic prophylaxis for the purpose of preventing bacterial infection; had received Ig replacement in the preceding 3 months; or had a current active infection requiring systemic antibiotics. Other exclusion criteria included contraindications to receiving trial antibiotics, such as severe renal impairment, pregnancy or breastfeeding, or prolonged significant cytopenia that would preclude trial antibiotics. The full list of the inclusion and exclusion criteria is provided in the supplemental Appendix.
Randomization, intervention, and blinding
Randomization was performed at a 1:2 (Ig:antibiotics) ratio according to a centrally prepared, computer-generated allocation sequence, stratified as per the site and balanced with blocks of varying, undisclosed sizes. Opaque, sealed envelopes were used to allocate the participants to the intervention arm.
The participants allocated to the prophylactic Ig arm received either IV or subcutaneous Ig replacement. IV Ig was administered monthly (every 4 weeks ± 1 week) at a dose of 0.4 g/kg, modified according to an IgG trough level of at least the lower limit of the age-specific serum IgG reference range. In the first month of therapy, if the IgG level was < 4 g/L, an additional (loading) dose of 0.4 g/kg was allowed. Subcutaneous Ig was administered at a dose of 100 mg/kg per week, modified to achieve an IgG steady state of at least the lower limit of the serum IgG reference range. Participants were allowed to transition from IV to subcutaneous Ig during the course of the trial, using a conversion factor of 1:1 for the total monthly IV to subcutaneous dosing.
Participants allocated to antibiotic prophylaxis received trimethoprim-sulfamethoxazole (cotrimoxazole) 160 mg/800 mg once daily. For participants with a sensitivity to trimethoprim-sulfamethoxazole, either at enrollment or during the course of the trial, doxycycline 100 mg once daily was allowed as an alternative antibiotic. The selection of antibiotics was based on a number of factors, including spectrum of activity, concerns about increases in antimicrobial resistance with the use of a fluoroquinolone, lack of availability of levofloxacin in Australasia, prior studies showing the efficacy of cotrimoxazole, and recommendations in national and international guidelines.9-11
The intervention in both arms was continued for 12 months. Participants allocated to prophylactic antibiotics who developed a grade 3 or higher infection (as per the Common Terminology Criteria for Adverse Events [CTCAE] version 5.0) were allowed to discontinue prophylactic antibiotics and commence Ig replacement at the discretion of the treating clinician. All other aspects of care, including other antimicrobial prophylaxis, vaccinations, and treatment of the hematological malignancy, were performed according to the usual care.
Participants, study staff, and treating clinicians were not blinded to the treatment allocation. However, all infection outcomes and adverse events were independently adjudicated by a blinded Outcome Adjudication Committee (OAC; see “Outcome adjudication”).
Data collection and monitoring
Participants were reviewed in person at study entry and 3, 6, 9, and 12 months after randomization. Participants were also provided with a daily patient diary to complete information on compliance with interventions, infection events (including whether they had recorded their temperature that day and whether it was elevated), and antibiotic use between the study visits. Data on participant demographics, underlying diseases and treatments, antimicrobial use, infections, hospitalizations, and quality of life were collected.
Outcomes
The primary outcome was the proportion of participants who were alive and on their assigned treatment 12 months after randomization. Participants in the prophylactic antibiotic arm who developed a grade 3 or higher infection and commenced Ig were not considered to be in their assigned treatment arm for assessment of the primary outcome.
Secondary outcomes included time to first major infection (defined as grade 3 or higher according to the CTCAE version 5.0 as assessed by the OAC), number of clinically documented infections (defined as presence of symptoms or signs of infection requiring systemic antimicrobial therapy), number of microbiologically confirmed infections (as confirmed by the OAC), susceptibility profile (proportion of resistant organisms as confirmed by the OAC), treatment-related adverse events (possibly, probably, or definitely related to the allocated treatment), time period free from hospitalization and IV antibiotics, trough IgG levels and quality of life (as measured by the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (QLQ-C30), Functional Assessment of Cancer Therapy (FACT)-Neutropenia, and EuroQol-5 dimension (EQ5D)-5L). In this article, only the QLQ-C30 is reported. EQ5D-5L and FACT-Neutropenia, including a cost-effectiveness analysis, will be reported in a subsequent manuscript.
Outcome adjudication
Infection outcomes and adverse events were reviewed by a blinded OAC, which comprised 2 infectious disease physicians and 1 hematologist. They were provided with case report forms, investigation results, and medical records. All supplied data had all information regarding treatment allocation removed. The OAC assigned CTCAE grades to the outcomes of infection, date of infection onset, and whether it met the definition of a major, clinically documented, or microbiologically confirmed infection. They also reviewed susceptibility profile of any isolated organisms. Each member of the OAC independently assessed and classified each outcome event, and the members met to resolve any discrepancies between their adjudicated outcomes. The majority decision determined the final classification of events.
Statistical analysis
The planned sample size (n = 60) was chosen based on feasibility considerations. With a sample size of 60 patients (40 in the antibiotic arm and 20 in the IV Ig arm), we estimated an overall adherence rate to the protocol of 80% with a 95% confidence interval (CI) of approximately ±10%.
Details of the prespecified statistical analysis plan are presented in the supplemental Appendix. The main population for all analyses was the intention-to-treat population, which included all randomized participants. The proportions of participants in each treatment arm who were alive and in their assigned treatment arm at 12 months were calculated together with 95% Clopper-Pearson Exact CIs.12 Comparison between the 2 arms was based on a Fisher exact test. We also reported the odds ratio and 95% CI from a logistic regression model, with the treatment group as the only covariate. The primary end point was also analyzed based on the per-protocol population, which excluded participants with a major protocol deviation. We used Kaplan-Meier estimates to present the time to first major infection and time to first microbiologically confirmed infection (any grade) of the 2 treatment groups and the log-rank test to formally compare the time distributions. The QLQ-C30 data were analyzed using a mixed-effects model, with fixed effects for time (included as a factor), treatment arm, and their interaction and random effects in patients and assessment of the patient. We assumed an unstructured structured formulation for the variance-covariance structure of random effects. Missing quality-of-life data were handled following the published guidelines.13
Analyses were performed with the statistical software R version 4.2.1 and STATA version 17 and validated using SAS version 9.4.
Results
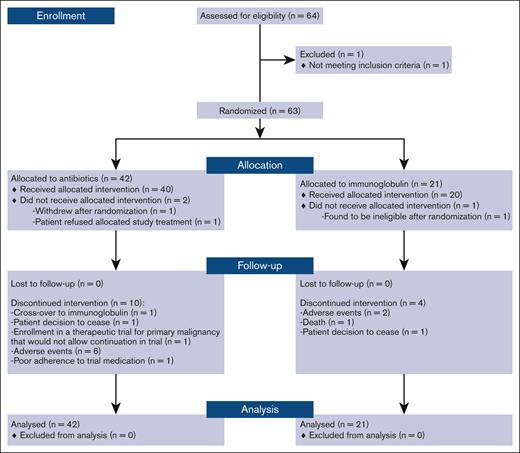
Between August 2017 and April 2019, 63 patients were enrolled and randomized. There were 42 participants allocated to the prophylactic antibiotics and 21 allocated to Ig. Participant flowchart is shown in Figure 1.
Participant characteristics
The participant characteristics are shown in Table 1. The mean age was 70 years (standard deviation, 8), 34 (54%) were female, 29 (46%) had CLL, 12 (19%) had MM, 20 (32%) had NHL, and 2 (3%) had another hematological malignancy. The median baseline IgG level was 4.1 g/L (interquartile range [IQR], 3.1-5.2), and 59 (94%) had a history of prior infection. With regard to disease therapy, 17 (27%) had never received systemic anticancer therapy, 15 (24%) were receiving systemic anticancer therapy at the time of randomization, 21 (33%) had previously received therapy and were in remission, and 8 (13%) had previously received therapy and had refractory or relapsed disease.
Participant characteristics
| Characteristic . | Overall, N = 63 . | IV Ig, N = 21 . | Oral antibiotics, N = 42 . |
|---|---|---|---|
| Age at registration (y) | |||
| Mean (SD) | 70 (8) | 72 (9) | 70 (8) |
| Median | 69 | 69 | 70 |
| Range | 48-87 | 57-87 | 48-87 |
| Sex, n (%) | |||
| Female | 34 (54.0) | 13 (61.9) | 21 (50.0) |
| Male | 29 (46.0) | 8 (38.1) | 21 (50.0) |
| ECOG performance status, n (%) | |||
| 0 | 27 (42.9) | 8 (38.1) | 19 (45.2) |
| 1 | 26 (41.3) | 11 (52.4) | 15 (35.7) |
| 2 | 9 (14.3) | 2 (9.5) | 7 (16.7) |
| Not performed | 1 (1.6) | 0 (0.0) | 1 (2.4) |
| Diagnosis at screening, n (%) | |||
| CLL | 29 (46.0) | 8 (38.1) | 21 (50.0) |
| MM | 12 (19.0) | 3 (14.3) | 9 (21.4) |
| NHL | 20 (31.7) | 8 (38.1) | 12 (28.6) |
| Other | 2 (3.2) | 2 (9.5) | 0 (0.0) |
| Disease therapy n (%) | |||
| Currently receiving systemic anticancer treatment | 15 (24.2) | 6 (28.6) | 9 (22.0) |
| In remission having previously received systemic anticancer treatment | 21 (33.9) | 8 (38.1) | 13 (31.7) |
| Never received systemic anticancer treatment | 17 (27.4) | 4 (19.0) | 13 (31.7) |
| Relapsed or refractory disease, having previously received systemic anticancer treatment | 8 (12.9) | 3 (14.3) | 5 (12.2) |
| Other | 1 (1.6) | 0 (0.0) | 1 (2.4) |
| Missing | 1 (1.6) | 0 | 1 (2.4) |
| IgG level at enrollment (g/L), median (IQR) | 4.1 (3.1-5.2) | 4.5 (3.1-5.8) | 4.0 (3.2-4.5) |
| IgG level < 4 g/L, n (%)∗ | 28 (45.2) | 8 (38.1) | 20 (48.8) |
| History of infection, n (%) | 59 (93.7) | 20 (95.2) | 39 (92.9) |
| Prior pneumococcal vaccination, n (%) | 29 (46) | 9 (43) | 20 (48) |
| Characteristic . | Overall, N = 63 . | IV Ig, N = 21 . | Oral antibiotics, N = 42 . |
|---|---|---|---|
| Age at registration (y) | |||
| Mean (SD) | 70 (8) | 72 (9) | 70 (8) |
| Median | 69 | 69 | 70 |
| Range | 48-87 | 57-87 | 48-87 |
| Sex, n (%) | |||
| Female | 34 (54.0) | 13 (61.9) | 21 (50.0) |
| Male | 29 (46.0) | 8 (38.1) | 21 (50.0) |
| ECOG performance status, n (%) | |||
| 0 | 27 (42.9) | 8 (38.1) | 19 (45.2) |
| 1 | 26 (41.3) | 11 (52.4) | 15 (35.7) |
| 2 | 9 (14.3) | 2 (9.5) | 7 (16.7) |
| Not performed | 1 (1.6) | 0 (0.0) | 1 (2.4) |
| Diagnosis at screening, n (%) | |||
| CLL | 29 (46.0) | 8 (38.1) | 21 (50.0) |
| MM | 12 (19.0) | 3 (14.3) | 9 (21.4) |
| NHL | 20 (31.7) | 8 (38.1) | 12 (28.6) |
| Other | 2 (3.2) | 2 (9.5) | 0 (0.0) |
| Disease therapy n (%) | |||
| Currently receiving systemic anticancer treatment | 15 (24.2) | 6 (28.6) | 9 (22.0) |
| In remission having previously received systemic anticancer treatment | 21 (33.9) | 8 (38.1) | 13 (31.7) |
| Never received systemic anticancer treatment | 17 (27.4) | 4 (19.0) | 13 (31.7) |
| Relapsed or refractory disease, having previously received systemic anticancer treatment | 8 (12.9) | 3 (14.3) | 5 (12.2) |
| Other | 1 (1.6) | 0 (0.0) | 1 (2.4) |
| Missing | 1 (1.6) | 0 | 1 (2.4) |
| IgG level at enrollment (g/L), median (IQR) | 4.1 (3.1-5.2) | 4.5 (3.1-5.8) | 4.0 (3.2-4.5) |
| IgG level < 4 g/L, n (%)∗ | 28 (45.2) | 8 (38.1) | 20 (48.8) |
| History of infection, n (%) | 59 (93.7) | 20 (95.2) | 39 (92.9) |
| Prior pneumococcal vaccination, n (%) | 29 (46) | 9 (43) | 20 (48) |
ECOG, European Cooperative Oncology Group; SD, standard deviation.
Missing data for 1 participant in the oral antibiotic arm.
Trial and concomitant therapies
Of the 63 randomized participants, 17 (27%) either did not commence or discontinued their allocated treatment within 12-months of study follow-up: 5 (23%) in the Ig arm and 12 (29%) in the prophylactic antibiotic arm (Figure 1). In the Ig arm, 1 patient was withdrawn early from the study, 2 discontinued therapy, and 2 died before the 12-month review. In the antibiotic arm, 2 were withdrawn early from the study and 10 discontinued therapy before the 12-month review. Nine participants in the antibiotic arm crossed over to Ig replacement after an infection.
Primary outcome
In the intention-to-treat analysis population, the proportion of participants alive who remained in their allocated treatment arm at 12 months was 76% (16 of 21; 95% CI, 53-92) in the Ig replacement arm and 71% (30 of 42; 95% CI, 55-84) in the prophylactic antibiotic arm (Fisher test P-value = .77; odds ratio [OR], 0.78; 95% CI, 0.22-2.52).
The analysis of the primary outcome in the per-protocol population was similar, with the proportion of participants in the assigned treatment arm at 12 months being 80% (16 of 20; 95% CI, 56-94) in the Ig arm and 75% (30 of 40; 95% CI, 58-87) in the prophylactic antibiotic arm (Fisher test P-value = .76; odds ratio [OR], 0.75; 95% CI, 0.18-2.65).
Secondary outcomes
The time periods to the first major infection (grade 3 or higher) and the first microbiologically confirmed infection (any grade) are shown in Figure 2. The median time points were not reached in either treatment arm. The lower quartile for time to first major infection was 11.1 months in the Ig replacement arm compared with 9.7 months in the prophylactic antibiotic arm (log-rank test P=.65). The lower quartile for time to first microbiologically confirmed infection was 6.3 months in the Ig replacement arm and not reached in the prophylactic antibiotic arm (log-rank test P = .17). The number of clinically documented infections and microbiologically documented infections are shown in Table 2, and a summary of the types of infection by treatment arm is reported in supplemental Appendix Tables 1 and 2. Clinically documented infections averaged 1.86 per participant in the Ig treatment arm and 1.33 per participant in the prophylactic antibiotic treatment arm. Microbiologically confirmed infections per participant averaged 0.52 and 0.38 respectively in the Ig and prophylactic antibiotic treatment arms. Two participants with resistant organisms were identified in the Ig replacement arm (cotrimoxazole resistant [n = 1] and extended-spectrum β-lactamase [n = 1]) and 3 in the prophylactic antibiotic arm (all multidrug resistant).
Time to infection events based on the treatment arm. First major infection (A) and first microbiologically confirmed infection (B).
Time to infection events based on the treatment arm. First major infection (A) and first microbiologically confirmed infection (B).
Secondary outcomes
| Characteristic . | IV Ig, N = 21 . | Oral antibiotics, N = 42 . |
|---|---|---|
| Number of clinically documented infections, n | 39 | 56 |
| Number of participants with clinically documented infections, n (%) | ||
| 0 infections | 3 (14) | 18 (43) |
| 1 infection | 5 (24) | 12 (29) |
| 2 infections | 8 (38) | 2 (5) |
| ≥ 3 infections | 5 (24) | 5 (24) |
| Number of microbiologically documented infections, n | 11 | 16 |
| Number of participants with microbiologically documented infection, n (%) | ||
| 0 infections | 13 (62) | 34 (81) |
| 1 infection | 6 (29) | 2 (5) |
| 2 infections | 1 (5) | 5 (12) |
| ≥ 3 infections | 1 (5) | 1 (2) |
| Participants with resistant organism identified, n | ||
| Cotrimoxazole resistant | 1 | 0 |
| Extended-spectrum β-lactamases | 1 | 0 |
| Multidrug resistant | 0 | 3 |
| Number of deaths, n (%) | 2 (9.5) | 1 (2.4) |
| Number of infection deaths, n (%) | 1 (4.8) | 1 (2.4) |
| IgG (g/L), median (IQR) | 9.1 (7.4-11.1) | 4.2 (3.3-5.6) |
| Number of treatment-related AEs∗, n | ||
| Grade 1 | 3 | 5 |
| Grade 2 | 2 | 11 |
| Grade 3 | 4 | 4 |
| Duration of IV antibiotics (d), median (IQR) | 2.0 (2.0-2.0) | 3.0 (2.5-3.5) |
| Hospital duration (d), median (IQR) | 2.0 (1.0-4.5) | 2.0 (1.0-4.0) |
| Characteristic . | IV Ig, N = 21 . | Oral antibiotics, N = 42 . |
|---|---|---|
| Number of clinically documented infections, n | 39 | 56 |
| Number of participants with clinically documented infections, n (%) | ||
| 0 infections | 3 (14) | 18 (43) |
| 1 infection | 5 (24) | 12 (29) |
| 2 infections | 8 (38) | 2 (5) |
| ≥ 3 infections | 5 (24) | 5 (24) |
| Number of microbiologically documented infections, n | 11 | 16 |
| Number of participants with microbiologically documented infection, n (%) | ||
| 0 infections | 13 (62) | 34 (81) |
| 1 infection | 6 (29) | 2 (5) |
| 2 infections | 1 (5) | 5 (12) |
| ≥ 3 infections | 1 (5) | 1 (2) |
| Participants with resistant organism identified, n | ||
| Cotrimoxazole resistant | 1 | 0 |
| Extended-spectrum β-lactamases | 1 | 0 |
| Multidrug resistant | 0 | 3 |
| Number of deaths, n (%) | 2 (9.5) | 1 (2.4) |
| Number of infection deaths, n (%) | 1 (4.8) | 1 (2.4) |
| IgG (g/L), median (IQR) | 9.1 (7.4-11.1) | 4.2 (3.3-5.6) |
| Number of treatment-related AEs∗, n | ||
| Grade 1 | 3 | 5 |
| Grade 2 | 2 | 11 |
| Grade 3 | 4 | 4 |
| Duration of IV antibiotics (d), median (IQR) | 2.0 (2.0-2.0) | 3.0 (2.5-3.5) |
| Hospital duration (d), median (IQR) | 2.0 (1.0-4.5) | 2.0 (1.0-4.0) |
AEs, adverse events.
No treatment-related grade 4 or 5 AEs reported.
There were 2 deaths in the Ig replacement arm within 12 months, including 1 infection-related death (defined as death within 7 days of the diagnosis of infection by microbiological means). One death occurred in the prophylactic antibiotic arm after 12.3 months and was after the report of a major infection, not microbiologically confirmed, at 11.7 months.
The durations of IV antibiotics and hospitalization are shown in Table 2. The number of treatment-related adverse events was similar between the 2 treatment arms (Table 2). The adverse event rates are shown in supplemental Appendix Tables 3 and 4. The median trough IgG level was higher in the Ig arm (9.1 g/L; IQR, 7.4-11.1) than in the prophylactic antibiotic arm (4.2 g/L; IQR, 3.3-5.6). The IgG levels in the treatment arm during the trial are shown in supplemental Figure 1.
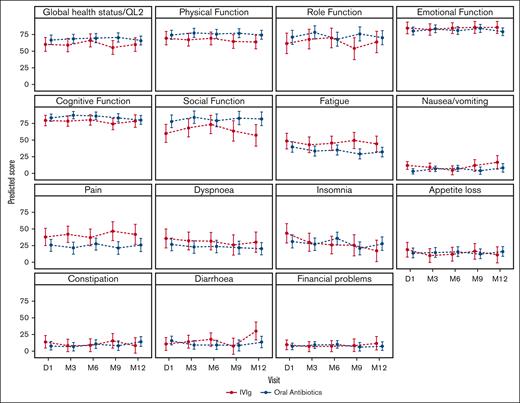
The predicted means for the domains of quality of life as measured by the QLQ-C30 are shown in Figure 3. There was no significant difference in the change in global quality of life or other domains over time between the treatment arms (supplemental Appendix Table 5).
Predicted means of the QLQ-C30 based on the treatment arm. Higher scores are better for global health status, physical function, role function, emotional function, cognitive function, and social function. Lower scores are better for fatigue, nausea/vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial problems. P values for the interaction between the intervention arm and quality-of-life domains are presented in the supplemental Appendix. The bars represent 95% CIs. D, day; M, month.
Predicted means of the QLQ-C30 based on the treatment arm. Higher scores are better for global health status, physical function, role function, emotional function, cognitive function, and social function. Lower scores are better for fatigue, nausea/vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial problems. P values for the interaction between the intervention arm and quality-of-life domains are presented in the supplemental Appendix. The bars represent 95% CIs. D, day; M, month.
Discussion
In this randomized feasibility trial for adults with acquired hypogammaglobulinemia secondary to hematological malignancies, we found a similar proportion of participants who remained on prophylactic antibiotics at 12 months as those on Ig replacement despite the provision of crossover from prophylactic antibiotics to Ig in the event of a major infection. There were similar rates of infection, time to first major infection, time to first microbiologically confirmed infection, and the number of antimicrobial-resistant organisms detected between the 2 treatment arms. The participants had a similar quality of life (as measured by the QLQ-C30) between the 2 treatment arms.
To our knowledge and according to a recent systematic review,4 this is the first clinical trial to directly compare the efficacy and safety of prophylactic antibiotics with Ig replacement for patients with secondary hypogammaglobulinemia. This is despite their common use for infection prevention in this patient population. In the most recent systematic review on this topic, 8 trials that evaluated Ig replacement for infection prevention (7 IV and 1 subcutaneous) were identified, all comparing Ig with either a placebo or standard of care. Most of the trials were published before 2000, and none of them specified the use of prophylactic antibiotics. The largest trial was of 81 patients with CLL, published in 1994.14 When pooled, in a total of 5 trials enrolling 267 patients, prophylactic Ig was associated with a reduced risk of clinically documented infection (relative risk, 0.74; 95% CI, 0.54-9.96) but not mortality.4 However, the difference in infection rate was not significant after trials with a high risk of bias were removed, and it is unclear whether these results are still generalizable to current practice. For example, of the 8 historical trials evaluating Ig replacement, the proportion of patients with 1 or more clinically documented infections in the standard-of-care group ranged from ∼60% to 90%, which was higher than that observed in either arm of the current trial.14-18 Changing treatment paradigms may account for this difference, because targeted agents such as monoclonal antibodies, Bruton tyrosine kinase inhibitors, and proteosome inhibitors have largely displaced cytotoxic chemotherapies.
Trials evaluating prophylactic antibiotics (including trimethoprim-sulfamethoxazole, levofloxacin, ciprofloxacin, and clarithromycin) for patients with MM have been conducted more recently, with 4 trials reported since 2012.6,19-21 One meta-analysis pooled 3 trials of prophylactic antibiotics in 664 newly diagnosed patients with myeloma and found a reduction in infection within 3 months (relative risk, 0.79; 95% CI, 0.62-1.00) but no difference in mortality.7 A more recent systematic review and meta-analysis included 5 trials of prophylactic antibiotics for patients with myeloma and found no significant difference in clinically documented infections (relative risk, 0.91; 95% CI, 0.78-1.08) or mortality.4 None of the included trials specified whether patients had hypogammaglobulinemia, and none reported on concomitant Ig use. The systematic review also did not identify any trials of prophylactic antibiotics for patients with CLL or NHL.4
Our study has limitations. As a phase 2 feasibility trial with a relatively small sample size that was not powered for noninferiority or equivalence, we cannot exclude a clinically important difference in infection rates between the 2 treatment arms. Our study was unblinded, although all infection outcomes were independently adjudicated by a committee of infectious disease physicians and hematologist blinded to treatment allocation. Participants in the prophylactic antibiotic group were allowed to crossover to Ig replacement after their first major infection, although this would not affect the primary or secondary outcomes of the time to first major infection and time to first microbiologically confirmed infection. We did not collect information on vaccination status other than for pneumococcus. Recently, a small observational study of the outcomes of hematology patients referred to a clinical immunology service for recurrent infections reported that of 75 consecutive patients, 45 (60%) had their infections controlled with prophylactic antibiotics for 6 months, and 30 (40%) were commenced on Ig replacement.22
However, to our knowledge, this is the first trial to evaluate prophylactic antibiotics as an alternative to Ig replacement for patients with acquired hypogammaglobulinemia secondary to hematological malignancies. Although prophylactic antibiotics are recommended in some jurisdictions before commencing Ig replacement, there is little evidence to support their efficacy for this indication. Given concerns about the global health threat of antimicrobial resistance,23 prophylactic antibiotic strategies should be evidence based. Our trial supports the feasibility of progressing to a larger phase 3 trial comparing prophylactic antibiotics with Ig replacement, powered for a patient-centered outcome incorporating survival and major infection risk. The choice of antibiotics and exclusion of a prophylaxis arm should be considered in future trial designs. Health economic analyses, quality of life, rates of antimicrobial resistance, and changes in the microbiome are also key outcomes to consider in future studies on these 2 infection prevention measures.
The increasing use of B-cell– and plasma cell–depleting therapies, such as anti-CD20 and anti-CD38 monoclonal antibodies, Bruton tyrosine kinase and B-cell lymphoma 2 (BCL2) inhibitors, and cellular therapies, is improving disease-specific survival in patients with B-cell malignancies but is likely to result in an increasing prevalence of acquired hypogammaglobulinemia. This will place a greater demand, which is already increasing globally, on the supply of Ig.24 In Australia, the demand for Ig has increased by >10% per year over the last decade, and patients with hematological malignancies are the largest single group using Ig products.25 There are considerable costs of providing Ig replacement. For example, based on the published cost of domestic IV Ig in Australia, the annual product cost for a 70 kg individual is ∼21 372 Australian dollars.26 Furthermore, there are barriers in countries to manufacture Ig preparations in order to achieve self-sufficiency for plasma products, including sufficient domestic plasma collection, cost, and access to commercial or not-for-profit manufacturers.27 Therefore, there is a pressing need to generate contemporary data regarding the comparative effectiveness and safety of Ig replacement in this patient population.
Conclusions
In this randomized feasibility trial, a similar proportion of participants remained on prophylactic antibiotics at 12 months as those on Ig replacement despite the provision of crossover from prophylactic antibiotics to Ig replacement in the event of grade 3 or higher infection. The rates of major infections were similar between the 2 treatment arms. Our findings support the feasibility of progressing to a larger phase 3 trial.
Acknowledgments
Z.K.M. is funded by a National Health and Medical Research Council (NHMRC) Emerging Leader Investigator grant (GNT1194811). E.M.W. is funded by a NHMRC investigator grant (GNT1177784). R.W. is funded by the Health Research Council of New Zealand Clinical Practitioner Research Fellowship (19/139). The trial was funded by a grant (ID124) from the Australian National Blood Authority, which had no role in the design, analysis, or reporting of the trial results.
Authorship
Contribution: Z.K.M., E.M.W., R.W., and C.O.M. conceived and designed the study; Z.K.M. drafted the protocol; J.R. and Z.K.M. wrote the statistical analysis plan; J.R. and L.T. analyzed the data; and all authors performed the research and revised the manuscript.
Conflict-of-interest disclosure: E.M.W. is a grant holder. E.M.W. and Z.K.M. received grant funding from CSL Behring, not related to this study. The remaining authors declare no competing financial interests.
A complete list of the participating sites and site investigators from the Australasian Leukaemia and Lymphoma Group appears in the supplemental Material.
Correspondence: Zoe K. McQuilten, Transfusion Research Unit, School of Public Health and Preventive Medicine, Monash University, 553 St Kilda Rd, Melbourne, VIC 3004, Australia; email: zoe.mcquilten@monash.edu.
References
Author notes
Deidentified participant data and study protocol are available with publication upon request from the corresponding author, Zoe K. McQuilten (zoe.mcquilten@monash.edu).
Investigators whose proposal has been reviewed and approved by the RATIONAL investigators and relevant ethical review committees will be able to undertake analyses to achieve the aims specified in the approved proposal by accessing data through a web-based data portal safe–haven based at Monash University, Melbourne, Australia.
The full-text version of this article contains a data supplement.