TO THE EDITOR:
Primary diffuse large B-cell lymphoma of the central nervous system (PCNSL) is a rare aggressive extranodal B-cell lymphoma.1,2 Despite intensive protocols such as methotrexate, cytarabine, thiotepa, and rituximab (MATRix) and consolidative thiotepa-based autologous stem cell transplant (ASCT), refractory and relapsed disease remains a major clinical problem and limited prognostic tools are available.3-5 Although PCNSL shares biological similarities with systemic de novo diffuse large B-cell lymphoma (DLBCL), the prognosis remains significantly worse with a median overall survival of 2 years, and only one-third of patients are alive at 5 years.6 The underlying drivers of poorer survival in PCNSL remain unclear but likely reflect biological differences in disease biology, challenges in treatment delivery, and a high burden of comorbidity at presentation. Although a number of clinical risk scores are available,7-9 these were developed before the adoption of intensive chemotherapy regimens such as MATRix and do not inform treatment decisions in contemporary clinical practice.10
In systemic DLBCL, double expression of MYC and BCL2 (double expressor [DE]) are associated with inferior clinical outcomes, but the prognostic impact of DE status in PCNSL has not been conclusively defined.11 We evaluated the prognostic value of BCL2 and MYC expression in a cohort of newly diagnosed patients with PCNSL contemporarily treated and demonstrate that DE status is associated with adverse clinical outcomes.
We retrospectively collected data from patients with histologically confirmed PCNSL diagnosed consecutively between 1 May 2015 and 31 May 2020, treated with high-dose methotrexate-based (HD-MTX) induction from 7 UK referral centers. Exclusion criteria included evidence of systemic disease at diagnosis, postmortem diagnosis and treatment with non–HD-MTX (3.5 g/m2) containing induction chemotherapy. Data were retrieved from local health records according to a standardized data collection proforma capturing baseline patient characteristics, treatment details and timings, diagnostic immunohistochemistry (IHC) assessment of BCL2, BCL6, MYC, CD10, and MUM1 as well as receipt and type of consolidation treatment. Positive expression of MYC and BCL2 was assessed in accordance with the World Health Organization (WHO) reporting criteria (MYC, nuclear stain positive in >40% of cells; BCL2, cytoplasmic stain positive in >50% of cells).12 Staining was performed as per each institution’s standard operating procedure. DE status was determined centrally and defined as IHC positivity (by WHO reporting criteria) for both MYC and BCL2.
Outcomes included end of treatment response rates, progression-free survival (PFS) and overall survival (OS). Cox regression for PFS and OS were used to determine baseline factors associated with response and survival. Patients were retrospectively categorized into 2 groups according to induction treatment received. A significant P value was defined as ≤.05. All patient data were anonymized at source and treated according to the principles of the Declaration of Helsinki and the UK Data Protection Act (1998).
Data from 260 patients were collected. Of these, 18 were excluded (11 diagnosed outside of the study period; 5 with insufficiently annotated clinical outcome data; and 2 with no diagnostic IHC data available), and in total, 242 patients were included for analysis (supplemental Figure 1). Key baseline patient characteristics and IHC data are summarized in Table 1. The median age was 65 years (interquartile range [IQR], 56-71), 60% were male, and 64% of patients had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 1. One hundred and seventy-four (72%) patients received treatment with MATRix chemoimmunotherapy, and 68 patients (28%) received treatment with other non-MATRix, HD-MTX–containing induction regimens. Patients who received MATRix chemotherapy (MATRix subgroup) were younger (median age, 62 vs 73 years; P < .01), had better baseline ECOG PS (PS ≤ 1, 75% vs 37%; P < .01), and higher rates of consolidation with BCNU-thiotepa ASCT (58% vs 7%; P < .01). Median follow-up was 3.0 years (IQR, 2.0-4.2) during which 34% (MATRix subgroup, 28%; non-MATRix subgroup, 50%) of patients relapsed and 45% (MATRix subgroup, 36%; non-MATRix subgroup, 66%) died.
Baseline clinical characteristics and surface marker expression in the entire cohort and both the MATRix-treated and non-MATRix–treated subgroups
| . | Entire cohort (n=242) . | MATRix (n=174) . | Non-MATRix (n=68) . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (%) . | Missing . | N (%) . | Missing . | N (%) . | Missing . | P value . | ||||
| Male | 144 | (60) | — | 108 | (62) | — | 36 | (53) | — | .24 |
| Age >60 y | 154 | (64) | — | 95 | (55) | — | 59 | (87) | — | <.01 |
| Median age | 65 | Range, 23-84 | 62 | Range, 23-77 | 73 | Range, 34-84 | — | |||
| PS ≤ 1 | 146 | (64) | 14 | 121 | (75) | 13 | 25 | (37) | 1 | <.01 |
| ASCT | 104 | (44) | 3 | 99 | (58) | 2 | 5 | (7) | 1 | <.01 |
| WBRT | 22 | (9) | 2 | 20 | (12) | 2 | 2 | (3) | — | <.01 |
| MYC positive (>40%) | 119 | (60) | 44 | 89 | (62) | 31 | 30 | (55) | 13 | — |
| BCL2 positive (>50%) | 126 | (59) | 28 | 82 | (54) | 21 | 44 | (72) | 7 | — |
| BCL6 positive | 199 | (88) | 15 | 153 | (93) | 10 | 46 | (73) | 5 | <.01 |
| CD10 positive | 61 | (27) | 14 | 42 | (25) | 8 | 19 | (31) | 6 | .65 |
| MUM1 positive | 216 | (94) | 12 | 154 | (94) | 10 | 62 | (97) | 4 | .71 |
| Double expressor | 67 | (40) | 73 | 46 | (40) | 58 | 21 | (40) | 15 | 1.00 |
| Overall response rate | 172 | (74) | 9 | 133 | (79) | 5 | 39 | (61) | 4 | .01 |
| . | Entire cohort (n=242) . | MATRix (n=174) . | Non-MATRix (n=68) . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (%) . | Missing . | N (%) . | Missing . | N (%) . | Missing . | P value . | ||||
| Male | 144 | (60) | — | 108 | (62) | — | 36 | (53) | — | .24 |
| Age >60 y | 154 | (64) | — | 95 | (55) | — | 59 | (87) | — | <.01 |
| Median age | 65 | Range, 23-84 | 62 | Range, 23-77 | 73 | Range, 34-84 | — | |||
| PS ≤ 1 | 146 | (64) | 14 | 121 | (75) | 13 | 25 | (37) | 1 | <.01 |
| ASCT | 104 | (44) | 3 | 99 | (58) | 2 | 5 | (7) | 1 | <.01 |
| WBRT | 22 | (9) | 2 | 20 | (12) | 2 | 2 | (3) | — | <.01 |
| MYC positive (>40%) | 119 | (60) | 44 | 89 | (62) | 31 | 30 | (55) | 13 | — |
| BCL2 positive (>50%) | 126 | (59) | 28 | 82 | (54) | 21 | 44 | (72) | 7 | — |
| BCL6 positive | 199 | (88) | 15 | 153 | (93) | 10 | 46 | (73) | 5 | <.01 |
| CD10 positive | 61 | (27) | 14 | 42 | (25) | 8 | 19 | (31) | 6 | .65 |
| MUM1 positive | 216 | (94) | 12 | 154 | (94) | 10 | 62 | (97) | 4 | .71 |
| Double expressor | 67 | (40) | 73 | 46 | (40) | 58 | 21 | (40) | 15 | 1.00 |
| Overall response rate | 172 | (74) | 9 | 133 | (79) | 5 | 39 | (61) | 4 | .01 |
P values refer to χ2 testing MATRix- vs non-MATRix–treated subgroup. Significant P values are indicated in bold.
WBRT, whole-brain radiotherapy.
Across the entire cohort, the overall response rate (ORR) after induction treatment was 74% with a 2-year PFS of 52% and 2-year OS of 60%. In the MATRix-treated subgroup, ORR was 79% with 2-year PFS and OS of 60% and 69%, respectively. The non-MATRix–treated subgroup ORR was 61% with 2-year PFS and OS of 30% and 37%, respectively. In total, 30% and 21% of cases in the entire cohort had incomplete BCL2 or MYC expression data, respectively, due to missing IHC expression data or not being reported in line with WHO criteria. In the entire cohort, 88% of cases were BCL6 positive, 59% were BCL2 positive, and 60% were MYC positive, consistent with previously reported rates.13,14
BCL6 positivity has been shown to be associated with favorable outcomes.15 In this study, BCL6 negativity by IHC (12% of cases) was associated with significantly shorter PFS and OS in the entire cohort. However, a significant association of BCL6 negativity with the receipt of non-MATRix induction therapy and inferior baseline PS confounds this association.
DE status was evaluable in 169 of 242 patients (69%). Clinical outcomes were comparable between DE-evaluable and DE-unevaluable (due to missing data) patients in the entire cohort (2-year PFS, 49% vs 57%; P = .28; and 2-year OS, 59% vs 61%; P = .58, respectively) and between the 2 treatment subgroups. Among DE-evaluable patients (n = 169 patients), 40% of patients had DE-PCNSL, higher than the estimates of DE prevalence in systemic DLBCL, which range between 20% and 30%.12 DE-positive cases were not significantly associated with older age at diagnosis (P = .99; median, 65 years; IQR, 53.5-71 years; vs median, 65 years; IQR, 57.25-71 years), poorer baseline PS (P = .44; ECOG PS ≤1, 58% vs 59%), or less intensive induction treatment (P = .77; MATRix receipt, 69% vs 69%) compared with DE-negative cases.
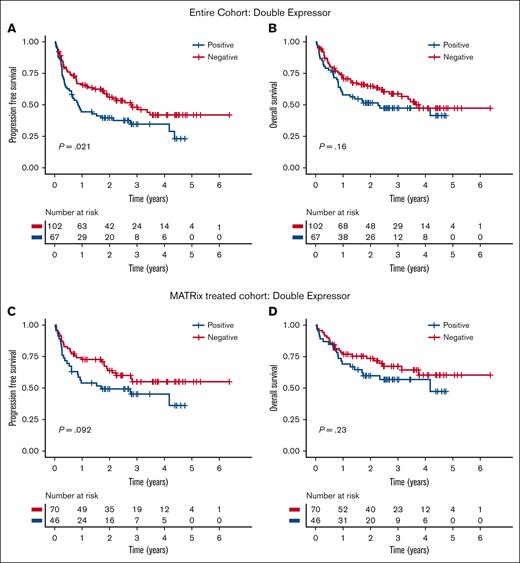
Clinical parameters associated with significantly longer PFS or OS included PS ≤1, receipt of MATRix induction chemotherapy, and receipt of ASCT consolidation. Neither BCL2 nor MYC expression positivity alone was independently associated with shorter PFS or OS in the entire cohort (supplemental Figure 2). However, DE status was associated with shorter PFS in the entire cohort than that of patients without DE (median, 0.86 vs 2.77 years; P = .021) but not shorter OS (Figure 1A-B). In a subgroup analysis restricted to patients treated with MATRix chemotherapy (n = 116 patients), there was a trend toward inferior PFS and OS, although this did not reach significance in a univariable analysis (Figure 1C-D).
Adverse clinical outcomes associated with double expressor status. Association of double expression of MYC (>40%) and BCL2 (>50%) with PFS and OS in the entire cohort (A-B) and in the MATRix-treated subgroup (C-D).
Adverse clinical outcomes associated with double expressor status. Association of double expression of MYC (>40%) and BCL2 (>50%) with PFS and OS in the entire cohort (A-B) and in the MATRix-treated subgroup (C-D).
In a multivariable Cox regression model accounting for age, baseline PS, induction treatment received, and DE status (supplemental Table 1), there was an independent association of DE status with shorter PFS for the entire cohort (n = 163) (hazard ratio, 1.78; 95% confidence interval, 1.17-2.71; P < .01). To investigate whether the association between DE status and shorter PFS was treatment dependent, we performed a further multivariate analysis with an additional interaction term between MATRix induction treatment and DE status. This term had no significant effect on PFS (P = .57), indicating that patients with DE-PCNSL have inferior outcomes irrespective of the type of induction treatment received. A trend toward an association between DE status and OS was noted, but this did not reach significance in either a univariable or multivariable analysis.
In conclusion, we demonstrate that double expression of MYC and BCL2 is associated with poorer clinical outcomes in a large, real-world, contemporary cohort of patients with PCNSL primarily treated with intensive induction therapy. Our data add clarity to the limited prior work in this area (including studies in single-center, noncontemporary cohorts and studies using alternative IHC thresholds for MYC and BCL2 positivity) in which the prognostic relevance was unclear.13,15-17 These findings have potential value in risk stratifying patients and guiding decisions around consolidation after induction chemotherapy. Validation in a prospective series is warranted, and more work to understand the disease biology of DE-PCNSL is needed to identify biologically rational therapies for this higher risk cohort.
Acknowledgments: E.P. is a recipient of a Cancer Research UK City of London Clinical Research Training Fellowship and Lymphoma Research Trust research training grant. J.O. is supported by a Cancer Research UK Clinician Scientist Fellowship (C57432/A22742) and Cancer Research UK Accelerator Award (C355/A26819).
Contribution: E.P., K.C., C.P.F., and J.O. conceived the study and design; E.P., E.C., J.D., C.P., D.H., P.R., T.O., N.T., E.J., P.G., S.C., P.M., J.S., T.A.E., and N.M.-C. collected clinical data; A. Akarca, S.P., T.M., and M.C. provided pathological data and interpretation; A. Ali and A.A.K. provided statistical support; E.P. and J.O. analyzed the data and wrote the first draft of the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: J.S. reports consulting or serving in an advisory role for AbbVie and AstraZeneca. T.A.E. reports consulting or serving in an advisory role for Roche, Gilead/Kite, Loxo Oncology, BeiGene, Incyte, Secura Bio, and Autolus; honoraria from Roche, Gilead/Kite, Janssen, AbbVie, and AstraZeneca; and research support from Gilead, AstraZeneca, and BeiGene. P.M. reports consulting or serving in an advisory role for Gilead/Kite, Incyte, Janssen, AbbVie, AstraZeneca, BeiGene, Celgene/Bristol Myers Squibb (BMS), Epizyme, Roche, and Takeda. S.C. reports consulting or serving in an advisory role for AbbVie, Adicet Bio, Atara Biotherapeutics, Gilead/Kite, Novartis, Orion Pharma, Pierre Fabre, Roche, and Takeda. K.C. reports consulting or serving in an advisory role for BeiGene, Roche, Celgene/BMS, Takeda, Gilead/Kite, Incyte, Atara, and Janssen. C.P.F. reports consulting or serving in an advisory role for Roche, BeiGene, Gilead/Kite, Incyte, Janssen, Roche, Takeda, AbbVie, AstraZeneca, Atara Bio, and Celgene/BMS, and research support from BeiGene. The remaining authors declare no competing financial interests.
Correspondence: Jessica Okosun, Centre for Haemato-Oncology, Barts Cancer Institute, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, United Kingdom; email: j.okosun@qmul.ac.uk.
References
Author notes
K.C., C.P.F., and J.O. contributed equally as joint senior authors.
Original data are available upon reasonable request from the corresponding author, Jessica Okosun (j.okosun@qmul.ac.uk).
The full-text version of this article contains a data supplement.