TO THE EDITOR:
Pure red cell aplasia (PRCA) is a potential long-lasting and devastating complication after allogeneic hematopoietic stem cell transplantation (allo-SCT), with significant morbidity. Patients who underwent transplantation with major ABO-incompatible stem cell grafts can develop PRCA because of antidonor AB isohemagglutinins (IsoHGs). Approximately 25% of patients receive a major ABO-mismatched allograft,1 of which up to 50% of patients develop PRCA.2-4 With >25 000 allo-SCTs reported annually by the European Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research,5,6 it is evident that hundreds of patients undergoing transplantation must suffer from PRCA each year.
Although multiple strategies to eradicate persisting donor-specific IsoHGs (such as rituximab, bortezomib, and donor lymphocyte infusion [DLI]) have been applied, the evidence for the efficacy of these interventions remains inconclusive.3,7,8 Recently, daratumumab has been explored as a treatment for PRCA, with the hypothesis that residual plasma cells of the recipient producing antidonor A or B IsoHGs are CD38 positive and that treatment with daratumumab will deplete these residual plasma cells. In 2018, successful treatment of refractory PRCA with the anti-CD38 monoclonal antibody daratumumab was described for the first time.9 Since then, 9 additional cases have been reported,10 which unanimously showed swift resolution of PRCA after 2 to 4 courses of daratumumab.
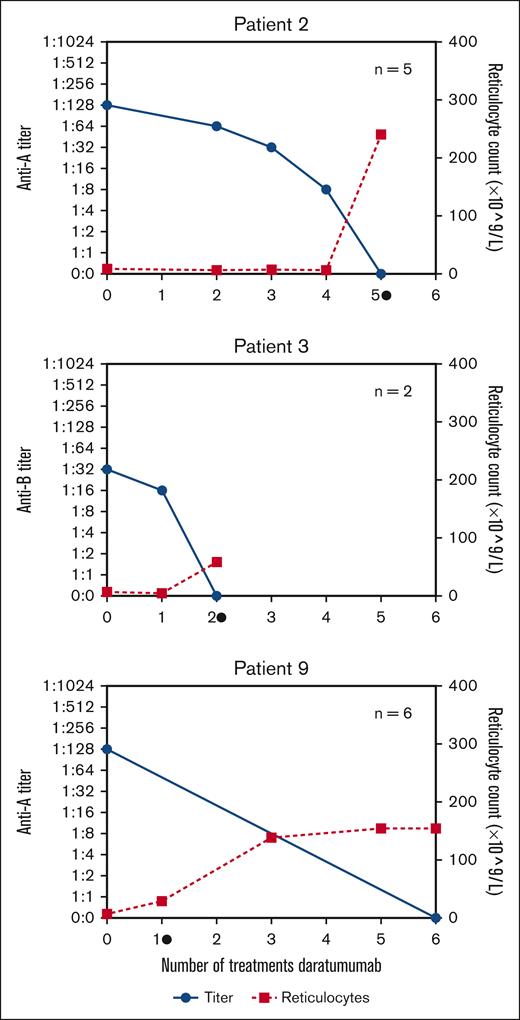
To overcome reporting bias through individual case reports, we identified in our “real-world” all patients (n = 14) with posttransplant PRCA treated with daratumumab within 7 Dutch bone marrow transplant centers from 2020 to 2023 (Table 1). Most patients had anti-A antibodies (86%) with IsoHG titers ranging from 1:4 to 1:1024 (supplemental Table 2) and all patients required erythrocyte support. Previously administered therapies consisted of rituximab (43%), DLI (29%), and bortezomib (7%), all of which were unsuccessful. The start of daratumumab (1800 mg s.c. per administration) was heterogeneous (range, day 60 to day 2898 after transplantation), as daratumumab is currently neither registered as a treatment for PRCA nor part of the (inter)national treatment guidelines. Successful treatment of PRCA was defined as the development of reticulocytosis and was achieved in 13 of 14 patients (93%, median time 14 days; Table 1). Patients received either a fixed number of daratumumab administrations (n = 7) or the treatment was stopped after the development of reticulocytosis (n = 6). Therefore, the median number of daratumumab administrations after which reticulocytosis developed was 2 (range 1-5), but the median number of total daratumumab administrations was higher (median 4, range 1-6) (supplemental Figure 1). In all patients, AB0-titers were normalized (Figure 1, supplemental Figure 1). In 2 patients (numbers 2 and 3) AB0-titers and reticulocyte numbers were evaluated at each daratumumab administration. In both patients, the disappearance of IsoHGs and development of reticulocytosis occurred simultaneously. Our data suggest that normalization of the AB0-titer and also the development of reticulocytosis can be used to determine the duration of daratumumab treatment. In this cohort, 1 patient (number 14) did not respond after 4 administrations of daratumumab. Potential explanations may be the very high titer of IsoHGs (1:1024) and/or other concomitant infections (the patient was febrile, a Parvo B19 infection was excluded) or that in some patients, >4 administrations of daratumumab are required (as patient number 2). None of the patients with resolution of PRCA developed a relapse (median time of follow-up, 383 days, range 105-1045; Table 1).
Details of 14 cases of patients with PRCA treated with daratumumab in the Netherlands
| No. . | Age . | Sex . | Underlying disease . | Donor type and match grade . | Stem cell source . | Conditioning intensity∗ . | AB0-titer† before treatment . | Donor chimerism . | Prior treatment . | Start daratumumab after SCT (d) . | Total number of doses given (7 day interval‡) . | Number of doses given at reticulocytosis§ . | Iso-hemaglutinnin disappeared . | Reported side-effects . | Ongoing response (d) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 63 | M | AML | MUD 10/10 | Peripheral blood | MAC | 1:16 Anti-A | T 100% nT 100% | Rituximab + DLI | 108 | 2 | 2 | Yes | None reported | 405 |
| 2 | 56 | M | MDS | MUD 10/10 | Peripheral blood | MAC | 1:128 Anti-A | T 98% nT 96% | Steroids + Rituximab | 140 | 5‡ | 5 | Yes | PML (death) | 191|| |
| 3 | 63 | M | PMF | MUD 9/10 | Peripheral blood | MAC | 1:32 Anti-B | T 100% nT 100% | Rituximab + DLI | 121 | 2‡ | 2 | Yes | None reported | 294 |
| 4 | 28 | M | Sickle cell disease | SIB 10/10 | Peripheral blood | NMA | 1:32 Anti-B | T 69% nT 100% | Rituximab | 60 | 4 | 3 | Yes | None reported | 456 |
| 5 | 61 | F | AML | MUD 10/10 | Peripheral blood | MAC | 1:512 Anti-A | Whole blood 100% | None | 210 | 2 | 2 | Yes | None reported | 383 |
| 6 | 60 | F | AML | MUD 10/10 | Peripheral blood | MAC | 1:64 Anti-A | Whole blood >95% | Rituximab | 460 | 6 | 4 | Yes | None reported | 1045 |
| 7 | 55 | M | AML | MUD 10/10 | Peripheral blood | RIC | 1:512 Anti-A | Whole blood >95% | Rituximab + DLI + BOR | 2898 | 4 | 2 | Yes | None reported | 957 |
| 8 | 45 | F | AML | MUD 10/10 | Peripheral blood | MAC | 1:16 Anti-A + B | T 99% nT 100% | None | 176 | 3 | 2 | Yes | Infuse reaction | 389 |
| 9 | 55 | F | MDS | MUD 10/10 | Peripheral blood | RIC | 1:128 Anti-A | T 96% nT 100% | None | 111 | 6 | 1 | Yes | None reported | 229 |
| 10 | 61 | M | MDS | SIB 10/10 | Peripheral blood | MAC | 1:512 Anti-A | T 99% nT 100% | None | 123 | 4 | 2 | Yes | None reported | 700 |
| 11 | 60 | M | AML | MUD 9/10 | Peripheral blood | RIC | 1:128 Anti-A | Whole blood 100% | DLI | 391 | 1 | 1 | Yes | None reported | 219 |
| 12 | 46 | M | AML | MUD 10/10 | Peripheral blood | RIC | 1:256 Anti-A | Whole blood >95% | None | 136 | 4 | 2 | Yes | None reported | 126 |
| 13 | 45 | M | AML | MUD 10/10 | Peripheral blood | MAC | 1:128 Anti-A | Whole blood > 95% | None | 160 | 4 | 3 | Yes | None reported | 105 |
| 14 | 58 | M | T-cell lymphoma¶ | MUD 10/10 | Peripheral blood | MAC | 1:512 Anti-A | Whole blood 99% | DLI | 123 | 4‡ | - | No | None reported | - |
| No. . | Age . | Sex . | Underlying disease . | Donor type and match grade . | Stem cell source . | Conditioning intensity∗ . | AB0-titer† before treatment . | Donor chimerism . | Prior treatment . | Start daratumumab after SCT (d) . | Total number of doses given (7 day interval‡) . | Number of doses given at reticulocytosis§ . | Iso-hemaglutinnin disappeared . | Reported side-effects . | Ongoing response (d) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 63 | M | AML | MUD 10/10 | Peripheral blood | MAC | 1:16 Anti-A | T 100% nT 100% | Rituximab + DLI | 108 | 2 | 2 | Yes | None reported | 405 |
| 2 | 56 | M | MDS | MUD 10/10 | Peripheral blood | MAC | 1:128 Anti-A | T 98% nT 96% | Steroids + Rituximab | 140 | 5‡ | 5 | Yes | PML (death) | 191|| |
| 3 | 63 | M | PMF | MUD 9/10 | Peripheral blood | MAC | 1:32 Anti-B | T 100% nT 100% | Rituximab + DLI | 121 | 2‡ | 2 | Yes | None reported | 294 |
| 4 | 28 | M | Sickle cell disease | SIB 10/10 | Peripheral blood | NMA | 1:32 Anti-B | T 69% nT 100% | Rituximab | 60 | 4 | 3 | Yes | None reported | 456 |
| 5 | 61 | F | AML | MUD 10/10 | Peripheral blood | MAC | 1:512 Anti-A | Whole blood 100% | None | 210 | 2 | 2 | Yes | None reported | 383 |
| 6 | 60 | F | AML | MUD 10/10 | Peripheral blood | MAC | 1:64 Anti-A | Whole blood >95% | Rituximab | 460 | 6 | 4 | Yes | None reported | 1045 |
| 7 | 55 | M | AML | MUD 10/10 | Peripheral blood | RIC | 1:512 Anti-A | Whole blood >95% | Rituximab + DLI + BOR | 2898 | 4 | 2 | Yes | None reported | 957 |
| 8 | 45 | F | AML | MUD 10/10 | Peripheral blood | MAC | 1:16 Anti-A + B | T 99% nT 100% | None | 176 | 3 | 2 | Yes | Infuse reaction | 389 |
| 9 | 55 | F | MDS | MUD 10/10 | Peripheral blood | RIC | 1:128 Anti-A | T 96% nT 100% | None | 111 | 6 | 1 | Yes | None reported | 229 |
| 10 | 61 | M | MDS | SIB 10/10 | Peripheral blood | MAC | 1:512 Anti-A | T 99% nT 100% | None | 123 | 4 | 2 | Yes | None reported | 700 |
| 11 | 60 | M | AML | MUD 9/10 | Peripheral blood | RIC | 1:128 Anti-A | Whole blood 100% | DLI | 391 | 1 | 1 | Yes | None reported | 219 |
| 12 | 46 | M | AML | MUD 10/10 | Peripheral blood | RIC | 1:256 Anti-A | Whole blood >95% | None | 136 | 4 | 2 | Yes | None reported | 126 |
| 13 | 45 | M | AML | MUD 10/10 | Peripheral blood | MAC | 1:128 Anti-A | Whole blood > 95% | None | 160 | 4 | 3 | Yes | None reported | 105 |
| 14 | 58 | M | T-cell lymphoma¶ | MUD 10/10 | Peripheral blood | MAC | 1:512 Anti-A | Whole blood 99% | DLI | 123 | 4‡ | - | No | None reported | - |
AML, acute myeloid leukemia; BOR, bortezomib; DLI, donor lymphocyte infusion; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; MUD, matched unrelated donor; NMA, nonmyeloablative conditioning; PMF, primary myelofibrosis; PML, progressive multifocal leukoencephalopathy; RIC, reduced intensity conditioning; SIB, matched related donor; T/nT, T cell/non–T-cell chimerism.
For details of the conditioning regimen, see supplemental Table 1.
Highest titer reported, for additional information about Ig subclass see supplemental Table 2.
In 3 patients the dose interval of daratumumab was 14 days.
Reticulocytosis is defined as recovery of normal values of absolute reticulocyte count (>25 × 109/L).
At time of death (day 191), ongoing response.
Hepatosplenic T-cell lymphoma.
Development of ABO titer (blue, left axis) and reticulocytes (red, right axis) related to the number of daratumumab administrations. Reticulocytosis was defined as reticulocyte count of 25 × 109/L. Please refer to supplemental Figure 1 for the development of ABO titer in all patients. • = occurrence of reticulocytosis, n = total number of given daratumumab treatments.
Development of ABO titer (blue, left axis) and reticulocytes (red, right axis) related to the number of daratumumab administrations. Reticulocytosis was defined as reticulocyte count of 25 × 109/L. Please refer to supplemental Figure 1 for the development of ABO titer in all patients. • = occurrence of reticulocytosis, n = total number of given daratumumab treatments.
We conclude that despite the retrospective nature of the study, we could report the largest real-world data cohort with an impressive overall response after a short treatment course. The importance of our observation is emphasized by the to date inconsistent results of other proposed treatment modalities such as rituximab or DLI.3,8 As PRCA can lead to long-lasting dependency on erythrocyte support and subsequent iron overload, it is evident that patients with severe and/or long-lasting PRCA might greatly benefit from a short course of daratumumab. Hereto, it is important to identify patients in whom PRCA will not resolve spontaneously and what is the optimal number of daratumumab administrations. Chimerism analysis of the lymphoid fraction could be used to demonstrate that the persistence of IsoHGs coincides with patient-derived plasma cells and that treatment with daratumumab results in elimination of these populations. In addition, standardization of methods to quantify and monitor IsoHGs is currently lacking,11 as also reflected in this cohort. It is assumed that IgM IsoHGs are the main contributors to PRCA,9 as for patient 6 in this study, in which after normalization of the anti-A IgM, the PRCA resolved despite the persistence of anti-A IgG (supplemental Table 2).
In general, transplantation with a major ABO antagonism does not result in inferior overall survival.8 Recent publications have shown that most cases of PRCA resolve spontaneously within 3 to 9 months.3,8 However, high (>1:64) or increasing IsoHG titers, high frequency of erythrocyte support, and/or absence of graft-versus-host disease are associated with prolonged duration of PRCA. In addition, patients with PRCA and pancytopenia are at risk of developing poor graft function or secondary graft failure.8 These patients are likely to benefit from early treatment with daratumumab.
CD38 is not only expressed on plasma cells but also on T, B, and natural killer cells, as well as on erythrocytes, neutrophils, and other myeloid subsets.12 Consequently, daratumumab treatment may lead to hypogammaglobulinemia, neutropenia, and lymphopenia, resulting in an increased susceptibility to infections.13 Experience with daratumumab treatment after allo-SCT mainly comes from patients with multiple myeloma, in which studies have shown no remarkable increase in infections or graft-versus-host disease.14,15 Although these results are reassuring, important safety concerns with regard to the development of infections remain unanswered for patients with posttransplant PRCA. Eight out of 14 patients were screened for hypogammaglobulinemia, of which 3 patients had a total IgG <5.0 g/L (data not shown). These patients received pretreatment with rituximab for PRCA, making it likely that multiple factors contributed to hypogammaglobulinemia. None of the patients developed severe bacterial infection. Patient 2 developed progressive multifocal leukoencephalopathy (PML). The first signs of PML started 9 months after allo-SCT, 7 months after rituximab, and 4 months after daratumumab treatment. As PML is only rarely associated with daratumumab and rituximab treatment and the allo-SCT procedure itself contains more well-known risk factors for developing PML, this case cannot link daratumumab treatment directly to the development of PML but neither can exclude a correlation.13
In summary, with the currently available data (14 patients presented here and 10 previously published cases), we propose that there are sufficient data to strongly consider treatment with daratumumab as early as 3 months after allo-SCT for patients with posttransplant PRCA with persisting high IsoHG titers (defined as ≥1:64) combined with a requirement of frequent erythrocyte support or PRCA with concomitant pancytopenia. In 9 of 14 patients, both the development of reticulocytosis and normalization of the AB0-titer were observed after 1 or 2 administrations of daratumumab, suggesting that most patients may only need a short course of daratumumab. However, patients without reticulocytosis and/or persisting IsoHGs are likely to require prolonged treatment. These assumptions need to be tested in larger (“real-world”) cohorts, in which predictors of spontaneous resolution of PRCA as well as the long-term safety profile of daratumumab need to be addressed.
Acknowledgments: Funding for this study was provided by the Dutch Cancer Society 2021-13493 to M.A.d.W. and Stichting Leukemie to M.A.d.W. and J.K.
Contribution: F.W. and C.N. collected data; F.W. and M.A.d.W. analyzed data; M.R., A.E.C.B., E.D., A.v.R., G.v.S., C.L.E.H., P.v.B., M.T.K., and M.A.d.W treated patients and provided data; F.W., E.N., J.K., and M.A.d.W. wrote the manuscript; M.R., A.E.C.B., K.M.K.d.V., and L.M. reviewed the data and manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Moniek de Witte, Department of Hematology, Universitair Medisch Centrum Utrecht, Huispostnummer B02.226, PO Box 85500, 3508 GA Utrecht, The Netherlands; email: m.a.dewitte-7@umcutrecht.nl.
References
Author notes
Data will be available upon reasonable request from the corresponding author, Moniek de Witte (m.a.dewitte@umcutrecht.nl).
The full-text version of this article contains a data supplement.