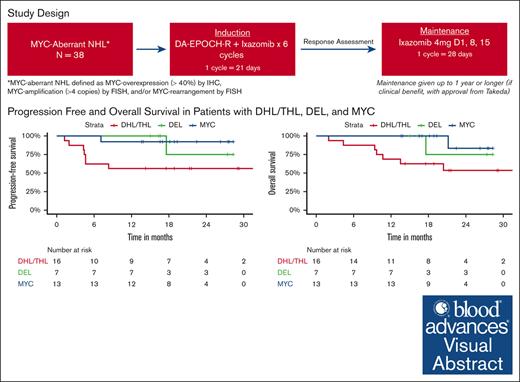
DA-EPOCH-R and adjunctive ixazomib as frontline therapy in aggressive MYC-aberrant NHL is most effective in patients with DEL.
Reasons for early discontinuation of ixazomib included peripheral neuropathy, patient preference, progression, and death.
Visual Abstract
MYC-aberrant non-Hodgkin lymphoma (NHL) is associated with poor outcomes with conventional chemotherapy. Ixazomib is an orally bioavailable proteasome inhibitor that targets drivers of MYC expression and has demonstrated preclinical activity in aggressive MYC-aberrant NHL. We conducted a phase 1/2 study evaluating the safety and efficacy of DA-EPOCH-R with adjunctive ixazomib in aggressive MYC-aberrant NHL. For induction, patients received 6 cycles of DA-EPOCH-R with ixazomib administered twice per 21-day cycle; responders continued weekly ixazomib maintenance for up to 1 year. Primary objectives were to determine the maximum tolerated dose in phase 1 and efficacy of DA-EPOCH-R with ixazomib as measured by 12-month progression-free survival (PFS) rate in phase 2. Thirty-six patients were evaluable for response. Median age was 63 years (range, 31-77) and 44% had double-hit lymphoma (DHL)/triple-hit lymphoma (THL). In phase 1, 3 mg of ixazomib was established as recommended phase 2 dose. Twenty-nine (76.3%) patients completed 6 cycles of DA-EPOCH-R and 25 (65.8%) underwent dose escalations. The ORR after induction was 97% (95% confidence interval, 81-100) with a CR rate of 69%. At median follow-up of 18.8 months, the 12-month PFS and overall survival (OS) rates were 78% and 86%, respectively. For DHL/THL vs dual expressor lymphomas (DEL), 12-month PFS rates were 53% vs 95% and 12-month OS rates were 65% vs 100%, respectively. Grade ≥3 toxicities were predominantly hematologic. Twenty-seven (75%) of patients experienced neuropathy, nearly all low-grade. DA-EPOCH-R induction with adjunctive ixazomib is feasible and appears effective in patients with DEL. This trial was registered at www.clinicaltrials.gov as #NCT02481310.
Introduction
In aggressive B-cell–non-Hodgkin lymphoma (NHL), alterations in a number of oncogenes and tumor suppressor genes have been identified as drivers of malignant transformation and inherent resistance to chemotherapy.1,2 A relatively common culprit is the MYC oncogene, a key regulator of cellular proliferation.2 Translocation of the c-MYC oncogene is the sine qua non mutation associated with Burkitt lymphoma, whereas c-MYC mutations and/or overexpression have been found in as many as one-third of other aggressive B-cell lymphomas including diffuse large B-cell lymphoma (DLBCL).3,4 Deregulation of MYC can result from chromosomal translocation or gene amplification, but it may also occur by transcriptional upregulation downstream of nuclear factor κB (NF-κB) pathway signaling.5-7
In DLBCL, the presence of MYC translocation and/or high MYC protein expression have been associated with an aggressive clinical course and diminished survival in patients treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).3,4,8,9 For those with MYC rearrangement as a sole abnormality or dual overexpression of MYC and BCL-2 proteins, data are conflicting but suggest a trend toward inferior survival.8-10 Long-term survival is less likely for patients with dual translocations of MYC and BCL2 or BCL6.10-12 The unique biology and poor outcomes of aggressive lymphoma with dual or triple translocations has earned it a distinct category in the recent World Health Organization Revision termed “DLBCL/High-Grade B-cell Lymphoma (HGBL) with MYC and BCL2 rearrangements.”13
There are compelling large retrospective data and several small prospective studies that suggest the dose-intensified regimen dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R) is beneficial in patients with MYC-aberrant aggressive B-cell NHLs including Burkitt and MYC-aberrant DLBCL.11,12,14 However, the advantage of dose intensification for MYC-aberrant DLBCL over R-CHOP has yet to be borne out in prospective randomized studies. Most recently, the Cancer and Leukemia Group B (CALGB) 50303 trial compared R-CHOP with DA-EPOCH-R in unselected patients with DLBCL. Although this trial showed lack of improvement in event-free survival as a primary end point and overall survival (OS), the trial was not powered to address differences in MYC-aberrant or dual expressor lymphomas (DEL) patient subsets.15 Moreover, outcomes with R-CHOP clearly remain suboptimal in these patients. Therefore, DA-EPOCH-R remains a consideration in clinical practice and the backbone for recent and ongoing trials seeking to augment the effects of chemoimmunotherapy in MYC-aberrant B-cell NHL (NCT03984448).16 Although clinical studies aim to improve outcomes for this population are needed, it is equally important to acknowledge the risk of increased toxicity when additional agents are combined to chemotherapy.17
Ixazomib is an orally bioavailable, potent, reversible, and specific inhibitor of the 20S proteasome that has demonstrated antitumor activity in preclinical NHL models and in early-phase trials for relapsed/refractory NHL as monotherapy.18-21 Ixazomib targets drivers of MYC expression such as CHK1, an important regulator of MYC expression, leading to dual inhibition of CHK1 and MYC resulting in synergistic cell death.18 Preclinical studies have also demonstrated that ixazomib induces apoptosis through activation of checkpoint kinase 2 signaling in DLBCL models, which included DLBCL-GCB and double-hit lymphoma (DHL) subtypes.7,18-20
Proteasome inhibitors, such as ixazomib, inhibit the NF-κB pathway by inhibiting the degradation of IκB, an inhibitor of NF-κB, ultimately preventing NF-κB from translocating to the nucleus. Given that constitutive activation of the NF-κB pathway is characteristic of the activated B-cell (ABC) subtype of DLBCL, early-phase clinical studies evaluated the efficacy of adding bortezomib to chemotherapy in R/R DLBCL. The authors reported improved responses in only the ABC subtype of DLBCL, suggesting its use as a potential therapeutic target requiring further exploration.22
Similarly, the REMoDL-B study was a phase 3 randomized controlled trial, investigating the role of adding bortezomib, a reversible proteasome inhibitor, to R-CHOP in the first-line treatment of DLBCL, stratified by molecular subtype. Although no overall benefit was reported in the recently published 5-year follow-up, the addition of bortezomib demonstrated differential efficacy with improvements in both progression-free survival (PFS) and OS in the ABC subtype of DLBCL. Furthermore, the authors highlighted the beneficial role of proteasomal inhibition in patients with MYC aberrancies. Those with high MYC and BCL2 mRNA showed an increase in PFS with RB-CHOP (hazard ratio, 0.61; 95% confidence interval [CI], 0.39-0.96).21,23,24
Collectively, this provides sound mechanistic rationale for its use as an adjunct to chemotherapy in MYC-driven malignancies. We hypothesized that the combination of DA-EPOCH-R plus ixazomib would be safe and effective, defined as an improvement in 1-year PFS by ∼20%, in patients with MYC-associated lymphoma.
Methods
This was an investigator-initiated multicenter, single-arm phase 1/2 study with patients enrolled between October 2015 and September 2019. Patients with untreated, MYC-aberrant aggressive B-cell NHL defined as MYC-overexpression (≥40%) by immunohistochemistry, MYC-amplification (>4 copies) as determined by fluorescence in situ hybridization (FISH), or MYC rearrangement as determined by FISH, were eligible.
Allowable histologies included DLBCL (including transformation from a previously indolent NHL, so long as no prior systemic treatment was given for the indolent NHL), B-cell lymphoma, unclassifiable, Burkitt lymphoma, or MYC+ plasmablastic lymphoma.
DHL were defined by MYC and BCL-2 and/or BCL-6 positivity by FISH and triple-hit lymphoma (THL) was defined by MYC and BCL-2 and BCL-6 positivity by FISH. DEL were defined by ≥40% MYC staining and ≥70% staining for BCL2 by immunohistochemistry without associated gene rearrangements. Eligible patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status ≥2, be ≥18 years of age, and have adequate liver, kidney, and bone marrow function. Patients with HIV were included provided they had no other AIDS-defining illness apart from lymphoma, a CD4 count of >350/mm3 and an undetectable viral load. Patients with known central nervous system (CNS) involvement or who were pregnant/nursing were excluded.
This study was performed in accordance with the Declaration of Helsinki with approval from the institutional review board at each site. Study drug and funding for this clinical trial was provided by Takeda Pharmaceuticals.
Treatment and response assessment
During induction, ixazomib was administered in combination with DA-EPOCH-R. Dose adjusted EPOCH-R followed established protocols.15 Ixazomib was administered twice per 21-day cycle on day 1 and either on day 8 or 15 depending on whether treatment criteria (absolute neutrophil count ≥1000/mm3, platelet ≥75 103/µL, and resolution of nonhematologic toxicity to ≤grade 1) were met. All patients received growth factor support with either filgrastim or peg-filgrastim within 72 hours of completion of each cycle of DA-EPOCH-R. CNS prophylaxis with intrathecal methotrexate for a minimum of 4 doses was encouraged but not required.
Responses were assessed after cycle 2 and after completion of induction therapy (before starting maintenance) and then at the discretion of the treating provider. Response was categorized as stable disease (SD), partial response (PR), or complete response (CR) by computed tomography (CT) or positron emission tomography-CT (PET-CT) according to the 2007 Revised Response Criteria for Malignant Lymphoma (modified).25 Patients were evaluable for a response if they completed at least 2 cycles of induction therapy and underwent a subsequent disease response assessment.
In patients with a response (either complete or partial response) and not then treated with consolidative hematopoietic cell transplant, ixazomib was continued as maintenance at a dose of 4 mg weekly for up to 1 year. Of note, rituximab was omitted in patients with plasmablastic lymphoma as these are uniformly CD20-negative.
Objectives
The primary objective of the phase 1 portion of this study was to evaluate the safety of ixazomib combined with DA-EPOCH in patients with aggressive MYC-aberrant lymphoid malignancies and to determine the recommended phase 2 dose (RP2D) of the combination as determined by the maximum tolerated dose (MTD).
The primary objective of the phase 2 portion was to evaluate the efficacy, as measured by 12-month PFS, of ixazomib given with DA-EPOCH-R in patients with aggressive, MYC-aberrant lymphoid malignancies. The 12-month PFS was defined as the percentage of patients alive and progression-free 12 months from start of therapy with censoring of patients who did not respond to follow-up.
Secondary objectives were extended to patients from phase 1 and phase 2 cohorts combined and included numerous factors: evaluation of frequency and severity of toxicity, evaluation of clinical efficacy as measured by response rate by CT of PET/CT, and OS.
Statistical design and toxicity assessment
During phase 1, an accelerated titration design was used to determine the MTD of ixazomib (doses of 2.3, 3, or 4 mg) in combination with DA-EPOCH-R. With the accelerated titration design, single-subject cohorts were enrolled until the MTD was encountered, after which expansion to 3-subject cohorts were undertaken. The National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03) was used to grade adverse events. The MTD was determined by the occurrence of dose-limiting toxicity (DLT), defined as any grade 3 or higher toxicity (using Clinical Terminology Criteria for Adverse Events v 4.03), except cytopenias, within the first cycle (3 weeks or 21 days) of study treatment. However, grade 5 cytopenias were also considered DLTs during the first cycle. There was no intrapatient dose escalation. The MTD from phase 1 was equated to RP2D and established as a dose of 3 mg of ixazomib given in conjunction with DA-EPOCH-R during induction. For maintenance, ixazomib was dosed at 4 mg weekly.
For the phase 2 portion of the trial, our null hypothesis was that DA-EPOCH-R plus ixazomib would yield no difference in 1-year PFS as compared to that observed in DHL, a rate we estimate at 40% based upon historical controls at the time of study inception.12,26 We felt that the combination of DA-EPOCH-R plus ixazomib would warrant further validation if we could demonstrate improvement in 1-year PFS by ∼20%, from a historical rate of 40% to a rate of 60%.12 We then used an optimal two-stage design to test our null hypothesis that P = .60, in which P represented 1-year PFS rate. We proposed error probability limits of α > 0.05 and β.
Results
Patients and disease characteristics
Demographic and clinical factors for our patients are shown in Table 1. In the phase 2 portion of the trial, 38 patients were enrolled and 36 were evaluable for response. Median age was 63 years (range 31-77) and 26 were male (72%). Histologic subtypes included 28 (78%) patients with DLBCL, 2 (6%) with B-cell lymphoma (unclassifiable), 3 (8%) with Burkitt lymphoma, 2 (6%) with HIV-associated, CD20-negative, plasmablastic lymphoma, and 1 (3%) with other. Seventy-eight percent had advanced stage, 94% had an international prognostic index (IPI) of 2 or higher, 36% had MYC aberrancy as a sole abnormality (either copy gain, or rearrangement, or overexpression), 19% had DEL, 25% had DHL, and 19% had THL.
Demographic and clinical characteristics of patients (N = 36)
| Characteristic . | N (%) . |
|---|---|
| Sex | |
| Female | 10 (27.8%) |
| Male | 26 (72.2%) |
| Age, median (range), y | 62.50 (31-77) |
| Stage | |
| Early stage | 8 (22.2%) |
| Advanced stage | 28 (77.8%) |
| ECOG PS category | |
| 0-1 | 25 (89.3%) |
| 2-3 | 3 (10.7%) |
| Unknown | 8 |
| IPI category | |
| 0-1(low risk) | 2 (5.7%) |
| 2-3 (low intermediate/high intermediate) | 7 (20%) |
| 4-5 (high risk) | 26 (74.3%) |
| Unknown | 1 |
| Histology | |
| DLBCL | 28 (77.8%) |
| B-cell lymphoma (unclassifiable) | 2 (5.6%) |
| Burkitt lymphoma | 3 (8.3%) |
| Plasmablastic lymphoma [both HIV (+)] | 2 (5.6%) |
| Other | 1 (2.8%) |
| MYC aberrancy | |
| DHL∗/THL† | 16 (44.4%) |
| DEL‡ | 7 (19.4%) |
| MYC single abnormality | 13 (36.1%) |
| Characteristic . | N (%) . |
|---|---|
| Sex | |
| Female | 10 (27.8%) |
| Male | 26 (72.2%) |
| Age, median (range), y | 62.50 (31-77) |
| Stage | |
| Early stage | 8 (22.2%) |
| Advanced stage | 28 (77.8%) |
| ECOG PS category | |
| 0-1 | 25 (89.3%) |
| 2-3 | 3 (10.7%) |
| Unknown | 8 |
| IPI category | |
| 0-1(low risk) | 2 (5.7%) |
| 2-3 (low intermediate/high intermediate) | 7 (20%) |
| 4-5 (high risk) | 26 (74.3%) |
| Unknown | 1 |
| Histology | |
| DLBCL | 28 (77.8%) |
| B-cell lymphoma (unclassifiable) | 2 (5.6%) |
| Burkitt lymphoma | 3 (8.3%) |
| Plasmablastic lymphoma [both HIV (+)] | 2 (5.6%) |
| Other | 1 (2.8%) |
| MYC aberrancy | |
| DHL∗/THL† | 16 (44.4%) |
| DEL‡ | 7 (19.4%) |
| MYC single abnormality | 13 (36.1%) |
Double-hit defined by MYC and BCL-2 and/or BCL-6 positivity by FISH.
Triple-hit defined by MYC and BCL-2 and BCL-6 positivity by FISH.
Defined as ≥40% MYC staining and ≥70% staining for BCL2 by immunohistochemistry without associated gene rearrangements.
Treatment and safety data
Treatment course details are shown in Table 2. Based on phase 1 results, 3 mg of ixazomib was chosen as the RP2D for phase 2. The DLT for the 4 mg dose was G3 duodenitis. The median time from diagnosis to first treatment was 14 days (range 0-49). Twenty-nine (76.3%) patients completed all 6 cycles of induction therapy with DA-EPOCH-R and 25 (65.8%) patients were able to undergo dose escalation of DA-EPOCH-R, 12 (31%) achieving a dose level ≥3. Two (5.6%) patients had ixazomib dose reductions during induction and 10 (26.3%) patients had discontinuation of ixazomib during induction. Twenty-one (58.3%) patients received at least 1 cycle of maintenance therapy. One patient received consolidation with autologous hematopoietic cell transplant and therefore did not proceed to maintenance ixazomib.
Treatment course details
| . | N (range) . |
|---|---|
| Median number of cycles during induction | 6 (1-6) |
| N (%) | |
| Patients who completed 6 cycles of DA-EPOCH-R | 29 (76.3) |
| Patients with DA-EPOCH dose escalation | 25 (65.8) |
| Patients with ixazomib dose reduction during induction | 2 (5.3) |
| Patients with discontinuation of ixazomib during induction | 10 (26.3) |
| Patients who received maintenance | 21 (58.3) |
| Patients with ixazomib dose reduction during maintenance | 5 (13.9) |
| Patients with discontinuation of ixazomib during maintenance | 9 (25) |
| N (range) | |
| Median number of cycles during maintenance | 5 (1-13) |
| . | N (range) . |
|---|---|
| Median number of cycles during induction | 6 (1-6) |
| N (%) | |
| Patients who completed 6 cycles of DA-EPOCH-R | 29 (76.3) |
| Patients with DA-EPOCH dose escalation | 25 (65.8) |
| Patients with ixazomib dose reduction during induction | 2 (5.3) |
| Patients with discontinuation of ixazomib during induction | 10 (26.3) |
| Patients who received maintenance | 21 (58.3) |
| Patients with ixazomib dose reduction during maintenance | 5 (13.9) |
| Patients with discontinuation of ixazomib during maintenance | 9 (25) |
| N (range) | |
| Median number of cycles during maintenance | 5 (1-13) |
Reasons for early discontinuation of ixazomib during induction included peripheral neuropathy in 3 (8.3%) and concomitant comorbidities in 3 (8.3%) patients. Early discontinuation of both ixazomib and DA-EPOCH-R during induction included progressive disease (PD) in 1 (2.8%) and death in 2 (5.6%) patients. One patient died of respiratory failure in the setting of sepsis, whereas the other experienced a cardiac arrest.
Treatment-related adverse events by cycles of therapy are noted in Table 3, whereas treatment-related adverse events of interest occurring in ≥ 20% of patients are noted in supplemental Table 1. During induction, the most common toxicities attributed to ixazomib were thrombocytopenia (41.7%), lymphopenia (38.9%), and anemia (36.1%). Furthermore, 7 (19.4%) patients had an infection, 9 (25%) experienced neuropathy and 5 (13.9%) had febrile neutropenia during induction. The majority of reported neuropathy was peripheral sensory neuropathy, which was observed after the first cycle of induction, with a total prevalence of ∼25% before initiation of maintenance therapy (Table 3; supplemental Table 1).
Treatment-related adverse events by cycles of therapy
| Characteristic . | Induction cycle 1, N = 351∗ . | Induction cycle 2, N = 272∗ . | Induction cycle 3, N = 271∗ . | Induction cycle 4, N = 303∗ . | Induction cycle 5, N = 327∗ . | Induction cycle 6, N = 290∗ . | Maintenance cycle 1, N = 99∗ . | Maintenance cycle 2, N = 34∗ . | Maintenance cycle 3, N = 16∗ . | Maintenance cycle 4, N = 9∗ . | Maintenance cycle 5, N = 6∗ . | Maintenance cycle 6, N = 5∗ . | Maintenance cycle 7, N = 5∗ . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AE term | |||||||||||||
| Alanine aminotransferase increased | 11 (3.1%) | 4 (1.5%) | 2 (0.7%) | 1 (0.3%) | 2 (0.6%) | 1 (0.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Anemia | 43 (12%) | 45 (17%) | 46 (17%) | 52 (17%) | 58 (18%) | 54 (19%) | 19 (19%) | 2 (5.9%) | 0 (0%) | 1 (11%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Aspartate aminotransferase increased | 10 (2.8%) | 2 (0.7%) | 0 (0%) | 1 (0.3%) | 1 (0.3%) | 0 (0%) | 1 (1.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (20%) |
| Constipation | 13 (3.7%) | 3 (1.1%) | 3 (1.1%) | 2 (0.7%) | 0 (0%) | 1 (0.3%) | 1 (1.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (20%) |
| Diarrhea | 3 (0.9%) | 1 (0.4%) | 3 (1.1%) | 2 (0.7%) | 0 (0%) | 4 (1.4%) | 2 (2.0%) | 2 (5.9%) | 0 (0%) | 1 (11%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Dizziness | 0 (0%) | 4 (1.5%) | 3 (1.1%) | 1 (0.3%) | 3 (0.9%) | 2 (0.7%) | 2 (2.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Dysgeusia | 3 (0.9%) | 2 (0.7%) | 4 (1.5%) | 1 (0.3%) | 3 (0.9%) | 3 (1.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Fatigue | 7 (2.0%) | 6 (2.2%) | 2 (0.7%) | 3 (1.0%) | 1 (0.3%) | 1 (0.3%) | 4 (4.0%) | 1 (2.9%) | 0 (0%) | 1 (11%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Febrile neutropenia | 4 (1.1%) | 1 (0.4%) | 3 (1.1%) | 0 (0%) | 3 (0.9%) | 3 (1.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) | 0 (0%) | 0 (0%) |
| Hyperglycemia | 11 (3.1%) | 7 (2.6%) | 6 (2.2%) | 3 (1.0%) | 4 (1.2%) | 8 (2.8%) | 1 (1.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hypoalbuminemia | 14 (4.0%) | 12 (4.4%) | 11 (4.1%) | 9 (3.0%) | 13 (4.0%) | 7 (2.4%) | 1 (1.0%) | 0 (0%) | 0 (0%) | 1 (11%) | 0 (0%) | 0 (0%) | 1 (20%) |
| Hypocalcemia | 16 (4.6%) | 9 (3.3%) | 2 (0.7%) | 5 (1.7%) | 8 (2.4%) | 3 (1.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hyponatremia | 7 (2.0%) | 0 (0%) | 2 (0.7%) | 2 (0.7%) | 4 (1.2%) | 2 (0.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (11%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Lymphocyte count decreased | 75 (21%) | 87 (32%) | 88 (32%) | 90 (30%) | 89 (27%) | 77 (27%) | 26 (26%) | 6 (18%) | 3 (19%) | 2 (22%) | 1 (17%) | 0 (0%) | 1 (20%) |
| Mucositis oral | 3 (0.9%) | 2 (0.7%) | 6 (2.2%) | 6 (2.0%) | 14 (4.3%) | 9 (3.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Nausea | 6 (1.7%) | 7 (2.6%) | 2 (0.7%) | 1 (0.3%) | 1 (0.3%) | 2 (0.7%) | 2 (2.0%) | 2 (5.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Neutrophil count decreased | 30 (8.5%) | 14 (5.1%) | 14 (5.2%) | 24 (7.9%) | 27 (8.3%) | 27 (9.3%) | 15 (15%) | 6 (18%) | 3 (19%) | 0 (0%) | 0 (0%) | 1 (20%) | 0 (0%) |
| Peripheral motor neuropathy | 0 (0%) | 0 (0%) | 0 (0%) | 2 (0.7%) | 1 (0.3%) | 1 (0.3%) | 1 (1.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Peripheral sensory neuropathy | 7 (2.0%) | 10 (3.7%) | 7 (2.6%) | 8 (2.6%) | 4 (1.2%) | 2 (0.7%) | 2 (2.0%) | 2 (5.9%) | 2 (13%) | 0 (0%) | 0 (0%) | 1 (20%) | 0 (0%) |
| Platelet count decreased | 45 (13%) | 31 (11%) | 36 (13%) | 51 (17%) | 52 (16%) | 47 (16%) | 5 (5.1%) | 3 (8.8%) | 1 (6.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Vomiting | 3 (0.9%) | 1 (0.4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (3.0%) | 2 (5.9%) | 0 (0%) | 0 (0%) | 1 (17%) | 0 (0%) | 0 (0%) |
| Weight loss | 1 (0.3%) | 4 (1.5%) | 1 (0.4%) | 1 (0.3%) | 1 (0.3%) | 2 (0.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) | 0 (0%) | 0 (0%) |
| White blood cell decreased | 39 (11%) | 20 (7.4%) | 30 (11%) | 38 (13%) | 38 (12%) | 34 (12%) | 14 (14%) | 8 (24%) | 7 (44%) | 2 (22%) | 2 (33%) | 3 (60%) | 1 (20%) |
| Characteristic . | Induction cycle 1, N = 351∗ . | Induction cycle 2, N = 272∗ . | Induction cycle 3, N = 271∗ . | Induction cycle 4, N = 303∗ . | Induction cycle 5, N = 327∗ . | Induction cycle 6, N = 290∗ . | Maintenance cycle 1, N = 99∗ . | Maintenance cycle 2, N = 34∗ . | Maintenance cycle 3, N = 16∗ . | Maintenance cycle 4, N = 9∗ . | Maintenance cycle 5, N = 6∗ . | Maintenance cycle 6, N = 5∗ . | Maintenance cycle 7, N = 5∗ . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AE term | |||||||||||||
| Alanine aminotransferase increased | 11 (3.1%) | 4 (1.5%) | 2 (0.7%) | 1 (0.3%) | 2 (0.6%) | 1 (0.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Anemia | 43 (12%) | 45 (17%) | 46 (17%) | 52 (17%) | 58 (18%) | 54 (19%) | 19 (19%) | 2 (5.9%) | 0 (0%) | 1 (11%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Aspartate aminotransferase increased | 10 (2.8%) | 2 (0.7%) | 0 (0%) | 1 (0.3%) | 1 (0.3%) | 0 (0%) | 1 (1.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (20%) |
| Constipation | 13 (3.7%) | 3 (1.1%) | 3 (1.1%) | 2 (0.7%) | 0 (0%) | 1 (0.3%) | 1 (1.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (20%) |
| Diarrhea | 3 (0.9%) | 1 (0.4%) | 3 (1.1%) | 2 (0.7%) | 0 (0%) | 4 (1.4%) | 2 (2.0%) | 2 (5.9%) | 0 (0%) | 1 (11%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Dizziness | 0 (0%) | 4 (1.5%) | 3 (1.1%) | 1 (0.3%) | 3 (0.9%) | 2 (0.7%) | 2 (2.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Dysgeusia | 3 (0.9%) | 2 (0.7%) | 4 (1.5%) | 1 (0.3%) | 3 (0.9%) | 3 (1.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Fatigue | 7 (2.0%) | 6 (2.2%) | 2 (0.7%) | 3 (1.0%) | 1 (0.3%) | 1 (0.3%) | 4 (4.0%) | 1 (2.9%) | 0 (0%) | 1 (11%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Febrile neutropenia | 4 (1.1%) | 1 (0.4%) | 3 (1.1%) | 0 (0%) | 3 (0.9%) | 3 (1.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) | 0 (0%) | 0 (0%) |
| Hyperglycemia | 11 (3.1%) | 7 (2.6%) | 6 (2.2%) | 3 (1.0%) | 4 (1.2%) | 8 (2.8%) | 1 (1.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hypoalbuminemia | 14 (4.0%) | 12 (4.4%) | 11 (4.1%) | 9 (3.0%) | 13 (4.0%) | 7 (2.4%) | 1 (1.0%) | 0 (0%) | 0 (0%) | 1 (11%) | 0 (0%) | 0 (0%) | 1 (20%) |
| Hypocalcemia | 16 (4.6%) | 9 (3.3%) | 2 (0.7%) | 5 (1.7%) | 8 (2.4%) | 3 (1.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hyponatremia | 7 (2.0%) | 0 (0%) | 2 (0.7%) | 2 (0.7%) | 4 (1.2%) | 2 (0.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (11%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Lymphocyte count decreased | 75 (21%) | 87 (32%) | 88 (32%) | 90 (30%) | 89 (27%) | 77 (27%) | 26 (26%) | 6 (18%) | 3 (19%) | 2 (22%) | 1 (17%) | 0 (0%) | 1 (20%) |
| Mucositis oral | 3 (0.9%) | 2 (0.7%) | 6 (2.2%) | 6 (2.0%) | 14 (4.3%) | 9 (3.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Nausea | 6 (1.7%) | 7 (2.6%) | 2 (0.7%) | 1 (0.3%) | 1 (0.3%) | 2 (0.7%) | 2 (2.0%) | 2 (5.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Neutrophil count decreased | 30 (8.5%) | 14 (5.1%) | 14 (5.2%) | 24 (7.9%) | 27 (8.3%) | 27 (9.3%) | 15 (15%) | 6 (18%) | 3 (19%) | 0 (0%) | 0 (0%) | 1 (20%) | 0 (0%) |
| Peripheral motor neuropathy | 0 (0%) | 0 (0%) | 0 (0%) | 2 (0.7%) | 1 (0.3%) | 1 (0.3%) | 1 (1.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Peripheral sensory neuropathy | 7 (2.0%) | 10 (3.7%) | 7 (2.6%) | 8 (2.6%) | 4 (1.2%) | 2 (0.7%) | 2 (2.0%) | 2 (5.9%) | 2 (13%) | 0 (0%) | 0 (0%) | 1 (20%) | 0 (0%) |
| Platelet count decreased | 45 (13%) | 31 (11%) | 36 (13%) | 51 (17%) | 52 (16%) | 47 (16%) | 5 (5.1%) | 3 (8.8%) | 1 (6.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Vomiting | 3 (0.9%) | 1 (0.4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (3.0%) | 2 (5.9%) | 0 (0%) | 0 (0%) | 1 (17%) | 0 (0%) | 0 (0%) |
| Weight loss | 1 (0.3%) | 4 (1.5%) | 1 (0.4%) | 1 (0.3%) | 1 (0.3%) | 2 (0.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) | 0 (0%) | 0 (0%) |
| White blood cell decreased | 39 (11%) | 20 (7.4%) | 30 (11%) | 38 (13%) | 38 (12%) | 34 (12%) | 14 (14%) | 8 (24%) | 7 (44%) | 2 (22%) | 2 (33%) | 3 (60%) | 1 (20%) |
AE, adverse event; N, total number of events.
n (%).
Of the 21 (58.3%) patients who received maintenance therapy, 5 (13.9%) had ixazomib dose reductions, and 9 (25%) had discontinuation of ixazomib (Table 2). Reasons for early discontinuation during maintenance included neuropathy in 1 (2.8%), patient preference in 3 (8.3%), and PD in 4 (11.1%) patients.
With maintenance, the most common toxicities attributed to ixazomib were thrombocytopenia (55.6%), leukopenia (52.8%), and anemia (52.8%, supplemental Table 1). Notably, 18 (50%) patients experienced neuropathy, all low-grade.
Lastly, 34 (94%) of 36 evaluable patients received intrathecal methotrexate (MTX), including 24 (67%) who received at least 4 doses of intrathecal MTX.
Efficacy
The ORR after induction was 97% (95% CI, 81-100) with an associated CR rate of 69%. Of the 21 patients who received maintenance, 16 had a CR, whereas 5 had a PR before initiating maintenance. Of note, patients could be restaged with either CT scans or PET/CT per physician discretion. With maintenance, 1 patient with PR converted to CR and 1 maintained a PR with ongoing response at last follow-up, thus leading to 17 patients with CR and 4 patients with PR.
At a median follow-up of 19 months (range, 2-43), 7 patients had PD. Five patients died, 2 from PD, 1 from respiratory failure, 1 from sepsis, and 1 from natural causes considered unrelated to study drug. Accordingly, the 12-month PFS and OS rates were 77.8% and 86.1%, respectively. The estimated 24-month PFS and OS rates were 73.7% (95% CI, 60.1-90.4) and 86.1% (95% CI, 75.5-98.2), respectively.
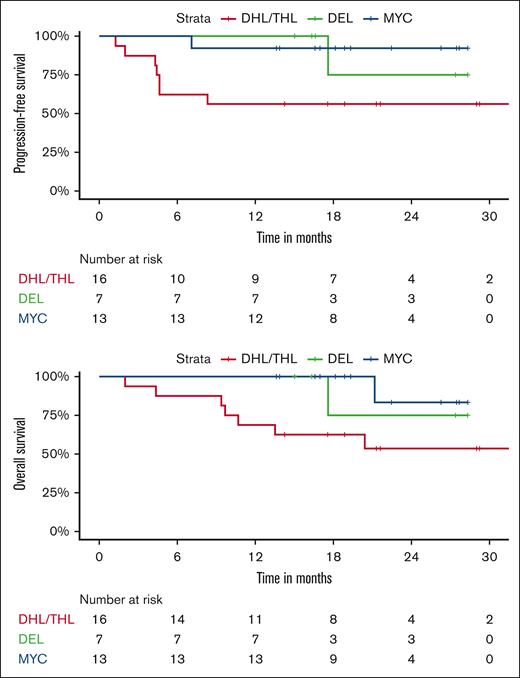
Twelve-month PFS rates were 53% for DHL/THL vs 95% for DEL vs 100% for patients with MYC aberrancy as a sole abnormality. Twelve-month OS rates were 65% for DHL/THL vs 100% for DEL vs 100% for patients with MYC aberrancy as a sole abnormality (Figure 1). All 3 patients with Burkitt lymphoma remain in remission, 2 with greater than 3 years of follow-up. For the 2 patients with plasmablastic lymphoma, 1 remains in remission and 1 progressed 1 month after completion of therapy and died shortly thereafter.
We evaluated sex, age, ECOG performance status, IPI, MYC status, and exposure to maintenance and correlated these parameters with PFS and OS. On univariate analysis, poor ECOG performance status was associated with worse PFS and OS (P < .0001). OS and PFS were also worse in DHL/THL as compared to DEL and patients with MYC aberrancy as a sole abnormality (P = .004 and P = .008, respectively) and in patients who did not receive at least 1 cycle of maintenance ixazomib. Differences between 12-month PFS rates without and with maintenance were not significant, (66.7% vs 80.4%; P = .18). Twelve-month OS rates were 73.3% without maintenance vs 95.2% with maintenance (P = .063). On multivariate analysis (MVA), none of these parameters predicted for worse PFS or OS taken in the context of a small sample size.
Discussion
To date, early data suggest standard R-CHOP remains suboptimal in MYC-aberrant aggressive B-cell lymphomas and in general, support the use of more intensive induction regimens such as DA-EPOCH-R in these patients.11,12 Prospective studies that have proposed intensification of R-CHOP either with chemotherapy or adjunctive agents were designed for all-comers and were not powered to address differences in MYC-aberrant patient subsets.15,27 Clinical data demonstrate that ixazomib exerts antitumor effects in MYC-aberrant lymphomas backed by clear biologic rationale.18-20 At the time of clinical trial design, there existed a large paucity of data regarding the effectiveness of maintenance or consolidative strategies utilizing novel targets in this high-risk population. Collectively, these observations prompted the current investigation of DA-EPOCH-R combined with ixazomib followed by ixazomib maintenance in this poor-risk population.
Historically, 2 year OS rates of 50% in MYC rearranged DLBCL as a sole abnormality, 50% to 60% in DEL lymphomas and 40% to 50% in DHL/THL lymphomas have been reported with R-CHOP.4,8,9,12 Clinical studies have evaluated the efficacy of consolidative therapies in efforts to improve outcomes in high-risk patients with aggressive large B-cell lymphomas.28,29 One large retrospective study evaluated the role of induction R-CHOP followed by consolidative HDT/ASCT in patients with untreated DHL. Of the 58 patients treated with intent for consolidative transplant, the majority received R-CHOP in the upfront setting, and ∼78% ultimately proceeded with high-dose therapy and autologous stem cell transplantation (HDT/ASCT). The study reported favorable outcomes with 4-year OS of 68% in the intention-to-treat population and 88% for those who ultimately underwent HDT/ASCT but also suggests that this intensive approach is not broadly applicable. For DHL/THL, intensification with DA-EPOCH-R has resulted in ORR response rates of ∼70% and an incremental improvement in 2-year OS in large retrospective data sets.11,12
Our results were less favorable in the DHL/THL, likely related to de-escalation and myelosuppression related to adjunctive ixazomib. However, for our patients with DEL, our results compare favorably to historical data achieving 2-year PFS and OS rates both of 100% in this subset. Compared with previous data suggesting a lack of benefit with DA-EPOCH-R over R-CHOP in patients with DEL, our study demonstrated high survival rates in this subgroup population suggesting a possible benefit with the addition of ixazomib.15 This is not surprising as DELs are typically activated B-cell in origin and characterized more than any other subset included in our study by constitutive NF-κB activation for pathogenesis, a primary target of ixazomib.7,30
All 3 of our patients with Burkitt lymphoma remain in clinical remission. For our 2 cases of plasmablastic lymphoma, 1 has remained in CR, whereas the other relapsed and died of disease progression, in keeping with what is typically expected for this extremely aggressive disease. Given the small number of patients, one cannot discern whether there is benefit to incorporating a proteasome inhibitor with dose intensive chemoimmunotherapy for these specific histologies. With 59 months of follow-up, Roschewski et al did show that DA-EPOCH-R alone was highly effective in patients with Burkitt lymphoma with event-free survival (EFS) and OS rates of 84.5% and 87.0%, respectively. By contrast, for plasmablastic lymphoma, intensive chemotherapy remains suboptimal.31 Clinical experience with EPOCH and bortezomib, an alternative proteasome inhibitor that inhibits NF-κB and down-regulates MYC-target genes much like ixazomib would suggest a rational benefit to pursuing the ixazomib DA-EPOCH combination in this population further.32-34
To our knowledge, only 1 other study, where data have matured, has prospectively examined and reported final results for a novel combination in the rituximab era in selected patients with MYC-aberrant lymphoma. This phase 1 study evaluated lenalidomide combined with DA-EPOCH-R followed by lenalidomide maintenance for 12 months in patients with initial response. The study included a small number of patients with DHL and DEL: 15 enrolled and 8 proceeded to maintenance. With a median of 28 months follow-up, 87% remained free of disease progression. Toxicities were mainly hematologic although peripheral neuropathy occurred in 53% of patients. Four patients (27%) developed venous thromboembolism despite low-dose aspirin prophylaxis and 1 patient developed t-MDS which raises concern about the risk/benefit profile of this combination.16
Another prospective phase 1 study evaluated venetoclax in combination with DA-EPOCH-R for aggressive B-cell lymphoma.35 Though not specific to MYC-aberrant lymphomas, the study was enriched for this population and laid the groundwork for the ongoing Alliance trial A051701 designed to evaluate DA-EPOCH-R ± venetoclax in DHL/THL and R-CHOP ± venetoclax in DEL (NCT 03984448). However, closure of the DA-EPOCH-R ± venetoclax arm was forced prematurely given concerning safety signals for the combination including myelosuppression with heightened infection risk. The R-CHOP ± venetoclax arm for DEL remains open and may provide therapeutic guidance for the latter population.
In our study, the most common toxicities associated with combination ixazomib and DA-EPOCH-R were hematologic. Peripheral neuropathy occurred in over 70% of patients, comparable to the over 80% incidence reported with DA-EPOCH-R alone in the CALGB/Alliance 50303 trial.15 Current practice standards, however, implement a 2 mg vincristine dose cap when giving DA-EPOCH-R which has significantly reduced the reported incidence of grade 2+ neuropathy to ∼40%.36 However, only 1 patient on our study incurred higher-grade neuropathy.
Of note, over half our patients were ≥65 years of age. Despite the inclusion of a substantial proportion of older patients, 76% of patients completed all 6 cycles of DA-EPOCH-R, 66% of patients underwent dose escalation of DA-EPOCH-R, and 72% of patients completed a full course of ixazomib during induction. The proportion of patients who completed 6 cycles of DA-EPOCH-R was comparable to the 82% completion rate reported in the CALGB/Alliance 50303 trial but modestly lower than what has been reported in more recent smaller studies.15,16,37 The proportion of patients who underwent dose escalation was similar to that reported in similar trials evaluating adjunctive agents but lower than what was prospectively reported with DA-EPOCH-R alone.14-16,37 Although this may be attributed to the cumulative hematologic toxicity of adjunctive ixazomib, it is also important to note the older age of our population, an independent factor known to be inversely correlated with dose intensification because of decreased hematopoietic reserve and/or decreased drug metabolism.38 Despite these findings, we were still able to demonstrate the feasibility of administering DA-EPOCH-R in combination with ixazomib across all age groups, including the majority of enrolled patients who were ≥65 years old.
The same was not true of maintenance in our study. Only 55% of our patients were able to initiate maintenance therapy and 7 (33%) completed a full 12-month course with equivocal results regarding clinical benefit. Although we demonstrated improved OS in patients who received at least 1 cycle of maintenance therapy on univariate analysis, this signal of benefit was not evident in multivariate analyses, perhaps a function of small sample size. Moreover, maintenance therapy exhibited notable toxicity leading to dose reductions in 5 (14%) of patients and complete discontinuation in 9 (25%) of patients. As a result, the modest adherence to maintenance may have compromised the efficacy of our approach.
Of interest, before and since the inception of our study, several other trials have shown no OS benefit to maintenance strategies in aggressive B-cell lymphomas.39,40 For example, The REMARC trial randomized 650 older patients with DLBCL with chemosensitive disease to maintenance lenalidomide at a dose of 25 mg per day vs placebo for 21 days of a 28-day cycle for 24 months. Although PFS was significantly improved for the patients treated with lenalidomide (59 months vs not reached; P = .01) there was no survival advantage noted, the reasons for which are unclear.39
In conclusion, DA-EPOCH-R and ixazomib therapy is feasible as frontline treatment in patients with MYC-aberrant lymphomas achieving an overall response rate of 97% with an estimated 2-year OS rate of 86%. The benefit of this combination is most evident in patients with DEL. Of note, this regimen did not demonstrate a benefit, with possible detriment, in patients with DHL/THL. Adjunctive ixazomib was feasible in patients of all ages but did result in high rates of peripheral neuropathy. Moreover, adjunctive ixazomib with DA-EPOCH-R was associated with significant rates of febrile neutropenia and infectious complications. The challenge of safely combining novel targeted adjuncts with intensive chemo-immunotherapy for the treatment of aggressive B-cell lymphomas remains a challenge.
We anticipate that longer follow-up with mature survival data will reflect an improved risk to benefit profile in our patients with DEL, a subset of patients otherwise likely to have poor outcomes with current standard treatments. Should this subgroup benefit be confirmed, further large randomized controlled trials will be needed to evaluate whether adjunctive ixazomib, perhaps without maintenance, can effectively improve outcomes in patients with DEL. Given the potentially prohibitive cumulative myelosuppression and neuropathy when paired with a dose-intensified regimen like DA-EPOCH-R, it may be reasonable to evaluate adjunctive ixazomib with R-CHOP, a less toxic regimen found to be noninferior to DA-EPOCH-R in the DEL subgroup population.15
Authorship
Contribution: R.K. and C.G. analyzed data and wrote the manuscript, and all authors performed the research and reviewed the manuscript before submission.
Conflict-of-interest disclosure: R.K. reports serving on advisory boards of BeiGene, Genentech/Roche, AstraZeneca, Miltenyi, Lilly, Calithera, Kite/Gilead, and Bristol Myers Squibb; serving on speakers' bureaus of BeiGene, AstraZeneca, and MorphoSys; and reports institutional research support from BeiGene, Takeda, Calithera, Kite/Gilead, and Bristol Myers Squibb. B.P. received honoraria from Seattle Genetics and Takeda, and is a consultant at BioSecura. The remaining authors declare no competing financial interests.
Correspondence: Reem Karmali, Division of Hematology/Oncology, Northwestern University Feinberg School of Medicine, Suite 850, 676 N St Clair St, Chicago, IL 60611; email: reem.karmali@northwestern.edu.
References
Author notes
Presented at the annual meeting of the American Society of Hematology, 5-8 December 2020, virtual.
Protocol and datasets may be shared upon reasonable request from the corresponding author, Reem Karmali (reem.karmali@northwestern.edu).
The full-text version of this article contains a data supplement.