In this issue of Blood Advances, Wurtzel et al1 report on the critical role of plasma growth factors in the maintenance of constitutive protein translation in platelets. Their findings suggest that plasma-borne components trigger the signaling pathways for initiation of translation, resulting in an altered platelet proteome, which promotes platelet reactivity.
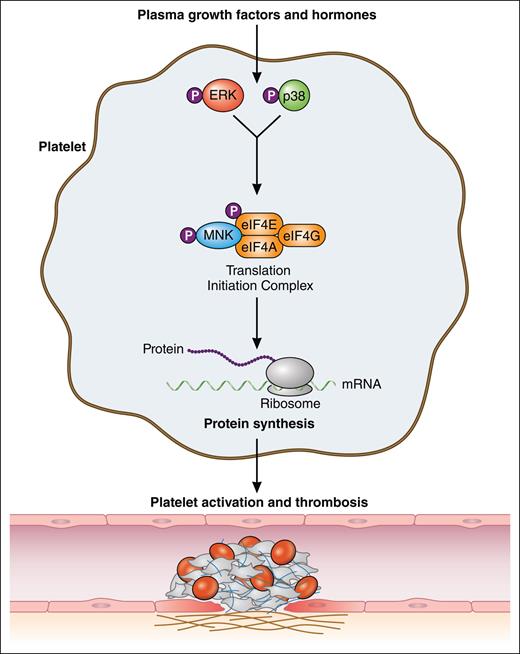
Despite being anucleate, platelets are capable of translating messenger RNA (mRNA) transcripts, largely inherited from megakaryocyte precursors, to synthesize new proteins. Although the concept of de novo protein synthesis by platelets has been known for several decades, most studies characterize this process in the context of platelet activation. Upon agonist stimulation, the platelet proteome undergoes significant changes with clear implications for platelet function (reviewed by Weyrich et al2). De novo protein synthesis generally requires binding of the eukaryotic initiation factor 4F (eIF4F) complex to the 5’ cap of mRNAs. This translation initiation complex consists of eIF4A, eIF4E, and eIF4G (see figure). The activity of eIF4E is regulated via both the mechanistic target of rapamycin and MAPK-interacting kinase (MNK) signaling pathways, both of which have been shown to control protein translation in platelets.3,4 In addition to signal-dependent translation, protein synthesis also occurs constitutively in platelets. Constitutive protein translation in platelets is eIF4E-mediated (as in most other cells) and results in the synthesis of numerous receptors, cargo, and other molecules in platelets.5-7 The molecular requirements and in vivo functional significance of constitutive protein synthesis in platelets remain incompletely understood. Wurtzel et al hypothesized that plasma-borne growth factors and hormones stimulate translation initiation signaling pathways in circulating platelets to maintain proteostasis and regulate platelet reactivity and thrombosis.
Plasma-borne growth factors and hormones drive constitutive translation in platelets through activation of MNK via the ERK/p38 signaling pathway. MNK activates the translation initiation complex, consisting of eukaryotic initiation factors (eIFs) eIF4A, eIF4G, and eIF4E, through phosphorylation of the latter. Plasma-driven constitutive protein synthesis modulates platelet reactivity and contributes to the overall thrombotic potential. Professional illustration by Patrick Lane, ScEYEnce Studios.
Plasma-borne growth factors and hormones drive constitutive translation in platelets through activation of MNK via the ERK/p38 signaling pathway. MNK activates the translation initiation complex, consisting of eukaryotic initiation factors (eIFs) eIF4A, eIF4G, and eIF4E, through phosphorylation of the latter. Plasma-driven constitutive protein synthesis modulates platelet reactivity and contributes to the overall thrombotic potential. Professional illustration by Patrick Lane, ScEYEnce Studios.
Constitutive translation in resting platelets was investigated using bio-orthogonal noncanonical amino acid tagging (BONCAT), a relatively new and innovative technology that metabolically labels newly synthesized proteins. BONCAT uses azidohomoalanine (AHA), which functions as a surrogate for methionine. As a result, AHA is incorporated in newly translating peptides. Through Cu2+-catalyzed click chemistry, the azide group of AHA is tagged in a highly selective manner, which enables the enrichment and visualization of newly synthesized proteins. Therefore, BONCAT represents an elegant, quantifiable technique to more comprehensively investigate the platelet translatome. Using BONCAT, Wurtzel et al demonstrated the necessity of plasma-borne components to support constitutive protein translation ex vivo in human platelets. Translation in platelets ceased gradually upon exposure to starvation buffer but rapidly resumed upon reintroduction to plasma. These findings suggest that nearly all de novo protein synthesis in platelets, even constitutively, is signal dependent in nature. Using western blot analysis for targeted validation assays, the authors demonstrated that plasma growth factors and hormones activate MNK through the p38/ERK pathway, which results in the activation of the translation initiation complex (see figure).
A major strength of the study by Wurtzel et al is the thoughtful use of in vivo murine experiments to complement their findings in ex vivo human platelets. Taking advantage of the BONCAT technology, the authors demonstrated the in vivo relevance of constitutive protein synthesis in circulating murine platelets. Not only did they visualize a robust translatome upon IV labeling with AHA, but they also enriched the newly translated proteins from the platelets for mass spectrometry analysis, confirming significant changes in numerous cytosolic signaling pathways. Additionally, the authors investigated the role of constitutive translation in platelet reactivity and overall thrombotic potential. IV administration of translation inhibitors (cycloheximide and puromycin) suppressed G protein–coupled receptor (GPCR)-stimulated platelet activation by blocking adenosine diphosphate–induced thromboxane A2 generation. The impairment of platelet reactivity in puromycin-treated mice expectedly also influenced hemostasis and thrombosis. Interestingly, puromycin-driven inhibition of translation was significantly protective in various platelet-dependent thrombosis models but had a much more modest effect on injury-induced bleeding. The authors also nicely demonstrated that the phenotype observed in thrombosis models is platelet specific by using adoptive platelet transfer to recapitulate their results. Finally, Wurtzel et al used the laser injury model in cremaster muscle arterioles to monitor platelet dynamics during thrombosis. The decreased thrombus size upon puromycin treatment was attributed to decreased platelet recruitment to the outer shell of the thrombus as the composition of the hemostatic core remained unaltered. Given the importance of GPCRs during platelet recruitment into a growing thrombus,8 these findings suggest that constitutive translation in platelets is indispensable for the growth and stabilization of large occlusive thrombi during thrombosis.
To confirm that the plasma-borne components supporting constitutive translation in platelets are actually growth factors, Wurtzel et al re-exposed starved platelet to purified growth factors and hormones. The authors studied 7 purified plasma growth factors and elucidated their relative contribution to activation of translation signaling pathways. Future studies may broaden these targeted investigations to further elucidate the relative contribution of other plasma growth factors and hormones to constitutive translation in platelets. Determining which growth factors most potently trigger translation initiation pathways could fill current knowledge gaps in how inflammatory diseases alter platelet functional responses. For example, many diseases in which either bleeding or thrombosis are increased are characterized by increased plasma levels of growth factors. It is therefore interesting to speculate whether disease-associated increases in plasma growth factors and hormones would upregulate translational pathways in platelets, leading to platelet activation and a heightened risk of thrombosis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.