Circulating, resting platelets maintain proteostasis by constitutive translation driven by plasma growth factors and hormones.
Constitutive protein synthesis is required for platelet reactivity and regulates the balance of hemostasis and thrombotic potential.
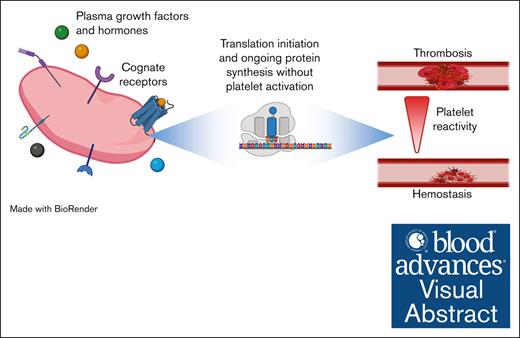
Visual Abstract
Mechanisms of proteostasis in anucleate circulating platelets are unknown and may regulate platelet function. We investigated the hypothesis that plasma–borne growth factors/hormones (GFHs) maintain constitutive translation in circulating platelets to facilitate reactivity. Bio-orthogonal noncanonical amino acid tagging (BONCAT) coupled with liquid chromatography–tandem mass spectrometry analysis revealed constitutive translation of a broad-spectrum translatome in human platelets dependent upon plasma or GFH exposure, and in murine circulation. Freshly isolated platelets from plasma showed homeostatic activation of translation-initiation signaling pathways: phosphorylation of p38/ERK upstream kinases, essential intermediate MNK1/2, and effectors eIF4E/4E-BP1. Plasma starvation led to loss of pathway phosphorylation, but it was fully restored with 5-minute stimulation by plasma or GFHs. Cycloheximide or puromycin infusion suppressed ex vivo platelet GpIIb/IIIa activation and P-selectin exposure with low thrombin concentrations and low-to-saturating concentrations of adenosine 5′-diphosphate (ADP) or thromboxane analog but not convulxin. ADP–induced thromboxane generation was blunted by translation inhibition, and secondary-wave aggregation was inhibited in a thromboxane-dependent manner. Intravenously administered puromycin reduced injury-induced clot size in cremaster muscle arterioles, and delayed primary hemostasis after tail tip amputation but did not delay neither final hemostasis after subsequent rebleeds, nor final hemostasis after jugular vein puncture. In contrast, these mice were protected from injury-induced arterial thrombosis and thrombin-induced pulmonary thromboembolism (PE), and adoptive transfer of translation-inhibited platelets into untreated mice inhibited arterial thrombosis and PE. Thus, constitutive plasma GFH-driven translation regulates platelet G protein–coupled receptor reactivity to balance hemostasis and thrombotic potential.
Introduction
The ability of anucleate platelets to translate nascent protein from their inherited messenger RNAs (mRNAs) was first demonstrated in 1967, and in 1987, translation was reported at elevated levels in immature platelets from patients with immune thrombocytopenia.1,2 Later studies supported these findings, but most groups have described that translation quickly ceases in ex vivo platelets, leading to a general presumption that platelet translation is not robust and, thus, of limited biological significance.3-7 More substantial effort has gone into characterizing translation after agonist stimulation of protease-activated receptors, collagen receptors, and other initiators of platelet “activation” pathways leading to conversion of inactive fibrinogen-binding integrins to the active high-affinity state, and degranulation; recent profiling studies confirmed >6000 inherited mRNAs in polysomes and association with inducible translation of cognate protein products in thrombin-stimulated human platelets.3,8-12 However, relevance of postactivation translation, a slow, rate-limiting step in cell response, to rapid hemostasis, is not clear. Surprisingly little attention has been given to translation in so-called “resting” platelets in circulation, despite the fact that platelets express the requisite components and show broad ribosome occupancy of mRNAs under resting conditions.3,13 Rosenwald et al were the first to show that ex vivo platelets in platelet-rich plasma require ongoing synthesis for reactivity, and Devine et al later confirmed that stored platelets do, in fact, continue to translate protein (integrins), with implications for reactivity of stored platelets.13,14 Platelets in those studies (and in general storage practice) were kept in plasma, in contrast to prior studies of “resting” platelet translation, in which ex vivo platelets were removed from their native plasma environment, and translation steadily came to a halt. We interpret these prior studies to suggest, instead, that platelets undergo translation constitutively in the presence of plasma, for example, in circulation, as a result of signaling, and that this expenditure of molecular resources and cellular energetics is critical for platelet proteostasis and functionality. However, roles of translation in reactivity of platelets in blood circulation, and the contributions of the plasma environment, remain unknown.
Platelets express receptors for many growth factors/hormones (GFHs). Plasma-circulating GFHs drive signal pathways for transcription in nucleated cells but also drive pathways initiating protein translation from mRNAs. Translation initiation, the rate-limiting step in translation, requires phosphorylation-driven formation and stabilization of the eIF4F protein complex leading to its binding to the 7-methylguanosine 5′ untranslated region cap in the majority of mRNAs, recruiting 43S ribosomes to initiate translation.15,16 Platelets harbor unspliced mRNAs that can become spliced upon agonist stimulation, and, particularly young platelets, also harbor transfer RNAs, a robust cohort of mature m7G-capped and 3′-polyadenylated mRNAs, and the components and regulators of the eIF4F complex.12,13,17-22 None of these pathways has been investigated in resting platelets exposed to plasma GFHs, and constitutive platelet translation and the regenerating proteome have not been explored. We hypothesized that GFHs in plasma stimulate translation-initiation signaling in circulating, inactive platelets to mediate constitutive protein synthesis, balancing ongoing protein degradation, and that this process regulates platelet reactivity and physiological function.
Methods
Bio-orthogonal noncanonical amino acid (ncAA) tagging
Platelets were incubated with 3 mM azidohomoalanine (AHA; Click Chemistry Tools, Scottsdale, AZ), or mice were given AHA intravenously at 30 mg/kg dissolved in sterile saline solution, for indicated times combined with inhibitors or vehicle (volume to volume). Platelets were collected from whole murine blood or human platelet suspensions, pelleted, lysed, and labeled with biotin-alkyne in the presence of Cu++ and triazole ligand using the Click iT Buffer Kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Proteins were precipitated with 5.5 N perchloric acid, separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and probed with streptavidin-IRDye680 conjugates, as described previously.23
Results
Constitutive translation in resting platelets
We adapted new technologies for metabolic labeling of newly synthesized proteins (NSPs) in human and murine platelets, which support visualization and identification of NSPs. We tested bio-orthogonal ncAA tagging, or BONCAT, in ex vivo platelets using the cell-permeable ncAA AHA, which can be charged onto methionine transfer RNAs and incorporated into nascent polypeptides; to date, no adverse effects have been observed on protein, cellular, developmental, or physiological function.24-26 We treated ex vivo human platelets in platelet-rich plasma with AHA, then isolated total protein and performed Cu++-catalyzed click chemistry reactions to biotinylate AHA-tagged NSPs. Western blot analysis with streptavidin conjugates revealed that plasma-exposed platelets underwent rapid translation of a broad proteome, consistent with prior indications, which furthermore was inhibited with translation inhibitor puromycin (Figure 1A). We hypothesized that this response requires GFH signaling from plasma. Removal of ex vivo platelets from plasma into serum-free defined media (starvation buffer [SB]: Tyrode buffer with 5 mM glucose, EDTA, and amino acids but no plasma proteins or hormones) suppressed translation. However, upon reintroduction of reserved autologous platelet-poor plasma, we observed rapid and robust resumption of translation of a broad-spectrum proteome in plasma-starved platelets, which was inhibited by puromycin, demonstrating that constitutive translation in resting human platelets requires stimulation by plasma factors (Figure 1B). Plasma starvation of platelets per se did not inhibit their ability to undergo enhanced translation after activating agonist stimulation, indicating that platelets can still support this function even in the absence of plasma factors (supplemental Figure 1). To confirm constitutive translation in resting platelets in circulation, we transfused AHA into the tail vein of wild-type mice,24,25 and isolated platelets after 1 hour. BONCAT biotin labeling and detection confirmed that circulating murine platelets undergo constitutive synthesis of a robust translatome, similar to that in ex vivo plasma-exposed human platelets (Figure 1C). As an alternative approach, we took advantage of the ability of puromycin to incorporate into nascent polypeptides during active translation, preventing further translation and resulting in enrichment of truncated nascent proteins. Within 2 hours of intravenous puromycin administration, puromycin–positive truncated polypeptides were observed in platelet lysates, and these polypeptides became further enriched at 4 hours (Figure 1D). Together, these results demonstrate constitutive protein synthesis in resting, circulating platelets.
Plasma-driven broad-spectrum protein synthesis in human platelets. (A) Platelet-rich plasma was treated with vehicle (−) or azidohomoalanine (AHA) in the absence (−) or presence (+) of puromycin (Puro, 10 μg/mL), and platelets were isolated at the indicated times, washed, and lysed. NSPs were labeled with biotin-alkyne and detected with streptavidin conjugate, and membranes were counter-stained with β-actin antibodies, as shown. (B) Washed human white blood cell/red blood cell–depleted platelets were either resuspended in autologous plasma–EDTA (platelet-poor plasma [PPP]; EDTA added to inhibit platelet activation) or SB followed by feedback with AHA and plasma or buffer as indicated, then harvested and processed for NSP detection as described earlier. (C) AHA (0.5 mg/g) with/without Puro (20 mg/kg) was injected into tail veins of wild-type (WT) mice, and blood was extracted after 1 hour for platelet isolation and platelet NSP detection. (D) Puromycin was administered as in panel C in the absence of AHA, platelets were collected at the indicated times, and platelet lysates were subject to immunoblotting with puromycin-specific antibodies. Each panel representative of 3 independent experiments.
Plasma-driven broad-spectrum protein synthesis in human platelets. (A) Platelet-rich plasma was treated with vehicle (−) or azidohomoalanine (AHA) in the absence (−) or presence (+) of puromycin (Puro, 10 μg/mL), and platelets were isolated at the indicated times, washed, and lysed. NSPs were labeled with biotin-alkyne and detected with streptavidin conjugate, and membranes were counter-stained with β-actin antibodies, as shown. (B) Washed human white blood cell/red blood cell–depleted platelets were either resuspended in autologous plasma–EDTA (platelet-poor plasma [PPP]; EDTA added to inhibit platelet activation) or SB followed by feedback with AHA and plasma or buffer as indicated, then harvested and processed for NSP detection as described earlier. (C) AHA (0.5 mg/g) with/without Puro (20 mg/kg) was injected into tail veins of wild-type (WT) mice, and blood was extracted after 1 hour for platelet isolation and platelet NSP detection. (D) Puromycin was administered as in panel C in the absence of AHA, platelets were collected at the indicated times, and platelet lysates were subject to immunoblotting with puromycin-specific antibodies. Each panel representative of 3 independent experiments.
Translation-initiation signaling by plasma GFHs in human platelets
Platelets express the components and regulators of the eIF4F complex, and use eIF4F complex-driven pathways to potentiate translation upon platelet stimulation12,27; however, little is known of their function in circulating platelets, and relationships to GFHs have not been explored. To begin to dissect connections in platelets, we investigated eIF4F complex signaling in human platelets starved in SB, and subsequently, with plasma added back. Consistent with suppression of translation in plasma-starved platelets (Figure 1), key components of the eIF4F complex necessary for cap-binding and ribosome recruitment, eIF4E and 4E-BP1, became steadily dephosphorylated in platelets maintained in SB, as did the requisite eIF4E kinase mitogen-activated protein kinase-interacting protein kinase (MNK), and the MNK cognate upstream kinases extracellular signal-regulated kinase (ERK) and p38 (Figure 2A). Reexposure to autologous platelet-poor plasma rapidly reactivated phosphorylation of eIF4F signaling (Figure 2B).
Translation-initiation signaling by plasma GFHs in human platelets. (A) Human washed platelets were resuspended in SB for the indicated times, and whole-platelet lysates were immunoblotted with antibodies as indicated. p, phosphorylated; t, total. (B) Platelets maintained in SB for 120 minutes, as in panel A, were pelleted by gentle centrifugation and resuspended in autologous platelet-poor plasma for 5 minutes, then pelleted, lysed, and subject to immunoblotting with the indicated antibodies. (C) Platelets were harvested directly from platelet-rich plasma (Fresh), or otherwise resuspended in SB for 120 minutes, then treated as in panel B for 5 minutes with platelet-poor plasma (PPP) or each indicated GFH, then pelleted for immunoblotting with the indicated antibodies. Vascular endothelial growth factor (VEGF), 100 pg/mL; insulin-like growth factor-1 (IGF-1), 200 ng/mL; insulin, 500 pM; EGF, 2 ng/mL; high mobility group box protein 1 (HMGB1), 2 ng/mL; epinephrine (Epi), 500 pM; serotonin (5-hydroxytryptamine [5-HT]), 1 nM. Fold change in phosphorylated (phospho) to total proteins from immunoblots as measured by densitometric analysis are shown to the right ± standard error of the mean, normalized to Fresh (1) and Starved (0). All were significantly increased over starved levels (P < .05) except for the following: IGF-1: p-4E-BP1; Epi: p-ERK, pAKT, p-eIF4E, p-4E-BP1; 5-HT: p-ERK, p-AKT, and p-4E-BP1; n = 5.
Translation-initiation signaling by plasma GFHs in human platelets. (A) Human washed platelets were resuspended in SB for the indicated times, and whole-platelet lysates were immunoblotted with antibodies as indicated. p, phosphorylated; t, total. (B) Platelets maintained in SB for 120 minutes, as in panel A, were pelleted by gentle centrifugation and resuspended in autologous platelet-poor plasma for 5 minutes, then pelleted, lysed, and subject to immunoblotting with the indicated antibodies. (C) Platelets were harvested directly from platelet-rich plasma (Fresh), or otherwise resuspended in SB for 120 minutes, then treated as in panel B for 5 minutes with platelet-poor plasma (PPP) or each indicated GFH, then pelleted for immunoblotting with the indicated antibodies. Vascular endothelial growth factor (VEGF), 100 pg/mL; insulin-like growth factor-1 (IGF-1), 200 ng/mL; insulin, 500 pM; EGF, 2 ng/mL; high mobility group box protein 1 (HMGB1), 2 ng/mL; epinephrine (Epi), 500 pM; serotonin (5-hydroxytryptamine [5-HT]), 1 nM. Fold change in phosphorylated (phospho) to total proteins from immunoblots as measured by densitometric analysis are shown to the right ± standard error of the mean, normalized to Fresh (1) and Starved (0). All were significantly increased over starved levels (P < .05) except for the following: IGF-1: p-4E-BP1; Epi: p-ERK, pAKT, p-eIF4E, p-4E-BP1; 5-HT: p-ERK, p-AKT, and p-4E-BP1; n = 5.
Platelets express a cohort of cognate receptors for GFHs abundant in plasma, several of which are implicated in initiation signaling. Feedback treatment of starved platelets with purified GFHs vascular endothelial growth factor (VEGF), insulin-like growth factor-1 (IGF-1), insulin, epidermal growth factor (EGF), high mobility group box protein 1 (HMGB1), epinephrine, or serotonin (5-hydroxytryptamine), at concentrations reflecting reported mean platelet-rich plasma levels in healthy individuals between meals28 was sufficient to stimulate eIF4F signaling, with partially but incompletely overlapping profiles. For example, treatment with either IGF-1 or insulin was sufficient to induce phosphorylation of each pathway protein tested, whereas epinephrine or 5-hydroxytryptamine each strongly induced p38 phosphorylation but did not induce robust phosphorylation of protein kinase B (AKT) or ERK (Figure 2C). GFH addition to starved platelets did not induce platelet activation (supplemental Figure 2A). Together, these results point to direct induction of translation-initiation signaling in resting platelets by plasma GFHs in the absence of activating stimuli.
Regulation of platelet agonist responses by plasma-driven translation
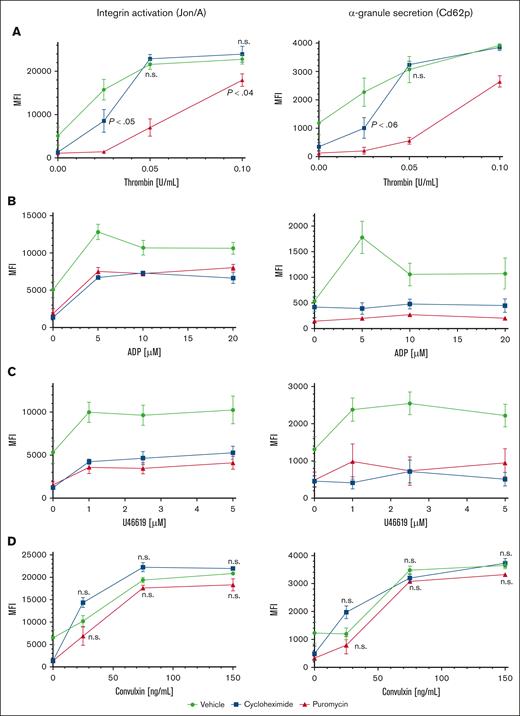
To investigate contributions of proteostasis to platelet function, we first tested agonist responses in human platelets maintained in plasma or in SB and observed modestly reduced platelet reactivity after 120 minutes in SB, and greatly reduced reactivity after 18 hours compared with platelets maintained in plasma (supplemental Figure 2B). However, these observations may reflect generalized degradation of platelet function under these conditions, including, but not limited to, suppressed translation. We considered effects of translation inhibitors on reactivity of ex vivo platelets to agonist stimulation. Although effects of translation inhibitors on platelet activities were tested many years ago, only acute effects were measured in short times, in treated platelets removed from plasma.1,13 We tested agonist responses in washed platelets extracted from mice infused with puromycin or cycloheximide. Integrin αIIbβ3 (GpIIb/IIIa) activation and α-granule secretion were both inhibited in platelets from puromycin- or cycloheximide-treated mice at low concentrations of thrombin compared with platelets from vehicle-treated mice. Activation responses were partially but not fully recovered in platelets from puromycin-treated mice with high concentrations of thrombin, suggesting modulation of overall platelet reactivity by translational suppression. Activation was also modulated with cycloheximide at the lowest thrombin concentration but appeared similar to that in controls at higher concentrations. This result may reflect suboptimal translational suppression, potentially faster drug clearance, or the reversible nature of translation inhibition by cycloheximide, allowing for resumption of translation under these specific treatment conditions (Figure 3A). Responses to ADP and thromboxane A2 analog U46619 were reduced in translation-inhibited platelets (both puromycin and cycloheximide) across a range of concentrations of each agonist including at saturating concentrations (Figure 3B-C). Translation inhibition did not increase surface exposure of phosphatidylserine nor activation of caspase-3, indicating that these treatments did not induce progression to apoptosis in the platelets (supplemental Figure 3). Thus, in vivo blockade of translation modulates platelet activation downstream of G protein–coupled receptor (GPCR) stimulation. In contrast, glycoprotein VI (GpVI)-mediated platelet activation with convulxin was unaltered with either puromycin or cycloheximide (Figure 3D), indicating selective roles for constitutive translation in GPCR but not GpVI receptor responses in platelets. Integrin surface expression in platelets from mice treated with either inhibitor was similar to that of controls (supplemental Figure 4). Together, these results demonstrate modulation but not ablation of platelet reactivity encompassing multiple GPCR agonist response pathways by in vivo translation inhibition.
Modulation of platelet reactivity by suppression of translation in circulating platelets. WT mice were dosed by tail vein injection with vehicle (saline, volume per volume), puromycin (20 mg/kg), or cycloheximide (120 mg/kg), 18 hours and again 4 hours before platelet collection. (A-D) Platelets from treated mice were subject to agonist stimulation as indicated in the presence of fluorophore-conjugated antibodies against activated αIIbβ3 integrin (Jon/A) and Cd62p, and measured by flow cytometry; n = 9. P < .03 compared with vehicle control except if indicated otherwise; other P values are shown compared with vehicle control; n.s., not significant compared with vehicle control; MFI, mean fluorescence intensity, shown ± standard error of the mean. Vehicle, green circles and green lines; puromycin, red triangles and red lines; cycloheximide, blue squares and blue lines.
Modulation of platelet reactivity by suppression of translation in circulating platelets. WT mice were dosed by tail vein injection with vehicle (saline, volume per volume), puromycin (20 mg/kg), or cycloheximide (120 mg/kg), 18 hours and again 4 hours before platelet collection. (A-D) Platelets from treated mice were subject to agonist stimulation as indicated in the presence of fluorophore-conjugated antibodies against activated αIIbβ3 integrin (Jon/A) and Cd62p, and measured by flow cytometry; n = 9. P < .03 compared with vehicle control except if indicated otherwise; other P values are shown compared with vehicle control; n.s., not significant compared with vehicle control; MFI, mean fluorescence intensity, shown ± standard error of the mean. Vehicle, green circles and green lines; puromycin, red triangles and red lines; cycloheximide, blue squares and blue lines.
Translation inhibition suppresses platelet aggregation by inhibition of thromboxane generation
Next, assessed ex vivo agonist-induced aggregation in platelets from treated mice. Whereas platelets from vehicle-treated mice showed the expected 2 waves of aggregation in response to stimulation with ADP, puromycin blunted the secondary wave (Figure 4A), suggesting defects in thromboxane A2 generation or response. In contrast, we did not observe altered convulxin-stimulated aggregation in puromycin-treated platelets (Figure 3B). To dissect the secondary-wave defect, we assessed aggregation with low-dose thrombin in the presence of apyrase, aspirin, or both combined. In the absence of inhibitors, overall aggregation was partially reduced in platelets from puromycin-treated mice compared with platelets from controls. As predicted, addition of apyrase or aspirin alone, or in combination, suppressed aggregation in control platelets. Interestingly, apyrase alone or combined with aspirin completely blocked aggregation induced by low-dose thrombin in translation-inhibited platelets (only shape change was observed) whereas suppressive effects of aspirin alone were similar in control and translation-inhibited platelets (Figure 4C). ADP–induced thromboxane production was blunted by in vivo puromycin treatment (Figure 4D). Together, these results demonstrate that translation inhibition in circulating platelets suppresses GPCR reactivity by blockade of ADP–induced thromboxane generation.
Translation inhibition in vivo suppresses murine platelet ex vivo aggregation by inhibition of thromboxane generation. (A-C) Platelet aggregation in response to agonists as indicated. In panel C, platelets were pretreated with vehicle, apyrase (1 U/mL), and/or aspirin (1 mM) for 30 minutes before stimulation with 0.0025 U/mL thrombin; n = 3; ∗P < .01, ∗∗P < .03. AUC, area under the curve. (D) Washed platelets (2 × 108) from vehicle- or puromycin-treated mice were suspended in 0.4 mL of buffer and stimulated with 10 μM ADP for 3 minutes, and thromboxane B2 in platelet-depleted releasates was measured as described in “Methods”; n = 5. Vehicle, green lines or bars; puromycin, red lines or bars.
Translation inhibition in vivo suppresses murine platelet ex vivo aggregation by inhibition of thromboxane generation. (A-C) Platelet aggregation in response to agonists as indicated. In panel C, platelets were pretreated with vehicle, apyrase (1 U/mL), and/or aspirin (1 mM) for 30 minutes before stimulation with 0.0025 U/mL thrombin; n = 3; ∗P < .01, ∗∗P < .03. AUC, area under the curve. (D) Washed platelets (2 × 108) from vehicle- or puromycin-treated mice were suspended in 0.4 mL of buffer and stimulated with 10 μM ADP for 3 minutes, and thromboxane B2 in platelet-depleted releasates was measured as described in “Methods”; n = 5. Vehicle, green lines or bars; puromycin, red lines or bars.
Contribution of plasma-driven translation in platelets to hemostasis in mice
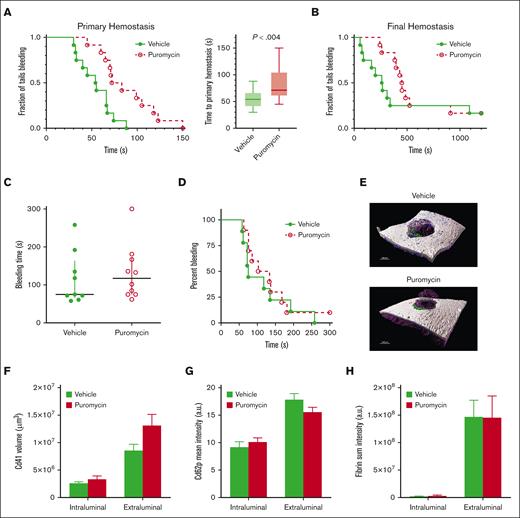
Inhibition of translation in vivo by puromycin in mice led to delayed time to initial occlusion after tail tip amputation, indicating suppression of platelet hemostatic function by blockade of constitutive translation (Figure 5A). However, hemostasis after subsequent rebleeds was not significantly altered, although a trend toward delayed hemostasis was evident (Figure 5B). Hemostasis after 150-μm needle puncture to the jugular vein29-31 was also not altered in puromycin-infused mice compared with that in controls, as measured by total bleeding times, platelet and fibrin deposition, and Cd62p exposure, as well as overall clot architectures (Figure 5C-H). Platelet counts and mean volumes and other blood cell counts were similar between the 2 groups, although a slight reduction in circulating lymphocytes after puromycin treatment was observed (supplemental Figure 5). Puromycin also had no apparent effects on platelet lifespan (supplemental Figure 6). Together, these results indicate that constitutive translation in circulating platelets, although essential for the full capacity of platelet reactivity, is not required for hemostasis in these models.
Inhibition of translation in circulating platelets delays but does not compromise hemostasis in mice. (A) Time to initial stoppage of bleeding after 1-mm diameter tail amputation in C57Bl/6J mice subject to 2 doses of either vehicle or puromycin as in Figure 3. Data are replotted as box and whisker plots to the right. (B) Time to hemostasis after rebleeds in the same mice as in panel A. The experiment was stopped at 1200 seconds; n = 9. P < .28 (not significant) for final hemostasis (log-rank test). (C-H) Bleeding times and clot features were monitored under microscopic observation in C57Bl/6J mice treated as earlier, after 150-μm puncture to the jugular vein; n = 10. (C) Time to hemostasis. The experiment was stopped at 300 seconds. (D) Replots of data from panel C. (E) Representative micrographs of intraluminal sides of injury sites, resected after the experimental end point, showing platelet volumes (Cd41, purple), Cd62p (P-selectin, green), and fibrin (blue); collagen is shown in gray. (F) Cd41 volumes. (G) Cd62p mean intensities. (H) Fibrin mean intensities. (C-H) no significant differences were observed. Vehicle, green closed circles and solid green lines or light green or green bars; puromycin, red open circles and dotted red lines or red bars.
Inhibition of translation in circulating platelets delays but does not compromise hemostasis in mice. (A) Time to initial stoppage of bleeding after 1-mm diameter tail amputation in C57Bl/6J mice subject to 2 doses of either vehicle or puromycin as in Figure 3. Data are replotted as box and whisker plots to the right. (B) Time to hemostasis after rebleeds in the same mice as in panel A. The experiment was stopped at 1200 seconds; n = 9. P < .28 (not significant) for final hemostasis (log-rank test). (C-H) Bleeding times and clot features were monitored under microscopic observation in C57Bl/6J mice treated as earlier, after 150-μm puncture to the jugular vein; n = 10. (C) Time to hemostasis. The experiment was stopped at 300 seconds. (D) Replots of data from panel C. (E) Representative micrographs of intraluminal sides of injury sites, resected after the experimental end point, showing platelet volumes (Cd41, purple), Cd62p (P-selectin, green), and fibrin (blue); collagen is shown in gray. (F) Cd41 volumes. (G) Cd62p mean intensities. (H) Fibrin mean intensities. (C-H) no significant differences were observed. Vehicle, green closed circles and solid green lines or light green or green bars; puromycin, red open circles and dotted red lines or red bars.
Inhibition of thrombosis by blockade of plasma-driven translation via decreased platelet accumulation
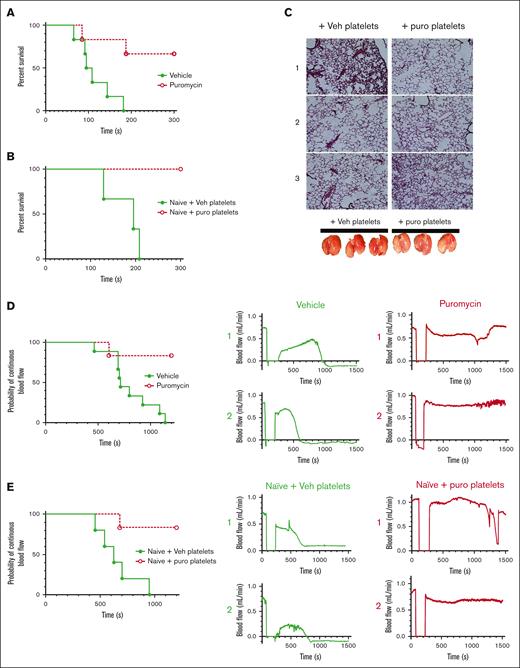
We considered effects of translation inhibition on thrombosis; first, in a model of pulmonary embolism (PE) by assessing time to cessation of respiration after a bolus intravenous injection of thrombin in treated mice.32,33 Whereas vehicle-treated mice ceased respiration within a few minutes, puromycin treatment led to delayed time to cessation of respiration (1 of 6) or outright survival (4 of 6) through the experimental time frame (Figure 6A). Although platelets are the primary cellular drivers of these effects, it is possible that injection of translation inhibitors may affect other contributors, particularly coagulation factors. We tested thrombin generation via extrinsic and intrinsic coagulation pathways in platelet-poor plasma from treated mice and found no significant differences in thrombin generation potential initiated by tissue factor or silica between saline-treated and puromycin-inhibited mice, and only marginal reduction in tissue factor–initiated thrombin generation with cycloheximide, which may reflect hepatotoxicity of this drug (supplemental Figure 7). These results are consistent with limited effects of translation inhibition on hemostasis as noted earlier and suggested a direct role for platelets as the primary drivers of the antithrombotic effects. To determine platelet-specific effects in PE in vivo, we transfused isolated platelets from inhibitor-treated mice into untreated recipient mice, followed immediately by thrombin administration. Whereas mice receiving platelets from vehicle-treated donors ceased respiration within a few minutes, mice receiving platelets from puromycin-treated mice survived throughout the experimental time frame (Figure 6B), and the lungs of these mice, removed after post-PE perfusion with saline, showed reduced thrombi and reduced entrapped blood in the lung vasculature compared with lungs from control mice (Figure 6C). To test contributions of constitutive translation to arterial thrombosis, we subjected vehicle- and puromycin-treated mice to FeCl3 injury to the carotid artery and monitored blood flow and vessel occlusion. Arteries in vehicle-treated mice consistently showed stable occlusion at 801.1 ± 71 seconds after injury. In contrast, arteries in puromycin-treated mice mostly did not occlude (5 of 6). In some cases, we observed a tendency toward occlusion, followed by restoration of blood flow reflecting clot instability (Figure 6D). To test platelet-specific effects of puromycin, we transfused platelets isolated from treated mice into naïve mice, followed by FeCl3 carotid arterial injury in the recipient mice. Whereas occlusive thrombosis occurred within typical time frames in mice receiving platelets from vehicle-treated donors, mice receiving platelets from puromycin-treated donors were protected from thrombosis in this model, in some cases showing clot formation followed by embolization and restoration of blood flow (Figure 6E). These results suggested that puromycin-treated weakly reactive platelets limited thrombus growth, or destabilized growing thrombi in multiple models of thrombosis.
Inhibition of thrombosis by blockade of plasma-driven translation in murine platelets. (A) Time to cessation of respiration in mice treated as indicated, after bolus administration of 900 U/kg thrombin to induce pulmonary thromboembolism; n = 6; P < .01. (B) Naïve mice were transfused with 1 × 108 washed platelets from vehicle- or puromycin-treated mice, and subject to thrombin administration and monitoring as in panel A. Time to cessation of respiration is shown. Veh, vehicle; puro, puromycin. n = 3; P < .03. (C) Representative images of hematoxylin/eosin-stained lung sections from mice from panel B, perfused with saline immediately after the experimental end point, before lung resection. Photographs of the resected lungs before sectioning are shown below. Lungs from mice receiving vehicle-treated platelets appeared blood-filled, indicating thrombosis preventing saline perfusion, whereas lungs from mice receiving puromycin-treated platelets appeared to contain less blood, indicating reduced thrombosis. (D) Carotid arteries in WT mice treated with vehicle or puromycin as indicated were injured with 7.5% FeCl3. Flow rates through the carotid artery downstream of the injury site were monitored with a Doppler probe. Time to cessation of blood flow is shown. Two representative tracings showing blood flow before, during, and after injury per treatment are shown to the right; n = 9 vehicle, n = 6 puromycin; P < .005. (E) WT untreated (naïve) mice transfused with 1 × 108 washed platelets freshly isolated from vehicle- or puromycin-treated mice were subject to FeCl3 carotid arterial injury as in panel D; n = 5 naïve + vehicle platelets, n = 6 naïve + puromycin platelets; P < .006. Vehicle or vehicle platelet-infused, green closed circles and solid green lines; puromycin or puro platelet-infused, red open circles and dotted red lines.
Inhibition of thrombosis by blockade of plasma-driven translation in murine platelets. (A) Time to cessation of respiration in mice treated as indicated, after bolus administration of 900 U/kg thrombin to induce pulmonary thromboembolism; n = 6; P < .01. (B) Naïve mice were transfused with 1 × 108 washed platelets from vehicle- or puromycin-treated mice, and subject to thrombin administration and monitoring as in panel A. Time to cessation of respiration is shown. Veh, vehicle; puro, puromycin. n = 3; P < .03. (C) Representative images of hematoxylin/eosin-stained lung sections from mice from panel B, perfused with saline immediately after the experimental end point, before lung resection. Photographs of the resected lungs before sectioning are shown below. Lungs from mice receiving vehicle-treated platelets appeared blood-filled, indicating thrombosis preventing saline perfusion, whereas lungs from mice receiving puromycin-treated platelets appeared to contain less blood, indicating reduced thrombosis. (D) Carotid arteries in WT mice treated with vehicle or puromycin as indicated were injured with 7.5% FeCl3. Flow rates through the carotid artery downstream of the injury site were monitored with a Doppler probe. Time to cessation of blood flow is shown. Two representative tracings showing blood flow before, during, and after injury per treatment are shown to the right; n = 9 vehicle, n = 6 puromycin; P < .005. (E) WT untreated (naïve) mice transfused with 1 × 108 washed platelets freshly isolated from vehicle- or puromycin-treated mice were subject to FeCl3 carotid arterial injury as in panel D; n = 5 naïve + vehicle platelets, n = 6 naïve + puromycin platelets; P < .006. Vehicle or vehicle platelet-infused, green closed circles and solid green lines; puromycin or puro platelet-infused, red open circles and dotted red lines.
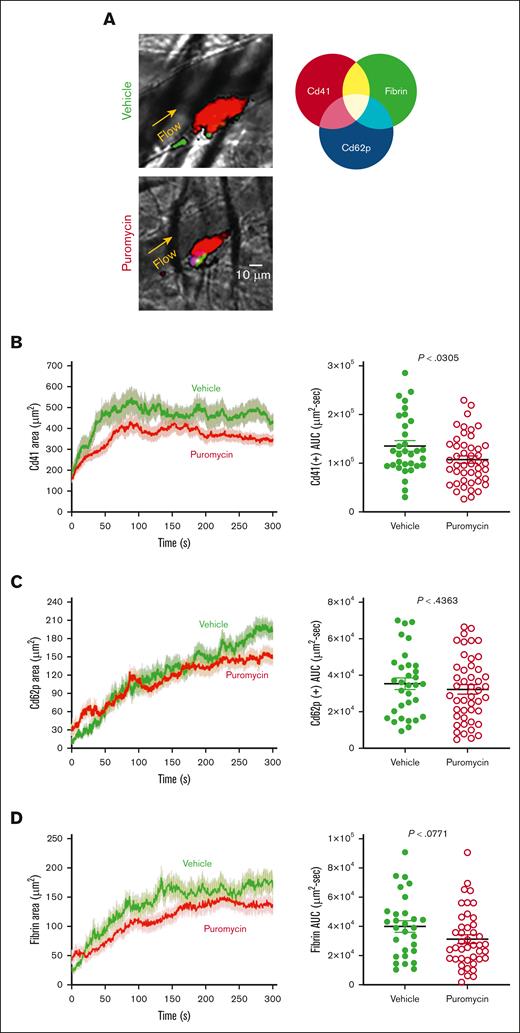
To investigate these possible mechanisms, we monitored platelet recruitment to thrombi and clot dynamics after laser injury in cremaster muscle arterioles in puromycin-treated mice. Thrombus size was decreased in puromycin-treated mice compared with controls, as evidenced by fewer Cd41+ platelets incorporated into the thrombi (Figure 7A-B). However, the hemostatic core of P-selectin–positive platelets was unaltered (Figure 7C). Fibrin deposition, also limited to the core region in this model,34,35 was also not significantly altered by puromycin, although a trend toward decreased fibrin was evident (Figure 7D). Together, these results demonstrate that ongoing, plasma-driven translation in platelets is necessary for growth and/or stabilization of loosely adherent platelets in the outer “shell” region of thrombi. Thus, constitutive translation in peripheral blood platelets is largely dispensable for hemostasis but contributes essentially to the growth and stability of larger occlusive clots leading to thrombosis in mice.
Clot dynamics after laser injury to cremaster muscle arterioles in puromycin-treated mice. (A) Representative images from hemostatic clots formed in vehicle- and puromycin-treated mice, 100 seconds after injury. Cd41, red; Cd62p, blue; fibrin, green. (B) Platelet accumulation, (C) Cd62p exposure, and (D) fibrin deposition were monitored by confocal intravital fluorescence microscopy; 32 injuries in 3 vehicle-treated mice, and 46 injuries in 3 puromycin-treated mice. Graphs in panels B-D shows the AUC for each. The lines and error bars indicate median and interquartile ranges. Vehicle, green lines and green closed circles; puromycin, red lines and red open circles.
Clot dynamics after laser injury to cremaster muscle arterioles in puromycin-treated mice. (A) Representative images from hemostatic clots formed in vehicle- and puromycin-treated mice, 100 seconds after injury. Cd41, red; Cd62p, blue; fibrin, green. (B) Platelet accumulation, (C) Cd62p exposure, and (D) fibrin deposition were monitored by confocal intravital fluorescence microscopy; 32 injuries in 3 vehicle-treated mice, and 46 injuries in 3 puromycin-treated mice. Graphs in panels B-D shows the AUC for each. The lines and error bars indicate median and interquartile ranges. Vehicle, green lines and green closed circles; puromycin, red lines and red open circles.
The constitutive translatome in circulating murine platelets
Next, we sought to map the plasma-driven translatome in circulating, inactive platelets, first by inhibiting translation in platelets in vivo. We predicted that prolonged blockade of translation would result in reduced expression of a broad array of proteins, as a result of lack of protein synthesis against a background of ongoing protein degradation. We selected cycloheximide as the translation inhibitor for these experiments, because puromycin creates truncated peptides that could confound data interpretation. Platelet counts were not altered between the treatment groups (supplemental Figure 8). Normalized leukocyte-/erythrocyte-depleted platelet samples isolated after treatment were lysed and subject to protein mapping by liquid chromatography–tandem mass spectrometry (LC-MS/MS) and analyzed by label-free quantitation of derived peptides. Although we predicted broad changes to the platelet proteome by in vivo translation inhibition, marked primarily by suppression of protein expression, the observed that effects were more subtle. A relatively small number of proteins were significantly suppressed in platelets from cycloheximide-treated mice compared with controls, as indicated by MS/MS results (supplemental Figure 9; supplemental Table 1). As discussed hereafter, we interpret these results to suggest that, despite ongoing translation of many proteins in circulating platelets, platelets may respond to pharmacological blockade of translation by reducing activity of the protein degradation machinery to maintain proteostasis.
Nonetheless, interesting patterns emerged when comparing cycloheximide-treated platelets with those from vehicle controls, including several downregulated proteins of special interest. Gene ontological clustering of the top 100 putatively downregulated proteins by molecular function (supplemental Figure 10A) and cellular process (supplemental Figure 10B) showed concentrations in translation-related functions such as ribosomal RNA binding, mRNA binding, structural constituents of ribosomes, and related designations (supplemental Table 2). Of particular note, we observed strong suppression of mammalian target of rapamycin (mTOR) after translation inhibition. We note that mTOR is a master regulator of translation of so-called 5′ terminal oligopyrimidine (TOP) mRNAs harboring 5′-TOP tracks, which comprise mostly ribosome-associated proteins, subject to additional levels of discriminatory regulation of translation driven by these tracks. We found that 21 of 95 classical mTOR-dependent 5′-TOP genes appeared suppressed in platelets from cycloheximide-treated mice (supplemental Table 1).36 We interpret these data to indicate that reduced expression of these 5′-TOP genes related to ribosome function may stem from reduced mTOR expression by in vivo translation inhibition.
We also noted apparent suppression of Akt2, although the degree of suppression did not reach significance in the MS/MS comparisons (supplemental Table 1; supplemental Figure 9). However, western blot analysis of platelet lysates from translation-inhibited mice revealed significant suppression of both mTOR and Akt2 (supplemental Figure 11), confirming suppressed expression of these proteins by translation inhibition in platelets in vivo. The Akt family including Akt2, has been associated with thromboxane synthesis and essential signaling leading to integrin activation and granule secretion in platelets,37-41 which we observed were reduced in translation-inhibited platelets.
Finally, an even smaller number of proteins appeared to be upregulated in platelets from cycloheximide-treated mice. Although most did not reach significance, one protein, Ces3a, was highly enriched in these platelets but was not detected in control platelets (supplemental Table 1). We note with interest that Ces3a has been reported to be a liver-specific protein,42 and an effect of cycloheximide (in rats) is to induce hepatocyte apoptosis, associated with hepatic toxicity.43 Our results suggest that cycloheximide-induced changes to the observed platelet proteome may also reflect internalized or surface-associated proteins derived from heterologous cells, in this case, potentially from destroyed hepatocytes.
As an alternative approach, we sought to map the plasma-driven translatome in circulating, inactive platelets in vivo, taking advantage of BONCAT labeling with AHA, by comparing proteomes from AHA- and vehicle-treated mice after biotinylation and neutravidin-sepharose affinity chromatography. The overall protein yield after these methods was considerably lower than for the aforementioned analyzed whole-platelet lysate samples, as might be expected with metabolic labeling of nascent proteins only, and sample loss during the click labeling, affinity purification, and protein isolation steps. Nonetheless, the overall pattern of BONCAT-labeled proteins identified by LC-MS/MS, which spanned an array of cellular processes mostly limited to cytosolic signaling mediators, aligned with aforementioned data comparing untreated platelets with translation-inhibited platelets (supplemental Table 3). Altogether, these results demonstrate that the constitutive translatome in circulating murine platelets includes various proteins that, through their combined activities, are necessary for optimal reactivity and physiological function.
Discussion
The results of this study demonstrate that circulating, resting platelets respond acutely to plasma-borne cues by propagating translation-initiation signaling pathways leading to constitutive synthesis of a broad-spectrum translatome, with essential roles in platelet function. The induction of translation-initiation signaling as a GFH response provides both a mechanistic basis for maintenance of proteostasis in anucleate circulating platelets, and a point of regulation with implications for disease. Imbalance in GFH homeostasis is a common feature of many disease states and aging, which can modulate platelet reactivity. In most cases increased GFHs have been associated with hyperreactivity, thrombosis, and thromboinflammation, whereas decreased GFHs have been associated with dysfunctional hemostasis, and, in some cases, GFH inhibition resulted in lower platelet receptor expression.44-58 Circulating EGF, which increases in patients with cancer because of heightened platelet activity but, along with VEGF-A, decreases with aging, primes platelets for hyperreactivity to thrombin via EGFR, its cognate receptor.56,59,60 Thus, release of GFHs stored in platelet granules is itself often associated with GFH imbalance. Similarly, the inflammatory cytokine HMGB1 is associated with platelet reactivity via the RAGE receptor.61 Signaling intermediates responsible for differential phosphorylation responses that we observed in the translation-initiation pathway after isolated GFH treatment are a subject for future investigation. Platelet reactivity effects have also been described in some cases to reflect in part metabolic or genetic programming in megakaryocytes; further investigation of megakaryocyte responses to GFHs related to platelet priming are warranted.50,53,62-65 Moreover, increased platelet reactivity is associated with viral infections, and increased eIF4F activation was recently shown in platelets of patients with COVID-19.27,66-68 Altogether, GFH-driven translation in circulating platelets is an important regulator of platelet reactivity with direct implications for disease, but other plasma-borne factors may also contribute, meriting further study.
Reduced reactivity in platelets from mice treated with translation inhibitors affected GPCR pathways most prominently, resulting from reduced ability to generate thromboxane aligned with suppressed expression of critical signaling intermediates including Akt2 and mTOR. Decreased mTOR expression levels may result from destabilized ribosome/chaperone complexes, for which mTOR is a major client, and may also relate to the apparent decreases that we observed in proteins encoded by mTOR-dependent 5′-TOP genes. However, despite a broad-spectrum translatome that appeared to regenerate constitutively in circulating platelets, as predicted, translation inhibition did not result in a broadly depleted proteome. We interpret these results to indicate that platelets respond to translation inhibition by suppressing degradation, through mechanisms yet to be determined. In addition, we cannot rule out false-negative results driven by limitations of LC-MS/MS in detecting moderate differences in low-abundance proteins. Nonetheless, we observed reduced levels of many proteins, including proteins critical for platelet reactivity. The Akt family has been associated with thromboxane synthesis and platelet reactivity. Akt3-deleted platelets showed very minor deficiencies in thrombin response with more significant deficiency in thromboxane response, whereas Akt1-deleted platelets were deficient in thrombin and collagen response. Akt2 deletion, in contrast, yielded moderately suppressed thrombin response and weakened aggregation, with unstable clot formation, similar to translation-inhibited platelets in our studies. Akt2 appears to be the predominant isoform phosphorylated in platelets after agonist stimulation.37-41 mTOR-deleted platelets harbor mild activation deficiencies to low doses of thrombin or collagen.69 However, we did not observe GpVI defects in translation-inhibited mice, which may reflect limited effects of translation inhibition on GpVI pathway signaling mediators in platelets as assessed by LC-MS/MS (eg, GpVI, Syk, Lat, Plcγ2). It is conceivable that these proteins show less dynamic synthesis and turnover in platelets compared with other signaling proteins; altogether, further investigation is needed to clarify this discrepancy. Mechanisms for specific decrease of Akt2 and not of Akt1/3 also remain to be determined. Thus, suppressed Akt2/mTOR expression provides partial mechanistic explanation for reduced thromboxane generation, and concomitant moderate blockade of GPCR-dependent platelet reactivity and aggregation, after in vivo translation inhibition. Ongoing synthesis of other platelet proteins balancing degradation may be involved in maintaining platelet reactivity in circulation. Additionally, mapping plasma-driven translatomes in disturbed GFH homeostasis will be important to investigate.
Reduced platelet reactivity in translation-inhibited mice correlated with delayed but not fully blocked hemostasis, and inhibition of pulmonary thromboembolism and arterial thrombosis in a platelet-dependent manner. These outcomes were reflected in reduced clot size; specifically, reduced formation or possibly decreased stability of the outer-shell region of loosely adherent platelets dependent on secondary activation responses, whereas the stable thrombus core of highly active, P-selectin–positive platelets was not significantly affected by inhibition of translation.34 These observations underscore our overall finding that ongoing translation in circulating platelets is primarily required for synthesis of proteins important for secondary signaling responses. We observed similar antithrombotic effects with intravenous administration of translation inhibitors as with adoptive transfer of platelets from treated donor mice, pointing to a dominant effect of translation inhibition in platelets as the main driver of those effects. However, we cannot rule out other, undefined alterations to platelets in the donor mice that may be influenced by translation inhibition in other cells. Altogether, a central conclusion from our findings is that constitutive plasma GFH-driven translation regulates platelet GPCR reactivity to balance hemostasis and thrombotic potential.
Although we observed moderate effects of translation blockade on hemostasis in mice, in clinical practice this may yield more robust effects on platelet function with potential for significant outcomes. Inhibition of translation is a widely used strategy in development and current clinical management of many diseases, most prominently malignancies and inflammatory conditions of many types.70-80 Emerging evidence points to inhibition of platelet function by many of these drugs, with associated bleeding complications. Bleeding is a common complication of everolimus (mTOR inhibitor) used in cancer treatment, stent-eluting surgeries, and other interventions, with propensities for severe bleeding events81-87; however, root causes may be manifold. Similarly, eIF4E antisense plus irinotecan showed significant minor bleeding and thrombocytopenia in phase 1/2 trials of solid cancers,88 and eIF4E antisense alone was associated with minor bleeding, thrombocytopenia, and prolonged prothrombin time in phase 1 trials.89 These drugs may modulate hemostasis at multiple levels including thrombopoiesis, and platelet reactivity via prolonged blockade of constitutive translation. MNK inhibitors are currently in development and in trials with unknown effects on hemostasis. Recently, tissue-specific MNK1 deletion in megakaryocytes was shown to result in defective thrombopoiesis as well as suppressed translation in megakaryocytes, although specific effects on translation in platelets could not be separated out.90-92 MNK inhibitors may therefore affect hemostasis related to platelet functional defects.70,72,74-77,93,94 Additionally, inhibitors for GFHs or their receptors have been associated with bleeding and/or thrombosis, as well as endocrine disorders.49,81,95-100 In total, available data indicate a clear need for investigation of the effects of translation inhibition on molecular, cellular, and physiological control of hemostasis, and contributions of translation-dependent platelet reactivity to thrombotic risk.
Acknowledgments
The authors gratefully acknowledge Hsin-Yao Tang and Thomas Beer at the Wistar Proteomics and Metabolomics Facility for LC-MS/MS and bioinformatics analysis.
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants R01HL159006 (L.E.G.), R01HL144574 (P.M.), P01HL139420 (T.J.S.), and P01HL139420, Project 2 (R.M.C.), American Heart Association grant 20TPA35490278 (L.E.G.), and the W.W. Smith Medical Research Award (P.M.).
Authorship
Contribution: J.G.T.W., S.L., S.A., X.Z., J.S., F.A., and J.M.V. performed the experiments; J.G.T.W., S.L., R.M.C., S.E.M., T.J.S., P.M., and L.E.G. designed the experiments and evaluated results; and J.G.T.W., S.L., and L.E.G. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lawrence E. Goldfinger, Division of Hematology, Department of Medicine, Thomas Jefferson University, 1020 Locust St, Philadelphia, PA 19107; email: lawrence.goldfinger@jefferson.edu.
References
Author notes
Data are available on request from the corresponding author, Lawrence E. Goldfinger (lawrence.goldfinger@jefferson.edu). Additional methods and proteomics data may be found in the supplement available with the online version of this article.
The full-text version of this article contains a data supplement.

![Plasma-driven broad-spectrum protein synthesis in human platelets. (A) Platelet-rich plasma was treated with vehicle (−) or azidohomoalanine (AHA) in the absence (−) or presence (+) of puromycin (Puro, 10 μg/mL), and platelets were isolated at the indicated times, washed, and lysed. NSPs were labeled with biotin-alkyne and detected with streptavidin conjugate, and membranes were counter-stained with β-actin antibodies, as shown. (B) Washed human white blood cell/red blood cell–depleted platelets were either resuspended in autologous plasma–EDTA (platelet-poor plasma [PPP]; EDTA added to inhibit platelet activation) or SB followed by feedback with AHA and plasma or buffer as indicated, then harvested and processed for NSP detection as described earlier. (C) AHA (0.5 mg/g) with/without Puro (20 mg/kg) was injected into tail veins of wild-type (WT) mice, and blood was extracted after 1 hour for platelet isolation and platelet NSP detection. (D) Puromycin was administered as in panel C in the absence of AHA, platelets were collected at the indicated times, and platelet lysates were subject to immunoblotting with puromycin-specific antibodies. Each panel representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/6/10.1182_bloodadvances.2023011734/1/m_blooda_adv-2023-011734-gr1.jpeg?Expires=1767705257&Signature=acyHUTxrtibDuVkCMvxb~S9DjT0LZSz--2DyQYakMOor~NgQm9midAB6N46FRWPxY2AF21THW~zto-eEfJwckpcyToHDqOt4E3frvZ4HKKNLQ0B5VOTzdJSaYENZPa-9KuvkFFL71n60b8Y83Dq0Y4crzgROu6uIQsAkta0-FjE1Uqo2jXxRn1FKNUb3eLmRblBTWXG5ueUO1uve-T4svLhf7YIPxOMuFaTdXNnhSBuoBy0xVIM4RwzDkXMk2MdYoA-TKFvCFrSWcfmWmjCg5-IHOiJMOKkCE7~GTaUB2topb7Rk5cNY-Iu5nsI4rNv~7UBV2lTtFedFxanh2Wcbxg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Translation-initiation signaling by plasma GFHs in human platelets. (A) Human washed platelets were resuspended in SB for the indicated times, and whole-platelet lysates were immunoblotted with antibodies as indicated. p, phosphorylated; t, total. (B) Platelets maintained in SB for 120 minutes, as in panel A, were pelleted by gentle centrifugation and resuspended in autologous platelet-poor plasma for 5 minutes, then pelleted, lysed, and subject to immunoblotting with the indicated antibodies. (C) Platelets were harvested directly from platelet-rich plasma (Fresh), or otherwise resuspended in SB for 120 minutes, then treated as in panel B for 5 minutes with platelet-poor plasma (PPP) or each indicated GFH, then pelleted for immunoblotting with the indicated antibodies. Vascular endothelial growth factor (VEGF), 100 pg/mL; insulin-like growth factor-1 (IGF-1), 200 ng/mL; insulin, 500 pM; EGF, 2 ng/mL; high mobility group box protein 1 (HMGB1), 2 ng/mL; epinephrine (Epi), 500 pM; serotonin (5-hydroxytryptamine [5-HT]), 1 nM. Fold change in phosphorylated (phospho) to total proteins from immunoblots as measured by densitometric analysis are shown to the right ± standard error of the mean, normalized to Fresh (1) and Starved (0). All were significantly increased over starved levels (P < .05) except for the following: IGF-1: p-4E-BP1; Epi: p-ERK, pAKT, p-eIF4E, p-4E-BP1; 5-HT: p-ERK, p-AKT, and p-4E-BP1; n = 5.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/6/10.1182_bloodadvances.2023011734/1/m_blooda_adv-2023-011734-gr2.jpeg?Expires=1767705257&Signature=UNnlioWLv~I9g6FJ3dXlDqRXLoj2X6h2w3lP8rW9uQf1NY8ZveZAX~UKbcR1glB0CxgHMKFIdOaQgJ7RaxtrY8QFAG1SS-IX~Jtzf-AFf~cPYEKpMhw7060HYxDDrN4T7HneDdQsnpFkvCO~OoZ5orkno9e1YdSXvhoMc2kdbCqpt~k2TUNEd4P7f~yR0Fssz0qBau90JxU3UAZ7cGOu5kKdO5r8mGR~bctnmb3lW5-~Jj3SdIa0w3RAnQwiF1EmA9W-22WvEOWW4ujYXn1~VFJIdzKxOIoXcNdaiinBrDHtNQ-2hZ2A2EGgqOdvcFxxVh-CQHzRyhJsfMqiEnQIzA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)