In patients with MCL receiving brexucabtagene autoleucel complicated by severe ICANS, 86% of patients demonstrated acute brain MRI findings.
Severe neurotoxicity did not influence progression-free survival or overall survival.
Visual Abstract
CD19 chimeric antigen receptor (CAR) T-cell therapy has proven highly effective for treating relapsed/refractory mantle cell lymphoma (MCL). However, immune effector cell–associated neurotoxicity syndrome (ICANS) remains a significant concern. This study aimed to evaluate the clinical, radiological, and laboratory correlatives associated with ICANS development after CD19 CAR T-cell therapy in patients with MCL. All patients (N = 26) who received standard-of-care brexucabtagene autoleucel until July 2022 at our institution were evaluated. Laboratory and radiographic correlatives including brain magnetic resonance imaging (MRI) and electroencephalogram (EEG) were evaluated to determine the clinical impact of ICANS. Seventeen (65%) patients experienced ICANS after treatment, with a median onset on day 6. Ten (38%) patients experienced severe (grade ≥3) ICANS. All patients with ICANS had antecedent cytokine release syndrome (CRS), but no correlation was observed between ICANS severity and CRS grade. Overall, 92% of EEGs revealed interictal changes; no patients experienced frank seizures because of ICANS. In total, 86% of patients with severe ICANS with postinfusion brain MRIs demonstrated acute neuroimaging findings not seen on pretreatment MRI. Severe ICANS was also associated with higher rates of cytopenia, coagulopathy, increased cumulative steroid exposure, and prolonged hospitalization. However, severe ICANS did not affect treatment outcomes of patients with MCL. Severe ICANS is frequently associated with a range of postinfusion brain MRI changes and abnormal EEG findings. Longer hospitalization was observed in patients with severe ICANS, especially those with abnormal acute MRI or EEG findings, but there was no discernible impact on overall treatment response and survival.
Introduction
Mantle cell lymphoma (MCL) is a rare subtype of non-Hodgkin lymphomas1 with a range of presentations from indolent to aggressive. Treatment options for MCL continue to evolve and now include chemoimmunotherapy, stem cell transplantation, Bruton tyrosine kinase inhibitors, and B-cell lymphoma 2 inhibitors. Despite these advances in therapeutic approaches, relapsed/refractory (r/r) MCL remains an incurable disease for the majority of patients.2 Chimeric antigen receptor (CAR) T-cell therapy has revolutionized the treatment of hematologic malignancies, with several US Food and Drug Administration approvals within the past few years, including brexucabtagene autoleucel (brexu-cel) for r/r MCL in 2020.2-7 In the pivotal ZUMA-2 trial, patients with heavily pretreated r/r MCL achieved durable long-term remissions after CD19 CAR T-cell therapy with a median progression-free survival (PFS) and overall survival (OS) of 25.8 and 46.6 months, respectively.8
Cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS) remain significant toxicities after CAR T-cell therapy, particularly for brexu-cel. Any grade and severe (grade ≥3) ICANS occurred in >60% and >30% of patients with MCL, respectively, after infusion of brexu-cel9,10 and occurs at a higher frequency in MCL than in other B-cell lymphoma subtypes undergoing similar CAR T-cell therapies.3-9 ICANS commonly manifests as a toxic encephalopathy with word-finding difficulty, confusion, aphasia, tremors, and impaired fine motor skills.11
Most of the existing research in ICANS come from patients with large B-cell lymphoma or pediatric B-cell acute lymphoblastic leukemia. Because CAR T-cell therapy neurotoxicity studies in MCL are limited, and particularly given the high rates of ICANS observed in this population, we chose to analyze the clinical and diagnostic features of a real-world single-institution cohort. This investigation aims to address several of these gaps in the field by analyzing clinical characteristics, computed tomography (CT), magnetic resonance imaging (MRI) and electroencephalogram (EEG) results, acute management strategies, and association with other adverse events to determine the clinical and neurological correlates of severe ICANS in MCL. These insights have the potential to improve future CAR T-cell therapy acute care for those with MCL and other hematologic malignancies.
Methods
Study population and data collection
This is a single-center, retrospective observational study that includes all adult patients with r/r MCL who received brexu-cel infusion from October 2020 to July 2022 at Stanford University. Patients with secondary central nervous system (CNS) involvement of MCL, a population usually excluded from clinical trials, are included in this study.9,12-14 Patients were staged with positron emission tomography–CT and brain MRI. After lymphodepleting chemotherapy, a single intravenous infusion of brexu-cel was administered on day 0 at a dose of 2 × 106 CAR+ T cells per kilogram of body weight. CRS and ICANS were managed similarly to ZUMA-1 cohort 4, except patients with persistent grade 1 CRS for >24 hours were given dexamethasone 10 mg once, and patients at the onset of grade 3 ICANS were given anakinra 100 mg subcutaneously every 6 hours. Baseline laboratory data were obtained before starting lymphodepletion chemotherapy. Clinical data, including laboratory data, neuroimaging review, and EEG results, were obtained retrospectively from chart review. CRS and ICANS were graded per American Society for Transplantation and Cellular Therapy consensus criteria.15 All patients, regardless of their serologic status, received prophylaxis for herpes simplex virus and varicella zoster virus from the day before initiation of lymphodepletion chemotherapy, and Pneumocystis jirovecii pneumonia prophylaxis commenced on day 28 and continued for 12 months after CAR T-cell infusion.16 This study was approved by the institutional review board of Stanford University.
Statistical analysis
Group comparison for categorical and continuous variables were performed with Fisher exact or χ2 tests, and Wilcoxon rank sum or Kruskall-Wallis tests, respectively. A log transformation using base 2 (log2) was applied to the Endothelial Activation and Stress Index (EASIX), simplified EASIX, and modified EASIX (mEASIX) scores; and base 10 (log10) was applied to ferritin level to reduce skewness. All tests were 2-tailed, and P < .05 was considered statistically significant. Treatment responses were evaluated according to Lugano response criteria.17 Duration of response (DOR) is calculated from the time of first objective response achievement to the last evaluable disease assessment date or disease progression or death. PFS is defined as the date from CAR T-cell infusion to the time of disease progression or death from any cause. OS is measured from the time of CAR T-cell infusion to the last contact date or death from any cause. Kaplan-Meier curves and log-rank test were used to analyze DOR, PFS, and OS. Nondisease-related mortality is modeled as a competing risk. The Cox proportional hazards model was used to compare the risk of DOR, PFS, and OS between patients with and without severe ICANS. Statistical analyses were performed using R version 4.2.1 (R Core Team, Vienna, Austria, 2022). Plots generated with GraphPad Prism version 9.5.1 (GraphPad Software, San Diego, CA, 2023).
Results
Patient and disease baseline characteristics
A total of 26 patients with r/r MCL, with a median age of 67 years (range, 50-82 years), and a male predominance (73%), who received brexu-cel infusion were evaluated (Table 1). The majority of patients had stage III or IV disease (85%) and had received at least 3 prior lines of therapy (85%). One patient (4%) had a medical history of epilepsy. Three patients (12%) had a history of secondary CNS involvement, and 1 patient (4%) had active CNS involvement at the time of lymphodepletion chemotherapy. All patients except 1 received lymphodepletion chemotherapy with fludarabine 30 mg/m2 and cyclophosphamide 500 mg/m2 daily between days −5 and −3, and the remaining patient received bendamustine 90 mg/m2 on days −4 and −3, because of a fludarabine shortage.18 Ultimately, 9 patients (35%) had no ICANS. ICANS grade 1 or 2 occurred in 7 patients (27%), and ICANS grade 3 occurred in 10 patients (38%). No patients died from direct complications of ICANS, but 1 fatality occurred due to human herpesvirus 6 (HHV-6) encephalitis ∼3 months after infusion. The median age, sex, MCL International Prognostic Index risk group, disease burden (as measured by serum lactate dehydrogenase level) at leukapheresis, high risk disease characteristics, and prior treatment history were similar between all subgroups (Table 1). We did not find any baseline patient, disease, or previous treatment characteristics that significantly associated with severe ICANS. For those patients with a history of CNS or active CNS involvement, only 1 patient developed severe ICANS.
Patient baseline characteristics
| . | ICANS grade 0-2 (n = 16) . | ICANS grade ≥3 (n = 10) . | Univariate P . |
|---|---|---|---|
| Age, median (range), y | 69.5 (50-82) | 64.5 (58-73) | .20 |
| Males, n (%) | 11 (69%) | 8 (80%) | .67 |
| Simplified MIPI intermediate or high risk, n (%) | 12 (75%) | 6 (60%) | .66 |
| MIPIc high-intermediate or high risk, n/total n (%) | 9/15 (60%) | 3/8 (38%) | .40 |
| History of epilepsy | 1 (6%) | 2 (20%) | .54 |
| Disease burden | |||
| Increased LDH at leukapheresis | 9 (56%) | 4 (40%) | .69 |
| Stage III-IV at leukapheresis | 14 (88%) | 8 (80%) | .63 |
| High-risk disease characteristic | |||
| Blastoid or pleomorphic morphologic characteristics of MCL, n (%) | 2 (13%) | 4 (40%) | .17 |
| Ki-67 Proliferation Index score of ≥30%, n/total n (%) | 9/15 (60%) | 4/8 (50%) | .53 |
| Known TP53 mutation or del(17p), n (%) | 2 (13%) | 4 (40%) | .17 |
| History of MCL with CNS involvement | 2 (13%) | 1 (10%) | 1.00 |
| Active MCL with CNS involvement status when starting LD chemotherapy | 1 (6%) | 0 | 1.00 |
| Previous therapies | |||
| Number of prior lines of therapy, median [range] | 4 [2-10] | 3.5 [2-8] | .61 |
| ≥3 prior lines of therapy, n (%) | 15 (94%) | 7 (70%) | .26 |
| Prior autologous SCT | 4 (25%) | 5 (50%) | .23 |
| Prior allogeneic SCT | 2 (13%) | 2 (20%) | 1.00 |
| Prior BTK inhibitor therapy, n (%) | 14 (88%) | 10 (100%) | .51 |
| r/r disease, n (%) | |||
| Refractory to most recent previous therapy | 5 (31%) | 5 (50%) | .42 |
| Relapse after most recent previous therapy | 11 (69%) | 5 (50%) | .42 |
| . | ICANS grade 0-2 (n = 16) . | ICANS grade ≥3 (n = 10) . | Univariate P . |
|---|---|---|---|
| Age, median (range), y | 69.5 (50-82) | 64.5 (58-73) | .20 |
| Males, n (%) | 11 (69%) | 8 (80%) | .67 |
| Simplified MIPI intermediate or high risk, n (%) | 12 (75%) | 6 (60%) | .66 |
| MIPIc high-intermediate or high risk, n/total n (%) | 9/15 (60%) | 3/8 (38%) | .40 |
| History of epilepsy | 1 (6%) | 2 (20%) | .54 |
| Disease burden | |||
| Increased LDH at leukapheresis | 9 (56%) | 4 (40%) | .69 |
| Stage III-IV at leukapheresis | 14 (88%) | 8 (80%) | .63 |
| High-risk disease characteristic | |||
| Blastoid or pleomorphic morphologic characteristics of MCL, n (%) | 2 (13%) | 4 (40%) | .17 |
| Ki-67 Proliferation Index score of ≥30%, n/total n (%) | 9/15 (60%) | 4/8 (50%) | .53 |
| Known TP53 mutation or del(17p), n (%) | 2 (13%) | 4 (40%) | .17 |
| History of MCL with CNS involvement | 2 (13%) | 1 (10%) | 1.00 |
| Active MCL with CNS involvement status when starting LD chemotherapy | 1 (6%) | 0 | 1.00 |
| Previous therapies | |||
| Number of prior lines of therapy, median [range] | 4 [2-10] | 3.5 [2-8] | .61 |
| ≥3 prior lines of therapy, n (%) | 15 (94%) | 7 (70%) | .26 |
| Prior autologous SCT | 4 (25%) | 5 (50%) | .23 |
| Prior allogeneic SCT | 2 (13%) | 2 (20%) | 1.00 |
| Prior BTK inhibitor therapy, n (%) | 14 (88%) | 10 (100%) | .51 |
| r/r disease, n (%) | |||
| Refractory to most recent previous therapy | 5 (31%) | 5 (50%) | .42 |
| Relapse after most recent previous therapy | 11 (69%) | 5 (50%) | .42 |
BTK, Bruton tyrosine kinase; LD, lymphodepletion; LDH, lactate dehydrogenase; MIPI, MCL International Prognostic Index; MIPIc, combined MIPI with Ki-67 Index; SCT, stem cell transplantation.
Antecedent CRS grade does not correlate with ICANS severity
CRS occurred in 25 of 26 patients (96%; Figure 1). The median CRS onset time was day +3 after infusion. All 17 patients who developed neurotoxicity had at least antecedent grade 1 CRS. However, there were no differences observed in the CRS severity, onset day, and duration among patients with different ICANS grades. ICANS occurred concurrently with CRS in 5 patients (29%) and after CRS in 12 patients (71%). The median time from infusion to ICANS onset was 6 days. The ICANS total duration trended toward longer in those who developed severe ICANS (10 vs 4 days, P = .06). One patient with preexisting cognitive impairment due to hemophagocytic lymphohistiocytosis–associated MCL did not significantly recover after developing brexu-cel–associated neurotoxicity after >12 months of follow-up. There were no cases of delayed neurotoxicity noted in the cohort.
Timeline of ICANS after brexu-cel infusion. Colors on the swimmer-lane plot indicate the highest grade of ICANS and CRS recorded on each day through the first 30 days after CAR T-cell infusion. Subgroups were compared using Kruskall-Wallis test.
Timeline of ICANS after brexu-cel infusion. Colors on the swimmer-lane plot indicate the highest grade of ICANS and CRS recorded on each day through the first 30 days after CAR T-cell infusion. Subgroups were compared using Kruskall-Wallis test.
Brain CT and MRI analyses
Of 26 patients, 15 (58%) underwent CT head imaging for grade ≥2 ICANS, with 5 of 26 patients (19%) undergoing CT head imaging on ≥2 occasions to evaluate for cerebral edema or intracranial abnormalities. No acute intracranial abnormalities including cerebral edema were seen by these routine noncontrast CT scans.
In contrast, MRI neuroimaging obtained in 7 patients with persistent neurological deficits demonstrated a high rate of acute imaging changes. The MRIs were performed at a median of 4 days after head CT. Of 7 patients with pretreatment and posttreatment MRI scans, 6 (86%) were found to have changes on MRI neuroimaging; 1 patient with grade 2 ICANS underwent a series follow-up brain MRI for MCL with CNS involvement after ICANS resolution. The patient with grade 3 ICANS shown in Figure 2E had follow-up imaging, which showed persistent white matter T2 hyperintensities 6 months after treatment. Otherwise, additional MRIs after ICANS resolution were not routinely performed if patients were neurologically stable at follow-up. Brain MRI changes during clinical ICANS include alterations across a range of MRI sequences, including diffusion weighted imaging (DWI), T1, T2 fluid attenuated inversion recovery (T2 FLAIR), gradient echo, and arterial spin label (ASL) sequences (Figure 2). One patient who had symptoms of perseveration and decreased speech output, left-right confusion, and left ankle clonus had marked T2 FLAIR hyperintensities in an area of right occipital cortex tissue adjacent to cerebrospinal fluid (CSF) and cortical fullness with additional abnormal T2 hyperintense signal along the right parietal-occipital cortical surface (Figure 2A-B). These imaging findings were asymmetric, affecting right more than left cerebral cortices. Another patient, who had expressive aphasia, decreased attention, and loss of orientation without other focal findings had a brain MRI revealing DWI abnormality that was punctate and in a nonvascular distribution (Figure 2C). In 2 other patients who presented with fluctuating mental status and aphasia, ASL sequences on MRI revealed asymmetric hyperemia in the left cerebral hemisphere (Figure 2D). In contrast, these patients did not have acute changes on T2 and DWI sequences. In 1 patient who had preexisting diffuse white matter lesions and underlying leukoencephalopathy of unknown etiology, the existing imaging abnormalities on T2 FLAIR significantly worsened during acute severe ICANS (Figure 2E). Additionally, he presented clinically with visual obscuration and additional evidence of bilateral optic nerve swelling on funduscopic examination, and supportive DWI hyperintensities were seen in optic nerves on neuroimaging (Figure 2F).
Brain MRI patterns after brexu-cel–associated ICANS. (A) MRI T2 FLAIR sequence showing focal hyperintensity at the right occipital horn. White arrow indicates the area of hyperintensity, visualized across axial (left), coronal (middle), and sagittal (right) cuts. The T2 hyperintense lesion is periventricular and located at the CSF and brain parenchymal border. (B) Additional MRI T2 FLAIR changes seen from patient examined in panel A. There is abnormal T2 hyperintensity and cortical fullness of right hemispheric parietal-occipital gyri. There is 1 area of notable T2 hyperintensity also on the contralateral side seen in the axial image to the right. As arrows indicate, these lesions contour along the meningeal surface of the brain. (C) MRI DWI sequence showing abnormal signal hyperintensity in the posterior right hemisphere. The multipunctate lesion has an atypical appearance at the juxtaposition of 3 cortical gyri and does not follow a specific vascular territory. (D) MRI ASL sequence from 2 patients with ICANS grade 3. Color maps further highlight the asymmetric hyperemia seen in the left hemisphere of both patients. (E) MRI T2 FLAIR sequence from a patient with preexisting white matter disease who then experienced grade 3 ICANS. The extension of white matter T2 hyperintensity increases between baseline before treatment (left column) and after treatment during ICANS (right column). Column images are axial views and arranged from rostral to caudal. (F) MRI DWI sequence from the same patient as in panel E, who experienced acute visual changes during grade 3 ICANS and found to have bilateral optic disk edema. White arrow indicates subtle increase in optic nerve DWI signal, left greater than right, that is seen in the postinfusion axial sequence (right) compared with baseline scan (left).
Brain MRI patterns after brexu-cel–associated ICANS. (A) MRI T2 FLAIR sequence showing focal hyperintensity at the right occipital horn. White arrow indicates the area of hyperintensity, visualized across axial (left), coronal (middle), and sagittal (right) cuts. The T2 hyperintense lesion is periventricular and located at the CSF and brain parenchymal border. (B) Additional MRI T2 FLAIR changes seen from patient examined in panel A. There is abnormal T2 hyperintensity and cortical fullness of right hemispheric parietal-occipital gyri. There is 1 area of notable T2 hyperintensity also on the contralateral side seen in the axial image to the right. As arrows indicate, these lesions contour along the meningeal surface of the brain. (C) MRI DWI sequence showing abnormal signal hyperintensity in the posterior right hemisphere. The multipunctate lesion has an atypical appearance at the juxtaposition of 3 cortical gyri and does not follow a specific vascular territory. (D) MRI ASL sequence from 2 patients with ICANS grade 3. Color maps further highlight the asymmetric hyperemia seen in the left hemisphere of both patients. (E) MRI T2 FLAIR sequence from a patient with preexisting white matter disease who then experienced grade 3 ICANS. The extension of white matter T2 hyperintensity increases between baseline before treatment (left column) and after treatment during ICANS (right column). Column images are axial views and arranged from rostral to caudal. (F) MRI DWI sequence from the same patient as in panel E, who experienced acute visual changes during grade 3 ICANS and found to have bilateral optic disk edema. White arrow indicates subtle increase in optic nerve DWI signal, left greater than right, that is seen in the postinfusion axial sequence (right) compared with baseline scan (left).
EEG analyses
EEGs were obtained for 11 patients during acute ICANS. Twelve of 13 EEGs were continuous studies, with an average recording time of 39 hours. Ten of 11 recordings were from patients with severe ICANS, obtained after clinical worsening of immune effector cell–associated encephalopathy score (Figure 3A). Features of slowing were identified in 8 of 11 (73%) patients, with moderate slowing reported most frequently at 55% of patients, compared with mild (18%) or severe (0%) slowing. Three patients (27%) had normal EEG results. Frequently identified EEG characteristics included generalized rhythmic delta activity, generalized periodic discharges, stimulus-induced rhythmic, periodic or ictal discharges, and triphasic waves (Figure 3A-C). The specific EEG findings from each recorded patient, along with relevant clinical features, are summarized in supplemental Table 1.
EEG after brexu-cel treatment. (A) Study characteristics from 13 total EEG recordings after CAR T-cell therapy. In total, 11 patients were studied. EEG characteristics broken down by occurrence. Characteristics found include: generalized rhythmic delta activity (GRDA); generalized periodic discharges (GPD); stimulus-induced rhythmic, periodic, or ictal discharges (SIRPIDs); and normal. Seizure and status epilepticus were found in 1 patient with HHV-6 encephalitis. (B) and (C) are representative EEG tracings of GPDs and GRDAs from patients with ICANS. In contrast, (D) and (E) show bilateral seizure activity found in 1 patient with HHV-6 encephalitis at >1 month after CAR T-cell therapy. Asterisks (∗) in panel A indicate the EEG data from this patients with HHV-6 encephalitis, with EEG recording performed outside of typical ICANS presentation.
EEG after brexu-cel treatment. (A) Study characteristics from 13 total EEG recordings after CAR T-cell therapy. In total, 11 patients were studied. EEG characteristics broken down by occurrence. Characteristics found include: generalized rhythmic delta activity (GRDA); generalized periodic discharges (GPD); stimulus-induced rhythmic, periodic, or ictal discharges (SIRPIDs); and normal. Seizure and status epilepticus were found in 1 patient with HHV-6 encephalitis. (B) and (C) are representative EEG tracings of GPDs and GRDAs from patients with ICANS. In contrast, (D) and (E) show bilateral seizure activity found in 1 patient with HHV-6 encephalitis at >1 month after CAR T-cell therapy. Asterisks (∗) in panel A indicate the EEG data from this patients with HHV-6 encephalitis, with EEG recording performed outside of typical ICANS presentation.
One patient, who had a history of ICANS grade 1 after CAR T-cell infusion, presented back at the hospital >28 days after CAR T-cell infusion with mental status changes, was found to have MRI brain imaging and infectious workup consistent with HHV-6 encephalitis, because HHV-6 polymerase chain reaction was positive in both CSF and plasma. Because of the HHV-6 infection, she had seizures in bilateral temporal lobes (Figure 3D-E). Apart from the patient with HHV-6 encephalitis, there were no other patients with seizures on EEG. Finally, abnormal EEG and MRI findings were correlated with a trend of longer hospitalization lengths (supplemental Figure 1).
EASIX and CAR-HEMATOTOX scores
Previous studies suggest that mEASIX score before lymphodepletion and early after CAR T-cell infusion may serve as a predictive tool for severe CRS and ICANS before the onset of severe symptoms.19 We calculated the EASIX, simplified EASIX, and mEASIX before lymphodepletion chemotherapy, on day 0, day +1, and day +3 (Figure 4A; supplemental Figure 2). In our cohort, these 3 scores did not correlate with the development of severe ICANS. We also calculated the CAR-HEMATOTOX score20 and found that high-risk (≥2) scores were associated with prolonged neutropenia, a higher incidence of thrombocytopenia, and anemia after CAR T-cell infusion. A high-risk CAR-HEMATOTOX score was associated with a significantly higher proportion of severe ICANS than a low-risk CAR-HEMATOTOX score (Figure 4B).
The EASIX and CAR-HEMATOTOX distribution by ICANS severity. (A) The EASIX, simplified EASIX (sEASIX), and mEASIX score calculated before starting lymphodepletion chemotherapy were similar between the patients with and without severe ICANS. (B) A significantly higher proportion of patients with severe ICANS were categorized in the CAR-HEMATOTOX high-risk group.
The EASIX and CAR-HEMATOTOX distribution by ICANS severity. (A) The EASIX, simplified EASIX (sEASIX), and mEASIX score calculated before starting lymphodepletion chemotherapy were similar between the patients with and without severe ICANS. (B) A significantly higher proportion of patients with severe ICANS were categorized in the CAR-HEMATOTOX high-risk group.
Thirty-day ICANS severity-associated laboratory evaluation
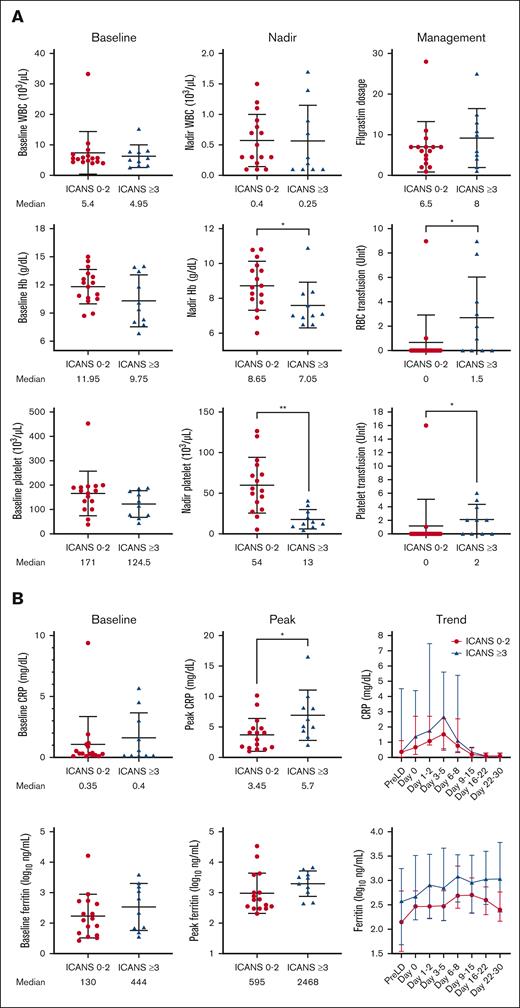
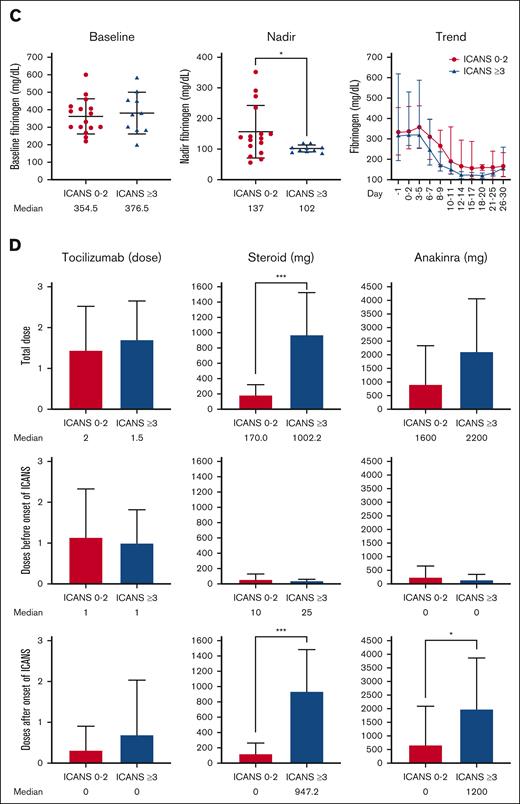
The baseline (just before starting lymphodepletion chemotherapy) and posttreatment levels of white blood cell count, absolute neutrophil count, absolute monocyte count, absolute lymphocyte count, hemoglobin, and platelet level were comparable in patients with different ICANS severity (Figure 5A; supplemental Figure 3A). Patients with severe ICANS had more pronounced anemia and thrombocytopenia (P = .047 and P = .002, respectively) within 30 days after brexu-cel infusion and higher packed red blood cell and platelet transfusion burden (P = .03 and P = .02, respectively). C-reactive protein (CRP) peaked concurrently with the onset of neurotoxicity, and higher peak CRP levels were noted in patients with severe ICANS than those with lower grade ICANS (median, 5.7 vs 3.45 mg/dL; P = .02; Figure 5B). Peak ferritin level tended to be higher in patients with severe ICANS but did not reach statistical significance. Patients with severe ICANS had a lower nadir fibrinogen level (Figure 5C), but the cryoprecipitate transfusion burden was similar between all subgroups. The peak serum lactate dehydrogenase level was found to be comparable among patients with different levels of ICANS severity (supplemental Figure 3B).
Severe ICANS is associated with cytopenia, coagulopathy, and increased inflammation. (A) The baseline (just before starting lymphodepletion chemotherapy) and posttreatment levels of white blood cell count, hemoglobin, and platelet count were comparable between patients with and without severe ICANS. Anemia, thrombocytopenia, and increased transfusion burden were associated in patients with severe ICANS. (B) The baseline CRP and ferritin were similar between the 2 groups. The peak CRP level peaked higher in patients with severe ICANS. (C) The baseline fibrinogen level was similar between the 2 groups. The fibrinogen level nadir was lower in patients with severe ICANS. (D) The cumulative dose of tocilizumab, steroid (equivalent dose of dexamethasone), and anakinra were compared between patients with and without severe ICANS. The tocilizumab total cumulative dose is similar between the 2 groups, and mainly used before ICANS onset. Patients with severe ICANS received more cumulative doses of steroid, mainly given after the onset of ICANS, and received higher cumulative doses of anakinra. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001; by Wilcox rank sum test (continuous) or Fisher exact test (categorical).
Severe ICANS is associated with cytopenia, coagulopathy, and increased inflammation. (A) The baseline (just before starting lymphodepletion chemotherapy) and posttreatment levels of white blood cell count, hemoglobin, and platelet count were comparable between patients with and without severe ICANS. Anemia, thrombocytopenia, and increased transfusion burden were associated in patients with severe ICANS. (B) The baseline CRP and ferritin were similar between the 2 groups. The peak CRP level peaked higher in patients with severe ICANS. (C) The baseline fibrinogen level was similar between the 2 groups. The fibrinogen level nadir was lower in patients with severe ICANS. (D) The cumulative dose of tocilizumab, steroid (equivalent dose of dexamethasone), and anakinra were compared between patients with and without severe ICANS. The tocilizumab total cumulative dose is similar between the 2 groups, and mainly used before ICANS onset. Patients with severe ICANS received more cumulative doses of steroid, mainly given after the onset of ICANS, and received higher cumulative doses of anakinra. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001; by Wilcox rank sum test (continuous) or Fisher exact test (categorical).
Among 10 patients with severe ICANS, 2 patients underwent lumbar puncture because of prolonged neurological symptoms. The white blood cell count in the CSF was 1 cell per μL and 3 cells per μL, respectively. One patient's CSF was sent for flow cytometry, revealing heterogeneous T cells predominantly composed of effector/effector memory subsets. B cells were not detected by CD19. The CSF glucose levels were within normal limits in both patients. However, the total protein concentration was mildly elevated, measuring 49 and 53 mg/dL (normal range: 15-45 mg/dL), respectively. Comprehensive CSF virus analysis, including varicella zoster virus, herpes simplex virus, HHV-6, cytomegalovirus, Epstein-Barr virus, enterovirus, and West Nile virus, yielded negative results. Furthermore, CSF cultures for both bacteria and fungi were negative, indicating an absence of microbial growth.
Management of ICANS
The cumulative doses of tocilizumab, corticosteroids (in dexamethasone equivalents), and anakinra were calculated and compared between patients with severe ICANS vs those with nonsevere ICANS (Figure 5D). The tocilizumab total cumulative dose was similar between the 2 groups, and mainly used before ICANS onset. Patients with severe ICANS received more total corticosteroids (median dose: 1002.2 vs 170 mg; P < .001), with the vast majority typically given after ICANS onset (median dose: 947.2 vs 0 mg; P < .001). Patients with severe ICANS also received a higher cumulative dose of anakinra (median dose: 1200 vs 0 mg, P = .02).
Four patients with severe ICANS were admitted to the intensive care unit. One of these patients experienced severe agitation requiring dexmedetomidine hydrochloride, and the remaining 3 patients predominantly needed airway monitoring. Two patients with ICANS grade 0 to 2 were admitted to the intensive care unit for grade 3 CRS with hypotension and needed vasopressor support and hemodynamic monitoring. Overall, patients with severe ICANS had a significantly longer hospitalization than patients with mild or no ICANS, with median 29 vs 15.5 days, respectively (P < .001).
ICANS and survival
The treatment response was similar in patients with severe vs nonsevere ICANS (Figure 6A). With a median follow-up time of 11.7 months, the median DOR was comparable between the groups (15.2 months vs not reached, log-rank P = .4; Figure 6B). The 12-month PFS (64.3% vs 68.7%, log-rank P = .2; Figure 6C) and OS (90% vs 93.8%, log-rank P = .7; Figure 6D) were not significantly different between the groups. The severity of ICANS was not significantly associated with the DOR, PFS, or OS.
ICANS severity and survival. The (A) optimal response rate, (B) DOR, (C) PFS, and (D) OS are similar between the patients with different ICANS severity. CI, confidence interval; CR, complete response; HR, hazard ratio; PD, progressive disease; PR, partial response.
ICANS severity and survival. The (A) optimal response rate, (B) DOR, (C) PFS, and (D) OS are similar between the patients with different ICANS severity. CI, confidence interval; CR, complete response; HR, hazard ratio; PD, progressive disease; PR, partial response.
Discussion
In this study, the clinical outcomes and incidence rates of ICANS were found to be similar to previously reported rates for patients with MCL treated with brexu-cel.9,10 In contrast to studies of other B-cell malignancies, we did not observe any statistically significant correlation between patient demographics, including age, tumor burden, or prior treatment characteristics, and the severity of ICANS. However, our observation is limited because of relative small sample size. Of note, we opted to include patients with epilepsy history, MCL with CNS involvement,21 and those with prior abnormal brain MRIs at baseline. Although several of these patients did experience severe ICANS with MRI and EEG changes after treatment, they were able to be successfully treated and maintained high overall clinical efficacy.
Head CT scans obtained during ICANS did not reveal intracranial abnormalities in any patient, including those who had brain MRI abnormalities. Nonetheless, we advise that head CT should not be omitted during acute neurological evaluation, given its ease of acquisition and exquisite sensitivity for ruling out intracranial bleed and cerebral edema.
In contrast, we identified multiple discrete patterns of acute brain MRI changes across several distinct MRI sequences, which we hypothesize may represent the diverse spectrum of pathophysiology underlying ICANS. For example, Figure 2A-B shows abnormalities at the meningeal and cortical surface, as well as in a periventricular location between CSF and brain tissue. These imaging findings at “brain-border” tissues may suggest treatment-related immune interactions occurring at the systemic circulation and CNS interface. Although DWI is most commonly used to identify acute stroke, it can also indicate areas of hypercellularity. Figure 2C shows DWI abnormalities that are not typical for stroke, and this hyperintensity also appeared at the cortical surface. Such changes could potentially represent hypercellularity or inflammation at the meninges. One hypothesis is that this finding represents treatment-related inflammation of meningeal tertiary lymphoid tissue. The abnormalities from 2 patients on ASL imaging (a proxy for cerebral perfusion and blood flow) shown in Figure 2D demonstrate hyperemia of the entire left hemisphere. Although the reason for such asymmetry is unknown, these findings coincided with ICANS-related language deficits, which, in most patients, localize to the left cerebral hemisphere. In a patient with preexisting white matter disease of unknown etiology and no history of methotrexate exposure, there was neuroimaging evidence of increased volume of the T2 signal abnormality during acute ICANS (Figure 2E), compared with the preinfusion brain MRI. He went on to make a full neurological recovery and his MCL remains in complete remission.
This study highlights the importance of obtaining preinfusion baseline brain MRIs so that acute MRI changes during ICANS could be accurately identified. Prior ICANS studies in other hematologic malignancies such as diffuse large B-cell lymphoma have reported MRI changes in ∼40% of patients with grade ≥3 ICANS.22 In contrast, Rubin et al, in a heterogenous CAR T-cell therapy cohort that included leukemia, lymphoma, multiple myeloma, and sarcoma, found 17% of patients who had undergone MRI had acute abnormalities. A French cohort reported minimal MRI changes from a subset of patients with grade ≥3 ICANS and suggest positron emission tomography scans may be of more utility.23 The discrepancies in neuroradiological findings reported in available literature may reflect intrinsic biological differences between ICANS presentations according to baseline tumor type, the specific CAR T-cell product, and/or the different number and types of MRI sequences obtained across centers worldwide. For example, this study includes the ASL imaging to measure CSF, and is a sequence not always obtained in all studies. Based on the higher rate of ICANS in MCL and associated MRI findings, our clinical practice is to obtain baseline brain MRIs for all patients with MCL before CAR T-cell therapy.
The EEG analyses demonstrated that seizures are relatively rare, in concordance with previously reported CAR T-cell therapy neurotoxicity studies.24 The only case of seizure within our cohort occurred in a patient who ultimately was diagnosed with HHV-6 encephalitis. She also had classic temporal lobe abnormalities on MRI consistent with this diagnosis. She experienced grade 1 ICANS, which occurred initially day +8, recurred on day +13, and fully resolved on posttreatment day +18. She then re-presented to the hospital on day +34 with worsened mental status and was then found to have bilateral focal nonmotor seizures on EEG at that time, well after the resolution of ICANS. This case highlights that after CAR T-cell therapy, individuals are at risk for infection, including CNS infections. Alternative etiologies for new neurological symptoms, such as encephalitis, should be seriously considered, particularly when symptoms develop later than 2 weeks after CAR T-cell infusion or do not rapidly improve with standard ICANS management.
Patients with acute MRI abnormalities during ICANS had longer posttreatment hospital stays than patients who had normal brain MRI during ICANS (Figure 2A). This difference was ∼10 days, which has direct implications for hospital resource allocation and systems level considerations for CAR T-cell therapy inpatient care. There was also a trend toward longer hospitalizations with abnormal EEG findings during acute ICANS (Figure 2B). This is consistent with a previous report on patients with r/r lymphoma undergoing CAR T-cell therapy with axicabtagene ciloleucel, in which ICANS was a major contributor to the higher use of hospital resources because of prolonged inpatient stays.25
In our study, baseline CRP, ferritin, and fibrinogen level were similar between patients who developed severe ICANS vs those who did not. This is in contrast to prior reports in which patients with a higher baseline inflammatory state (increased levels of CRP, ferritin, D-dimer and proinflammatory cytokines) were shown to have increased risk of developing CRS and ICANS after axicabtagene ciloleucel.26 However, this cohort size may limit our ability to detect significant associations between baseline CRP, ferritin, and fibrinogen, and severe ICANS. Our cohort recapitulated the previous association between the development of severe ICANS and higher peak CRP, hypofibrinogenemia, and severe anemia and thrombocytopenia.27 Given these findings, patients who develop severe ICANS should be considered at higher risk for bleeding complications, and should be monitored frequently to maintain appropriate platelet, hemoglobin, and fibrinogen levels.
Limitations of our study include the retrospective single-center design and limited patient numbers. Cytokine analyses were not routinely done in this cohort, but, in the future, it would be helpful for a better understanding of ICANS pathophysiology. Pretreatment neurofilament light chain levels has been identified in multiple studies in adults to be a predictive biomarker for the development of ICANS,28,29 although in a separate pediatric study this was not found.30 Future studies should determine whether this biomarker is clinical useful in patients with MCL undergoing CAR T-cell therapy. Additionally, we have not yet been able to conduct longer-term surveillance MRI brain imaging to evaluate for the resolution of the MRI brain abnormalities in most patients. Approaches are underway to develop risk-mitigation strategies for individuals at high risk of developing CAR T cell–associated neurotoxicity.31-33 These strategies include the prophylactic use of steroids or anakinra and the use of Janus kinase inhibitors or dasatinib.33-35 Because severe ICANS also varies among CAR T-cell products, the use of alternative CAR T-cell constructs to treat MCL are being investigated. Notably, in patients with MCL treated with lisocabtagene maraleucel or a CD19-CD20 CAR T-cell therapy, severe ICANS was reported in 9% and 0% of patients, respectively, significantly lower than that observed for breu-xel.9,36,37 Although high-dose corticosteroids are commonly used as initial intervention for the treatment of severe ICANS, steroids are known to have pleiotropic effects and can worsen neuropsychiatric side effects. Thus, it is imperative to investigate additional novel or targeted therapeutic approaches to enhance clinical management. We have plans to address these important issues and conduct further investigations into these additional topics in future studies.
In conclusion, we found a range of acute brain MRI changes that occur in the setting of severe ICANS, including abnormalities in the brain-border tissues not previously reported in ICANS neuroradiology. On EEG studies, interictal EEG patterns were common whereas seizure was rare. Thus, when seizures or other neurologic changes occur outside of clinical ICANS, a full diagnostic workup should be obtained to rule out other seizure etiologies such as CNS infection. Abnormal EEG and MRI findings correlate with presence of severe ICANS and with longer acute hospital length of stay. Preexisting nervous system diagnoses or lymphoma with CNS involvement were not associated with a worse neurological outcome. Patients with MCL who developed severe ICANS were found to be at higher risk for anemia, thrombocytopenia, and hyperfibrinogenemia. However, ICANS severity did not affect the overall treatment response, and survival of patients with MCL was observed. Overall, these emerging insights into ICANS and its clinical correlatives will contribute to the improvement of acute neurological and oncological care for patients undergoing CAR T-cell therapy.
Acknowledgments
The authors thank the members of the Stanford University Blood and Marrow Transplantation and Cellular Therapy Program and the members of the Neurology Program for their tireless work caring for the patients involved in this study.
Authorship
Contribution: E.H.N., Y.J.S., and M.J.F. designed the study; E.H.N. and Y.J.S. did the primary data analysis; E.H.N. and Y.J.S. wrote the first version of the manuscript; S.D., D.B.M., J.H.B., S.B., B.J.S., and J.E.D. provided critical input and contributed to the writing of select sections within the manuscript; and all authors contributed to reviewing the final manuscript and have agreed to be co-authors.
Conflict-of-interest disclosure: E.H.N. reports consulting or serving in an advisory role for Acorn AI Labs and Medidata; and reports research support from Roche/Genentech. M.H.H. reports consulting or serving in an advisory role for Roche/Genentech and Alexion. J.E.D. reports consulting or serving in an advisory role for Alexion, Janssen, Bristol Myers Squibb, and Genzyme. L.B.K. reports research support from Roche/Genentech. D.B.M. reports consulting for Kite/Gilead, Juno/Celgene, and Novartis; and reports research support from Kite/Gilead. M.J.F. reports consulting or serving in an advisory role for Kite (a Gilead company), Adaptive Biotechnologies, and Allogene Therapeutics; and reports research support from Adaptive Biotechnologies and Allogene Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Matthew J. Frank, Division of Blood and Marrow Transplantation and Cellular Therapy, Stanford University School of Medicine, Room H0145, 300 Pasteur Dr, Stanford, CA 94305; email: mjfrank@stanford.edu.
References
Author notes
E.H.N. and Y.J.S. contributed equally to this study.
Data are available on request from the corresponding author, Matthew J. Frank (mjfrank@stanford.edu).
The full-text version of this article contains a data supplement.