Hepatic injury is common in individuals with FA.
Risk of persistent liver injury was increased in patients who had received total body irradiation during transplant but not androgens.
Visual Abstract
Liver disease has not been well described in patients with Fanconi anemia (FA). Improvements in outcomes of transplant mean that more individuals with FA are reaching adulthood and new features of the FA phenotype are being discovered. We performed a retrospective review of liver function in a cohort of 97 patients with FA followed-up for at least 10 years at a single center. We identified a high frequency of transaminitis (n = 31, 32%) without elevation of bilirubin and with no evidence of structural hepatic abnormality in patients with FA. Transaminitis was persistent in many cases, sometimes lasting more than a decade without clinical manifestation, although 2 patients with prolonged transaminitis are deceased from liver failure, indicating important long-term clinical consequences. Transaminitis was found in patients who had and had not received transplant but was more frequent in recipients of transplant. Exposure to total body irradiation increased risk (odds ratio, 15.5 [95% confidence interval, 2.44-304.54]; P = .01), whereas treatment with androgens did not. Review of limited numbers of liver biopsies and autopsy material showed a cholestatic pattern of liver injury, with progressive fibrosis, in the majority of patients. Occurrence in cases without transplant as well as cases with transplant argues against a potential diagnosis of atypical liver graft-versus-host disease. Limited data regarding therapy suggest no benefit from treatment with steroids or other immune suppressive medications or ursodeoxycholic acid. Our data show that liver disease is common in patients with FA, and because most children with FA now reach adulthood, end-stage liver disease in young adulthood means systematic testing of potential therapies is urgently needed.
Introduction
Fanconi anemia (FA) is the most common inherited bone marrow failure syndrome, characterized by a range of physical abnormalities, bone marrow failure, and cancer predisposition.1-4 Physical anomalies can include abnormal skin pigmentation, skeletal abnormalities of the upper and lower extremities, and short stature.5 Mutations in at least 20 genes in pathways involved in DNA damage sensing and repair have been described in association with a Fanconi anemia phenotype.6-8 Progressive bone marrow failure with pancytopenia typically presents within the first decade of life, commonly requiring hematopoietic stem cell transplantation (HSCT).9 Individuals with FA are at markedly increased risk of hematologic malignancy (acute myeloid leukemia) and solid tumors of the head and neck, genitourinary tract, and skin.1-4,10-12
Chronic liver disease in patients with FA is not well described. Hepatic abnormalities previously described in individuals with FA include the consequences of androgen exposure, acute and late complications of HSCT, and predisposition to benign and malignant liver tumors.13-17 Androgens transiently improve peripheral blood counts in ∼50% of FA cases, but only 10% to 20% of these patients are long-term responders and they require close monitoring for hepatotoxicity.13-15 In the only previous study examining longitudinal liver abnormalities, Masserot-Lureau et al described a cohort of 64 adult and pediatric patients with FA who had persistent liver abnormalities.18 In the aforementioned study, 7 of 44 (15.9%) patients who had prior androgen treatment developed significant liver abnormalities. However, significant liver enzyme abnormalities were also observed in patients (5/20, 25%) who had never been treated with androgens, indicating that significant liver injury occurs outside the setting of androgen treatment.18 However, in this valuable study, analysis was restricted to liver function testing before the start of a transplant conditioning regimen, leaving a major gap in understanding of how hepatic abnormalities evolve over time in the post-HSCT period. Improvements in HSCT strategies over the last 20 years mean that a majority of children who receive transplantation for FA survive transplantation, and many more children with FA are reaching adulthood than in the past. This larger number of older patients with FA allows observation of new aspects of the FA phenotype.
Our objectives in this study were to describe the frequency, clinical characteristics, management, and outcomes of liver impairment in patients with FA. We also identify clinical features predictive of persistent liver injury (PLI) and describe outcomes of patients with FA-related liver disease.
Methods
Patient demographics and data collection
We performed a retrospective cohort analysis of all patients with FA with at least 10 years of observation, seen at Cincinnati Children’s Hospital Medical Center (CCHMC). We included only those with 10 years of observation as our goal was to identify persistent, not transient, liver injury. We did not limit data analysis to 10 years if data for a longer period were available. The study was approved by the CCHMC institutional review board. All available clinical, laboratory, and radiological data related to the liver were systematically reviewed for each patient.
HSCT procedures
Transplant procedures were performed per uniform institutional review board–approved institutional protocols and all research procedures were performed in accordance with the Declaration of Helsinki. Before 2009, CCHMC used a low-dose radiation–based (450 cGy) HSCT preparative regimen for all patients with FA. Starting in 2009, radiation was eliminated from the protocol and replaced with a busulfan-based preparative regimen.19,20
At our institution, once weekly monitoring for viral reactivation for adenovirus, BK virus, cytomegalovirus, and Epstein Barr Virus began at the completion of the conditioning regimen. More frequent monitoring of viral reactivation occurred as clinically indicated, such as in the case of transaminase elevations.
Monitoring of patients with FA who have not received HSCT
Patients with FA were monitored with complete blood counts and other laboratory testing as clinically indicated, approximately quarterly, and annual bone marrow aspirate and biopsies to monitor for progression toward myelodysplastic syndrome and acute myelogenous leukemia. Multidisciplinary care services were recommended depending on the clinical phenotype of each patient.
Diagnosis of PLI
Diagnosis of PLI was made based on a previous published definition to allow for comparison of findings.18 Patients were defined as having PLI if both serum aminotransferases (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]) were above the upper limit of normal on at least 3 different blood draws, >1 month apart. Serum bilirubin levels were typically normal until the development of end-stage liver disease, in agreement with the previous report, and those data are not reported here. Results were interpreted as elevated using established age- and sex-dependent reference intervals at the time of the result. For analysis, to determine changes in serum AST and ALT rounded to multiples of the upper limit of normal values, we used established pediatric reference ranges established by the National Health and Nutrition Examination Survey, 1996 to 2006. Using a cutoff of 25 U/L, we calculated times above the limit of the normal range for AST and ALT. We excluded any liver function testing performed from the start of the conditioning regimen to 6 months after HSCT in recipients of HSCT because of the known hepatotoxicity associated with transplant-related procedures and medications. Our institution’s hepatic function panel includes measurements of AST, ALT, total protein, albumin and bilirubin, and not γ-glutamyl transpeptidase (GGT). However, all available GGT levels were recorded if obtained on each patient. All recipients of HSCT received aggressively T-cell depleted stem cell grafts, with a very low to negligible frequency of graft-versus-host disease (GVHD).19
Available radiographic imaging, which included visualization of the liver and biliary system (ultrasound, computed tomography, or magnetic resonance imaging) was reviewed for all patients. We reviewed patients’ medical records for clinical signs of liver disease including hepatosplenomegaly, symptoms of portal hypertension, jaundice, pruritus, hepatic encephalopathy, and history of gastrointestinal bleeding as a consequence of severe liver disease. A pediatric hepatologist also reviewed all clinical and radiographic findings for each patient to ensure agreement of final diagnosis of PLI. Given the known liver abnormalities associated with androgens, information on treatment with androgen therapy for hematopoiesis was specifically sought and recorded for each patient.
We additionally collected any other information on diagnostic workup that was included to evaluate PLI for each patient. This included but was not limited to viral reactivation, evidence of iron overload, evidence of nonalcoholic fatty liver disease by imaging, and autoimmune hepatitis. Viral reactivation within 2 months before or after maximal liver injury was evaluated to determine if this was contributing to liver injury.
In the graphs detailing the progression of liver injury in all patients, we used a smoothing function to illustrate trends and change in the liver function tests over long periods of time. For the smoothing function, we averaged 3 values (either ALT or AST) and used a fourth-order smoothing polynomial to create these graphs. GGT was plotted against time without using any smoothing features because these values were checked less frequently. No statistical analysis was performed on the smoothed plots graphing the time course of ALT, AST, and GGT as the purpose of these graphs is to illustrate temporal trends over long periods of time.
Statistical analysis
Median (interquartile range [IQR]) and frequencies (percent) were used to describe continuous and categorical variables. Differences by group for continuous and categorical variables were determined using Fisher exact and Wilcoxon tests, respectively. Nonparametric Mann-Whitney U test was used to compare 2 unpaired groups. Log-rank tests were used to assess the difference in overall survival by group. Odds ratios and corresponding P values were obtained using a multivariable logistic regression model. Analyses were performed using R version 3.1.3 and GraphPad Prism version 9.4.1. All statistical tests were 2-sided, and significance was assessed at P < .05.
Results
Patient demographics and clinical characteristics
We identified a total of 119 individuals with FA having at least 10 years of follow-up, 68 of whom had received allogeneic-HSCT (HSCT group) and 51 who had not received HSCT (non-HSCT group; Figure 1). Eighteen patients in the HSCT group and 4 in the non-HSCT group were excluded because of insufficient follow-up data or death from causes unrelated to the liver, resulting in an overall final cohort of 97 patients. Twenty-one patients in the HSCT group and 10 patients in the non-HSCT group met our definition of PLI. The median time of follow-up in the entire cohort was 13.7 years (IQR, 10.37-16.0). Eight of 21 recipients of HSCT with PLI developed PLI preceding transplant, and 13 patients developed new PLI >6 months after HSCT. In the HSCT group, 3 patients were deceased at the time of analysis, with 2 patients dying from liver failure. In the non-HSCT group, 1 patient died from multiorgan failure associated with hepatocellular carcinoma.
Patient demographics and transplant-related characteristics of patients with FA who received HSCT are described in Table 1. There were no significant differences between patients who received HSCT with and without PLI, with the exception of year of HSCT, likely related to the use of a total body irradiation (TBI) in the conditioning regimen in earlier years. Of note, exposure to androgens was similar in recipients of HSCT with and without PLI (33% vs 21%, P = .35). To examine whether these patients had direct transplant-related injury that may have contributed to future development of PLI, we evaluated incidence of veno-occlusive disease, acute GVHD and chronic GVHD (cGVHD). We saw a higher incidence of cGVHD in those with PLI than in those without, however the incidence of liver cGVHD in this group was 1 in those with PLI compared with 0 in those without PLI, which makes any role in the etiology of PLI highly questionable. Furthermore, 3 of the 6 patients with cGVHD already had PLI preceding HSCT.
Patient demographics and transplant-related characteristics of patients with FA who underwent HSCT
| . | HSCT, PLI (n = 21) . | HSCT, no PLI (n = 29) . | P value . |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 12 (57.1) | 19 (65.5) | 1.00 |
| Male | 9 (42.9) | 10 (34.5) | |
| Race, n (%) | |||
| Caucasian | 17 (81) | 23 (79.3) | .57 |
| African American | 3 (14.3) | 4 (13.8) | |
| Hispanic | 0 | 2 (6.9) | |
| Asian | 1 (4.7) | 0 | |
| Age (y) at HSCT, median (IQR) | 6.7 (5.5-10.3) | 10.1 (6.2-12.8) | .32 |
| FANC complementation group, n (%) | |||
| FANCA | 5 (23.8) | 12 (41.4) | .47 |
| Non-FANCA | 12 (57.1) | 10 (34.5) | |
| Unknown | 4 (19.1) | 7 (24.1) | |
| Androgen use before HSCT, n (%) | 7 (33.3) | 6 (20.7) | .35 |
| Year of HSCT, n (%) | |||
| 2006-2010 | 10 (47.6) | 5 (17.2) | .03 |
| 2011-2014 | 11 (52.4) | 24 (82.8) | |
| Stem cell source, n (%) | |||
| Bone marrow | 2 (9.5) | 5 (17.2) | .19 |
| PBSC | 17 (81) | 24 (82.8) | |
| Cord | 2 (9.5) | 0 | |
| Degree of match, n (%) | |||
| Fully matched | 18 (85.7) | 26 (89.7) | 1.00 |
| Mismatched | 3 (14.3) | 3 (10.3) | |
| Donor type, n (%) | |||
| Related | 2 (9.5) | 8 (27.6) | .16 |
| Unrelated | 19 (90.5) | 21 (72.4) | |
| Conditioning regimen, n (%) | |||
| ATG/Bu/Cy/Flu | 12 (57.1) | 24 (82.8) | .09 |
| ATG/Cy/Flu | 2 (9.5) | 3 (10.4) | |
| ATG/Cy/Flu/TBI | 7 (33.2) | 1 (3.4) | |
| ATG/Flu | 0 | 1 (3.4) | |
| GVHD prophylaxis, n (%) | |||
| CNI-based only | 11 (52.4) | 12 (41.4) | .52 |
| TCD only | 2 (9.5) | 4 (13.8) | |
| CNI/TCD | 8 (38.1) | 13 (44.8) | |
| VOD, n (%) | 1 (4.8) | 2 (6.9) | 1.0 |
| TA-TMA, n (%) | 4 (19) | 13 (44.8) | .009 |
| Day-100 acute GVHD, n (%) | |||
| Grades 2-4 | 0 | 2 (6.9)∗ | .13 |
| Grades 3-4 | 0 | 2 (6.9) | |
| Acute GVHD with liver involvement | 0 | 0 | |
| cGVHD, n (%) | 6 (28.6) | 0 | .0034 |
| cGVHD with liver involvement | 1 (16.7) | 0 | |
| Survival, n (%) | |||
| Alive | 18 (85.7) | 19 (65.5) | .19 |
| . | HSCT, PLI (n = 21) . | HSCT, no PLI (n = 29) . | P value . |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 12 (57.1) | 19 (65.5) | 1.00 |
| Male | 9 (42.9) | 10 (34.5) | |
| Race, n (%) | |||
| Caucasian | 17 (81) | 23 (79.3) | .57 |
| African American | 3 (14.3) | 4 (13.8) | |
| Hispanic | 0 | 2 (6.9) | |
| Asian | 1 (4.7) | 0 | |
| Age (y) at HSCT, median (IQR) | 6.7 (5.5-10.3) | 10.1 (6.2-12.8) | .32 |
| FANC complementation group, n (%) | |||
| FANCA | 5 (23.8) | 12 (41.4) | .47 |
| Non-FANCA | 12 (57.1) | 10 (34.5) | |
| Unknown | 4 (19.1) | 7 (24.1) | |
| Androgen use before HSCT, n (%) | 7 (33.3) | 6 (20.7) | .35 |
| Year of HSCT, n (%) | |||
| 2006-2010 | 10 (47.6) | 5 (17.2) | .03 |
| 2011-2014 | 11 (52.4) | 24 (82.8) | |
| Stem cell source, n (%) | |||
| Bone marrow | 2 (9.5) | 5 (17.2) | .19 |
| PBSC | 17 (81) | 24 (82.8) | |
| Cord | 2 (9.5) | 0 | |
| Degree of match, n (%) | |||
| Fully matched | 18 (85.7) | 26 (89.7) | 1.00 |
| Mismatched | 3 (14.3) | 3 (10.3) | |
| Donor type, n (%) | |||
| Related | 2 (9.5) | 8 (27.6) | .16 |
| Unrelated | 19 (90.5) | 21 (72.4) | |
| Conditioning regimen, n (%) | |||
| ATG/Bu/Cy/Flu | 12 (57.1) | 24 (82.8) | .09 |
| ATG/Cy/Flu | 2 (9.5) | 3 (10.4) | |
| ATG/Cy/Flu/TBI | 7 (33.2) | 1 (3.4) | |
| ATG/Flu | 0 | 1 (3.4) | |
| GVHD prophylaxis, n (%) | |||
| CNI-based only | 11 (52.4) | 12 (41.4) | .52 |
| TCD only | 2 (9.5) | 4 (13.8) | |
| CNI/TCD | 8 (38.1) | 13 (44.8) | |
| VOD, n (%) | 1 (4.8) | 2 (6.9) | 1.0 |
| TA-TMA, n (%) | 4 (19) | 13 (44.8) | .009 |
| Day-100 acute GVHD, n (%) | |||
| Grades 2-4 | 0 | 2 (6.9)∗ | .13 |
| Grades 3-4 | 0 | 2 (6.9) | |
| Acute GVHD with liver involvement | 0 | 0 | |
| cGVHD, n (%) | 6 (28.6) | 0 | .0034 |
| cGVHD with liver involvement | 1 (16.7) | 0 | |
| Survival, n (%) | |||
| Alive | 18 (85.7) | 19 (65.5) | .19 |
ATG, antithymocyte globulin; Bu, busulfan; CNI, calcineurin inhibitor; Cy, cyclophosphamide; Flu, fludarabine; PBSC, peripheral blood stem cell; TCD, T-cell depleted; VOD, veno-occlusive disease;
Both cases of grade 3 GVHD with gut and gut/skin but no liver involvement.
Characteristics of patients who had not received HSCT are shown in Table 2. Similar to the HSCT cohort, the frequency of patients with PLI treated with androgens (n = 2; 20%) was not different from those without PLI (n = 2; 5.4%; P = .19), although numbers are small. Additionally, mutations in non-FANCA complementation groups were more frequent in patients with PLI (n = 6; 60%) compared with in patients without PLI (n = 7; 18.9%; P = .045), although the sample size is too small to draw any firm conclusion.
Patient demographics and characteristics of patients with FA who did not undergo HSCT
| . | Non-HSCT, PLI (n = 10) . | Non-HSCT, no PLI (n = 37) . | P value . |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 6 (60) | 17 (45.9) | .49 |
| Male | 4 (40) | 20 (54.1) | |
| Race, n (%) | |||
| Caucasian | 10 (100) | 36 (97.3) | 1.0 |
| African American | 0 | 0 | |
| Hispanic | 0 | 1 (2.7) | |
| Asian | 0 | 0 | |
| Age (y) at first evaluation, median (IQR) | 11.9 (2.5-15.7) | 4.2 (2.6-10.5) | .28 |
| FANC complementation group, n (%) | |||
| FANCA | 4 (40) | 29 (78.3) | .045 |
| Non-FANCA | 6 (60) | 7 (18.9) | |
| Unknown | 0 | 1 (2.8) | |
| Prior androgen use, n (%) | 2 (20) | 2 (5.4) | .19 |
| Survival, n (%) | |||
| Alive | 9 (90%) | 37 (100) | .26 |
| . | Non-HSCT, PLI (n = 10) . | Non-HSCT, no PLI (n = 37) . | P value . |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 6 (60) | 17 (45.9) | .49 |
| Male | 4 (40) | 20 (54.1) | |
| Race, n (%) | |||
| Caucasian | 10 (100) | 36 (97.3) | 1.0 |
| African American | 0 | 0 | |
| Hispanic | 0 | 1 (2.7) | |
| Asian | 0 | 0 | |
| Age (y) at first evaluation, median (IQR) | 11.9 (2.5-15.7) | 4.2 (2.6-10.5) | .28 |
| FANC complementation group, n (%) | |||
| FANCA | 4 (40) | 29 (78.3) | .045 |
| Non-FANCA | 6 (60) | 7 (18.9) | |
| Unknown | 0 | 1 (2.8) | |
| Prior androgen use, n (%) | 2 (20) | 2 (5.4) | .19 |
| Survival, n (%) | |||
| Alive | 9 (90%) | 37 (100) | .26 |
Clinical and imaging characteristics associated with liver disease
Results of liver imaging and clinical findings associated with liver disease in patients with PLI are shown in Table 3. In the majority of cases, no imaging abnormalities were seen in either the HSCT cohort (n = 11; 55%) or the non-HSCT cohort (n = 5; 62.5%). Hepatosplenomegaly was observed in 2 (9.5%) patients in the HSCT group. Sequelae of portal hypertension were observed in 2 (9.5%) patients who received HSCT and 1 (10%) patient who did not receive HSCT. Hepatic imaging was available for 37 patients without PLI in the HSCT group, and 14 patients in the non-HSCT group. Only 1 patient without PLI in the HSCT group exhibited a 5-mm hepatic nodule. The remaining patients had normal liver on imaging. Gastrointestinal bleeding from varices occurred in 1 recipient of HSCT, and 1 patient who did not receive HSCT.
Liver imaging characteristics in HSCT and non-HSCT cohorts with PLI
| . | HSCT cohort (n = 21) . | Non-HSCT cohort (n = 10) . |
|---|---|---|
| Imaging findings, n (%) | n = 20 with imaging | n = 8 with imaging |
| Normal liver | 11 (55) | 5 (62.5) |
| Biliary duct dilatation | 2 (10) | 1 (12.5) |
| Coarse/cirrhotic liver | 0 | 1 (12.5) |
| Steatosis | 1 (5) | 1∗ (6.3) |
| Periportal edema | 3 (15) | 0 |
| Hepatomegaly | 0 | 0 |
| Splenomegaly | 0 | 0 |
| Nodules/discrete lesions | 1 (5) | 0 |
| Increased stiffness | 2 (10) | 1∗ (6.2) |
| Hepatosplenomegaly, n (%) | 2 (9.5) | 0 |
| Evidence of portal hypertension, n (%) | n=2 | n=1 |
| Ascites | 1 | 1 |
| Splenomegaly | 2 | 0 |
| Thrombocytopenia | 1 | 0 |
| Varices | 0 | 0 |
| Jaundice, n (%) | 4 (19) | 1 (10) |
| Pruritus, n (%) | 2 (9.5) | 0 |
| Encephalopathy, n (%) | 0 | 0 |
| History of gastrointestinal bleeding, n (%) | 1 (4.8) | 1 (10) |
| . | HSCT cohort (n = 21) . | Non-HSCT cohort (n = 10) . |
|---|---|---|
| Imaging findings, n (%) | n = 20 with imaging | n = 8 with imaging |
| Normal liver | 11 (55) | 5 (62.5) |
| Biliary duct dilatation | 2 (10) | 1 (12.5) |
| Coarse/cirrhotic liver | 0 | 1 (12.5) |
| Steatosis | 1 (5) | 1∗ (6.3) |
| Periportal edema | 3 (15) | 0 |
| Hepatomegaly | 0 | 0 |
| Splenomegaly | 0 | 0 |
| Nodules/discrete lesions | 1 (5) | 0 |
| Increased stiffness | 2 (10) | 1∗ (6.2) |
| Hepatosplenomegaly, n (%) | 2 (9.5) | 0 |
| Evidence of portal hypertension, n (%) | n=2 | n=1 |
| Ascites | 1 | 1 |
| Splenomegaly | 2 | 0 |
| Thrombocytopenia | 1 | 0 |
| Varices | 0 | 0 |
| Jaundice, n (%) | 4 (19) | 1 (10) |
| Pruritus, n (%) | 2 (9.5) | 0 |
| Encephalopathy, n (%) | 0 | 0 |
| History of gastrointestinal bleeding, n (%) | 1 (4.8) | 1 (10) |
Same patient had both steatosis and increased stiffness on ultrasound.
Patients with PLI did not have evidence of viral reactivation in the blood. Four patients had high-level BK viremia proximal to the time of maximal liver injury. Two patients had prior history of transfusion-related iron overload in the liver however this preceded HSCT and was appropriately treated with chelation and phlebotomy. Four patients had evaluation for autoimmune hepatitis and was negative.
One patient had evidence of hepatic steatosis seen on ultrasound.
PLI in patients with FA treated with HSCT
The median age liver function tests (LFTs) were first checked was 7.1 years (IQR, 5.9-9.3) in those in the HSCT cohort who met criteria for PLI (n = 21). The median age of first abnormal LFT was also 7.1 years (IQR, 6.1-9.3). The median maximum ALT was 180 U/L (7.2× above normal); IQR, 109-302 (4.4× to 12× above normal), and median maximum AST was 162 U/L (6.5× above normal); IQR, 109-295 (4.4× to 11.8× above normal). The median age at time of highest liver enzyme elevation was 13.4 years (IQR, 8.7-16.5).
PLI in patients with FA who did not undergo HSCT
In total, 10 patients who did not undergo HSCT met criteria for PLI. The median age that LFTs were first checked was 11.8 years (IQR, 1.93-18.3). The first abnormal LFTs were identified at a median age of 12.3 years (IQR, 2.8-15.7 years). In this group, the median maximum ALT was 197.5 U/L (7.9× above normal); IQR, 132.5-420 (5.3× to 16.8× above normal), and median maximum AST 97 U/L (3.9× above normal); IQR, 78-242 (3.1× to 9.7× above normal). The median age at time of highest liver enzyme elevation was 23 years (IQR, 6.5-24.8).
Patients with PLI did not have evidence of transfusion-related iron overload in the liver. In the non-HSCT cohort, only 1 patient had evidence of hepatic steatosis visualized on imaging.
Progressive changes in liver enzymes in patients with FA with PLI
Figure 2A-D show representative graphs that illustrate some of the characteristics of PLI in patients with FA. The prolonged duration of transaminitis is illustrated, commonly having been observed for more than a decade. In addition, graphs are quite similar in those who had and those who had not received HSCT, supporting the hypothesis that PLI is a consequence of FA, not of HSCT.
Individual changes in liver enzymes in patients with FA. The average of 3 values for ALT (U/mL) and AST (U/mL) graphed against the patient’s age in years using a fourth-order smoothing polynomial. Actual values of GGT (U/L) were plotted against the patient’s age in years (green). Shown are representative examples of changes in ALT (U/mL), AST (U/mL), and GGT (U/L) in (A) a patient who received HSCT at age 10.3 years; and (B) a patient who received HSCT at age 5.1 years. (C) A patient who never received HSCT and had liver enzyme elevations starting at birth. (D) A patient who had received androgen therapy and liver enzyme abnormalities were first observed at age 14.3 years.
Individual changes in liver enzymes in patients with FA. The average of 3 values for ALT (U/mL) and AST (U/mL) graphed against the patient’s age in years using a fourth-order smoothing polynomial. Actual values of GGT (U/L) were plotted against the patient’s age in years (green). Shown are representative examples of changes in ALT (U/mL), AST (U/mL), and GGT (U/L) in (A) a patient who received HSCT at age 10.3 years; and (B) a patient who received HSCT at age 5.1 years. (C) A patient who never received HSCT and had liver enzyme elevations starting at birth. (D) A patient who had received androgen therapy and liver enzyme abnormalities were first observed at age 14.3 years.
Temporal changes in liver enzymes in persons with FA with PLI
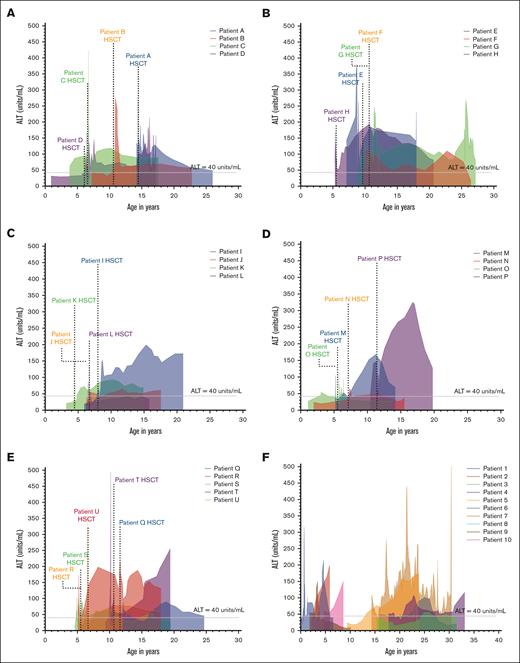
We plotted the average ALT (U/L) against patient age at the time the ALT was performed to further illustrate how liver injury for each patient fluctuated at different ages (Figure 3A-E). As expected, there was a temporary period of elevation in ALT in the immediate peritransplant period in recipients of HSCT. Notably, there were elevations in ALT present in some recipients of HSCT years before transplant, as seen in patients C, D, O, and M (Figure 3A,D); all 4 patients had ALT elevations first recorded prior to the age of 5 years. Outside of the peri-HSCT period, the majority of patients had variable chronic increases in ALT, which, for some patients, stabilized at an abnormal level (patient K and L; Figure 3C) or continued to worsen (patient I, Figure 3C; and patients M and P, Figure 3D). In patients with PLI who did not received HSCT (Figure 3F), there was a suggestion of a bimodal distribution of first observation of liver injury, with a subset of patients developing liver enzyme abnormalities early on in life, starting at birth or shortly thereafter (Patients 1, 2, and 10). In contrast, other patients developed ALT elevations starting in adolescent years (Patients 3-9). However, within both subsets of patients, the pattern of liver injury was quite variable.
Patterns of ALT variation in patients with FA with PLI. Changes in ALT in patients with FA with PLI are graphed and shown. Four patients are shown per graph for ease of comparison. The average of 3 values for ALT (U/mL) were used to graph these values against the patient’s age in years using a fourth-order smoothing polynomial. (A-E) ALT changes in each patient with FA who underwent HSCT and met criteria for PLI. The area under the curve is shaded for each patient and the time of stem cell infusion in recipients of HSCT are noted. (F) ALT changes in patients with PLI who did not receive HSCT.
Patterns of ALT variation in patients with FA with PLI. Changes in ALT in patients with FA with PLI are graphed and shown. Four patients are shown per graph for ease of comparison. The average of 3 values for ALT (U/mL) were used to graph these values against the patient’s age in years using a fourth-order smoothing polynomial. (A-E) ALT changes in each patient with FA who underwent HSCT and met criteria for PLI. The area under the curve is shaded for each patient and the time of stem cell infusion in recipients of HSCT are noted. (F) ALT changes in patients with PLI who did not receive HSCT.
Univariate and multivariate analyses of risk factors for PLI in recipients of HSCT
Univariate analysis identified exposure to TBI as the only variable associated with increased risk of PLI (P = .009) in recipients of HSCT (Table 4). Prior androgen exposure was not associated with PLI (P = .50).
Univariate analysis of clinically significant liver injury in individuals with FA who received HSCT
| Variable . | PLI∗ (n = 21) . | No PLI (n = 29) . | P value . |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 12 (57.1) | 19 (65.5) | .76 |
| Male | 9 (42.9) | 10 (34.5) | |
| Race, n (%) | |||
| Caucasian | 17 (81) | 23 (79.3) | .42 |
| African American | 3 (14.3) | 4 (13.8) | |
| Hispanic | 0 | 2 (6.9) | |
| Asian | 1 (4.8) | 0 | |
| Age (y) at HSCT, median (IQR) | 6.7 (5.5-10.3) | 10.1 (6.3-12.8) | .05 |
| Complementation group, n (%) | |||
| FANCA | 5 (29.4) | 12 (54.5) | .21 |
| Non-FANCA | 12 (70.6) | 10 (45.5) | |
| Androgen use, n (%) | |||
| Yes | 7 (33.3) | 6 (20.7) | .50 |
| No | 14 (66.7) | 23 (79.3) | |
| Donor type, n (%) | |||
| Related | 2 (9.5) | 9 (28.1) | .20 |
| Unrelated | 19 (90.5) | 23 (71.9) | |
| Degree of match, n (%) | |||
| Fully matched | 12 (57.1) | 16 (55.2) | .82 |
| Mismatched | In year 9 (42.9) | 13 (44.8) | |
| Donor source, n (%) | |||
| Bone marrow | 2 (9.5) | 5 (17.2) | .19 |
| PBSC | 17 (81) | 24 (82.8) | |
| Cord | 2 (9.5) | 0 | |
| TBI in conditioning regimen, n (%) | |||
| Yes | 14 (66.7) | 31 (96.9) | .009 |
| No | 7 (33.3) | 1 (3.1) | |
| GVHD prophylaxis, n (%) | |||
| CNI based | 11 (52.4) | 12 (37.5) | .65 |
| TCD | 2 (9.5) | 4 (12.5) | |
| Both CNI and TCD | 8 (38.1) | 15 (46.9) | |
| Other | 0 | 1 (3.1) | |
| Acute GVHD score at day 100, n (%) | |||
| Grades 1-4 | 0 | 2 (6.2) | .67 |
| GVHD with liver involvement, n (%) | 0 | 1 (3.1) | 1.00 |
| TA-TMA, n (%) | 4 (19.0) | 15 (46.9) | .08 |
| Variable . | PLI∗ (n = 21) . | No PLI (n = 29) . | P value . |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 12 (57.1) | 19 (65.5) | .76 |
| Male | 9 (42.9) | 10 (34.5) | |
| Race, n (%) | |||
| Caucasian | 17 (81) | 23 (79.3) | .42 |
| African American | 3 (14.3) | 4 (13.8) | |
| Hispanic | 0 | 2 (6.9) | |
| Asian | 1 (4.8) | 0 | |
| Age (y) at HSCT, median (IQR) | 6.7 (5.5-10.3) | 10.1 (6.3-12.8) | .05 |
| Complementation group, n (%) | |||
| FANCA | 5 (29.4) | 12 (54.5) | .21 |
| Non-FANCA | 12 (70.6) | 10 (45.5) | |
| Androgen use, n (%) | |||
| Yes | 7 (33.3) | 6 (20.7) | .50 |
| No | 14 (66.7) | 23 (79.3) | |
| Donor type, n (%) | |||
| Related | 2 (9.5) | 9 (28.1) | .20 |
| Unrelated | 19 (90.5) | 23 (71.9) | |
| Degree of match, n (%) | |||
| Fully matched | 12 (57.1) | 16 (55.2) | .82 |
| Mismatched | In year 9 (42.9) | 13 (44.8) | |
| Donor source, n (%) | |||
| Bone marrow | 2 (9.5) | 5 (17.2) | .19 |
| PBSC | 17 (81) | 24 (82.8) | |
| Cord | 2 (9.5) | 0 | |
| TBI in conditioning regimen, n (%) | |||
| Yes | 14 (66.7) | 31 (96.9) | .009 |
| No | 7 (33.3) | 1 (3.1) | |
| GVHD prophylaxis, n (%) | |||
| CNI based | 11 (52.4) | 12 (37.5) | .65 |
| TCD | 2 (9.5) | 4 (12.5) | |
| Both CNI and TCD | 8 (38.1) | 15 (46.9) | |
| Other | 0 | 1 (3.1) | |
| Acute GVHD score at day 100, n (%) | |||
| Grades 1-4 | 0 | 2 (6.2) | .67 |
| GVHD with liver involvement, n (%) | 0 | 1 (3.1) | 1.00 |
| TA-TMA, n (%) | 4 (19.0) | 15 (46.9) | .08 |
CNI, calcineurin inhibitor; PBSC, peripheral blood stem cell; TCD, T-cell depletion.
Persistent liver injury.
In multivariate analysis adjusted for sex, race, stem cell source, and donor age, younger age at HSCT was associated with reduced risk of PLI (odds ratio [OR], 0.84; 95% confidence interval [CI], 0.7-0.97; P = .04) (Table 5). An HSCT conditioning regimen incorporating TBI was significantly associated with increased risk of PLI (OR, 15.5; 95% CI, 2.44-304.54; P = .01). Mutations in non-FANCA complementation groups were not associated with increased risk of PLI. Patients with a history of transplant-associated thrombotic microangiopathy (TA-TMA) during transplant had reduced risk of PLI (OR, 0.27; 95% CI, 0.07-0.91; P = .045).
Multivariate analysis of PLI in individuals with FA who received HSCT
| Variable . | OR . | P value . |
|---|---|---|
| Age (y) at HSCT, median (IQR) | 0.84 (0.7-0.97) | .04 |
| Complementation group | ||
| Non-FANCA | 2.88 (0.78-11.76) | .12 |
| FANCA | 1 | |
| Conditioning regimen with TBI | ||
| Yes | 15.5 (2.44-304.54) | .01 |
| No | 1 | |
| TA-TMA | ||
| Yes | 0.27 (0.07-0.91) | .045 |
| No | 1 |
| Variable . | OR . | P value . |
|---|---|---|
| Age (y) at HSCT, median (IQR) | 0.84 (0.7-0.97) | .04 |
| Complementation group | ||
| Non-FANCA | 2.88 (0.78-11.76) | .12 |
| FANCA | 1 | |
| Conditioning regimen with TBI | ||
| Yes | 15.5 (2.44-304.54) | .01 |
| No | 1 | |
| TA-TMA | ||
| Yes | 0.27 (0.07-0.91) | .045 |
| No | 1 |
Univariate analysis of PLI in patients that did not receive HSCT
Univariate analysis was performed separately for patients that did not receive HSCT to distinguish liver injury in these patients because exposures to HSCT procedures can alter the natural time course of liver enzyme fluctuations (Table 6). Univariate analysis in the 10 patients with PLI that did not receive HSCT showed no association of sex, race, complementation group, and previous androgen use with risk of developing PLI. Multivariate analysis was not performed in this group because there were no findings in the univariate analysis and sample size is small.
Univariate analysis of clinically significant liver injury in individuals with FA who did not receive HSCT
| Variable . | PLI∗ (n = 10) . | No PLI (n = 37) . | P value . |
|---|---|---|---|
| Sex, n (%) | .67 | ||
| Female | 6 (60) | 17 (45.9) | |
| Male | 4 (40) | 20 (54.1) | |
| Race, n (%) | |||
| Caucasian | 10 (100) | 35 (94.6) | .75 |
| African American | 0 | 0 | |
| Hispanic | 0 | 1 (2.7) | |
| Asian | 0 | 1 (2.7) | |
| Complementation group, n (%) | |||
| FANCA | 5 (55.6) | 29 (80.6) | .26 |
| Non-FANCA | 4 (44.4) | 7 (19.4) | |
| Androgen use, n (%) | |||
| Yes | 8 (80) | 35 (94.6) | .41 |
| No | 2 (20) | 2 (5.4) |
| Variable . | PLI∗ (n = 10) . | No PLI (n = 37) . | P value . |
|---|---|---|---|
| Sex, n (%) | .67 | ||
| Female | 6 (60) | 17 (45.9) | |
| Male | 4 (40) | 20 (54.1) | |
| Race, n (%) | |||
| Caucasian | 10 (100) | 35 (94.6) | .75 |
| African American | 0 | 0 | |
| Hispanic | 0 | 1 (2.7) | |
| Asian | 0 | 1 (2.7) | |
| Complementation group, n (%) | |||
| FANCA | 5 (55.6) | 29 (80.6) | .26 |
| Non-FANCA | 4 (44.4) | 7 (19.4) | |
| Androgen use, n (%) | |||
| Yes | 8 (80) | 35 (94.6) | .41 |
| No | 2 (20) | 2 (5.4) |
Persistent liver injury.
End-stage liver disease
Two patients, both recipients of HSCT, are deceased from liver failure. The first patient died at another institution and data other than cause of death are limited. The second patient, shown in Figure 4, developed chronic cholestatic liver disease at the age of 23 years, 17 years after an uneventful HSCT. During the immediate post-HSCT period, the patient did not have any evidence of infectious or noninfectious liver injury including viral reactivation, veno-occlusive disease, thrombotic microangiopathy, or GVHD. Serial liver biopsies showed portal inflammation and bland cholestasis with no evidence of sclerosing cholangitis or GVHD. There was no biochemical or clinical response to trials of therapy with steroids, oral vancomycin, rifaximin, ursodeoxycholic acid, or cholestyramine, and she died of complications of end-stage liver disease, including hepatic hydrothorax and hepatorenal syndrome, at the age of 27 years. At autopsy she had biliary cirrhosis with segmental areas of bile duct paucity. The patient had received TBI (450 cGY) as part of her HSCT conditioning regimen.
Liver biopsy and postmortem findings in a recipient of HSCT with FA and with PLI. Shown are representative images from serial liver biopsy samples and postmortem analysis of liver sample in a recipient of HSCT with FA and with PLI. The patient had a liver biopsy at 15 years after HSCT to investigate transaminitis. (A-B) H&E staining shows inflammation of the portal tracts with associated cholangitis and pericholangitis and reactive changes in the bile ducts. Bridging fibrosis and focal nodule formation are seen. The patient had a repeat liver biopsy at 16-years post-HSCT. (C-D) Hematoxylin and eosin staining demonstrates acute cholangitis and pericholangitis with lobular cholestasis. Extensive fibrosis of the portal tracts is again seen. Postmortem examination of the liver, 1 year later (E-F) shows marked nodular hyperplasia, fibrosis, and ischemia. The portal tracts show reactive expansion secondary to mononuclear infiltrates and associated cholangitis and pericholangitis. (G-H) Trichrome staining shows portal to portal bridging fibrosis with a prominent perisinusoidal component. (I-J) CK7 immunostaining shows ductular proliferation with ectopic staining of hepatocytes.
Liver biopsy and postmortem findings in a recipient of HSCT with FA and with PLI. Shown are representative images from serial liver biopsy samples and postmortem analysis of liver sample in a recipient of HSCT with FA and with PLI. The patient had a liver biopsy at 15 years after HSCT to investigate transaminitis. (A-B) H&E staining shows inflammation of the portal tracts with associated cholangitis and pericholangitis and reactive changes in the bile ducts. Bridging fibrosis and focal nodule formation are seen. The patient had a repeat liver biopsy at 16-years post-HSCT. (C-D) Hematoxylin and eosin staining demonstrates acute cholangitis and pericholangitis with lobular cholestasis. Extensive fibrosis of the portal tracts is again seen. Postmortem examination of the liver, 1 year later (E-F) shows marked nodular hyperplasia, fibrosis, and ischemia. The portal tracts show reactive expansion secondary to mononuclear infiltrates and associated cholangitis and pericholangitis. (G-H) Trichrome staining shows portal to portal bridging fibrosis with a prominent perisinusoidal component. (I-J) CK7 immunostaining shows ductular proliferation with ectopic staining of hepatocytes.
Figure 4 shows liver biopsies and postmortem liver pathology of this patient. Liver biopsy performed for transaminitis 6 years after HSCT was interpreted as being consistent with cGVHD with loss of bile ducts in ∼50% of the portal tracts, a mixed inflammatory portal infiltrate, and focal periportal fibrosis (images not shown). The patient had no response to treatment with steroids or ursodeoxycholic acid. She continued to have PLI, and liver biopsy was repeated 15 years after HSCT. The liver biopsy again showed marked portal tract inflammation with neutrophil infiltrates. Surrounding bile ducts had reactive epithelium with hepatocellular canalicular cholestasis and reactive changes in hepatocytes. Hepatic portal fibrosis with bridging and focal nodule formation was also present (Figure 4A-B). This biopsy was interpreted as not being consistent with cGVHD and due to chronic liver disease of unclear etiology.
The patient progressed to end-stage liver disease 16 years after HSCT and liver biopsy was repeated. Mononuclear and neutrophilic infiltrates in the portal tracts with reactive injury to the bile ducts were again seen in the liver (Figure 4C-D). The patient ultimately died from multiorgan failure, a consequence of liver failure. Postmortem analysis of the liver showed extensive nodular hyperplasia, ischemia, and fibrosis, with mononuclear inflammatory infiltrates. The bile ducts showed damage and significant reactive epithelial changes (Figure 4E-F). Trichrome staining of the liver showed extensive fibrosis of the portal tracts and perisinusoidal regions (Figure 4G-H). CK7, which stains the bile ducts, showed relative bile ductopenia (Figure 4I-J). Iron stain was negative and viral inclusions (cytomegalovirus, JC virus, herpes simplex virus, and simian virus 40) were absent.
Discussion
We report frequent PLI in individuals with FA, including those who did and those who did not receive HSCT, which may be a newly identified aspect of the FA phenotype. We found that liver enzyme abnormalities are frequent in patients with FA, before, after, and without HSCT, and can be remarkably prolonged, observed for >15 years in some of our patients. In our overall cohort, 32% of all patients with FA had significant liver injury. For patients who received HSCT, PLI was seen in 42% of patients compared with 27% in the non-HSCT cohort. Transaminitis can start at young ages (even at birth), and progress throughout a patient’s life without a clear etiology other than the presence of FA, and can peak at up to 8 times the upper limit of normal regardless of HSCT status. Masserot-Lureau et al published the only previous report describing longitudinal changes in liver enzymes in FA and identified 12 of 64 patients (18.8%) with significant liver injury. These authors did not see increased risk of liver injury in those treated with anabolic steroids, in agreement with our findings. We were surprised that androgen exposure was not associated with increased risk of PLI in either study despite significant evidence in the literature that androgens are associated with liver injury.13,15 Our institution has used oxandrolone for treatment of bone marrow failure instead of the androgens danazol or oxymethalone, and oxandrolone may be associated with reduced liver toxicity.14,15,21 Improvements in HSCT outcomes mean that androgens are now less frequently used in FA.
We found increased risk for PLI in recipients of HSCT with exposure to TBI, likely reflecting the known increased sensitivity to radiation toxicity in persons with FA. In recent years, radiation has been largely eliminated from the conditioning regimen used for FA, commonly being replaced with busulfan.19 This might be attributable to the fact that busulfan can be monitored with pharmacokinetic levels to achieve both stable donor engraftment but avoid excessive toxicity in patients with FA, whereas TBI cannot. At our institution, we target an optimal busulfan steady-state concentration level of ≤350 ng/mL for patients with FA undergoing HSCT, which is significantly lower than the optimal target for patients without FA who receive full myeloablative busulfan doses ranging between 817 and 1050 ng/mL.22 Further work is urgently needed to identify the mechanism of liver injury and to develop treatment and preventive therapies, and we are embarking on those studies.
The reduced risk of PLI associated with occurrence of TA-TMA is puzzling and needs to be replicated, because there may be unrecognized confounding factors. It is important to recognize that transaminitis occurred before, as well as after, HSCT and in those who had not received HSCT, so although TBI exposure increases risk it does not explain all, or even most, of the transaminitis that seems more likely to reflect the diagnosis of FA itself. One potential hypothesis for this observation that TA-TMA is associated with less PLI might be a surrogate marker for receiving a preparative regimen including TBI, because TBI and busulfan are mutually exclusive. If this was correct, we would expect TA-TMA to be more frequent in those who received busulfan compared with in those that received TBI. In this cohort, none of the patients who received TBI developed TA-TMA; however, 47.2% of those who received busulfan developed TA-TMA after HSCT.
Interestingly, we did not observe any increased incidence of liver injury in recipients of HSCT who received busulfan as part of their conditioning regimen. Busulfan has known hepatotoxicity and has been associated with increased rates of veno-occlusive disease in recipients of HSCT.23 Our institution has adopted a radiation-free conditioning regimen using a T-cell–depleted graft to eliminate radiation exposure and minimize early and late toxicities of transplant.19,24 The reduced risk of PLI in patients who received busulfan may be attributable to the use of busulfan-based precision pharmacokinetic dosing to target a lower steady-state concentration of ≤350 ng/mL, which still leads to successful engraftment rates and limits toxicity after HSCT in patients with FA.20
The success of transplantation has increased survival into adulthood for patients with FA, and it appears likely that clinical consequences of liver dysfunction will be observed more frequently as the adult FA population expands. We have tried immune suppression (largely steroids), antibiotics for possible cholangitis, and ursodiol without success, although numbers of cases are currently small. Liver transplant could alleviate the morbidity and mortality of liver failure in patients but is not routinely offered to patients with FA because of cancer predisposition and additional comorbidities. However, there has been 1 successful case of sequential liver transplant with HSCT reported in a pediatric patient with FANCD2 mutation.25 An iterative approach to testing possible therapies, ideally starting early in the course of liver injury, is needed to identify effective therapy. Patients with FA would benefit from regular annual visits with a bone marrow failure specialist familiar with FA, which should include laboratory evaluations of hepatic transaminases. Persistent elevations in transaminases would warrant a referral to a gastroenterologist who may provide further guidance on screening, frequency of monitoring, and other diagnostic modalities including imaging or liver biopsy.
The progressive liver injury seen in several of our patients that led to end-stage liver disease and death in 2 of our cohort, seems to be centered on the intrahepatic bile ducts. Clinically, patients have cholestatic liver disease, with only modest elevation in serum aminotransferases and no increase in bilirubin until late in the disease. As the disease progresses, patients experience the symptomatology of patients with cholestatic liver disease: deep jaundice, intense pruritis, fat soluble vitamin deficiency, and fat malabsorption. Several liver biopsies in our cohort were interpreted as “consistent with chronic liver GVHD,” but on closer inspection and/or repeat biopsy this interpretation changed. Clinical context is crucial, the lack of other organ involvement, the long period since transplant, and our own experience with failure of treatment directed toward GVHD makes an alternative etiology for progressive cholangiopathy likely in this population.
Mechanisms leading to liver disease and dysfunction in patients with FA are not well characterized. FANCD2-deficient mice fed a diet enriched in fat, cholesterol, and cholic acid (Paigen diet) developed extensive hepatobiliary disease and had decreased survival compared with non–FAND2-deficient mice fed a high-fat diet.26 Male mice fed the Paigen diet had marked biliary hyperplasia, elevated serum bile acid concentrations, and hepatic inflammation compared with mice fed a high-fat diet. Female mice lacking FANCD2 had significant hepatobiliary disease regardless of diet.26 These data suggest that the FA pathway may be involved in hepatobiliary metabolism, which may contribute to the liver disease in individuals with FA. It is not known whether this observation is specific to mice with FANCD2 mutations. Our data show no clear association of complementation group and risk of developing PLI in either the HSCT or non-HSCT cohorts. However, our analysis is limited by the number of patients, with the majority of patients having mutations in the FANCA complementation group.
We also considered whether the incidence of PLI was only specific to patients with FA. We reviewed our institution’s experience over the last 20 years for transplants for acquired aplastic anemia. During this time period, 87 patients completed transplant and 15 patients died. Only 2 of these patients died from multiorgan failure involving the kidney, lungs, and heart, secondary to overwhelming sepsis and TA-TMA. These data do not support the hypothesis that PLI is caused by aplastic anemia outside of the setting of FA.
Our work has strengths and limitations. The strengths include the relatively large number of patients with FA treated with HSCT at a single institution and the long length of observation. The retrospective nature of the study is a limitation and available data vary among patients, some of whom traveled to Cincinnati from great distances. Systematic prospective observational and interventional studies will be key to better understanding and treatment of PLI in FA.
In conclusion, our data show that PLI is frequent in patients with FA. Patients with FA and associated PLI require expert multidisciplinary care and management. Collaborative multicenter strategies and trials to better describe and treat PLI in FA are needed.
Acknowledgments
The authors thank Leann Mount, Michelle Harris, Kaitlin Brooks, Melissa Hunter, and Alison O’Conner for their contribution and dedication toward the patients’ care.
Authorship
Contribution: S.M.D., K.C.M., and J.K. were responsible for study concept; A.J.S., S.M.D., J.K., K.M., K.M.C., and P.A.M. were responsible for the study methodology; A.J.S. and J.K. performed investigation; S.M.D. and J.K. were responsible for visualization; S.M.D., J.K., K.M., K.M.C., and P.A.M. were responsible for project administration; S.M.D., K.M., and J.K. supervised the study; and J.K., S.M.D., K.M., A.J.S., P.A.M., and K.M.C. wrote the original draft, and reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jane Koo, Division of Bone Marrow Transplantation and Immune Deficiency, Cancer and Blood Disease Institute, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, MLC 11027, Cincinnati, OH 45229; email: jane.koo@cchmc.org.
References
Author notes
Data are available on request from the corresponding author, Jane Koo (jane.koo@cchmc.org).