We developed the CoBRA score.
The CoBRA score could predict the NRM risk as well as OS after UCBT.
Visual Abstract
Higher rate of nonrelapse mortality (NRM) remains yet to be resolved in umbilical cord blood transplantation (UCBT). Considering that UCBT has some unique features compared with allogeneic hematopoietic cell transplantation from other graft sources, a UCBT-specific NRM risk assessment system is required. Thus, in this study, we sought to develop a UCBT-specific NRM Risk Assessment (CoBRA) score. Using a nationwide registry database, we retrospectively analyzed 4437 recipients who had received their first single-unit UCBT. Using the backward elimination method, we constructed the CoBRA score in a training cohort (n = 2687), which consisted of recipients age ≥55 years (score 2), hematopoietic cell transplantation–specific comorbidity index ≥3 (score 2), male recipient, graft-versus-host disease prophylaxis other than tacrolimus in combination with methotrexate, performance status (PS) 2 to 4, HLA allele mismatch ≥ 2, refined Disease Risk Index high risk, myeloablative conditioning, and CD34+ cell doses < 0.82 × 105/kg (score 1 in each). The recipients were categorized into 3 groups: low (0-4 points), intermediate (5-7 points), and high (8-11 points) groups according to the CoBRA score. In the validation cohort (n = 1750), the cumulative incidence of NRM at 2 years was 14.9%, 25.5%, and 47.1% (P < .001), and 2-year overall survival (OS) was 74.2%, 52.7%, and 26.3% (P < .001) in the low, intermediate, and high groups, respectively. In summary, the CoBRA score could predict the NRM risk as well as OS after UCBT. Further external validation will be needed to confirm the significance of the CoBRA score.
Introduction
Umbilical cord blood (UCB) is used as an alternative donor source when an HLA-matched donor is unavailable or urgent transplantation is needed.1,2 Although comparable overall survival (OS) could be expected in umbilical cord blood transplantation (UCBT) compared with in bone marrow transplantation (BMT) and peripheral blood stem cell transplantation (PBSCT), the rate of nonrelapse mortality (NRM) in UCBT is still very high.3-6 Various risk factors for NRM in UCBT have been proposed, and previous studies have evaluated the impact of each risk factor.7-12 However, recipients in the real world often have multiple risk factors, and it is not yet clear how different combinations of risk factors affect clinical outcomes. In contrast to BMT or PBSCT, UCBT has unique features, such as limited cell doses, a lower risk of acute and chronic graft-versus-host disease (GVHD), tolerance of HLA mismatch, and a higher risk of graft failure.1,13-15 However, previous risk assessment systems for NRM after allogeneic hematopoietic cell transplantation (allo-HCT) were not limited to UCBT.16-21 Thus, we focused on UCBT and aimed to evaluate the risks for NRM using a nationwide registry database. Finally, we propose a UCBT-specific NRM Risk Assessment (CoBRA) score.
Methods
Recipients
We retrospectively analyzed clinical outcomes for recipients with acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), myelodysplastic syndrome, myeloproliferative neoplasms, and adult T-cell leukemia-lymphoma (ATL). We only included those who were aged 16 years or older and who received their first allo-HCT from single-unit UCB between 2008 and 2020 (n = 8540). In this period, the cumulative incidences of NRM did not change according to transplant year, whereas OS, progression-free survival (PFS), and the cumulative incidences of relapse were improved with time (supplemental Figure 1). We excluded those who received both methotrexate (MTX) and mycofenolate mofetil (MMF) with a calcineurin inhibitor, or calcineurin inhibitor alone for GVHD prophylaxis (n = 785). We also excluded recipients in the very high–risk group according to the refined Disease Risk Index (DRI; n = 858).21 Because the original refined DRI does not include ATL, we defined ATL in complete remission as high risk. ATL in noncomplete remission was considered to be very high risk and was excluded from this analysis.22 In addition, those with missing data for HLA genotypes (n = 911), donor (n = 740) and recipient sex (n = 3), CD34+ cell doses (n = 343), granulocyte-monocyte colony forming unit (CFU-GM; n = 233), clinical outcomes (n = 94), hematopoietic cell transplantation–specific comorbidity index (HCT-CI; n = 58), GVHD prophylaxis (n = 45), refined DRI (n = 26), or performance status (PS; n = 7) were excluded. As a result, 4437 cases met the inclusion criteria (supplemental Figure 2; Table 1). Clinical data were obtained from the nationwide registry database of the Japan Society for Transplantation and Cellular Therapy (JSTCT).23 This study was approved by the Institutional Review Board of Jichi Medical University Saitama Medical Center in accordance with the Declaration of Helsinki.
Clinical characteristics
| Variable . | Training cohort . | Validation cohort . | P value∗ . |
|---|---|---|---|
| n = 2687 . | n = 1750 . | ||
| Age (%) | |||
| <55 y | 1220 (45.4) | 786 (44.9) | .76 |
| ≥55 y | 1467 (54.6) | 964 (55.1) | |
| Recipient sex (%) | |||
| Male | 1538 (57.2) | 970 (55.4) | .24 |
| Female | 1149 (42.8) | 780 (44.6) | |
| Disease (%) | |||
| AML | 1524 (56.7) | 964 (55.1) | .27 |
| ALL | 403 (15.0) | 291 (16.6) | |
| MDS | 423 (15.7) | 304 (17.4) | |
| MPN | 107 (4.0) | 76 (4.3) | |
| ATL | 230 (8.6) | 115 (6.6) | |
| Refined DRI (%) | |||
| Low | 153 (5.7) | 68 (3.9) | .002 |
| Intermediate | 1277 (47.5) | 905 (51.7) | |
| High | 1257 (46.8) | 777 (44.4) | |
| GVHD prophylaxis (%) | |||
| Tac/MTX | 1056 (39.3) | 680 (38.9) | <.001 |
| Tac/MMF | 965 (35.9) | 780 (44.6) | |
| CsA/MTX | 590 (22.0) | 238 (13.6) | |
| CsA/MMF | 76 (2.8) | 52 (3.0) | |
| HCT-CI (%) | |||
| 0-2 | 2099 (78.1) | 1359 (77.7) | .74 |
| ≥3 | 588 (21.9) | 391 (22.3) | |
| HLA mismatch (%) | |||
| 0-1 | 234 (8.7) | 169 (9.7) | .29 |
| ≥2 | 2453 (91.3) | 1581 (90.3) | |
| PS (%) | |||
| 0-1 | 2425 (90.2) | 1631 (93.2) | .001 |
| 2-4 | 262 (9.8) | 119 (6.8) | |
| Conditioning (%) | |||
| Myeloablative | 1766 (65.7) | 1099 (62.8) | .05 |
| Reduced intensity | 921 (34.3) | 651 (37.2) | |
| CD34+cell doses (%) | |||
| ≥0.82 × 105/kg | 1350 (50.2) | 945 (54.0) | .015 |
| <0.82 × 105/kg | 1337 (49.8) | 805 (46.0) | |
| CFU-GM (%) | |||
| ≥25 × 103/kg | 1361 (50.7) | 853 (48.7) | .22 |
| <25 × 103/kg | 1326 (49.3) | 897 (51.3) | |
| TBI (%) | 2053 (76.4) | 1097 (62.7) | <.001 |
| Variable . | Training cohort . | Validation cohort . | P value∗ . |
|---|---|---|---|
| n = 2687 . | n = 1750 . | ||
| Age (%) | |||
| <55 y | 1220 (45.4) | 786 (44.9) | .76 |
| ≥55 y | 1467 (54.6) | 964 (55.1) | |
| Recipient sex (%) | |||
| Male | 1538 (57.2) | 970 (55.4) | .24 |
| Female | 1149 (42.8) | 780 (44.6) | |
| Disease (%) | |||
| AML | 1524 (56.7) | 964 (55.1) | .27 |
| ALL | 403 (15.0) | 291 (16.6) | |
| MDS | 423 (15.7) | 304 (17.4) | |
| MPN | 107 (4.0) | 76 (4.3) | |
| ATL | 230 (8.6) | 115 (6.6) | |
| Refined DRI (%) | |||
| Low | 153 (5.7) | 68 (3.9) | .002 |
| Intermediate | 1277 (47.5) | 905 (51.7) | |
| High | 1257 (46.8) | 777 (44.4) | |
| GVHD prophylaxis (%) | |||
| Tac/MTX | 1056 (39.3) | 680 (38.9) | <.001 |
| Tac/MMF | 965 (35.9) | 780 (44.6) | |
| CsA/MTX | 590 (22.0) | 238 (13.6) | |
| CsA/MMF | 76 (2.8) | 52 (3.0) | |
| HCT-CI (%) | |||
| 0-2 | 2099 (78.1) | 1359 (77.7) | .74 |
| ≥3 | 588 (21.9) | 391 (22.3) | |
| HLA mismatch (%) | |||
| 0-1 | 234 (8.7) | 169 (9.7) | .29 |
| ≥2 | 2453 (91.3) | 1581 (90.3) | |
| PS (%) | |||
| 0-1 | 2425 (90.2) | 1631 (93.2) | .001 |
| 2-4 | 262 (9.8) | 119 (6.8) | |
| Conditioning (%) | |||
| Myeloablative | 1766 (65.7) | 1099 (62.8) | .05 |
| Reduced intensity | 921 (34.3) | 651 (37.2) | |
| CD34+cell doses (%) | |||
| ≥0.82 × 105/kg | 1350 (50.2) | 945 (54.0) | .015 |
| <0.82 × 105/kg | 1337 (49.8) | 805 (46.0) | |
| CFU-GM (%) | |||
| ≥25 × 103/kg | 1361 (50.7) | 853 (48.7) | .22 |
| <25 × 103/kg | 1326 (49.3) | 897 (51.3) | |
| TBI (%) | 2053 (76.4) | 1097 (62.7) | <.001 |
MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasms.
To calculate the P value, Fisher exact test and χ2 test were used to compare 2 groups and ≥3 groups between the training and validation cohort, respectively.
Definitions of clinical outcomes
The primary end point was NRM, and the secondary end point was OS. Exploratory end points included relapse, neutrophil engraftment, platelet recovery, and acute and chronic GVHD. NRM was defined as death from any cause in the sustained status of complete response. OS was defined as the time from the day of UCBT to death from any cause. PFS was calculated from the day of UCBT to disease progression or death from any cause. Relapse was defined as the onset of hematologic recurrence. Neutrophil engraftment was defined as the first of 3 consecutive days when the absolute neutrophil count was >0.5 × 109/L. Platelet recovery was defined as the first of 7 consecutive days when the platelet count was >50 × 109/L since the last platelet transfusion. The diagnosis and severity of acute GVHD were reported based on traditional grading scores, whereas those of chronic GVHD were reported based on the classical Seattle criteria.24,25 Recipients were censored at the time of last follow-up. Conditioning regimens were classified as myeloablative conditioning (MAC) or reduced-intensity conditioning (RIC) based on the report by Giralt et al.26 Briefly, conditioning regimens that included total body irradiation (TBI) >8 Gy, melphalan ≥ 140 mg/m2, or oral busulfan ≥ 9 mg/kg (IV busulfan ≥ 7.2 mg/kg) were classified as MAC, and other regimens were classified as RIC. HCT-CI was calculated according to a previous report.20 HLA matching was evaluated using the HLA-A, HLA-B, HLA-C, and HLA-DRB1 genotypes. The causes of death were determined based on the primary cause of death reported by the attending physicians. When the secondary cause of death was GVHD, relapse, or graft failure, the cause of death was reclassified as GVHD, relapse, or graft failure, respectively.
Statistical analysis
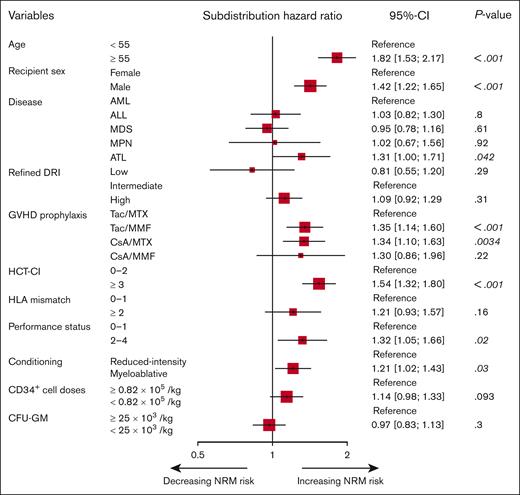
Categorical variables were compared using Fisher exact test. OS was calculated by the Kaplan-Meier method with a 95% confidence interval (CI) and compared using the log-rank test. The cumulative incidences of NRM, relapse, neutrophil and platelet engraftment, and acute, chronic, and extensive chronic GVHD were compared using Gray test to accommodate competing risks. NRM was found to be a competing risk for relapse and vice versa. Competing risks for neutrophil engraftment and platelet recovery were defined as death from any cause before each event occurred and that for GVHD was defined as relapse and death from any cause before the occurrence of GVHD. The chronic GVHD analyses included recipients who survived >100 days without relapse. Based on clinical significance, we included predetermined all covariates in a multivariable analysis. A Fine and Gray proportional hazards regression model for NRM was applied using the following clinical variables: age (<55 vs ≥ 55 years), recipient sex (female vs male), disease (AML vs ALL vs myelodysplastic syndrome vs myeloproliferative neoplasms vs ATL), PS (0-1 vs 2-4), HCT-CI (0-2 vs ≥3), refined DRI (low- vs intermediate vs high risk), GVHD prophylaxis (tacrolimus [Tac] in combination with MTX [Tac/MTX] vs cyclosporine A [CsA]/MTX vs Tac/MMF vs CsA/MMF), HLA allele mismatch (0-1 vs ≥2), conditioning regimen (MAC vs RIC), CD34+ cell doses (<0.82 × 105/kg vs ≥0.82 × 105/kg), and CFU-GM (<25 × 103/kg vs ≥25 × 103/kg). Because recipient sex seemed more influential on NRM compared with recipient and donor sex incompatibility, we used recipient sex as a covariate for multivariable analyses (supplemental Figure 3). Age, CD34+ cell doses, and CFU-GM were divided according to the value around the median. In the multivariable analysis for developing the CoBRA score, we summarized variables regarding refined DRI (low- and intermediate- vs high risk) and GVHD prophylaxis (Tac/MTX vs CsA/MTX, Tac/MMF, and CsA/MMF) based on the result of the multivariable analysis for NRM performed in advance (Figure 1).
Multivariable analysis for NRM in the training cohort. A Fine and Gray proportional hazards regression model was used to evaluate the effects of covariates on the cumulative incidence of NRM. MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasms.
Multivariable analysis for NRM in the training cohort. A Fine and Gray proportional hazards regression model was used to evaluate the effects of covariates on the cumulative incidence of NRM. MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasms.
The CoBRA score was developed as follows: first, using a training cohort of allo-HCT between 2008 and 2017, we selected covariates based on the multivariable analysis for NRM. Modeling fitness was compared using Akaike information criterion (AIC) because AIC-based model selection would be expected to have the lowest error in the validation cohort.27 Second, we rounded subdistribution hazard ratio, assigned adverse points to the variables, and developed the CoBRA score. Finally, the score was temporally validated using a validation cohort of recent allo-HCT between 2018 and 2020. Using the calibration plot, we also assessed the agreement between the observed NRM at 2 years after UCBT and the prediction by the CoBRA score.28 When a 2-tailed P value < .05 was obtained, we considered that this indicated statistical significance. Data manipulations and statistical approaches were conducted using EZR version 1.61 (Jichi Medical University at http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmed.html).29 This study adhered to the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis statement for reporting.30
Results
Clinical characteristics in the training cohort
In the training cohort (n = 2687), AML was the most common disease (56.7%). Regarding refined DRI, most of the recipients were in the intermediate- (47.5%) or high-risk group (46.8%). Tac/MTX (39.3%) was commonly used for GVHD prophylaxis. MAC was used in more than half of the recipients (65.7%), especially in those who were aged <55 years (86.6%) in contrast to those who were aged ≥55 years (48.4%). Three-quarters (76.4%) of the recipients received TBI in the conditioning regimen.
Development of the CoBRA score
The cumulative incidence of NRM in the training cohort at 2 years after UCBT was 26.7% (95% CI, 25.0-28.4). In a multivariable analysis, older age, male recipient, ATL, GVHD prophylaxis with Tac/MMF or CsA/MTX, higher HCT-CI score, worse PS, and MAC were significantly associated with NRM. Lower CD34+ cell doses and multiple HLA mismatches also tended to be associated with a higher incidence of NRM (Figure 1).
Using the backward elimination method based on AIC, we constructed the CoBRA score. Because eliminating disease and CFU-GM from the multivariable model with all aforementioned variables could result in a decreased AIC, we determined to exclude these factors from the model. Thereafter, we again performed a multivariable analysis for NRM using the remaining covariates other than disease and CFU-GM and assigned the weight for the CoBRA score based on the final model (Table 2). The CoBRA score was calculated as the sum of the subdistribution hazard ratio-weighted score, which comprised age ≥55 years (score 2), HCT-CI ≥ 3 (score 2), male recipient (score 1), GVHD prophylaxis other than Tac/MTX (score 1), PS 2-4 (score 1), HLA mismatch ≥ 2 (score 1), refined DRI high-risk (score 1), MAC (score 1), and CD34+ cell doses < 0.82 × 105/kg (score 1). Recipients were subsequently assigned a sum of each score: 0 to 3 (n = 466), 4 (n = 516), 5 (n = 552), 6 (n = 451), 7 (n = 319), 8 (n = 198), and 9-11 (n = 185). The cumulative incidence of NRM at 2 years was 13.9% (10.9-17.3), 18.0% (14.8-21.5), 22.3% (18.9-25.9), 29.8% (25.6-34.1), 37.2% (31.9-42.6), 42.6% (35.5-49.4), and 52.9% (45.4-59.9), respectively (P < .001; supplemental Table 1). Thus, the recipients were categorized into 3 risk groups based on the margin of differences in 2-year NRM: low 0 to 4 points (<20%), intermediate 5 to 7 points (20%-40%), and high 8 to 11 points (>40%) in the CoBRA score.
SHR-weighted scoring for the development of the CoBRA score
| . | SHR . | 95% CI . | P value . | Scoring∗ . |
|---|---|---|---|---|
| Age ≥55 y | 1.81 | 1.54-2.14 | <.001 | 2 |
| HCT-CI ≥3 | 1.51 | 1.29-1.76 | <.001 | 2 |
| Male recipient | 1.40 | 1.21-1.63 | <.001 | 1 |
| GVHD prophylaxis other than Tac/MTX | 1.36 | 1.16-1.58 | <.001 | 1 |
| PS 2-4 | 1.29 | 1.03-1.62 | .028 | 1 |
| HLA mismatch ≥2 | 1.22 | 0.94-1.59 | .14 | 1 |
| Refined DRI high-risk | 1.18 | 1.02-1.36 | .027 | 1 |
| MAC | 1.15 | 0.98-1.35 | .088 | 1 |
| CD34+ cell doses <0.82 × 105/kg | 1.12 | 0.98-1.29 | .10 | 1 |
| . | SHR . | 95% CI . | P value . | Scoring∗ . |
|---|---|---|---|---|
| Age ≥55 y | 1.81 | 1.54-2.14 | <.001 | 2 |
| HCT-CI ≥3 | 1.51 | 1.29-1.76 | <.001 | 2 |
| Male recipient | 1.40 | 1.21-1.63 | <.001 | 1 |
| GVHD prophylaxis other than Tac/MTX | 1.36 | 1.16-1.58 | <.001 | 1 |
| PS 2-4 | 1.29 | 1.03-1.62 | .028 | 1 |
| HLA mismatch ≥2 | 1.22 | 0.94-1.59 | .14 | 1 |
| Refined DRI high-risk | 1.18 | 1.02-1.36 | .027 | 1 |
| MAC | 1.15 | 0.98-1.35 | .088 | 1 |
| CD34+ cell doses <0.82 × 105/kg | 1.12 | 0.98-1.29 | .10 | 1 |
SHR, subdistribution hazard ratio.
We constructed the CoBRA score using the backward elimination method. Eliminating disease and CFU-GM from the multivariable model with all aforementioned variables could result in a decreased AIC. Thus, we determined to exclude these factors from the model, and performed a multivariable analysis for NRM using the remaining covariates. Finally, SHR-weighted scoring was assigned to the variables.
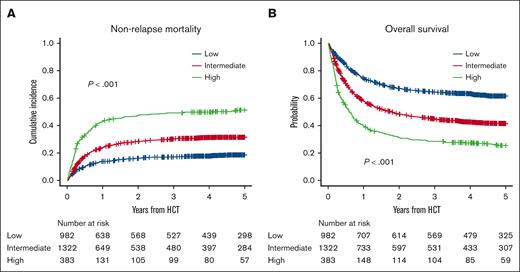
In the training cohort, the cumulative incidence of NRM at 2 years after UCBT was 16.1% (95% CI, 13.9-18.5) in the low, 28.5% (95% CI, 26.1-31.0) in the intermediate, and 47.6% (95% CI, 42.4-52.5) in the high CoBRA score group, respectively (P < .001; Figure 2A). The 2-year OS was 67.0% (95% CI, 63.9-69.9) in the low, 48.3% (95% CI, 45.5-51.0) in the intermediate, and 31.3% (26.6-36.0) in the high CoBRA score group, respectively (P < .001; Figure 2B). The 2-year PFS was 61.1% (95% CI, 58.0-64.1) in the low, 42.9% (95% CI, 40.2-45.6) in the intermediate, and 29.0% (24.4-33.6) in the high CoBRA score group, respectively (P < .001; supplemental Figure 4A). The cumulative incidence of relapse at 2 years after UCBT was higher in the intermediate group (28.6% [95% CI, 26.2-31.1]), whereas those in the low (22.8% [95% CI, 20.2-25.5]) and high CoBRA score groups (23.5% [95% CI, 19.3-27.9]) seemed to be similar (P = .0045; supplemental Figure 4B).
NRM and OS in the training cohort. (A) The cumulative incidence of NRM and (B) OS in the low-, intermediate-, and high-score groups.
NRM and OS in the training cohort. (A) The cumulative incidence of NRM and (B) OS in the low-, intermediate-, and high-score groups.
Validation of the CoBRA score
The validation cohort (n = 1750) included slightly more recipients with a refined DRI intermediate risk compared with the training cohort (51.7% vs 47.5%; P = .002). More recipients received GVHD prophylaxis with Tac/MMF (44.6% vs 35.9%), whereas fewer received CsA/MTX (13.6% vs 22.0%; P < .001). In addition, the validation cohort included more recipients with better PS (93.2% vs 90.2%, P = .001), those with infused CD34+ cell doses ≥0.82 × 105/kg (54.0% vs 50.2%; P = .015), and a lower frequency of TBI (62.7 vs 76.4%; P < .001; Table 1).
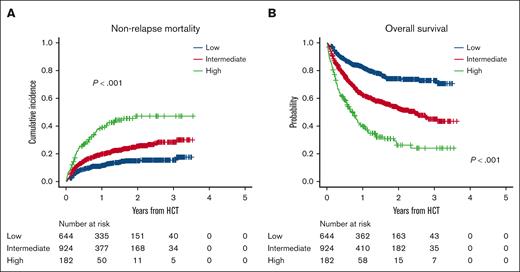
In this cohort, 644 (36.8%), 924 (52.8%), and 182 (10.4%) patients were categorized into the low, intermediate, and high CoBRA score groups, respectively. The CoBRA score could well stratify NRM in the validation cohort: the cumulative incidence of NRM at 2 years after UCBT was 14.9% (95% CI, 12.0-18.2) in the low, 25.5% (95% CI, 22.4-28.8) in the intermediate, and 47.1% (95% CI, 38.6-55.2) in the high CoBRA score groups, respectively (P < .001; Figure 3A; supplemental Table 2). Similarly, OS could also be stratified according to the CoBRA score in the validation cohort. The 2-year OS was 72.4% (95% CI, 69.9-77.9) in the low, 52.7% (95% CI, 48.8-56.5) in the intermediate, and 26.3% (95% CI, 18.6-34.5) in the high CoBRA score groups, respectively (P < .001; Figure 3B). The 2-year PFS was 69.7% (95% CI, 65.5-73.5) in the low, 47.9% (95% CI, 44.1-51.6) in the intermediate, and 21.4% (95% CI, 14.4-29.3) in the high CoBRA score group, respectively (P < .001; supplemental Figure 4C). The cumulative incidence of relapse at 2 years after UCBT showed a different trend compared with the training cohort. The cumulative incidence of relapse was lower in the low CoBRA score group (15.4% [95% CI, 12.4-18.6]), whereas those in the intermediate (26.6% [95% CI, 23.5-29.7]) and high groups (31.5% [95% CI, 24.0-39.3]) were similar (P < .001; supplemental Figure 4D). NRM and OS were well stratified by the CoBRA score in the validation cohort, excluding ATL (n = 1635; supplemental Figure 5).
NRM and OS in the validation cohort. (A) The cumulative incidence of NRM and (B) OS in the low-, intermediate-, and high-score groups.
NRM and OS in the validation cohort. (A) The cumulative incidence of NRM and (B) OS in the low-, intermediate-, and high-score groups.
The calibration plot suggested that the CoBRA score was well fitted to the prediction of NRM in the validation cohort, whereas this model slightly underestimated and overestimated the NRM risk in the intermediate and high groups, respectively (supplemental Figure 6).
Conditioning-based interaction analysis
In the entire cohort, the multivariable Cox regression analysis for NRM showed a weak interaction between refined DRI high risk or PS between 2 and 4 and conditioning regimen (supplemental Figure 7). The adverse impact of refined DRI high risk or PS between 2 and 4 might be strong in the RIC group.
Engraftment, GVHD, and causes of death
The cumulative incidence of neutrophil engraftment 30 days after UCBT was significantly lower in the high CoBRA score group (76.6% [95% CI, 72.9-79.9]) compared with the low (82.5% [95% CI, 80.6-84.3]) and intermediate groups (81.1% [95% CI, 79.5-82.7]; supplemental Figure 8A). Similarly, the cumulative incidence of platelet engraftment 100 days after UCBT was significantly lower in the high CoBRA score group (47.1% [95% CI, 42.9-51.1]) than in the low (75.0% [95% CI, 72.8-77.1]) and intermediate groups (65.5% [95% CI, 63.5-67.4]; supplemental Figure 8B).
The cumulative incidence of grade 2 to 4 acute GVHD 100 days after UCBT in the high CoBRA score group was 42.5% (95% CI, 38.4-46.6), which was higher than that in the low group (33.4% [95% CI, 31.1-35.7]) and comparable with that in the intermediate group (40.5% [95% CI, 38.5-42.6]; P < .001; supplemental Figure 9A). The cumulative incidence of grade 3 to 4 acute GVHD 100 days after UCBT was also higher in the high CoBRA score group (17.0% [95% CI, 14.0-20.2]) than in the low (7.8% [95% CI, 6.5-9.1]) and intermediate groups (12.3% [95% CI, 11.0-13.7]; P < .001; supplemental Figure 9B). In contrast, the cumulative incidences of chronic and extensive chronic GVHD were not different between the CoBRA score groups (P = .39 for chronic GVHD and P = .44 for extensive chronic GVHD; supplemental Figure 9C-D).
Regarding causes of death, fatal infections were more likely in the high CoBRA score group (28.5%) than in the low (13.9%) and intermediate groups (16.5%), whereas the percentage of deaths due to graft failure were lower in the high CoBRA score group (3.1%) than in the low (6.5%) and intermediate groups (5.4%, Table 3).
Causes of death in the entire cohort
| . | CoBRA score . | P value∗ . | ||
|---|---|---|---|---|
| Causes of death . | Low . | Intermediate . | High . | |
| n = 504 . | n = 1120 . | n = 390 . | ||
| Infection | 70 (13.9) | 185 (16.5) | 111 (28.5) | <.001 |
| Bacterial | 30 (6.0) | 93 (8.3) | 56 (14.4) | |
| Fungal | 17 (3.4) | 44 (3.9) | 23 (5.9) | |
| Viral | 14 (2.8) | 27 (2.4) | 14 (3.6) | |
| Unknown | 9 (1.8) | 21 (1.9) | 18 (4.6) | |
| Relapse | 210 (41.7) | 467 (41.7) | 106 (27.2) | |
| Organ failure | 41 (8.1) | 87 (7.8) | 42 (10.8) | |
| GVHD | 46 (9.1) | 114 (10.2) | 36 (9.2) | |
| Noninfectious lung disease | 25 (5.0) | 53 (4.7) | 24 (6.2) | |
| Graft failure | 33 (6.5) | 61 (5.4) | 12 (3.1) | |
| Hemorrhage | 21 (4.2) | 32 (2.9) | 12 (3.1) | |
| Others | 58 (11.5) | 121 (10.8) | 47 (12.1) | |
| . | CoBRA score . | P value∗ . | ||
|---|---|---|---|---|
| Causes of death . | Low . | Intermediate . | High . | |
| n = 504 . | n = 1120 . | n = 390 . | ||
| Infection | 70 (13.9) | 185 (16.5) | 111 (28.5) | <.001 |
| Bacterial | 30 (6.0) | 93 (8.3) | 56 (14.4) | |
| Fungal | 17 (3.4) | 44 (3.9) | 23 (5.9) | |
| Viral | 14 (2.8) | 27 (2.4) | 14 (3.6) | |
| Unknown | 9 (1.8) | 21 (1.9) | 18 (4.6) | |
| Relapse | 210 (41.7) | 467 (41.7) | 106 (27.2) | |
| Organ failure | 41 (8.1) | 87 (7.8) | 42 (10.8) | |
| GVHD | 46 (9.1) | 114 (10.2) | 36 (9.2) | |
| Noninfectious lung disease | 25 (5.0) | 53 (4.7) | 24 (6.2) | |
| Graft failure | 33 (6.5) | 61 (5.4) | 12 (3.1) | |
| Hemorrhage | 21 (4.2) | 32 (2.9) | 12 (3.1) | |
| Others | 58 (11.5) | 121 (10.8) | 47 (12.1) | |
χ2 test was used to calculate the P value.
Discussion
Using pretransplant variables, we developed a CoBRA score. The score was well validated and was also associated with OS. To the best of our knowledge, this is the first study to address the NRM risk score focusing on UCBT.
In general, HCT-CI has been most widely used for predicting NRM risk after allo-HCT.20 HCT-CI is objective and easy to evaluate but was based on data from BMT and PBSCT. Compared with BMT and PBSCT, UCBT has the advantage of higher tolerance to HLA mismatch instead of the disadvantage of a higher risk of graft failure. Thus, a CoBRA system that incorporates these features was required. Compared with HCT-CI alone, the CoBRA score contains essential factors for the assessment of NRM in the UCBT setting in addition to the common variables. As a result, the CoBRA score showed better predictive performance than HCT-CI alone. Moreover, the CoBRA score may help physicians modify GVHD prophylaxis or conditioning intensity to decrease the NRM risk.
Intensifying immunosuppression is a strategy for reducing NRM risk derived from allogeneic immune reactions.7,31 Actually, severe pre-engraftment immune reactions tend to result in hemophagocytic syndrome and an increased incidence of NRM.32,33 Corticosteroid is commonly used for the treatment of pre-engraftment immune reactions or GVHD, which could increase the risk of life-threatening infection,34,35 and lead to a higher incidence of NRM. In the CoBRA score, 1-point was assigned to GVHD prophylaxis other than Tac/MTX. Because CsA compared with Tac and MMF compared with MTX were reported to increase the incidences of GVHD and NRM,8,36 Tac/MTX is considered to be more immunosuppressive. Thus, it seems reasonable to add the score associated with GVHD prophylaxis other than Tac/MTX.
Amelioration of the conditioning regimen also helps to reduce graft failure and NRM.37-39 Regimen-related toxicity based on intensifying conditioning has been reported to increase NRM in BMT and PBSCT.40,41 Indeed, MAC might have a potential to increase the NRM risk compared with RIC especially for older recipients.42,43 Although the effects of the conditioning intensity in UCBT have been inconclusive,44-46 MAC was found to be significantly associated with an increased risk of NRM in this study and considering the conditioning intensity could significantly improve AIC and modeling fitness.
The impact of the infused CD34+ cell dose in UCBT is a matter of debate.10,47-50 Based on the value around the median, we adopted 0.82 × 105/kg as a cutoff for the CD34+ cell dose, and <0.82 × 105/kg UCB cells tended to be associated with higher NRM in the current study. Recent studies from JSTCT also used a cutoff value for the CD34+ cell dose around 0.8 × 105/kg, and a lower CD34+ cell dose was associated with higher mortality.6,12,51 In contrast, guidelines from the National Marrow Donor Program/Center for International Blood and Marrow Transplant Research recommended ≥1.5 × 105/kg in single-unit UCBT and ≥1.0 × 105/kg in double-unit UCBT.47 Indeed, notable variations in CD34+ cells or lymphocytes in UCB have been reported among ethnic groups.52 The cutoff CD34+ cell dose might need to be validated in other than Asian recipients. Moreover, the CoBRA score should be externally validated although internal validation was performed in the current study.
The allele-level typing for HLA-A, -B, -C, and -DRB1 in UCB units is also recommended in the guidelines from National Marrow Donor Program/Center for International Blood and Marrow Transplant Research, whereas the impact of allele-level HLA disparity on NRM is still controversial.11,53-55 Based on the Japanese registry database, Kanda et al reported that HLA-DRB1 double mismatch was associated with a high risk of NRM.55 Eapen et al showed that the incidence of NRM was higher with HLA-A, -C, and -DRB1 mismatched units compared with HLA-matched units.11 The discrepancy of the impact between HLA locus mismatches on NRM might be because of the different population, whereas both studies suggested that the incidence of NRM was higher because the number of HLA allele mismatches increased. Thus, we focused on the number of HLA allele mismatches in this study. Although an HLA mismatch was not significantly associated with an increased risk of NRM in the current cohort, it has been widely known as a risk factor of NRM. Adding the number of HLA mismatches could improve the modeling fitness in our model.
Although the low and intermediate CoBRA score groups have a lower NRM risk, their higher rate of relapse in causes of death is another problem. Similar to HCT from other graft sources, a decrease in the NRM risk is associated with an increase in the relapse risk in UCBT.56,57 HLA 8/8 allele match, RIC, and Tac/MTX have been reported to increase the incidence of relapse in UCBT.8,12,57 These factors are considered not to increase the NRM risk in the CoBRA score, whereas physicians also need to consider the relapse risk according to the background disease status of each recipient.
This study had some limitations because of its retrospective nature. First, this study was based on the nationwide registry database of JSTCT. Although the result was validated in the Japanese cohort, the impact of transplant variables and events might be different between Japanese and other populations.58 For example, GVHD prophylaxis with MTX has been widely applied in Japan, whereas it is uncommon in Unite States and European countries because of its negative impact for hematopoietic recovery.59,60 Moreover, the score was developed with a cohort including ATL because UCBT is commonly used against ATL in Japan. However, the CoBRA score was also validated in a cohort excluding ATL and, thus, is expected to be a useful tool in other countries. Further external validation studies are needed to apply the CoBRA score to other ethnic groups. However, external validation might be challenging because double UCB units have been widely used in other countries,1,61 whereas the CoBRA score was developed based on single-unit UCBT. Second, we did not consider total nucleated cells of UCB units. Instead, considering the results of previous studies, we used CD34+ cell dose to reflect the quality of UCB units.10,62 Third, we also did not consider anti-HLA antibodies. The screening of anti-HLA antibodies in Japan started in 2018, and most of the recipients in this study did not have information about anti-HLA antibodies. However, we now commonly do not select UCB units when the recipients have donor-specific anti-HLA antibodies with a high mean fluorescence intensity.63 Fourth, we excluded large proportion of the recipients for missing data. This might limit the validity and robustness of the CoBRA score.
In conclusion, in this study we constructed the CoBRA score, which is a UCBT-specific NRM risk assessment score. The CoBRA score could predict both the NRM risk and OS after UCBT. Further validations and prospective studies will be needed to confirm the significance of the CoBRA score.
Acknowledgments
The authors are grateful for the contributions of all of the participating patients and donors, and for the work of all of the physicians and data managers at the centers that contributed valuable data on transplantation to the JSTCT. The authors thank all of the members of the Transplant Registry Unified Management committees at JSTCT for their dedicated data management.
Authorship
Contribution: Y.O. designed the study, analyzed data, and wrote the manuscript; Y. Usui, H.H., M.N., T.T., and J.K. designed the study or advised on methods and wrote the manuscript; N.U., M.T., M.O., S.T., N.D., Y. Uehara, Y.M., K.I., T.K., M.S., and T.E. collected data and revised the manuscript; F.I., K.K., and T.F. collected data, revised the manuscript, and were responsible for data management at JSTCT; Y.A. managed the unified registry database and revised the manuscript; and K.Y. and H.N. designed the study, advised on the methods, revised the manuscript, and were responsible for the project of JSTCT Donor/Source and Transplant Complication Working Groups.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hideki Nakasone, Jichi Medical University, 3311-1 Yakushiji, Shimotsuke, 329-0498, Japan; email: nakasone-tky@umin.ac.jp.
References
Author notes
The data of this study are not publicly available because of the ethical restriction that it exceeds the scope of the recipient/donor’s consent for research use in the registry of the Japan Society for Transplantation and Cellular Therapy (JSTCT).
The full-text version of this article contains a data supplement.