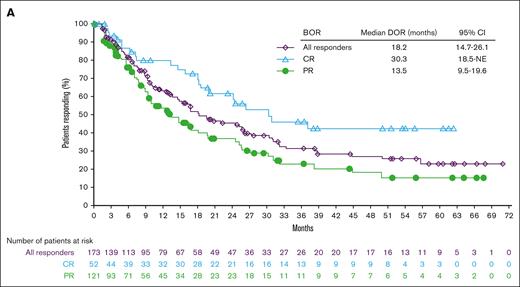
Page 6269: In the table in Figure 1A, under “BOR,” “Progressive disease” should read “PR.” Beneath the line graph, under “Number of patients at risk,” “Progressive disease” should read “PR.”
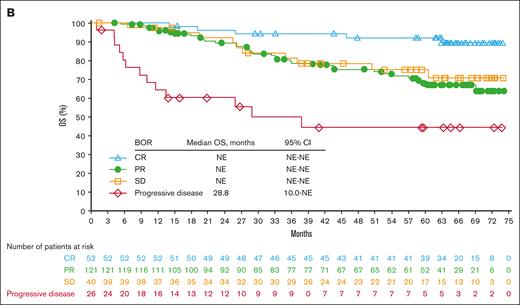
Page 6270: In the table for Figure 1C, “Median DOR (months)” should read “Median PFS (months)”; the y-axis label “Patients responding (%)” should read “PFS (%).”
Efficacy endpoints: duration of response and progression-free survival. DOR according to the BOR (A), PFS according to the cohort (B), and PFS according to the BOR (C). NE, not estimable; SD, stable disease.
Efficacy endpoints: duration of response and progression-free survival. DOR according to the BOR (A), PFS according to the cohort (B), and PFS according to the BOR (C). NE, not estimable; SD, stable disease.
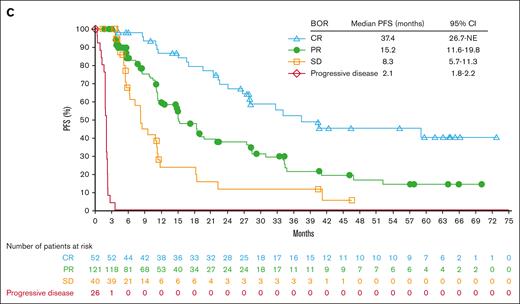
Page 6271: In the table in Figure 2B, “Median DOR, months” should read “Median OS, months.” Under this heading, the “CR” reading “37.4” should be “NE”; the “PR” reading “15.2” should be “NE”; the “SD” reading “8.3” should be “NE”; and the “Progressive disease” reading “2.1” should be “28.8.” Under “95% CI,” the “CR” reading “26.7-NE” should be “NE-NE”; the “PR” reading “11.6-19.8” should be “NE-NE”; the “SD” reading “5.7-11.3” should be “NE-NE”; and the “Progressive disease” reading “1.8-2.2” should be “10.0-NE.”
Efficacy endpoints: Overall survival and best overall responses. OS according to the cohort (A) and BOR (B). Of the 65 deaths, causes included disease progression (n = 36), graft-versus-host disease (n = 5), sepsis and/or septic shock (n = 3), pneumonia (n = 3), cardiac arrest (n = 2), multiple organ failure (n = 2), lung cancer (n = 1), Epstein-Barr virus-positive T-cell lymphoma with multiple organ failure (n = 1), adverse reaction to allo-HCT (n = 1), allo-HCT complicated by graft-versus-host disease (n = 1), heart failure (n = 1), post-transplant complications (n = 1), respiratory infection (n = 1), hemorrhagic cystitis (n = 1), acute hypoxemic respiratory failure secondary to pneumocystic pneumonia (n = 1), and unknown causes (n = 5).
Efficacy endpoints: Overall survival and best overall responses. OS according to the cohort (A) and BOR (B). Of the 65 deaths, causes included disease progression (n = 36), graft-versus-host disease (n = 5), sepsis and/or septic shock (n = 3), pneumonia (n = 3), cardiac arrest (n = 2), multiple organ failure (n = 2), lung cancer (n = 1), Epstein-Barr virus-positive T-cell lymphoma with multiple organ failure (n = 1), adverse reaction to allo-HCT (n = 1), allo-HCT complicated by graft-versus-host disease (n = 1), heart failure (n = 1), post-transplant complications (n = 1), respiratory infection (n = 1), hemorrhagic cystitis (n = 1), acute hypoxemic respiratory failure secondary to pneumocystic pneumonia (n = 1), and unknown causes (n = 5).
The corrected Figure 2B is shown below:
The errors do not affect the results or the conclusions of the study.