TO THE EDITOR:
Agent Orange (AO) was an herbicide mixture that was contaminated with the carcinogenic 2,3,7,8-tetrachlorodibenzo-p-dioxin.1 Between 1962 and 1971, AO was widely sprayed throughout Vietnam and the demilitarized zone in Korea to destroy vegetation that hid or fed enemy forces during operation Ranch Hand.2 According to the National Academies of Sciences, Engineering, and Medicine’s report on Veterans and Agent Orange, AO exposure has been associated with the development of lymphoid malignancies, such as lymphoma and chronic lymphocytic leukemia (CLL).1 Here, we evaluate outcomes among Vietnam veterans who were diagnosed with lymphoid malignancies using data from the VA Central Cancer Registry.
This study was approved by the institutional review boards at the VA Long Beach Healthcare System and the University of California, Irvine and was conducted in accordance with the principles of the Declaration of Helsinki.
The study compared survival between AO-exposed and AO-unexposed veterans who served between 9 January 1962 and 7 May 1974 and were diagnosed with lymphoid malignancies between 1 October 1998 and 31 December 2020. Participants were identified from the VA Central Cancer Registry and included if diagnosed with Burkitt lymphoma, CLL, cutaneous T-cell lymphoma, diffuse large B-cell lymphoma (DLBCL), follicular lymphoma, Hodgkin lymphoma (HL), lymphoplasmacytic lymphoma, mantle cell lymphoma (MCL), mature T-cell and natural killer cell lymphoma, marginal zone lymphoma (MZL), and lymphomas not otherwise specified (NOS). Clinical variables were extracted from the VA Corporate Data Warehouse (the central data repository) using the VA Informatics and Computing Infrastructure.3 Age at diagnosis was calculated from date of birth and earliest date of lymphoid malignancy diagnosis. Analytic variables included AO exposure, combat exposure, tobacco use, military branch, and cancer stage at diagnosis. Race/ethnicity, education, rank, and number of clinic visits were obtained through the US Veterans Eligibility Trends and Statistics.4 Causes and dates of death were confirmed using the National Death Index (NDI) through 31 December 2020.5 We excluded male patients with incomplete clinical data and all women, who comprised <1% of patients (supplemental Figure 1).
Patient and disease characteristics were summarized using descriptive statistics. The χ2 test and Wilcoxon rank sum tests were used to compare the association between categorical and continuous variables, respectively. Patients with no record of death before 31 December 2020 were censored at date of last contact or 31 December 2020. Overall survival (OS) was calculated using time from diagnosis to any death. Disease-specific survival was modeled using the time from diagnosis to death from a hematologic malignancy, with death from other causes as censored. Kaplan-Meier curves were used to estimate survival by AO exposure and compared by log-rank tests. P values were reported for 2-sided tests. Hazard ratios were estimated using Cox proportional hazard models adjusted for race, tobacco history, military branch, rank, combat history, year of birth, year of diagnosis, age at diagnosis, and stage at diagnosis.
Characteristics of the 7392 veterans included in the study are summarized in supplemental Table 1. Patients exposed to AO were more often White (82% vs 78%; P < .0001), enlisted in the army vs other branches (62% vs 52%; P < .0001), and older at diagnosis (median age, 65 vs 63 years; P < .0001) than patients who were unexposed. More patients exposed to AO than those unexposed had been diagnosed with CLL (20% vs 18%) and follicular lymphoma (19% vs 17%; P < .0001), whereas more patients unexposed to AO had been diagnosed with DLBCL (27% vs 24%; P < .0001). More patients exposed to AO with CLL were diagnosed with stage 0 disease (11% vs 9%); fewer patients with CLL and lymphoma with AO exposure had stage 4 disease (31% vs 34%; P = .0460).
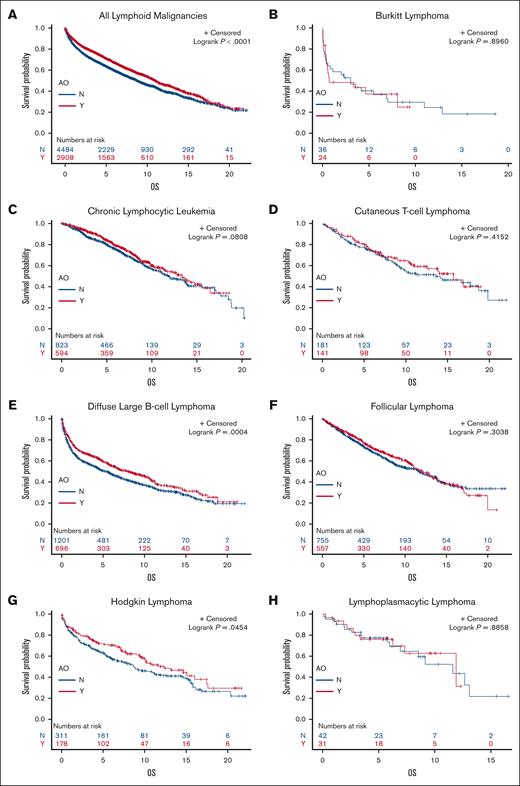
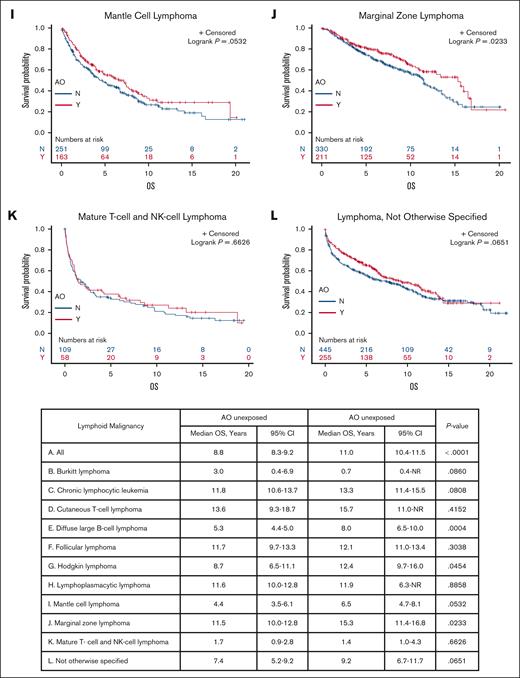
Veterans with DLBCL, HL, and MZL exposed to AO had a longer median OS than those who were unexposed (8.0 years vs 5.3 years [P = .0004], 12.4 years vs 8.7 years [P = .0454], and 15.3 years vs 11.5 years [P = .0233], respectively); AO exposure did not seem to affect the survival of patients with other subtypes on univariate analysis (Figure 1). Multivariate Cox regression of OS revealed that AO exposure was independently associated with a longer survival in patients with DLBCL, HL, MCL, MZL, and NOS (Table 1). To explore why patients exposed to AO may have a longer median OS and earlier stage at diagnosis, we evaluated health care utilization leading to earlier diagnosis. By subtype, patients with MCL with AO exposure had more visits in the 2 years before year of diagnosis (16 vs 12 visits; P = .0246), whereas those with DLBCL, HL, MZL, and NOS did not (15 vs 13 [P = .0910]; 14 vs 12 [P = .2120]; 14.5 vs 14 [P = .2391]; and 13 vs 13 [P = .3756], respectively). There were no differences in alcohol use or number of comorbidities between the patients who were exposed and those unexposed (data not shown).
Kaplan-Meier curves showing OS of patients exposed to AO (red) and those unexposed to AO (blue) with each histology. OS curves for each lymphoid malignancy subtype in veterans with and without AO exposure. BL, Burkitt lymphoma; CI, confidence interval; CTCL, cutaneous T-cell lymphoma; FL, follicular lymphoma; HL, Hodgkin lymphoma; LPL, lymphoplasmacytic lymphoma; MCL, mantle cell lymphoma; MTNKL, mature T-cell and natural killer cell lymphoma; NR, not reached.
Kaplan-Meier curves showing OS of patients exposed to AO (red) and those unexposed to AO (blue) with each histology. OS curves for each lymphoid malignancy subtype in veterans with and without AO exposure. BL, Burkitt lymphoma; CI, confidence interval; CTCL, cutaneous T-cell lymphoma; FL, follicular lymphoma; HL, Hodgkin lymphoma; LPL, lymphoplasmacytic lymphoma; MCL, mantle cell lymphoma; MTNKL, mature T-cell and natural killer cell lymphoma; NR, not reached.
HR estimates from multivariate∗Cox regression of OS and disease-free survival on AO exposure history
| Histology . | AO-OS . | AO disease–specific survival . | ||||||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% confidence limits . | P value . | HR . | 95% confidence limits . | P value . | |||
| BL | 0.855 | 0.320 | 2.285 | .6808 | 0.557 | 0.070 | 4.413 | .5792 |
| CLL | 0.813 | 0.658 | 1.004 | .0544 | 1.334 | 0.936 | 1.902 | .1110 |
| CTCL | 0.718 | 0.482 | 1.071 | .1045 | 0.758 | 0.328 | 1.755 | .5177 |
| DLBCL | 0.748 | 0.653 | 0.857 | <.0001 | 0.926 | 0.772 | 1.110 | .4060 |
| FL | 0.876 | 0.730 | 1.050 | .1313 | 0.796 | 0.591 | 1.071 | .1321 |
| HL | 0.768 | 0.571 | 1.034 | .0353 | 0.739 | 0.468 | 1.166 | .1933 |
| LPL | 0.918 | 0.252 | 3.349 | .8974 | † | |||
| MCL | 0.710 | 0.532 | 0.946 | .0195 | 0.728 | 0.503 | 1.054 | .0925 |
| MTNKL | 0.696 | 0.436 | 1.113 | .1303 | 0.966 | 0.523 | 1.783 | .9121 |
| MZL | 0.624 | 0.459 | 0.850 | .0028 | † | |||
| NOS | 0.737 | 0.586 | 0.928 | .0093 | 0.765 | 0.554 | 1.058 | .1059 |
| Histology . | AO-OS . | AO disease–specific survival . | ||||||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% confidence limits . | P value . | HR . | 95% confidence limits . | P value . | |||
| BL | 0.855 | 0.320 | 2.285 | .6808 | 0.557 | 0.070 | 4.413 | .5792 |
| CLL | 0.813 | 0.658 | 1.004 | .0544 | 1.334 | 0.936 | 1.902 | .1110 |
| CTCL | 0.718 | 0.482 | 1.071 | .1045 | 0.758 | 0.328 | 1.755 | .5177 |
| DLBCL | 0.748 | 0.653 | 0.857 | <.0001 | 0.926 | 0.772 | 1.110 | .4060 |
| FL | 0.876 | 0.730 | 1.050 | .1313 | 0.796 | 0.591 | 1.071 | .1321 |
| HL | 0.768 | 0.571 | 1.034 | .0353 | 0.739 | 0.468 | 1.166 | .1933 |
| LPL | 0.918 | 0.252 | 3.349 | .8974 | † | |||
| MCL | 0.710 | 0.532 | 0.946 | .0195 | 0.728 | 0.503 | 1.054 | .0925 |
| MTNKL | 0.696 | 0.436 | 1.113 | .1303 | 0.966 | 0.523 | 1.783 | .9121 |
| MZL | 0.624 | 0.459 | 0.850 | .0028 | † | |||
| NOS | 0.737 | 0.586 | 0.928 | .0093 | 0.765 | 0.554 | 1.058 | .1059 |
BL, Burkitt lymphoma; CTCL, cutaneous T-cell lymphoma; DSS, disease-specific survival; FL, follicular lymphoma; HL, Hodgkin lymphoma; HR, hazard ratio; LPL, lymphoplasmacytic lymphoma; MCL, mantle cell lymphoma; MTNKL, mature T-cell and natural killer cell lymphoma.
Adjusted for race, tobacco history, military branch, rank, combat history, year of birth, age at diagnosis, year of diagnosis, and stage at diagnosis.
Number of events not adequate for multivariate analysis.
Within histologic subtypes, there were no differences in disease-specific survival between patients exposed to AO and those unexposed by multivariate Cox regression (Table 1).
By the cutoff date, 31 December 2020, a total of 3481 patients (47%) had died. There were more deaths in the AO-unexposed group than AO-exposed group (50% vs 42%; P < .0001). Analyzing deaths due to hematologic neoplasm, other neoplasm, cardiovascular disease, infection, other cause, or unknown cause identified no statistically significant difference in the cause of death between exposure groups (P = .1872; supplemental Table 2).
In our study, veterans exposed to AO with DLBCL, HL, MCL, MZL, and NOS had longer median OS compared with those who were unexposed. To assess possible reasons for longer survival in the AO-exposed group, we evaluated health care utilization in the 2 years before the year of diagnosis because of the possibility that veterans who were aware of toxic effects of AO exposure may have frequent clinic visits due to concern for developing cancer or had already developed chronic diseases. Patients exposed to AO with MCL, but not the other subtypes, had a higher median number of clinic visits 2 years before diagnosis.
Limitations include changing lymphoma classifications and treatment paradigm, constrained measurement of potential confounders, missing data, and unavailability of data from early years after AO exposure. We could not address confounding by unrecognized factors that may influence the course of lymphoid malignancy or the risk of death due to other causes; and the binary measure of tobacco use (never vs former/current) may have resulted in residual confounding by influences of tobacco. The practice of excluding patients with incomplete data may have selected for veterans who were more likely to establish care at the VA and to report clinical information, such as race and tobacco use; this could introduce participation bias of unpredictable direction if AO-survival associations differed between patients with and without complete data. Patients with more aggressive disease may have been diagnosed and died before electronic documentation that determined the start date of the study in 1998. Participants in this research developed malignancies at later ages; biology of their disease may be associated with more favorable outcomes than disease with earlier onset.
Among VA patients diagnosed with lymphoid malignancies 40 to 60 years after operation Ranch Hand, history of AO exposure was not associated with shorter survival or increased cancer-related deaths. This pattern may reflect the biology of disease diagnosed in later decades of life, influences of participation bias, and/or residual confounding by unknown factors. Our findings indicate that history of remote AO exposure does not define a distinct risk stratum for Vietnam-era veterans with lymphoid malignancies. They also illustrate the need for prospective data collection, beginning immediately after service members encounter military exposures, to quantify potential impacts and determine causation.
Acknowledgments: H.M. received a VA Career Development Award (1IK2CX002437-01A1), Lymphoma Research Foundation Lymphoma Scientific Research Mentoring Program Award.
Contribution: H.M. designed the research, collected data, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript. J.Y.W. analyzed the data and wrote the manuscript. V.K.C., P.G., and W.C. designed the research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Helen Ma, VA Long Beach Healthcare System, Health Sciences Assistant Clinical Professor, University of California, Irvine, 5901 East 7th St, Long Beach, California 90822; email: helen.ma@va.gov.
References
Author notes
Due to the restricted access to veteran records, patient level data sharing is not available.
The full-text version of this article contains a data supplement.