Key Points
Two fractions of low-dose RT enhance antitumor responses in both irradiated and nonirradiated sites when combined with CART-19 therapy.
Enhanced responses from two fractions of RT are linked to activation of the STING pathway, TAA cross-priming, and epitope spreading.
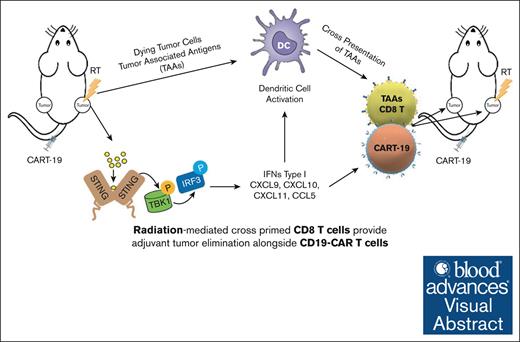
Visual Abstract
Chimeric antigen receptor (CAR) T-cell therapy targeting CD19 (CART-19) represents a significant advance in the treatment of patients with relapsed or refractory CD19+ B-cell lymphomas. However, a significant portion of patients either relapse or fail to respond. Moreover, many patients have symptomatic disease, requiring bridging radiation therapy (RT) during the period of CAR T-cell manufacturing. To investigate the impact of 1 to 2 fractions of low-dose RT on CART-19 treatment response, we developed a mouse model using A20 lymphoma cells for CART-19 therapy. We found that low-dose fractionated RT had a positive effect on generating abscopal systemic antitumor responses beyond the irradiated site. The combination of RT with CART-19 therapy resulted in additive effects on tumor growth in irradiated masses. Notably, a significant additional increase in antitumor effect was observed in nonirradiated tumors. Mechanistically, our results validate activation of the cyclic guanosine adenosine synthetase/stimulator of interferon genes pathway, tumor-associated antigen crosspriming, and elicitation of epitope spreading. Collectively, our findings suggest that RT may serve as an optimal priming and bridging modality for CAR T-cell therapy, overcoming treatment resistance and improving clinical outcomes in patients with CD19+ hematologic malignancies.
Introduction
The advent of CD19-targeted chimeric antigen receptor (CAR) T cells (CART-19) has brought about a significant transformation in the management of patients with relapsed or refractory B-cell lymphomas (BCLs),1-3 leading to the approval of 4 CART-19 therapies by the US Food and Drug Administration.4-6 However, despite its clinical success, a significant subset (50%-60%) of patients who receive CART-19 cell therapy fail to respond or eventually relapse.7-11 Additionally, many patients eligible for CART-19 therapy often have progressive or symptomatic disease, requiring alternative treatment options to bridge them during the weeks between leukapheresis and CART-19 cell infusion.12,13 Finding effective interim therapies to manage chemorefractory disease during this manufacturing period and to improve antitumor efficacy is crucial for the overall success of the treatment.14,15
Radiotherapy (RT) is a cornerstone of cancer treatment with approximately half of patients with solid tumor receiving RT sometime during the disease course.16 RT induces tumor cell death through mechanisms such as mitotic catastrophe, apoptosis, senescence, autophagy, and necrosis.16,17 Specific threshold doses and regimens trigger distinct damage signaling pathways, leading to immunogenic tumor cell death and immune-modulating effects.18,19 RT’s immune-modulatory activity involves releasing of danger/damage-associated molecular patterns pathways, activating the innate immune system by interacting with pattern recognition receptors.20-22 These, in turn, activate the interferon (IFN) response, potentially promoting out-of-RT field antitumor or “abscopal” effects.23,24
In response to the evolving landscape of non-Hodgkin lymphoma treatment, it is important to acknowledge that although the use of RT has declined with advances in systemic therapies, its role remains critical in the relapsed or refractory setting. Particularly, RT serves as an effective bridging therapy for patients preparing for CAR T-cell therapy, especially those with bulky disease sites.1,2,25 The continued relevance of RT in this context is well supported by various real-world evidence data sets, underscoring its utility in optimizing patient condition for subsequent CAR T-cell treatment.26
Given these properties of RT, we proposed that combining it with CART-19 therapy could be a promising approach. To test our hypothesis, we developed an A20 lymphoma CART-19 therapy mouse model, using the inherent expression of CD19 in A20 cells27 and evaluated 2 low-dose RT regimens, 4-Gy × 2 fractions or 8-Gy × 1 fraction, mimicking clinical practices for large BCL. After identifying the more effective regimen, we combined it with CART-19 cell therapy in a dual-tumor mouse model, treating 1 tumor with RT. The combination showed additive effects on both irradiated and nonirradiated tumors, with a more pronounced impact on abscopal tumors. The combination treatment also enhanced the infiltration of CART-19 and total CD8+ T cells in both tumors compared with individual therapies. These outcomes were driven by the activation of the stimulator of interferon genes (STING) pathway, which subsequently triggered crosspresentation and initiated a systemic effector T-cell response.
Collectively, our findings demonstrate that RT serves as an effective immune adjuvant therapy when used in conjunction with CART-19 cell therapy. This combination promotes the release of damage-associated molecular patterns pathways and the activation of the STING pathway, which in turn increases CART-19 cell infiltration and enhances the crosspresentation of tumor-associated antigens (TAAs). The synergistic effects of RT and CART-19 therapy initiate a robust autologous antitumor response against specific TAAs, significantly improving therapeutic outcomes.
Methods
Please see the supplemental Methods for additional methods, including mice strains, cell lines, tumor challenge, irradiation, lymphodepleting chemotherapy, CART-19 cell manufacturing, adoptive T-cell transfer, cell isolation, flow cytometry, immunoblotting, and enzyme-linked immunospot.
Statistics
Two-way analysis of variance was used to compare tumor progression. One-tailed Mann-Whitney test or 2-tailed Student t tests were used to compare data sets when indicated. A P value <.05 was considered significant (∗P < .05; ∗∗P < .01; ∗∗∗P < .001). All statistical analysis were conducted using GraphPad Prism 8.
All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) and the University Laboratory Animal Resources at the University of Pennsylvania. Mice were treated in accordance with University of Pennsylvania guidelines.
Results
Enhanced abscopal antitumor effects in A20 lymphoma model with fractionated RT
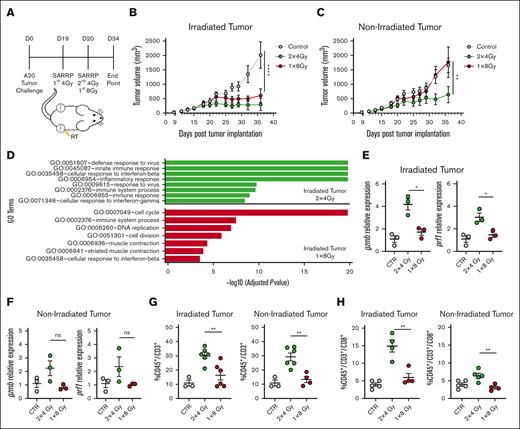
Given the radiosensitivity of lymphomas, doses as low as 4 to 8 Gy are commonly used for palliation, and both 1 and 2 fraction regimens are used.28 To investigate the use of RT in the A20 lymphoma model, we tested 2 clinically relevant low-dose RT regimens, 8-Gy × 1 fraction and 4-Gy × 2 fractions. A total of 2 × 106 of A20 tumor cells were injected subcutaneously in both sides of mice, and 19 days later, 1 of the 2 tumors was irradiated with either 8-Gy × 1 fraction or 4-Gy × 2 fractions on consecutive days (Figure 1A). Regardless of fractionation, the effect of RT alone on the irradiated tumor was similar (Figure 1B). However, 4-Gy × 2 fraction RT significantly increased the antitumor response in nonirradiated tumors, whereas a single dose of 8-Gy RT did not produce a similar effect (Figure 1C). Previously, Demaria's research demonstrated that RT doses can influence the expression of the endonuclease TREX1.29 TREX1 degrades double-stranded DNA produced by RT-induced radicals, thereby inhibiting activation of the cyclic guanosine adenosine synthetase (cGAS)/STING pathway. Despite testing this pathway, we did not observe induction of TREX1 at any of the doses used (supplemental Figure 1A). Further RNA sequencing (RNA seq) analysis from both irradiated and nonirradiated tumors 1 week after RT revealed a robustly active immune system in the group that received fractionated RT compared with the untreated tumors, whereas the single-dose–treated group did not present such a heightened immune activation (Figure 1D; supplemental Figure 1B). Quantitative polymerase chain reaction analysis revealed that both tumors of the fractionated RT group showed significant upregulation of prf1 and gzmb, unlike the single-dose RT group (Figure 1E-F). RNA seq analysis confirmed the elevated expression of the cytotoxic T lymphocyte (CTL) effector molecules ifng, gzmb, gzma, and prf1 in both irradiated and nonirradiated tumors of mice treated with fractionated RT (supplemental Figure 1C-D). Fluorescence-activated cell sorting analysis showed that mice treated with fractionated RT showed a significant increase in CD3+ and CD8+ T-cell infiltration in both the irradiated and nonirradiated tumors compared with those treated with a single dose of RT (Figure 1G-H). Upregulation of essential chemokines for tumor infiltration such as CCL5, CXCL9, CXCL11, CXCL10, and CCR7 was also noted in the RNA seq data, further correlating with increased T-cell infiltration and activity in the fractionated RT group (supplemental Figure 1E-F). Considering the interesting observed abscopal effect and our usual clinical practice to use at least 2 fractions, we used fractionated RT for subsequent experiments.
Enhanced abscopal antitumor effects in A20 lymphoma model with fractionated RT. (A) Working model: time line and schematic representation of in vivo A20 tumor–bearing mice treated with 2 × 4-Gy or 1 × 8-Gy RT in 1 of the 2 tumors. (B-C) A20 tumor growth from irradiated and nonirradiated abscopal tumor as followed by caliper measurements (n = 5-6 mice). (D) Gene ontology terms that are significantly enriched with an adjusted P value <.05 in the differentially expressed gene sets. Data represent irradiated tumors from the fractionated- or single-dose–treated groups. (E-F) Gene expression of granzyme B (gzmb) and perforin 1 (prf1) in irradiated and nonirradiated tumor 5 days after RT. (G-H) CD45+/CD3+ and CD45+/CD3+/CD8+ T-cell infiltration in irradiated and abscopal tumors 34 days after tumor implantation. Graphs show the mean ± standard error of the mean (SEM). ∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Data are representative of 3 independent experiments. CTR, control; ns, not significant; SARRP, Small Animal Radiation Research Platform.
Enhanced abscopal antitumor effects in A20 lymphoma model with fractionated RT. (A) Working model: time line and schematic representation of in vivo A20 tumor–bearing mice treated with 2 × 4-Gy or 1 × 8-Gy RT in 1 of the 2 tumors. (B-C) A20 tumor growth from irradiated and nonirradiated abscopal tumor as followed by caliper measurements (n = 5-6 mice). (D) Gene ontology terms that are significantly enriched with an adjusted P value <.05 in the differentially expressed gene sets. Data represent irradiated tumors from the fractionated- or single-dose–treated groups. (E-F) Gene expression of granzyme B (gzmb) and perforin 1 (prf1) in irradiated and nonirradiated tumor 5 days after RT. (G-H) CD45+/CD3+ and CD45+/CD3+/CD8+ T-cell infiltration in irradiated and abscopal tumors 34 days after tumor implantation. Graphs show the mean ± standard error of the mean (SEM). ∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Data are representative of 3 independent experiments. CTR, control; ns, not significant; SARRP, Small Animal Radiation Research Platform.
Enhancing CART-19 cell infiltration and antitumor response through preceding RT in vivo
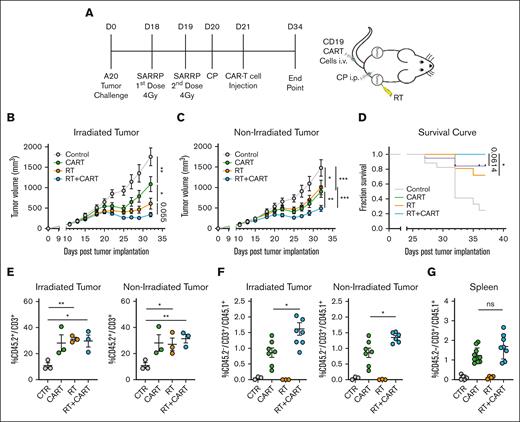
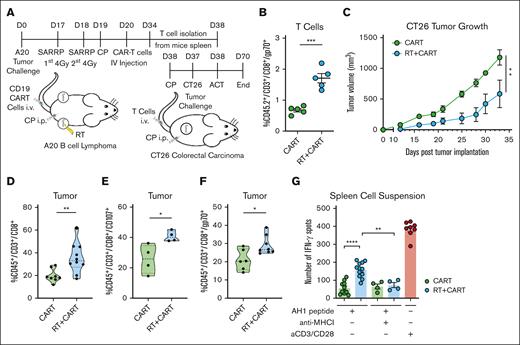
To investigate how RT affects CART-19 therapy, we injected 2 × 106 A20 tumor cells in both sides of the mice as previously and, 19 days later, treated one of the tumors with RT, followed by lymphodepletion with cyclophosphamide and IV injection of 1 × 106 CART-19 cells (Figure 2A; supplemental Figure 2A). As expected, both RT and CART-19 therapies alone significantly affected tumor progression in both irradiated and nonirradiated tumors (Figure 2B-C). Interestingly, the combination of both treatments further reduced the growth of both irradiated and nonirradiated tumors (Figure 2B-C). Consistently, RT before CART-19 therapy significantly improved the overall survival of the mice compared with those treated with RT alone (Figure 2D).
Enhancing CART-19 cell infiltration and antitumor response through preceding RT in vivo. (A) Working model: time line and schematic representation of in vivo A20 tumor–bearing mice treated with RT followed by CART-19 cell injection. All mice of all groups were treated with the same cyclophosphamide (CP) lymphodepletion regimen. (B-C) A20 tumor growth from irradiated and abscopal tumor (n = 17-21). (D) Survival curve after treatment administration. (E) CD45.2+/CD3+ T-cell infiltration in irradiated and abscopal tumors. (F) CD45.2–/CD3+/CD45.1+ T-cell infiltration in irradiated and abscopal tumor representing CART-19 cells. (G) CD45.2–/CD3+/CD45.1+ T-cell infiltration in the spleen representing CART-19 cells. Graphs show the mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Data are representative of 3 independent experiments. CTR, control.
Enhancing CART-19 cell infiltration and antitumor response through preceding RT in vivo. (A) Working model: time line and schematic representation of in vivo A20 tumor–bearing mice treated with RT followed by CART-19 cell injection. All mice of all groups were treated with the same cyclophosphamide (CP) lymphodepletion regimen. (B-C) A20 tumor growth from irradiated and abscopal tumor (n = 17-21). (D) Survival curve after treatment administration. (E) CD45.2+/CD3+ T-cell infiltration in irradiated and abscopal tumors. (F) CD45.2–/CD3+/CD45.1+ T-cell infiltration in irradiated and abscopal tumor representing CART-19 cells. (G) CD45.2–/CD3+/CD45.1+ T-cell infiltration in the spleen representing CART-19 cells. Graphs show the mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Data are representative of 3 independent experiments. CTR, control.
Fluorescence-activated cell sorting analysis revealed a higher infiltration by CD3+ T cells in both the irradiated and nonirradiated tumors across treatment groups (Figure 2E). Although more CD8 T cells infiltrated the tumors of both groups that received RT (supplemental Figure 2B), only the group that received the combination treatment demonstrated a significant increase in cytotoxic CD107+ T cells (supplemental Figure 2C). CART-19 cells were generated from CD45.1 congenic mice, and the CD45.1 marker was used to track the adoptively transferred CART-19 cells. A significantly higher CD45.1+ T-cell population was identified in both irradiated and nonirradiated tumors of mice previously treated with RT followed by CAR T cells (Figure 2F; supplemental Figure 2D). A similar trend of a higher CD45.1+ T-cell population was observed in the spleens of the same group (Figure 2G). These data show that RT significantly boosts CART-19 therapy effectiveness in both irradiated and nonirradiated tumors. This enhancement is tied to the restructuring and heightened activation of infiltrating T cells.
RT/CART-19 combination treatment enhances crosspresentation of TAAs and T-cell responses to TAAs
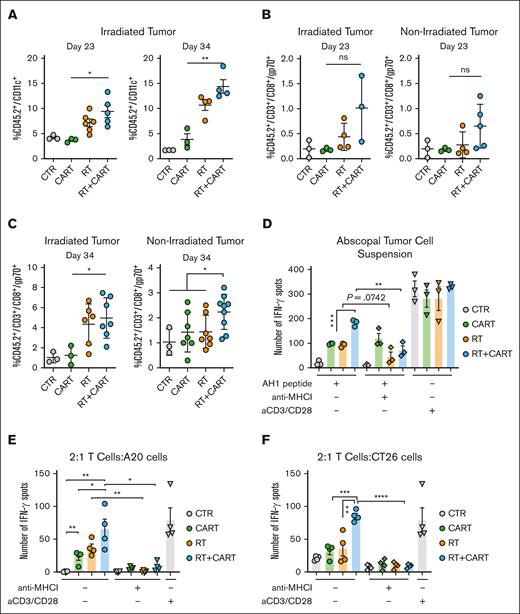
RT demonstrates robust immune-modulatory effects by directly activating and recruiting crosspresenting dendritic cells (DCs), facilitating the crosspriming of TAAs, resulting in the activation of antitumor CD8+ T cells and the induction of abscopal effects.19,23,30 In our study, RT treatment significantly increased the infiltration of DCs only in the irradiated tumors, compared with the group that received only CART-19 cells (Figure 3A; supplemental Figure 3A). We also examined the expression levels of critical genes involved in the antigen presentation pathway, such as batf3, batf2, cd86, xcr1, and b2m,31-33 and noted their upregulation in RT-treated tumors (supplemental Figure 3B-C). Notably, the A20 model expresses both CD19 (the target of CAR T cells) and the TAA gp70, a product of the env gene of endogenous murine leukemia virus, which contains the immune-dominant epitope AH1 (SPSYVYHQF).34 AH1 tetramer staining in the various treatment groups revealed a notable increase in AH1-specific T-cell infiltration in RT-treated tumors, as well as in nonirradiated tumors (Figure 3B-C).
RT/CART-19 combination treatment enhances crosspresentation of TAAs and T-cell responses to TAAs. (A) CD45.2+/CD11c+ DC infiltration in irradiated tumor on day 23 and day 34 after tumor challenge. (B-C) AH1-specific T-cell infiltration in irradiated and abscopal tumors on day 23 and day 34 after tumor challenge. (D) IFN-γ spots of an enzyme-linked immunospot (ELISPOT) assay after stimulation of tumor cell suspensions with AH1 peptide. (E) CD45.2+/CD3+ T cells isolated from treated mice stimulated with AH1 peptide and incubated with A20 tumor cells. (F) CD45.2+/CD3+ T cells isolated from treated mice stimulated with AH1 peptide and incubated with CT26 tumor cells. Graphs show the mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. CTR, control.
RT/CART-19 combination treatment enhances crosspresentation of TAAs and T-cell responses to TAAs. (A) CD45.2+/CD11c+ DC infiltration in irradiated tumor on day 23 and day 34 after tumor challenge. (B-C) AH1-specific T-cell infiltration in irradiated and abscopal tumors on day 23 and day 34 after tumor challenge. (D) IFN-γ spots of an enzyme-linked immunospot (ELISPOT) assay after stimulation of tumor cell suspensions with AH1 peptide. (E) CD45.2+/CD3+ T cells isolated from treated mice stimulated with AH1 peptide and incubated with A20 tumor cells. (F) CD45.2+/CD3+ T cells isolated from treated mice stimulated with AH1 peptide and incubated with CT26 tumor cells. Graphs show the mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. CTR, control.
Stimulation of tumor cell suspensions from all groups with AH1 peptide resulted in significantly higher production of IFN-γ in the group treated with RT and CAR T cells. These effects were abrogated in the presence of an anti–major histocompatibility complex I (MHC I) antibody (Figure 3D). To validate the recognition of AH1 peptide also on tumor cells by in vivo primed CD8 T cells, we isolated T cells from the spleens of the different treatment groups and cocultured in vitro with A20 tumor cells in the presence of anti–MHC I antibody. Only the T cells from the group treated with RT and CAR T cells showed a significant increase in IFN-γ spots, which was diminished when cell interactions were blocked with an anti–MHC I antibody (Figure 3E).
To further prove crosspresentation, we used the CT26 cell line, a syngeneic BALB/c-derived colorectal carcinoma cell line, which, similar to A20 cells, expresses the endogenous TAA gp70 but lacks CD19 expression. Stimulation of T cells from the combination-treated group with the AH1 peptide and coculturing them with CT26 cells resulted in increased reactivity and a higher number of IFN-γ spots, which was dependent on MHC I (Figure 3F). These findings demonstrating the upregulation of crosspresentation of TAAs, epitope spreading, and consequent elicitation of an autologous anti-gp70 T-cell response further support the additive effects of RT and CAR T-cell therapy.
Enhanced tumor protection in a colorectal model through adoptive transfer of CD3+ T cells from mice treated with RT plus CART-19
To functionally validate crosspresentation as a mechanism of enhancing the antitumor effect of CART-19 after RT, we used an adoptive T-cell therapy approach. Isolated CD3+ T cells from the spleens of A20 tumor–bearing mice that had received treatment with either CART-19 cells alone or CART-19 cells in combination with RT were expanded ex vivo for 3 days and then transferred into recipient mice bearing CT26 tumors. (Figure 4A). The specificity of the gp70 TAA of the T cells after expansion was confirmed by AH1 tetramer staining before transfer (Figure 4B). Lymphodepleted CT26 tumor–bearing mice that received T cells from donors treated with RT followed by CART-19 cells exhibited significantly slower tumor progression than the group that received T cells from donors treated only with CART-19 cells (Figure 4C). Moreover, the group that received T cells from the combination-treated donors showed increased infiltration by CD8+ T cells (Figure 4D) and cytotoxic T cells within the tumor (Figure 4E). Importantly, the group that received T cells from donors treated with combination therapy demonstrated significantly higher infiltration of AH1-specific CD8+ T cells into the tumor (Figure 4F). When spleen cell suspensions were stimulated overnight with AH1 peptide, significantly more IFN-γ spots were recorded from the mice that previously received T cells from RT plus CART-19 cell–treated donors, with this immune reaction being mediated by MHC I (Figure 4G). In summary, these results validate that the combination of RT with CART-19 promotes the crosspresentation of TAAs, thereby eliciting an additional autologous T-cell immune response that enhances the antitumor efficacy of CART-19 therapy when administered in combination with RT.
Enhanced tumor protection in a colorectal model through adoptive transfer of CD3+ T cells from mice treated with RT plus CART-19. (A) Working model: time line and schematic representation of in vivo A20 tumor–bearing mice treated with 2 × 4-Gy RT followed by CAR T cells and adoptive T-cell transfer to CT26 tumor–bearing mice. All mice of all groups were treated with the same CP lymphodepletion regimen. (B) AH1-specific T cells from donor mice after ex vivo expansion, before ACT. (C) CT26 tumor growth of recipient mice (n > 4). (D) CD45+/CD3+/CD8+ T-cell tumor infiltration. (E) CD45+/CD3+/CD8+/CD107+ T-cell tumor infiltration. (F) CD45+/CD3+/CD8+/AH1-specific T-cell tumor infiltration. (G) IFN-γ spots of an ELISPOT assay after AH1 peptide stimulation of splenocytes from mice that received ACT. Graphs show the mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Data are representative of 2 independent experiments. ACT, Adoptive Cell Therapy.
Enhanced tumor protection in a colorectal model through adoptive transfer of CD3+ T cells from mice treated with RT plus CART-19. (A) Working model: time line and schematic representation of in vivo A20 tumor–bearing mice treated with 2 × 4-Gy RT followed by CAR T cells and adoptive T-cell transfer to CT26 tumor–bearing mice. All mice of all groups were treated with the same CP lymphodepletion regimen. (B) AH1-specific T cells from donor mice after ex vivo expansion, before ACT. (C) CT26 tumor growth of recipient mice (n > 4). (D) CD45+/CD3+/CD8+ T-cell tumor infiltration. (E) CD45+/CD3+/CD8+/CD107+ T-cell tumor infiltration. (F) CD45+/CD3+/CD8+/AH1-specific T-cell tumor infiltration. (G) IFN-γ spots of an ELISPOT assay after AH1 peptide stimulation of splenocytes from mice that received ACT. Graphs show the mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Data are representative of 2 independent experiments. ACT, Adoptive Cell Therapy.
RT-induced activation of the cGAS/STING pathway promotes IFN type I gene and chemokine expression upregulation in A20 tumor cells
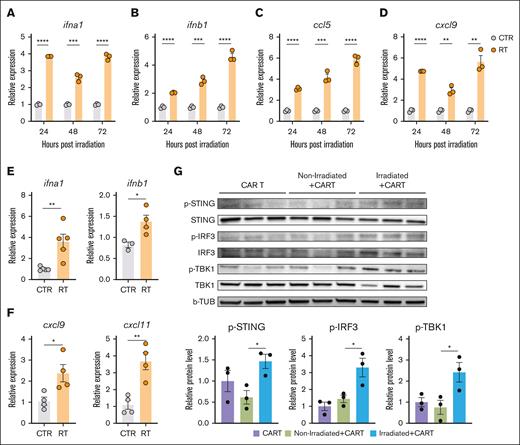
To investigate the enhanced crosspresentation and CART-19 tumor infiltration achieved by the combination of RT and CART-19 cell therapy, we tested the impact of RT on the expression of critical cytokines responsible for crosspriming and immune cell trafficking within tumors.35 A20 tumor cells were exposed to 4-Gy × 2 in vitro, and the expression of ifna1, ifnb1, ccl5, and cxcl9 was measured 24, 48, and 72 hours after RT. IFN type I genes (Figure 5A-B) and ccl5 and cxcl9 (Figure 5C-D) were significantly upregulated after RT. These consistent findings were also observed in vivo (Figure 5E-F). Type I interferons initiate a variety of responses including DC activation and maturation, as well as CD8+ T-cell activation and differentiation.35
RT-induced activation of the cGAS/STING pathway promotes IFN type I gene and chemokine expression upregulation in A20 tumor cells. (A-D) Expression of ifna1, ifnb1, ccl5, and cxcl9 after 24, 48, and 72 hours of RT in vitro. (E-F) Expression of ifna1, inb1, cxcl9, and cxcl11 48 hours post in vivo RT. (G) Activation of STING pathway in A20 tumors treated with CART-19 alone or combination of CART-19 and RT. Graphs show the mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. b-TUB, b-tubulin; RT, Radiation Therapy; p-IRF3, phosphorylated-Interferon Regulatory Factor 3; p-STING, phosphorylated-Stimulator of interferon genes; p-TBK1, phosphorylated-TANK-binding kinase 1.
RT-induced activation of the cGAS/STING pathway promotes IFN type I gene and chemokine expression upregulation in A20 tumor cells. (A-D) Expression of ifna1, ifnb1, ccl5, and cxcl9 after 24, 48, and 72 hours of RT in vitro. (E-F) Expression of ifna1, inb1, cxcl9, and cxcl11 48 hours post in vivo RT. (G) Activation of STING pathway in A20 tumors treated with CART-19 alone or combination of CART-19 and RT. Graphs show the mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. b-TUB, b-tubulin; RT, Radiation Therapy; p-IRF3, phosphorylated-Interferon Regulatory Factor 3; p-STING, phosphorylated-Stimulator of interferon genes; p-TBK1, phosphorylated-TANK-binding kinase 1.
Upregulation of the IFN type I genes and the induction of crosspresentation led to the hypothesis that the cGAS/STING pathway is relevant to the results observed.23,30,36 Indeed, we observed a significant enhancement of the STING pathway activation in the irradiated tumors (Figure 5G; supplemental Figure 4A). These findings suggest that STING activation is involved in the adjuvant effects of RT on TAA crosspresentation, whereas CXCL9 and CCL5 may play a crucial role in facilitating lymphocyte infiltration within the Tumor Microenvironment (TME), including CART-19 cells.
RT enhances CAR T-cell therapy through STING activation
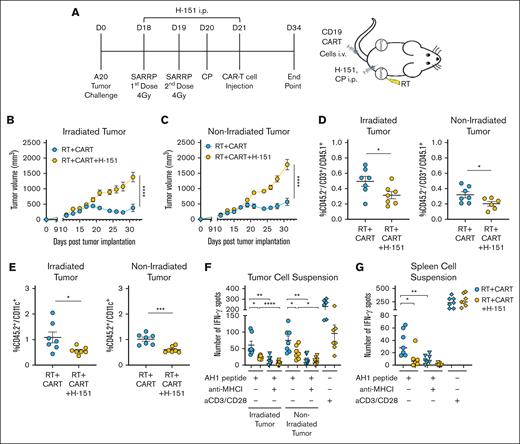
To validate the relevance of the STING pathway in the additive effects of RT with CART-19 cells in vivo, we blocked STING using H-151, a well characterized STING antagonist.37,38 A20 tumor–bearing BALB/c mice were treated with RT as previously described, followed by CART-19 cell therapy with or without H-151 (Figure 6A). Inhibition of the cGAS/STING pathway significantly abolished the antitumor effects of RT on the irradiated tumor and the additive effect of the 2 treatments on the nonirradiated tumor (Figure 6B-C). Evaluation of the TME revealed that fewer endogenous T cells tended to infiltrate the spleen, as well as both irradiated and nonirradiated tumors, of the mice that were treated with the STING antagonist (supplemental Figure 5A-B). Importantly, the STING antagonist caused a significant decrease in the infiltration of CART-19 cells and DCs in both irradiated and nonirradiated tumors (Figure 6D-E). To confirm the involvement of STING in the observed RT-induced crosspresentation against the gp70 AH1 epitope, we stimulated tumor and spleen suspensions. A significant reduction in IFN-γ spots was observed in the tumors and spleens of mice treated with H-151, compared with those that received combination therapy. The addition of the anti–MHC I antibody decreased IFN-γ spots specifically in the RT plus CAR T-cell group, as expected (Figure 6F-G). To further validate and characterize the relevance of the STING pathway in the observed crosspresentation, we generated a chimeric mouse model by knocking down STING specifically in the bone marrow and, consequently, in the immune system. This approach demonstrated that STING activation in leukocyte populations is essential for RT-induced crosspresentation and the enhanced antitumor effects observed with the combined RT and CAR T-cell therapy (supplemental Figure 6A-F). These findings provide strong evidence that STING is a crucial mediator in RT-enhanced antitumor effects of CART-19 cell therapy.
RT enhances CAR T-cell therapy through STING activation. (A) Working model: time line and schematic representation of in vivo A20 tumor–bearing mice treated with RT followed by CART-19 cell injection with or without the STING antagonist H-151. All mice of all groups were treated with the same CP lymphodepletion regimen. (B-C) Tumor growth from irradiated and abscopal tumors (n = 8). (D) CD45.2–/CD3+/CD45.1+ T-cell infiltration in irradiated and abscopal tumors, representing the CART-19 cells. (E) CD45.2+/CD11c+ DC infiltration in irradiated and abscopal tumors. (F-G) IFN-γ spots after overnight stimulation of tumor or spleen cell suspensions with AH1 peptide with or without anti–MHC I antibody. Graphs show the mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Data are representative of 1 experiment.
RT enhances CAR T-cell therapy through STING activation. (A) Working model: time line and schematic representation of in vivo A20 tumor–bearing mice treated with RT followed by CART-19 cell injection with or without the STING antagonist H-151. All mice of all groups were treated with the same CP lymphodepletion regimen. (B-C) Tumor growth from irradiated and abscopal tumors (n = 8). (D) CD45.2–/CD3+/CD45.1+ T-cell infiltration in irradiated and abscopal tumors, representing the CART-19 cells. (E) CD45.2+/CD11c+ DC infiltration in irradiated and abscopal tumors. (F-G) IFN-γ spots after overnight stimulation of tumor or spleen cell suspensions with AH1 peptide with or without anti–MHC I antibody. Graphs show the mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Data are representative of 1 experiment.
Enhancing sensitivity of target cells to CTL function through RT: implications for CAR T-cell efficacy and antigen escape
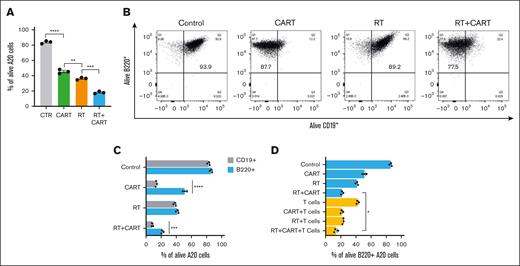
To understand the interaction between RT and CTL function in the context of CART-19 cell therapy, we aimed to determine whether RT could make target cells more susceptible to CTL cytotoxicity39 and investigate RT’s potential impact on antigen escape. A20 tumor cells were irradiated with 4-Gy × 2 and cocultured with CART-19 cells. Beyond the expected effects on crosspresentation, RT also significantly increased the vulnerability of target cells to CART-19 cell–mediated cytotoxicity (Figure 7A). Interestingly, we observed a subset of A20 cells that escaped CART-19 attack, characterized by a loss of CD19 with sustained expression of B220 (Figure 7B-C). This phenomenon, consistent with the well-documented antigen loss or downregulation mechanism, often underpins resistance to CAR T-cell therapy.
Enhancing sensitivity of target cells to CTL function through RT: implications for CAR T-cell efficacy and antigen escape. (A) Percentage of A20 tumor cells alive after RT, CAR T-cell, and RT and CAR T-cell treatments. (B) Flow cytometry for B220 and CD19 markers after in vitro treatment of A20 tumor cells with RT, CAR T-cell, and RT and CAR T-cell treatments. (C) B220+ and CD19+ alive cells after treatments. (D) B220+ alive cells after treatment with RT, CAR T cells, and T cells isolated from CT26 tumor–bearing mouse adoptively transferred with T cells from RT plus CAR T-cell donor. Graphs show the mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. CTR, control.
Enhancing sensitivity of target cells to CTL function through RT: implications for CAR T-cell efficacy and antigen escape. (A) Percentage of A20 tumor cells alive after RT, CAR T-cell, and RT and CAR T-cell treatments. (B) Flow cytometry for B220 and CD19 markers after in vitro treatment of A20 tumor cells with RT, CAR T-cell, and RT and CAR T-cell treatments. (C) B220+ and CD19+ alive cells after treatments. (D) B220+ alive cells after treatment with RT, CAR T cells, and T cells isolated from CT26 tumor–bearing mouse adoptively transferred with T cells from RT plus CAR T-cell donor. Graphs show the mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. CTR, control.
Then, we investigated the susceptibility of these CD19–, B220+ cells to endogenous T cells targeting TAAs. Using CD3 T cells from mice with rejected CT26 tumors after combined RT and CART-19 therapy, we cocultured them with irradiated A20 cells and CART-19 cells. Regardless of CD19 or B220 expression, endogenous T cells had a consistent impact. However, when both CART-19 and endogenous T cells were present, there was a significant elimination of the CD19–, B220+ subset within the A20 cells (Figure 7D). This indicates that crossprimed endogenous T cells, induced by RT's crosspresentation, collaborate with CART-19 cells to prevent antigen escape.
Discussion
The interplay between RT and the immune responses has become an area of interest within cancer therapeutics. Bridging RT has been safely used in the clinic to control tumor growth in patients who are scheduled for CAR T-cell therapy, and to date, it has not been shown to negatively affect outcome.11,25,26,40-43 In preclinical models, DeSelm et al used low-dose immunogenic RT to sensitize pancreatic tumor cells to TRAIL-mediated death in response to CAR T cells.44 In a glioblastoma mouse model, RT was shown to synergize with CAR T cells by increasing their antitumor effectiveness and infiltration to the tumor site.45 Our study is the first, to our knowledge, to investigate the use of RT before CAR T-cell therapy in a murine lymphoma model, aiming to understand how this combination enhances antitumor effects.
It has been hypothesized that fractionated RT could enhance antitumor immunity while minimizing adverse effects.30 Variability in tumor responses to different RT regimens is well documented, with each inducing unique cellular responses such as mitotic catastrophe, apoptosis, senescence, autophagy, ferroptosis, and necrosis.46-48 These processes can promote or inhibit immunogenic cell death and affect critical enzyme expression.20,29 Our study demonstrates that a fractionated dose of 2 × 4-Gy RT enhances antigen presentation and epitope spreading more effectively than a single 8-Gy dose, improving the control of A20 murine lymphoma. This is observed both at the irradiation site and systemically, enhancing the abscopal effect and controlling distant, nonirradiated tumors. Furthermore, RNA seq analysis of treated mouse tumors revealed significantly divergent findings between the 2 regimens. The gene ontology analysis for a tumor treated with a single dose of 8 Gy revealed significant upregulation in genes associated with critical cellular processes such as the cell cycle, cell division, and DNA replication and repair, whereas treatment with 2 fractions of 4 Gy showed a distinct shift toward immune-related processes. Hence, it appears that a single higher dose induces more extensive DNA damage, promoting cell-intrinsic repair mechanisms and direct damage responses, whereas fractionating the same dose into 2 smaller doses results in a less overall cell damage, yet still produces sufficient signals to promote a more robust immune-mediated response.49-51 Our findings confirmed that RT can serve as an effective immune adjuvant to CART-19 therapy. The combined treatment resulted in significant increase in both CART-19 positive and nonpositive effector T-cell infiltration and antitumor effects, leading to a significant reduction in the growth of both irradiated and nonirradiated tumors. These outcomes align with studies demonstrating that RT can augment the infiltration and activation of T cells within certain TMEs.52
RT also enhanced the expression of specific chemokines, such as CCL5 and CXCL9, which are pivotal for orchestrating T-cell infiltration into the tumor site.53,54 Investigating the increased infiltration of autologous T cells, we demonstrate the involvement of crosspresentation and epitope spreading. In line with previous works, our findings reveal that fractionated RT induces an inflammatory process that promotes the recruitment and activation of DCs within the TME. This process, in turn, augments the crosspresentation of TAAs and epitope spreading.55,56 This phenomenon is supported by the upregulated expression of genes related to antigen presentation and a marked increase in AH1-specific T-cell infiltration, indicative of epitope spreading. To validate the relevance of the antitumor crossprimed T-cell response in the combination, we conducted an experiment involving T-cell adoptive transfer in the CT26 colorectal cancer model. Unlike the A20 model, CT26 does not express CD19 but does express gp70, which can be recognized by gp70/AH1–specific T cells. This experiment demonstrated that the transfer of T cells from the A20 model treated with RT plus CART-19 provided significant protection against CT26 tumors, compared with the T cells from the CART-19 alone treatment. These results underscore the critical role of crosspresentation and epitope spreading in enhancing the antitumor efficacy of CAR T-cell therapy when combined with RT.
The cGAS-STING pathway is crucial for detecting cytoplasmic double-stranded DNA and initiating immune responses. This pathway is activated by RT-induced DNA damage in tumor cells, which leads to the production of type I IFN and cytokines, thereby enhancing the immune response.23,57,58 Our data confirm the activation of the STING pathway in irradiated tumors. When the STING pathway activation is inhibited, the antitumor effects of both RT and CART-19 cell therapy are significantly impaired. To further characterize the importance of the STING pathway, we generated bone marrow chimeras depleted of STING in the leukocyte compartment. This experimental approach aligns with other published works, underscoring the critical role of the STING pathway in leukocytes for the efficacy of combined RT and immunotherapy. Our results emphasize the pathway's essential function in orchestrating a robust immune response and enhancing the therapeutic effects of the combined treatment.59
Antigen escape poses a significant challenge in CAR T-cell therapy, often leading to relapse.3,60 We observed that after radiation, cancer cells become more sensitive to attack by CTLs. However, in our experiments, we also identified a subpopulation of CD19– cells that were resistant to CAR T-cell therapy. Importantly, these resistant cells were effectively eliminated by endogenous TAA-specific T cells. This finding underscores the crucial role of endogenous TAA-specific T cells in overcoming challenges such as antigen escape when combining RT with CAR T-cell therapy. By facilitating the targeting and elimination of tumor cells that have lost the CAR T-cell target antigen, endogenous TAA-specific T cells address issues related to tumor heterogeneity and metastatic spread. This combination therapy not only enhances the initial antitumor response but also provides a broader immune surveillance mechanism to target and eliminate resistant tumor cell populations, thereby reducing the likelihood of relapse.
In the clinical treatment of large BCL with CART-19, bridging RT aims to obtain durable local control, often using 30 to 40 Gy given in 2 to 2.5 Gy daily fractions, or to more rapidly relieve symptoms, using 20 to 30 Gy in 3 to 4 Gy daily fractions.61 Our study explored the role of a very low dose of radiation in mice, suggesting a compelling mechanism for RT priming and crosspriming before CAR T-cell infusion. We acknowledge the limitations of using a syngeneic murine model, including species differences in immune response and tumor microenvironment complexity.62,63 Despite the inherent differences between mouse and human immune systems, our findings are potentially clinically relevant, because we demonstrate that low-dose RT can effectively prime the immune response, enhancing the efficacy of CAR T cells. If low doses prove effective within and outside RT fields in clinical settings, this strategy could offer a comprehensive treatment approach for various tumor types. The use of low-dose RT may reduce toxicity compared with higher doses, potentially offering a more favorable toxicity profile while maintaining therapeutic efficacy. Prospective human clinical trials are essential to validate this concept and determine the optimal RT dosing and scheduling in combination with CAR T-cell therapy.
In conclusion, although our murine model provides a valuable proof of concept, the results underscore the potential of integrating low-dose RT with CAR T-cell therapy. Further research in human participants will be crucial to fully realize the benefits of this combinatorial approach, potentially improving treatment outcomes for patients with large BCLs and other cancers.
Acknowledgments
The authors thank Norma Carretta for the life career advice and financial support.
This work was supported by the National Cancer Institute, National Institutes of Health (grant 5R01CA219871).
Authorship
Contribution: N.K. initiated the project, designed and performed all the experiments, acquired and analyzed the data, and drafted the manuscript; R.P.-L., F.C., S.B., and E.K. helped perform the experiments and analyze the data; D.G. performed the irradiation of the tumors; S.J.S., M.J.L., and J.P.P. gave suggestions for the manuscript and provided clinical advice on the irradiation dose that was used; G.T. and E.A.C. gave suggestions for the manuscript; C.K. advised on experimental design, helped with critical advice on radiation protocols, and reviewed the manuscript; and A.F. conceived the project, designed the study, supervised project planning and execution, and wrote the manuscript. A.M. gave suggestions on the RT regimens and helped in the manuscript revision.
Conflict-of-interest disclosure: E.A.C. reports funding from Genmab, Genentech/Roche, AstraZeneca, CARGO, Juno Therapeutics, Novartis, Lymphoma Research Foundation, and Kite Pharma; and advisory board from AstraZeneca and Beigene. S.J.S. reports research funding from TG Therapeutics, Incyte, Adaptive Biotechnologies, Pharmacyclics, Merck, Genmab, Genentech/Roche, AstraZeneca, Cargo, AbbVie, and Lymphoma Research Foundation; consultancy fees and research funding from Genentech/Roche, Juno Therapeutics, and AbbVie; consultancy fees from Tessa Therapeutics, Loxo Oncology, BeiGene, Alimera Sciences, Acerta Pharma/AstraZeneca, and Nordic Nanovector; consultancy fees, honoraria, patents and royalties, and research funding from Novartis; consultancy fees, honoraria, and research funding from Celgene; and advisory board fees from AstraZeneca and BeiGene. The remaining authors declare no competing financial interests.
Correspondence: Andrea Facciabene, Smilow Center for Translational Research, Department of Radiation Oncology and the Ovarian Cancer Research Center, member of the Center of Cellular Immunotherapy, University of Pennsylvania Perelman School of Medicine, Room 8-133, 3400 Civic Center Blvd 421, Philadelphia, PA 19104; email: facciabe@pennmedicine.upenn.edu.
References
Author notes
The data reported in this article has been deposited in NCBI Gene Expression Omnibus (GEO) (accession number: GSE281695).
Author verified drug dosages: Radiation Therapy: 4Gyx2, 8GyCD19-CART Cells therapy: 1 million/mouseLymphodepleting Chemotherapy: 100mg/kgH-151 Inhibitor: 10mg/kg/day
The full-text version of this article contains a data supplement.