Key Points
The 6-month change in pain interference did not significantly differ between the digital CBT and digital pain/SCD education arms.
Health coach–supported, digital CBT and pain/SCD education are equally effective adjunct treatments for chronic pain in SCD.
Visual Abstract
Despite the burden of chronic pain in sickle cell disease (SCD), nonpharmacological approaches remain limited. This multisite, randomized trial compared digital cognitive behavioral therapy (CBT) with a digital pain/SCD education program (“Education”) for managing pain and related symptoms. Participants were recruited virtually from seven SCD centers and community organizations in the United States. Adults (aged ≥18 years) with SCD-related chronic pain and/or daily opioid use were assigned to receive either CBT or Education for 12 weeks. Both groups used an app with interactive chatbot lessons and received personalized health coach support. The primary outcome was the change in pain interference at six months, with secondary outcomes including pain intensity, depression, anxiety, quality of life, and self-efficacy. Of 453 screened participants, 359 (79%) were randomized to CBT (n = 181) or Education (n = 178); 92% were Black African American, and 66.3% were female. At six months, 250 participants (70%) completed follow-up assessments, with 16 (4%) withdrawals. Engagement with the chatbot varied, with 76% connecting and 48% completing at least one lesson, but 80% of participants completed at least one health coach session. Both groups showed significant within-group improvements in pain interference (CBT: −2.13; Education: −2.66), but no significant difference was observed between them (mean difference, 0.54; P = .57). There were no between-group differences in pain intensity, depression, anxiety, or quality of life. High engagement with health coaching and variable engagement with digital components may explain the similar outcomes between interventions in this diverse, hard-to-reach population.
Background
Sickle cell disease (SCD), a rare genetic blood disorder affecting ∼100 000 Black Americans,1,2 is characterized by acute complications and chronic pain that impair daily functioning and quality of life. Recurrent vaso-occlusive episodes often require emergency care,3 and both acute and chronic pain and depression increase health care use and mortality risk.4-9 Despite the substantial burden, current SCD pain management standards are inadequate,10,11 with overreliance on opioids that have limited long-term efficacy data and known adverse effects.6,12-16 Effective nonpharmacological pain treatments are needed.17
Cognitive behavioral therapy (CBT) is a type of psychotherapy that has shown effectiveness in treating various pain conditions. It focuses on identifying and changing negative thought patterns and behaviors that may contribute to a person’s pain experience. CBT has been proven effective in helping individuals develop coping strategies and more adaptive ways of thinking about and managing their pain.18-22 However, it remains underutilized for SCD, with reported barriers including limited availability or access, cost, and stigma related to seeking mental health services.23-26 Emerging mobile health interventions could expand access to CBT and improve health outcomes.27-29 However, large-scale trials demonstrating digital CBT’s effectiveness and scalability, particularly in minority populations, are lacking.17,24-26
Access barriers to CBT persist, but high-quality SCD and pain education materials are readily available through patient communities. Thus, there is benefit in comparing effectiveness between CBT and educational programs that patients may already be using routinely. Although limited data exist on psychoeducation for SCD pain specifically, evidence in other chronic pain conditions suggests that educational interventions can improve pain and coping.30,31 Given the promising evidence and accessible resources, structured educational interventions have the potential to improve outcomes in SCD. Both CBT and education are viable nonpharmacological options, but evidence is needed to guide providers on feasible and acceptable nonpharmacological SCD pain management.
The CaRISMA trial compared digital CBT and digital pain/SCD education (hereafter referred to as “Education”) mobile interventions for reducing pain interference (primary outcome) in adults with SCD at 6-month follow-up. We hypothesized that digital CBT would lead to greater improvement in pain interference, intensity, depression, anxiety, quality of life, and self-efficacy compared with Education. Secondary aims assessed baseline depression as a potential moderator of the effect on the primary outcome.
Methods
Participants
English-speaking adults (aged ≥18 years) with SCD and chronic pain were recruited from 7 comprehensive sickle cell centers and partnering community-based organizations (CBOs) from 19 August 2020 through 12 April 2022. In-person and web-based recruitment identified eligible patients using screening tools, best practice alerts, and study/CBO websites. The inclusion criteria were (1) SCD diagnosis, (2) chronic pain (≥4 d/wk for ≥3 months) and/or prescribed daily opioids for pain, and (3) smartphone access. To verify SCD diagnoses, we required the virtual enrollees to provide recent official medical documentation (such as hospital records, laboratory tests, or doctors’ letters) or government forms that clearly stated their full name and confirmed their SCD diagnosis or treatment. The exclusion criteria were (1) cognitive dysfunction or low literacy, (2) inability to read, speak, and write English, and (3) unwillingness to participate in either intervention arm.
Community organizations were integral partners throughout the CaRISMA study, contributing to protocol development, intervention creation, and health coach identification. They played a crucial role in recruitment efforts across various platforms including web, social media, and community events. Additionally, these organizations provided valuable feedback on study results throughout the project, ensuring that the research remained responsive to community needs and experiences.
Design
This multisite, randomized, pragmatic, comparative effectiveness trial assigned participants in a 1:1 ratio to either a digital CBT or an SCD and pain education (Education) intervention delivered via Facebook Messenger. Upon confirmation of eligibility, participants were randomly assigned to either digital CBT or Education. Permuted block randomization (varying block sizes of 4, 6, and 8) was stratified by study center to control for site-specific disease education and treatment approaches. The randomization schema was created using Stata (StataCorp) by the lead statistician (K.Z.A.) in the data coordinating center and integrated into the web-based data capture system to ensure allocation concealment. Upon confirmation of eligibility, the web-based system sent a unique code via an automated email and/or text to study participants to link them to their assigned chatbot intervention.
Both interventions were cocreated with and tailored for adults with SCD, providing scripted chat interactions and video lessons. The digital CBT focused on behavioral coping skills, whereas Education emphasized self-management through learning about pain and SCD. Participants completed online assessments of pain, self-efficacy, quality of life, depression, and anxiety at 3-, 6-, and 12-month follow-ups, and daily pain diaries between follow-ups. Qualitative interviews were conducted with 48 participants at baseline and 6 months. The qualitative data and 12-month outcomes will be reported in a separate article.
The study was approved by the Institutional Review Board of the University of Pittsburgh, and all participants provided written informed consent. Detailed methods are published in the trial protocol.32
Study outcomes and measures
PROMIS Pain Interference - Short Form 8a
The PROMIS Pain Interference - Short Form 8a measures the impact of pain on social, cognitive, emotional, physical, and recreational activities.33 Higher scores indicate greater pain interference. Raw scores (range, 8-40) are normalized to a T-score (mean = 50, standard deviation = 10) based on the general US population. Studies in pain populations show a minimally important difference ranging from 2.0 to 3.0 points.34
Electronic diary: daily pain intensity and mood
Participants entered daily pain scores (0-10 scale), opioid use, and mood via a mobile web application (app). At each follow-up period (baseline, 3, 6, and 12 months), participants received daily reminders for 2 weeks to assess pain but could continue entering data throughout the 365-day study. The mean pain intensity during each 2-week assessment period was used for analyses.
PHQ
The Patient Health Questionnaire (PHQ)-8/9 assesses depression severity. The PHQ-9, administered only at clinical sites, includes an additional item on suicidality compared with the PHQ-8.35 Participants with PHQ-2 scores >0 completed the full PHQ-8 or PHQ-9. Items are scored on a 0 to 3 scale, with total scores ranging from 0 to 24 (PHQ-8) or 0 to 27 (PHQ-9). Higher scores indicate more severe depression, with cutoffs of 5 (mild), 10 (moderate), 15 (moderately severe), and 20 (severe).36
GAD-7
The Generalized Anxiety Disorder scale (GAD)-7 assesses generalized anxiety disorder severity.37 Participants with GAD-2 scores >0 completed the full GAD-7. Items are scored on a 0 to 3 scale, with total scores ranging from 0 to 21. Higher scores indicate more severe anxiety, with cutoffs of 5 (mild), 10 (moderate), and 15 (severe).
ASCQ-Me
The ASCQ-Me (Adult Sickle Cell Quality of Life Measurement Information System) assesses health care experiences, stress responses, and social functioning in SCD. Higher scores represent worse SCD-related quality of life. We examined the social functioning and emotional impact subscales.38
SCSES
The 9-item Sickle Cell Self-Efficacy Scale (SCSES) measures self-perceived ability to manage day-to-day medical, mental, and emotional aspects of SCD. Items are scored on a 1 to 5 scale, with higher summed scores indicating greater self-efficacy.39
Intervention components
The CaRISMA interventions consisted of a chatbot app accessed through Facebook Messenger, and a health coach.32 The chatbot was fully scripted and followed prewritten conversation trees codeveloped with the research team and CBO partners. It provided customized responses, educational written content, both instructional and testimonial videos featuring adults with SCD, GIFs, and images based on participant responses.
CBT arm
The digital CBT arm taught participants how to recognize negative thoughts and emotions, use cognitive skills and problem-solving, and apply coping behaviors. It emphasized skills acquisition through practice, homework assignments, challenges, and check-ins with a health coach. Participants also had access to a study-associated Facebook page for peer support.
Education arm
The digital Education arm focused on pain and SCD education, teaching users about chronic pain, the physiology and neuroscience underpinning SCD pain, healthy lifestyle tips, and facts about SCD including genetic inheritance. It emphasized knowledge acquisition through brief quizzes and discussion with the health coach and the participant’s social network.
Health coach
Human support may enhance user engagement and optimize the impact of digital interventions.40,41 Thus, both study arms had access to trained lay health coaches, primarily peers with SCD or caregivers, who provided weekly emotional and informational support for 12 weeks to reinforce skills and intervention use. Health coaches underwent an initial 8-hour intensive training session covering health coaching principles, study protocol, communication skills, motivational interviewing, CBT overview, and SCD education. Following this, they received ongoing support through weekly 1-hour supervision meetings and continued training sessions. Adherence to the protocol was maintained through weekly supervision by a master’s-level psychologist or behavioral specialist and the study principal investigator, and monitoring of text message communications. Training time varied but involved at least 6 hours of live video instruction.
Statistical analysis
Our planned sample size of 350 participants provided 80% power to detect a 0.37 standard deviation difference between study arms in 6-month changes, accounting for 15% attrition. Descriptive statistics were calculated for all variables. The missing data mechanism was characterized by comparing attrition rates between study arms and baseline characteristics of withdrawn and remaining participants, and was determined to be nonignorable.
Intention-to-treat analyses were conducted using linear mixed models with time, study arm, their interaction, study site, and baseline depression level as fixed effects, and subject-level random effects. Contrasts assessed the impact on 6-month improvements. Sensitivity analyses of the primary outcome were restricted to participants with confirmed SCD diagnoses. For the depression outcome, sensitivity analyses omitted the ninth PHQ question for all participants.
Subgroup analysis examined the heterogeneity of treatment effects on pain interference between high (PHQ-9 >10) and low (PHQ-9 ≤10) baseline depression levels using a 3-way interaction (time × study arm × baseline depression level).
Exploratory analyses investigated the impact of intervention engagement on outcomes by restricting to participants who started at least 1 lesson and conducting “intensity-adjusted” analyses based on the proportion of completed chatbot sessions and health coach interactions.
Primary and secondary hypotheses were tested using a 5% type I error rate. Analyses were conducted using SAS 9.4 (SAS Institute).
Results
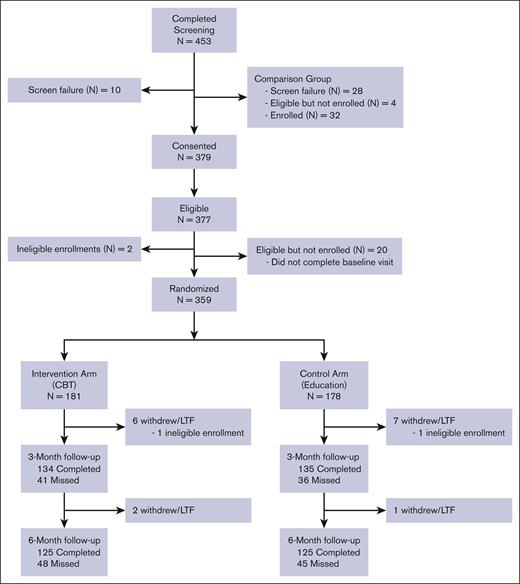
Of the 453 participants screened, 359 (79.2%) were randomly assigned to the CBT (n = 181) and Education (n = 178) groups (Figure 1). As shown in Table 1, the sample was predominantly clinic-enrolled (n = 265; 73.8%), female (66.3%), and Black/African American (92.5%). The mean age was 36.3 years. Most (74.7%) had at least some college, 36.8% were employed, and 45.7% were receiving disability benefits. Apart from a slightly higher proportion of males in the CBT group, demographics appeared balanced between arms. No group differences in suicidal ideation were observed.
CONSORT flow diagram of CaRISMA trial. Participant flow through screening, randomization, allocation, and follow-up. Numbers of participants screened and randomly assigned to CBT or Education arms, completed assessments, missed follow-ups, and withdrawals at 3 and 6 months are shown. LTF, lost to follow-up. For potential participants that were not interested in the intervention study, they were offered enrollment in the "Comparison Group" that required participants complete questionnaires only. Data for comparison group is not reported. LTF, Lost to follow up.
CONSORT flow diagram of CaRISMA trial. Participant flow through screening, randomization, allocation, and follow-up. Numbers of participants screened and randomly assigned to CBT or Education arms, completed assessments, missed follow-ups, and withdrawals at 3 and 6 months are shown. LTF, lost to follow-up. For potential participants that were not interested in the intervention study, they were offered enrollment in the "Comparison Group" that required participants complete questionnaires only. Data for comparison group is not reported. LTF, Lost to follow up.
Baseline demographic and clinical characteristics of CaRISMA trial participants
| Characteristic . | Total (N = 359) . | Intervention arm . | |
|---|---|---|---|
| CBT (n = 181) . | Education (n = 178) . | ||
| Enrolled via | |||
| Clinic | 265 (73.8%) | 133 (73.5%) | 132 (74.2%) |
| Virtual | 94 (26.2%) | 48 (26.5%) | 46 (25.8%) |
| Age at baseline (y) | |||
| Mean (SD) | 36 (10) | 36 (10) | 36 (11) |
| Gender | |||
| Male | 119 (33.1%) | 70 (38.7%) | 49 (27.5%) |
| Female | 238 (66.3%) | 110 (60.8%) | 128 (71.9%) |
| Prefer not to answer | 2 (0.6%) | 1 (0.6%) | 1 (0.6%) |
| Race | |||
| Black/African American | 332 (92.5%) | 169 (93.4%) | 163 (91.6%) |
| White | 2 (0.6%) | 0 | 2 (1.1%) |
| American Indian/Alaska Native | 1 (0.3%) | 0 | 1 (0.6%) |
| Mixed/other | 15 (4.2%) | 7 (3.9%) | 8 (4.5%) |
| Prefer not to answer | 9 (2.5%) | 5 (2.8%) | 4 (2.2%) |
| Education | |||
| Some high school | 22 (6.1%) | 12 (6.6%) | 10 (5.6%) |
| Completed high school or equivalent | 69 (19.2%) | 33 (18.2%) | 36 (20.2%) |
| Some college | 160 (44.6%) | 83 (45.9%) | 77 (43.3%) |
| Completed college | 61 (17.0%) | 31 (17.1%) | 30 (16.9%) |
| Graduate studies | 47 (13.1%) | 22 (12.2%) | 25 (14.0%) |
| Employment status | |||
| Yes, employed | 132 (36.8%) | 67 (37.0%) | 65 (36.5%) |
| No, not employed | 63 (17.5%) | 31 (17.1%) | 32 (18.0%) |
| Disability | 164 (45.7%) | 83 (45.9%) | 81 (45.5%) |
| Pain interference (PROMIS-8a) | |||
| Mean (SD) | 62.6 (7.1) | 62.2 (7.3) | 62.9 (7.0) |
| Proportion of days in happy mood (2 wk) | |||
| Mean (SD) | 0.1 (0.2) | 0.1 (0.2) | 0.1 (0.2) |
| Average daily pain intensity (2 wk) | |||
| Mean (SD) | 4.4 (2.5) | 4.2 (2.5) | 4.5 (2.5) |
| PHQ-9 depressive symptomseverity∗ | |||
| Minimal (0-4) | 35 (12.0%) | 13 (9.0%) | 22 (14.9%) |
| Mild (5-9) | 116 (39.7%) | 60 (41.7%) | 56 (37.8%) |
| Moderate (10-14) | 76 (26.0%) | 34 (23.6%) | 42 (28.4%) |
| Moderately severe (15-19) | 50 (17.1%) | 29 (20.1%) | 21 (14.2%) |
| Severe (20-27) | 15 (5.1%) | 8 (5.6%) | 7 (4.7%) |
| Did not complete | 67 | 37 | 30 |
| Suicidal ideation (PHQ) item 9 | |||
| No | 175 (85.4%) | 84 (84.8%) | 91 (85.8%) |
| Yes | 30 (14.6%) | 15 (15.2%) | 15 (14.2%) |
| GAD-7 anxiety symptom severity† | |||
| Minimal (0-4) | 46 (18.3%) | 23 (17.6%) | 23 (19.2%) |
| Mild (5-9) | 113 (45.0%) | 61 (46.6%) | 52 (43.3%) |
| Moderate (10-14) | 62 (24.7%) | 31 (23.7%) | 31 (25.8%) |
| Severe (15-21) | 30 (12.0%) | 16 (12.2%) | 14 (11.7%) |
| Did not complete | 108 | 50 | 58 |
| ASCQ-Me: pain episode frequency | |||
| Mean (SD) | 48.3 (12.3) | 48.2 (12.1) | 48.3 (12.6) |
| ASCQ-Me: pain episode severity | |||
| Mean (SD) | 47.3 (13.3) | 47.7 (13.3) | 46.8 (13.4) |
| ASCQ-Me: social functioning impact | |||
| Mean (SD) | 47.3 (7.9) | 47.4 (8.6) | 47.1 (7.1) |
| ASCQ-Me: emotional impact | |||
| Mean (SD) | 48.0 (8.8) | 47.2 (8.8) | 48.8 (8.8) |
| Pain catastrophizing scale score | |||
| Mean (SD) | 9 (4.0) | 9 (4.0) | 9 (4.0) |
| Sickle Cell Self-Efficacy Scale (SCSES) | |||
| Mean (SD) | 29 (7.0) | 28 (6.0) | 29 (7.0) |
| Characteristic . | Total (N = 359) . | Intervention arm . | |
|---|---|---|---|
| CBT (n = 181) . | Education (n = 178) . | ||
| Enrolled via | |||
| Clinic | 265 (73.8%) | 133 (73.5%) | 132 (74.2%) |
| Virtual | 94 (26.2%) | 48 (26.5%) | 46 (25.8%) |
| Age at baseline (y) | |||
| Mean (SD) | 36 (10) | 36 (10) | 36 (11) |
| Gender | |||
| Male | 119 (33.1%) | 70 (38.7%) | 49 (27.5%) |
| Female | 238 (66.3%) | 110 (60.8%) | 128 (71.9%) |
| Prefer not to answer | 2 (0.6%) | 1 (0.6%) | 1 (0.6%) |
| Race | |||
| Black/African American | 332 (92.5%) | 169 (93.4%) | 163 (91.6%) |
| White | 2 (0.6%) | 0 | 2 (1.1%) |
| American Indian/Alaska Native | 1 (0.3%) | 0 | 1 (0.6%) |
| Mixed/other | 15 (4.2%) | 7 (3.9%) | 8 (4.5%) |
| Prefer not to answer | 9 (2.5%) | 5 (2.8%) | 4 (2.2%) |
| Education | |||
| Some high school | 22 (6.1%) | 12 (6.6%) | 10 (5.6%) |
| Completed high school or equivalent | 69 (19.2%) | 33 (18.2%) | 36 (20.2%) |
| Some college | 160 (44.6%) | 83 (45.9%) | 77 (43.3%) |
| Completed college | 61 (17.0%) | 31 (17.1%) | 30 (16.9%) |
| Graduate studies | 47 (13.1%) | 22 (12.2%) | 25 (14.0%) |
| Employment status | |||
| Yes, employed | 132 (36.8%) | 67 (37.0%) | 65 (36.5%) |
| No, not employed | 63 (17.5%) | 31 (17.1%) | 32 (18.0%) |
| Disability | 164 (45.7%) | 83 (45.9%) | 81 (45.5%) |
| Pain interference (PROMIS-8a) | |||
| Mean (SD) | 62.6 (7.1) | 62.2 (7.3) | 62.9 (7.0) |
| Proportion of days in happy mood (2 wk) | |||
| Mean (SD) | 0.1 (0.2) | 0.1 (0.2) | 0.1 (0.2) |
| Average daily pain intensity (2 wk) | |||
| Mean (SD) | 4.4 (2.5) | 4.2 (2.5) | 4.5 (2.5) |
| PHQ-9 depressive symptomseverity∗ | |||
| Minimal (0-4) | 35 (12.0%) | 13 (9.0%) | 22 (14.9%) |
| Mild (5-9) | 116 (39.7%) | 60 (41.7%) | 56 (37.8%) |
| Moderate (10-14) | 76 (26.0%) | 34 (23.6%) | 42 (28.4%) |
| Moderately severe (15-19) | 50 (17.1%) | 29 (20.1%) | 21 (14.2%) |
| Severe (20-27) | 15 (5.1%) | 8 (5.6%) | 7 (4.7%) |
| Did not complete | 67 | 37 | 30 |
| Suicidal ideation (PHQ) item 9 | |||
| No | 175 (85.4%) | 84 (84.8%) | 91 (85.8%) |
| Yes | 30 (14.6%) | 15 (15.2%) | 15 (14.2%) |
| GAD-7 anxiety symptom severity† | |||
| Minimal (0-4) | 46 (18.3%) | 23 (17.6%) | 23 (19.2%) |
| Mild (5-9) | 113 (45.0%) | 61 (46.6%) | 52 (43.3%) |
| Moderate (10-14) | 62 (24.7%) | 31 (23.7%) | 31 (25.8%) |
| Severe (15-21) | 30 (12.0%) | 16 (12.2%) | 14 (11.7%) |
| Did not complete | 108 | 50 | 58 |
| ASCQ-Me: pain episode frequency | |||
| Mean (SD) | 48.3 (12.3) | 48.2 (12.1) | 48.3 (12.6) |
| ASCQ-Me: pain episode severity | |||
| Mean (SD) | 47.3 (13.3) | 47.7 (13.3) | 46.8 (13.4) |
| ASCQ-Me: social functioning impact | |||
| Mean (SD) | 47.3 (7.9) | 47.4 (8.6) | 47.1 (7.1) |
| ASCQ-Me: emotional impact | |||
| Mean (SD) | 48.0 (8.8) | 47.2 (8.8) | 48.8 (8.8) |
| Pain catastrophizing scale score | |||
| Mean (SD) | 9 (4.0) | 9 (4.0) | 9 (4.0) |
| Sickle Cell Self-Efficacy Scale (SCSES) | |||
| Mean (SD) | 29 (7.0) | 28 (6.0) | 29 (7.0) |
SD, standard deviation.
PHQ-9 for clinic participants and PHQ-8 for virtual participants with PHQ-2 >0.
GAD-7 for all participants with GAD-2 >0.
At 3 months, 269 (75%) participants completed assessments; at 6 months, 250 (70%) completed assessments. In the CBT arm, 8 participants withdrew or were ineligible, and 41 (23.4%) and 48 (27.7%) missed 3- and 6-month follow-ups, respectively. In the Education arm, 8 withdrew or were ineligible, and 36 (21.1%) and 45 (26.5%) missed 3- and 6-month follow-ups, respectively.
CBT and Education groups had similar withdrawal rates (CBT, n = 8 [4.4%] vs Education, n = 8 [4.5%]; P > .99) and 6-month retention rates (CBT, n = 125 [69.1%] vs Education, n = 125 [70.2%]; P = .82) (Figure 1).
Primary and secondary outcomes
There were no significant differences between CBT and Education arms in the effect of the intervention on pain interference (0.54; −1.30 to 2.37; P = .57) (Table 2). Restricting the analysis to the 346 participants with confirmed SCD yielded negligible differences (0.50; −1.34 to 2.35; P = .59). Similarly, there were no between-arm differences in the effects on daily pain intensity; PHQ depression, GAD anxiety, and ASCQ-Me social functioning scores (all P > .30); or self-efficacy (P = .12). For the ASCQ-Me emotional impact scores, the between-group difference approached significance, with CBT being associated with 1.72-point greater improvements (95% confidence interval [CI], −0.03 to 3.46; P = .05). When we restricted our primary analysis to the 346 participants with confirmed SCD diagnosis, the difference in results was negligible (0.50; −1.34 to 2.35; P = .59).
Primary and secondary outcomes at 6 months in the CaRISMA trial
| Outcome . | Estimated 6-month change . | CBT vs Education . | Cohen d . | |
|---|---|---|---|---|
| CBT . | Education . | |||
| Primary | ||||
| Pain interference (PROMIS-8a) | −2.13 (−3.42 to −0.84) | −2.66 (−3.97 to −1.36) | 0.54 (−1.30 to 2.37) P = .57 | 0.06 (−0.15 to 0.27) |
| Secondary | ||||
| Average daily pain intensity | 0.20 (−0.18 to 0.58) | 0.13 (−0.25 to 0.51) | 0.07 (−0.46 to 0.61) P = .79 | 0.05 (−0.37 to 0.49) |
| PHQ∗ | −1.33 (−2.28 to −0.39) | −1.12 (−2.06 to −0.18) | −0.21 (−1.55 to 1.12) P = .75 | 0.04 (−0.27 to 0.19) |
| GAD-7† | −0.85 (−1.68 to −0.01) | −1.49 (−2.40 to −0.58) | 0.64 (−0.60 to 1.87) P = .31 | 0.13 (−0.12 to 0.37) |
| ASCQ-Me: social functioning | 2.45 (1.13-3.76) | 2.34 (1.00-3.68) | 0.11 (−1.77 to 1.99) P = .91 | 0.01 (−0.19 to 0.22) |
| ASCQ-Me: emotional impact | 3.51 (2.29-4.73) | 1.79 (0.55-3.04) | 1.72 (−0.03 to 3.46) P = .05 | 0.20 (−0.004 to 0.41) |
| SCSES | 1.45 (0.44-2.46) | 0.32 (−0.70 to 1.35) | 1.13 (−0.31 to 2.56) P = .12 | 0.16 (−0.04 to 0.37) |
| Outcome . | Estimated 6-month change . | CBT vs Education . | Cohen d . | |
|---|---|---|---|---|
| CBT . | Education . | |||
| Primary | ||||
| Pain interference (PROMIS-8a) | −2.13 (−3.42 to −0.84) | −2.66 (−3.97 to −1.36) | 0.54 (−1.30 to 2.37) P = .57 | 0.06 (−0.15 to 0.27) |
| Secondary | ||||
| Average daily pain intensity | 0.20 (−0.18 to 0.58) | 0.13 (−0.25 to 0.51) | 0.07 (−0.46 to 0.61) P = .79 | 0.05 (−0.37 to 0.49) |
| PHQ∗ | −1.33 (−2.28 to −0.39) | −1.12 (−2.06 to −0.18) | −0.21 (−1.55 to 1.12) P = .75 | 0.04 (−0.27 to 0.19) |
| GAD-7† | −0.85 (−1.68 to −0.01) | −1.49 (−2.40 to −0.58) | 0.64 (−0.60 to 1.87) P = .31 | 0.13 (−0.12 to 0.37) |
| ASCQ-Me: social functioning | 2.45 (1.13-3.76) | 2.34 (1.00-3.68) | 0.11 (−1.77 to 1.99) P = .91 | 0.01 (−0.19 to 0.22) |
| ASCQ-Me: emotional impact | 3.51 (2.29-4.73) | 1.79 (0.55-3.04) | 1.72 (−0.03 to 3.46) P = .05 | 0.20 (−0.004 to 0.41) |
| SCSES | 1.45 (0.44-2.46) | 0.32 (−0.70 to 1.35) | 1.13 (−0.31 to 2.56) P = .12 | 0.16 (−0.04 to 0.37) |
PHQ-9 for clinic participants and PHQ-8 for virtual participants with baseline PHQ-2 >0.
GAD-7 for all participants with baseline GAD-2 >0.
Significant improvements in PROMIS Pain Interference scores were found in both CBT (−2.13 [95% CI −3.42 to −0.84]) and Education (−2.66 [−3.97 to −1.36]) arms at 6 months; however, this did not apply to daily pain intensity. Within-arm improvements were observed for PHQ depression (CBT, −1.33 [−2.28 to −0.39]; Education, −1.12 [−2.06 to −0.18]), GAD-7 anxiety (CBT, −0.85 [−1.68 to −0.01]; Education, −1.49 [−2.40 to −0.58]), ASCQ-Me social functioning (CBT, 2.45 [1.13-3.76]; Education, 2.34 [1.00-3.68]), and ASCQ-Me emotional impact scores (CBT, 3.51 [2.29-4.73]; Education, 1.79 [0.55-3.04]). For SCSES self-efficacy scores, only CBT showed significant improvement (1.45 [0.44-2.46]).
Heterogeneity of treatment effects
Baseline PHQ depression score (≥10 vs <10) did not moderate the effect of treatments on pain interference at 6 months. The effect of digital CBT was similar between participants with low (0.93 [95% CI, −1.42 to −3.28]) and high (0.10 [−2.87 to 3.07]) depression scores (P = .52).
Digital intervention engagement
As shown in Table 3, a similar proportion of participants in the CBT (24%) and Education (23%) arms never connected to the chatbot. Reasons for not connecting included not wanting to be on Facebook, difficulty with passwords, and lack of reminders. A higher percentage of CBT participants (19%) connected but never started lessons compared with Education participants (9%). Completion rates for Lesson 1 were 45% (CBT) and 51% (Education), but these rates decreased for subsequent lessons, with 18% (CBT) and 32% (Education) completing all lessons.
Digital intervention engagement metrics in the CaRISMA trial
| HC sessions . | CBT (N = 181)∗ . | Education (N = 178)† . | ||
|---|---|---|---|---|
| n . | mean (SD) . | n . | mean (SD) . | |
| Number within 3 months of enrollment | 140 | 4.1 (2.8) | 146 | 4.3 (3.0) |
| Lesson Number | Started n | Finished (% randomized) | Started n | Finished (% randomized) |
| Never connected to chatbot | − | 44 (24%) | − | 41 (23%) |
| Connected to chatbot, but never started | − | 35 (19%) | − | 16 (9%) |
| 1 | 102 | 81 (45%) | 120 | 91 (51%) |
| 2 | 72 | 52 (29%) | 78 | 70 (39%) |
| 3 | 49 | 43 (24%) | 67 | 61 (34%) |
| 4 | 41 | 36 (20%) | 60 | 57 (32%) |
| 5 | 32 | 32 (18%) | − | − |
| HC sessions . | CBT (N = 181)∗ . | Education (N = 178)† . | ||
|---|---|---|---|---|
| n . | mean (SD) . | n . | mean (SD) . | |
| Number within 3 months of enrollment | 140 | 4.1 (2.8) | 146 | 4.3 (3.0) |
| Lesson Number | Started n | Finished (% randomized) | Started n | Finished (% randomized) |
| Never connected to chatbot | − | 44 (24%) | − | 41 (23%) |
| Connected to chatbot, but never started | − | 35 (19%) | − | 16 (9%) |
| 1 | 102 | 81 (45%) | 120 | 91 (51%) |
| 2 | 72 | 52 (29%) | 78 | 70 (39%) |
| 3 | 49 | 43 (24%) | 67 | 61 (34%) |
| 4 | 41 | 36 (20%) | 60 | 57 (32%) |
| 5 | 32 | 32 (18%) | − | − |
HC, Health Coach.
No CBT participants used the wrong branch.
Three Education participants used the wrong branch (1 of whom never started lessons); 1 among 178 was excluded because of illogical chatbot entries.
Health coach engagement
Of all the randomly assigned participants, 286 (79.7%) completed at least 1 health coach session within 3 months of enrollment. The mean number of health coach sessions was 4.2 out of 12 overall, with 4.1 sessions in the CBT arm and 4.3 sessions in the Education arm. Health coach sessions were mostly conducted by telephone (62%-93%), with a mean session length of 25 minutes. For example, in Session 1, 93% of CBT contacts and 94% of Education contacts were conducted by telephone, with mean durations of 27 and 26 minutes, respectively. Text-based contacts accounted for 4% (CBT) and 3% (Education). The CBT and Education arms had similar numbers and durations of health coach contacts.
Exploratory and post hoc analyses
Treatment engagement intensity effect
In the subset of participants who started at least 1 chat session, the intervention effect on pain interference was more attenuated (−0.05 [95% CI, −2.29 to 2.20]; P = .97) than in the overall cohort. Further, the proportion of lessons completed was not associated with changes in pain interference (−0.13 points per each 10% increase in lessons completed [95% CI, −0.37 to 0.12]; P = .31) (Table 4), with estimated gains ranging from −1.83 points (0% completed) to −3.09 points (100% completed).
Association between intervention engagement and pain interference outcomes
| Intervention . | Pain interference (PROMIS-8a T-score) mean change to 6 months . | Estimate (95% CI)∗ . | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 completed . | 10% completed . | 25% completed . | 50% completed . | 75% completed . | 90% completed . | 100% completed . | ||
| Proportion of HC sessions within 3 months of enrollment | −1.65 | −1.89 | −2.26 | −2.87 | −3.48 | −3.85 | −4.10 | −0.024 (−0.059 to 0.010) P = .17 |
| Proportion of chatbot lessons completed | −1.83 | −1.96 | −2.15 | −2.46 | −2.78 | −2.96 | −3.09 | −0.013 (−0.037 to 0.012) P = .31 |
| Mean of proportion of HC sessions and chatbot lessons completed | −1.51 | −1.74 | −2.09 | −2.68 | −3.27 | −3.62 | −3.85 | −0.023 (−0.057 to 0.010) P = .17 |
| Intervention . | Pain interference (PROMIS-8a T-score) mean change to 6 months . | Estimate (95% CI)∗ . | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 completed . | 10% completed . | 25% completed . | 50% completed . | 75% completed . | 90% completed . | 100% completed . | ||
| Proportion of HC sessions within 3 months of enrollment | −1.65 | −1.89 | −2.26 | −2.87 | −3.48 | −3.85 | −4.10 | −0.024 (−0.059 to 0.010) P = .17 |
| Proportion of chatbot lessons completed | −1.83 | −1.96 | −2.15 | −2.46 | −2.78 | −2.96 | −3.09 | −0.013 (−0.037 to 0.012) P = .31 |
| Mean of proportion of HC sessions and chatbot lessons completed | −1.51 | −1.74 | −2.09 | −2.68 | −3.27 | −3.62 | −3.85 | −0.023 (−0.057 to 0.010) P = .17 |
For each % increase in intervention completed, estimated decline in pain interference at 6 months.
No relationship was found between the proportion of completed health coach sessions and reductions in pain interference at 6 months (−0.24 points per each 10% increase in health coach sessions completed [95% CI, −0.59 to 0.10]; P = .17) (Table 4). Estimated mean improvements ranged from −1.65 points among those completing 0% of health coach sessions to −4.10 points with 100% health coach session completion.
After combining the chatbot and health coach engagement measures into an average value, no relationship was found between the proportion of sessions completed and reductions in pain interference at 6 months (−0.23 points per each 10% increase in average sessions/lessons completed [95% CI, −0.57 to 0.10]; P = .17) (Table 4).
Discussion
The CaRISMA study represents a significant milestone in SCD research, which, to our knowledge, is the largest behavioral intervention trial to date, with a strong emphasis on community engagement. Conducted during the COVID-19 pandemic, this comparative effectiveness study of digital CBT and education met its enrollment goal of 350 participants with a relatively low dropout rate, underscoring the potential of community-engaged approaches in underserved populations.
Contrary to our hypothesis, this adequately powered study found that digital education with health coach support was as effective as digital CBT with the same support. Until now, there have been few large-scale trials of nonpharmacological SCD interventions. Although some studies suggest digital CBT’s effectiveness for pain and mental health in SCD,42-44 they are limited by small samples, heterogeneous interventions, lack of control groups, short follow-up periods, and reliance on self-report measures.27,45 Only one-third of nonpharmacological SCD interventions have shown effectiveness, with inconclusive evidence for self-management interventions.46
However, the existing data on CBT specifically for SCD pain are encouraging. Schatz et al47 found that increased practice of digital CBT coping skills was associated with next-day pain reductions among pediatric participants. Sil et al48 found some CBT-related improvements in health care utilization among children and adolescents with SCD. Palermo et al49 demonstrated medium-sized effects on pain in adolescents with SCD. Although CBT is effective for pain compared with control or usual care,50 the current study is, to our knowledge, the first to compare digital CBT with another evidence-based nonpharmacological intervention such as health coach–supported psychoeducation in adults.
The CaRISMA study addresses a key limitation of prior research by integrating the community at all stages, including intervention delivery. Previous SCD intervention research often lacked real community partnership in design, delivery, and trial conduct.45 Few studies involved people with SCD in developing self-management interventions, primarily in pediatrics,29,51 with limited participation in needs assessment or treatment delivery.46 To our knowledge, this is the first study in adults with SCD to involve the target population in intervention cocreation, which is crucial for engagement.52
Our findings suggest that community engagement facilitates enrollment, and peer support from community members is a particularly attractive aspect of the intervention. This underscores the need for more community-engaged work in SCD research and intervention development.
However, the study revealed important challenges in digital engagement. Despite successful enrollment, participants’ interaction with the digital components was lower than anticipated, which may explain the modest effect sizes and lack of significant differences between the CBT and Education arms. Trials typically find that less than half (44.2%) of participants complete all treatment modules,53 with poorer engagement among minority populations.54 Despite implementing codesign, tailoring, health coach support, and community involvement, engagement remained challenging.
Factors potentially contributing to lack of intervention uptake include Facebook platform restrictions preventing push notifications; lack of trust in Facebook and shifts in social media popularity55; complex password and identification code verification process; inconsistent participant payments due to logistical challenges; and perceived redundancy of intervention information for some participants.
Facebook was initially recommended by patients and family members during the study design phase,50 but subsequent data breaches and privacy concerns may have soured perceptions.55 Some participants refused to engage with Facebook, even when offered dummy accounts.
Despite the barriers to engagement, the CaRISMA trial had 2 promising findings that should drive future research. First, this study highlighted the potential of peer health coaches in enhancing digital therapeutics’ effectiveness. Although digital engagement was low, participants showed higher interaction with peer health coaches, suggesting that this human element may be crucial in bridging the gap between digital interventions and patient needs.
Second, CBT showed promise in improving self-efficacy, whereas the Education arm did not exhibit any change in self-efficacy. This suggests that CBT may be affecting the intended treatment mechanism,56,57 potentially contributing to positive outcomes if engagement is optimized. Future studies should evaluate whether CBT is more effective than education alone for patients with low self-efficacy.
The trial did not find differences between the study arms on the daily 0 to 10 numeric pain rating scale at 6 months. The discrepancy may be due to the limitations of this unidimensional measure, which only assesses pain intensity and fails to account for the affective and cognitive aspects of pain and the multidimensional nature of the pain experience.58,59 Furthermore, the scale’s simplicity may lead to a lack of sensitivity in detecting subtle changes in pain perception over time.
Limitations
This study has some limitations. First, technical challenges may have contributed to poor intervention engagement and participants’ ability to use the study apps as intended. Second, variability in health coaching quality and adherence to the protocol was observed due to unexpected demand and the number of coaches involved. Health coach interventions that rely on volunteers may be less effective.60 Third the attrition rate was greater than the proposed 15% for which this study was powered. Finally, the inclusion of a standard-of-care control group would have helped explain improvements in symptoms.
Conclusion
In this study, which, to our knowledge, is the largest behavioral trial in SCD and one of the largest digital health trials in a minority population, we demonstrated the feasibility of a community-engaged, decentralized approach. Both health coach–supported digital interventions effectively improved pain interference, mental health, and quality of life in adults with SCD. Our findings suggest that digital CBT, or pain education, when combined with centralized health coach support, may serve as a scalable and low-cost method for delivering high-quality behavioral care to underserved communities, addressing the challenges of chronic pain and mental health in individuals with SCD and other marginalized populations.
Acknowledgments
Editing was provided by Andrea Ball.
Research reported in this publication was funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (CER-2018C2-13320) (principal investigators: C.R.J. and K.Z.A.). J.A.O.’s work on this publication was supported by T32NR008857 from the National Institute of Nursing Research.
The statements in this publication are solely the responsibility of the authors and do not necessarily represent the views of PCORI or its Board of Governors or Methodology Committee.
Authorship
Contribution: C.R.J. and K.Z.A., as primary investigators, oversaw the execution and design of the study, outlined the manuscript and its coherence with research objectives, and actively contributed to data analysis; C.M.L. contributed to data analysis and analytical methods, and the interpretation of results and findings; and all authors contributed to the design and conduct of the study, including collection of study-related data, and reviewed the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Charles R. Jonassaint, Department of Medicine, University of Pittsburgh, 230 McKee Pl, Suite 600, Pittsburgh, PA 15213; email: cjonassaint@pitt.edu.
References
Author notes
Original data are available on request from the corresponding author, Charles R. Jonassaint (cjonassaint@pitt.edu).