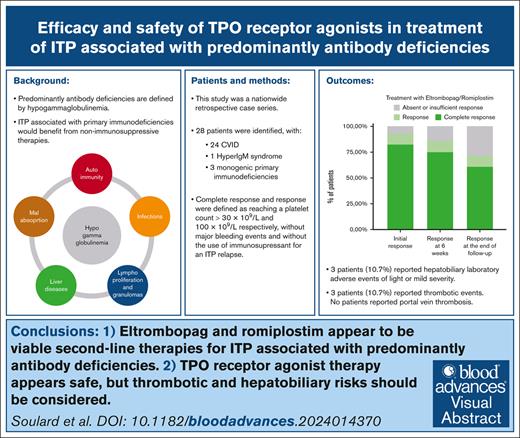
Key Points
After 6 weeks of treatment, response was achieved in 24 of 28 patients (85.7%).
Thrombotic events were reported for 3 patients (10.7%), none of which exhibited bleeding complications.
Visual Abstract
Predominantly antibody deficiencies have an estimated prevalence of >1 in 25 000. Their classical phenotype entails the association of autoimmune manifestations with increased susceptibility to infections. Up to 8% of these patients ultimately develop immune thrombocytopenic purpura (ITP). Reducing the risk for infections and considering nonimmunosuppressive treatments, such as thrombopoietin receptor agonists (TPO-RAs), are important considerations for these patients. This nationwide retrospective case series assessed the outcomes and safety of TPO-RAs as treatment for ITP in adults diagnosed with predominantly antibody deficiencies. Response and complete response to treatment were defined as platelet count reaching 30 × 109/L and 100 × 109/L, respectively. We analyzed data from 28 patients. The median follow-up time after introduction of the first TPO-RAs was 33 months (range, 2 weeks to 10.6 years). After 6 weeks of follow-up, response was achieved in 24 of the 28 patients (85.7%), and among those, 21 patients (75%) displayed a complete response. At the last available follow-up visit, only 7 patients (25%) needed second-line therapies for ITP, and among those, only 5 patients (17.9%) received immunosuppressants. Only 3 patients (10.7%) reported laboratory-confirmed hepatobiliary adverse events of light or mild severity and 3 patients (10.7%) reported thrombotic events. In conclusion, TPO-RAs seemed to be an effective and safe option of treatment in these case series. Our results suggest that eltrombopag or romiplostim should be considered as second-line therapy for ITP related to predominantly antibody deficiencies.
Introduction
Primary immunodeficiencies (PIDs) encompass a heterogenous group of nearly 500 disorders.1 The most prevalent PIDs are predominantly antibody deficiencies, characterized by hypogammaglobulinemia. Among them, common variable immunodeficiency disorders (CVIDs) are characterized by a significant decrease in immunoglobulin G (IgG) and IgA serum levels with or without low IgM levels and have an estimated prevalence of 1 in 25 000. The classical phenotype of predominantly antibody deficiencies entails an increased susceptibility to infections, autoimmune manifestations, granulomatous diseases, and unexplained polyclonal lymphoproliferation.2,3(p2) About 20% to 30% of patients diagnosed with CVIDs have autoimmune manifestations, with immune thrombocytopenic purpura (ITP) being the most prevalent manifestation, affecting up to 8% of patients with CVIDs.3(p2),4-6 ITP is categorized as primary ITP or as secondary ITP when it is associated with an underlying disorder, such as PIDs.7
Although immunosuppressive therapies remain the cornerstone of ITP treatment, management of primary ITP has been impacted significantly by the approval of thrombopoietin receptor agonists (TPO-RAs) in 2007, namely eltrombopag and romiplostim.8-14 The management of secondary ITP in the context of PIDs mirrors that of primary ITP and involve corticosteroids as first-line treatment. However, second-line therapeutic strategy lacks robust evidence and is based on limited published data.15 Although retrospective studies have explored the use of rituximab16 and splenectomy17 in patients with ITP and PIDs, the use of TPO-RAs has only been documented in 1 case report and an observational study on eltrombopag in secondary ITP that included data from 12 patients diagnosed with CVID.18,19
In patients with PIDs, the primary health burden is related to infectious diseases, underscoring the importance of minimizing the use of immunosuppressants. TPO-RAs have emerged as a promising alternative and detailed specification of their effectiveness and safety in this context is still lacking. This retrospective assessment of case series through a nationwide investigation based on real-life data aimed to provide additional insights on the effectiveness and safety of TPO-RAs in patients with ITP and PIDs.
Methods
Inclusion and exclusion criteria
The inclusion criteria were as follows:
1. A diagnosis of PID with predominantly antibody deficiency, defined as a clinical history consistent with CVIDs and associated with an IgG level below 500 mg/dL.2 Given their shared initial clinical presentation, patients with late-onset combined immune deficiency or monogenic causes of this CVID-like phenotype were also included.20,21
2. A diagnosis of secondary ITP, defined according to international working group criteria, with a platelet count threshold of 100 × 109/L.7
3. TPO-RA therapy (eltrombopag and/or romiplostim) introduced after the age of 18 years.
The exclusion criteria included the use of B-depleting therapies (such as rituximab) before the diagnosis of PID, except in patients for whom a monogenic cause of PID had been identified.
Data collection and study design
The French network Center of Reference for Hereditary Immunodeficiency Disorders (CEREDIH)22 facilitated the identification of practitioners who had reported cases of patients with PIDs and ITP from January 2007 to June 2023, among which featured members of the Department of Clinical Immunology, and Immunodeficiencies Center. The Center of Reference for Autoimmune Cytopenias in Adults (CeReCaI), a national referral network for adult immune cytopenias, launched a call for case reports to all clinicians from the network. In parallel, every French university hospital was contacted between October 2022 and June 2023 to identify patients who met the inclusion criteria.
In 2009, the French national registry of PIDs recorded 504 patients with predominantly antibody deficiencies22 with studies indicating that 5% to 10% were expected to develop ITP over time3(p2),4-6; this suggested that ∼50 patients in France were expected to have both conditions. We did not have an estimate of the percentage of patients who might have been treated with TPO-RA.
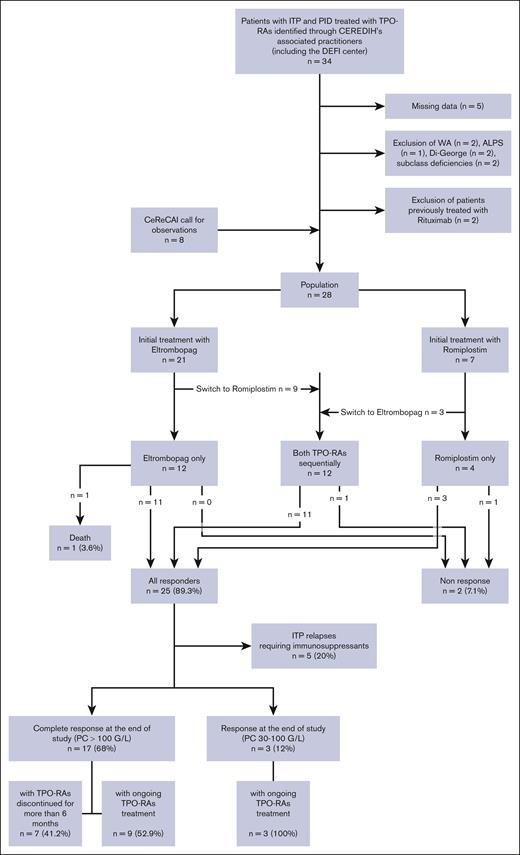
Figure 1 provides the flowchart for the 28 patients who were finally included from 12 centers. Retrospective data collection was then carried out using a standardized case report form. Each patient received an information letter, and none objected to data collection. This retrospective study was approved by the local ethics committee n°2324 from the Rennes University Hospital. Descriptive statistics for quantitative variables were presented as median (range, minimum-maximum) and qualitative variables were presented as frequency (percentage).
Flow chart: study population. ALPS, autoimmune lymphoproliferative syndrome; CeReCaI, Center of Reference for Autoimmune Cytopenias in Adults; CEREDIH, Center of Reference for Hereditary Immunodeficiency Disorders; Di-Georges, Di-Georges syndrome; PC, platelet count; WA, Wiskott-Aldrich syndrome.
Flow chart: study population. ALPS, autoimmune lymphoproliferative syndrome; CeReCaI, Center of Reference for Autoimmune Cytopenias in Adults; CEREDIH, Center of Reference for Hereditary Immunodeficiency Disorders; Di-Georges, Di-Georges syndrome; PC, platelet count; WA, Wiskott-Aldrich syndrome.
Definitions
Rescue treatments of ITP included high-dose of intravenous immunoglobulin at 1 to 2 g/kg (IVIG) or short courses of corticosteroids (prednisone at 0.5-2.0 mg/kg per day, for <6 weeks, including initial course and tapering, or dexamethasone at 40 mg per day for 4 days). Second-line treatments included immunomodulating therapies (hydroxychloroquine, dapsone, danazol, vinca alkaloids) and immunosuppressive approaches (corticosteroids for >6 weeks, rituximab, cyclosporin, cyclophosphamide, sirolimus, mycophenolate mofetil, and splenectomy).10 ITP duration was categorized according to the international working group criteria.7 Bleeding events were reported using the Khellaf score; the threshold of 8 was used because it is commonly associated with the indication for IVIG.23
TPO-RA introduction was defined as the time eltrombopag or romiplostim was introduced, whichever occurred first. Because eltrombopag and romiplostim can be used interchangeably as second-line ITP treatments, they were considered together for some analyses and referred to as any TPO-RAs.
For patients treated sequentially on-demand as reported in the REPEAT trial (Repeated Short-Term Use of Eltrombopag in Patients with Chronic ITP),24 treatment duration was measured from the first to the last day of any exposure to any TPO-RAs. Bleeding events during these off-treatment periods were still recorded as adverse events of TPO-RAs.
Lymphadenopathy was recorded based on either clinical, ultrasound, or computed tomography (CT) scan assessments. Splenomegaly was defined as a palpable spleen upon clinical examination or ultrasound/scan caudal measurement exceeding 12 cm.25 Bronchiectasis was defined based on the radiologist’s judgment from a chest CT scan. Granulomas included biopsy-proven granulomatous diseases and granulomatous-lymphocytic interstitial lung disease on chest CT scan. The diagnosis of porto-sinusoidal vascular disease (PSVD) was based on histologic considerations.26
Treatment response
Complete response and response were defined as achieving a platelet count greater than 100 × 109/L and 30 × 109/L, respectively, without bleeding events with a Khellaf score >8. We considered response to be incomplete when concomitant immunosuppressive approaches were introduced because of an ITP relapse. Nonresponders were patients who never achieved response despite TPO-RAs therapy. Treatment response was assessed as initial response (in the days following TPO-RAs introduction), after 6 weeks of treatment, and at the end of study.
Results
Patients characteristics
| Characteristics . | N = 28 . | |
|---|---|---|
| Male | 17 | (60.7%) |
| Age at PID diagnosis (y) | 30 | (14-68) |
| Age at ITP diagnosis (y) | 31 | (5-68) |
| Follow-up duration after ITP diagnosis (mo) | 81 | (11-324) |
| Type of PID | ||
| CVID | 24 | (85.7%) |
| Including LOCID | 3 | (10.7%) |
| HIGM | 1 | (3.6%) |
| CTLA4 deficiency | 1 | (3.6%) |
| PI3KCD mutation | 1 | (3.6%) |
| KMT2D mutation (Kabuki syndrome) | 1 | (3.6%) |
| Immunoglobulin serum levels at the time of PID diagnosis (mg/dL) | ||
| IgG | 326 | (85-486) |
| IgA | 27 | (0-146) |
| IgM | 39 | (4-500) |
| Characteristics . | N = 28 . | |
|---|---|---|
| Male | 17 | (60.7%) |
| Age at PID diagnosis (y) | 30 | (14-68) |
| Age at ITP diagnosis (y) | 31 | (5-68) |
| Follow-up duration after ITP diagnosis (mo) | 81 | (11-324) |
| Type of PID | ||
| CVID | 24 | (85.7%) |
| Including LOCID | 3 | (10.7%) |
| HIGM | 1 | (3.6%) |
| CTLA4 deficiency | 1 | (3.6%) |
| PI3KCD mutation | 1 | (3.6%) |
| KMT2D mutation (Kabuki syndrome) | 1 | (3.6%) |
| Immunoglobulin serum levels at the time of PID diagnosis (mg/dL) | ||
| IgG | 326 | (85-486) |
| IgA | 27 | (0-146) |
| IgM | 39 | (4-500) |
Normal values (ranges): IgG, 650 to 1400 mg/dL; IgA, 70 to 320 mg/dL; IgM, 50 to 350 mg/dL. Quantitative variables are presented as median (range, minimum to maximum) and qualitative variables as frequency (percentage).
HIGM, hyper IgM syndrome; LOCID, late-onset combined immune deficiency.
Population characteristics at the time of TPO-RAs introduction
| Patient and sex . | ITP duration, mo . | Age at diagnosis of PID/ITP/introduction of TPO-RA, y . | IgG/IgA/IgM levels at PID diagnosis, mg/dL∗ . | Type of PID . | Recurrent infections†/IRT . | Other PID-related manifestations . | ||
|---|---|---|---|---|---|---|---|---|
| 1 | M | 294 | 16/5/29 | 321/37/12 | LOCID | Yes | Yes | Lymphadenopathy, splenomegaly, GLILD, bronchiectasis, AIHA, HPV infections, PSVD |
| 2 | F | <1 | 19/19/19 | 250/15/28 | CVID | Yes | No | Splenomegaly, autoimmunity, hepatomegaly |
| 3 | M | 168 | 24/9/23 | 150/0/500 | HIGM | Yes | No | Lymphadenopathy, granulomas, MALT lymphoma |
| 4 | M | 295 | 33/12/37 | 85/0/4 | LOCID | Yes | Yes | Lymphadenopathy, splenomegaly, granulomas, bronchiectasis, HPV infections, PSVD |
| 5 | F | 3 | 49/48/48 | 300/18/8 | CVID | Yes | No | Lymphadenopathy, splenomegaly, GLILD |
| 6 | M | 5 | 22/22/23 | 460/33/39 | CVID | Yes | No | Autoimmunity, enteropathy |
| 7 | F | 127 | 14/14/25 | 250/50/50 | CVID | Yes | Yes | Lymphadenopathy, splenomegaly, AIHA |
| 8 | M | <1 | 28/30/30 | <500/‡ /‡ | CVID | Yes | Yes | Lymphadenopathy, splenomegaly, granulomas, autoimmunity |
| 9 | M | <1 | 16/29/29 | <500/‡/‡ | CVID | Yes | Yes | Lymphadenopathy, splenomegaly, granulomas, bronchiectasis, autoimmunity, enteropathy |
| 10 | F | ‡ | 33/34/34 | 320/5/25 | CVID | Yes | Yes | Splenomegaly, bronchiectasis |
| 11 | F | 55 | 68/68/72 | 438/146/33 | CVID | Yes | No | Lymphoma, bronchiectasis, ANCA associated vasculitis |
| 12 | F | 110 | 25/26/35 | 223/6/48 | LOCID | Yes | Yes | Splenomegaly, GLILD, lymphoid hyperplasia, AIHA, AIN, allergies, basal cell carcinoma, enteropathy, PSVD |
| 13 | M | 188 | ‡/32/47 | <500/‡/‡ | CVID | No | No | AIHA |
| 14 | F | 167 | 46/31/44 | 486/40/46 | CTLA4 | Yes | No | Lymphadenopathy, psoriasis, AIHA, allergies, HPV infections and related cancer, celiac disease |
| 15 | M | 64 | 20/19/25 | 450/42/47 | CVID | Yes | No | Lymphadenopathy, splenomegaly, autoimmunity |
| 16 | M | 62 | 26/26/31 | 450/47/‡ | CVID | Yes | No | Lymphoid hyperplasia, granulomas, AIHA, enteropathy |
| 17 | F | 27 | 32/31/34 | 430/26/45 | CVID | Yes | No | Lymphadenopathy, granulomas, psoriasis, AIHA, B12 deficiency |
| 18 | F | 191 | 50/50/66 | 426/104/109 | CVID | Yes | Yes | Lymphadenopathy, GLILD, LGL leukemia, bronchiectasis, AIHA and AIN |
| 19 | M | 21 | 43/43/45 | 350/20/45 | CVID | Yes | Yes | Allergies, AIN |
| 20 | M | 1 | 27/34/35 | 130/0/37 | CVID | Yes | No | Lymphadenopathy, splenomegaly, AIHA |
| 21 | M | 249 | 51/33/54 | 187/32/126 | PI3KD | No | No | AIHA and AIN |
| 22 | F | 1 | 18/18/18 | 396/25/33 | CVID | No | No | Lymphadenopathy, splenomegaly |
| 23 | F | 48 | ‡/55/59 | 197/28/17 | CVID | Yes | Yes | - |
| 24 | M | 4 | 42/42/42 | 452/32/40 | CVID | No | No | - |
| 25 | M | 54 | 52/53/57 | 302/6/17 | CVID | No | No | Splenomegaly, autoimmunity |
| 26 | M | 47 | 36/39/43 | <500/‡/‡ | CVID | No | No | - |
| 27 | M | 13 | 56/58/59 | 330/69/30 | CVID | Yes | No | - |
| 28 | M | 111 | 15/13/22 | 398/17/60 | Kabuki | No | Yes | AIHA and AIN, cholesteatoma |
| Patient and sex . | ITP duration, mo . | Age at diagnosis of PID/ITP/introduction of TPO-RA, y . | IgG/IgA/IgM levels at PID diagnosis, mg/dL∗ . | Type of PID . | Recurrent infections†/IRT . | Other PID-related manifestations . | ||
|---|---|---|---|---|---|---|---|---|
| 1 | M | 294 | 16/5/29 | 321/37/12 | LOCID | Yes | Yes | Lymphadenopathy, splenomegaly, GLILD, bronchiectasis, AIHA, HPV infections, PSVD |
| 2 | F | <1 | 19/19/19 | 250/15/28 | CVID | Yes | No | Splenomegaly, autoimmunity, hepatomegaly |
| 3 | M | 168 | 24/9/23 | 150/0/500 | HIGM | Yes | No | Lymphadenopathy, granulomas, MALT lymphoma |
| 4 | M | 295 | 33/12/37 | 85/0/4 | LOCID | Yes | Yes | Lymphadenopathy, splenomegaly, granulomas, bronchiectasis, HPV infections, PSVD |
| 5 | F | 3 | 49/48/48 | 300/18/8 | CVID | Yes | No | Lymphadenopathy, splenomegaly, GLILD |
| 6 | M | 5 | 22/22/23 | 460/33/39 | CVID | Yes | No | Autoimmunity, enteropathy |
| 7 | F | 127 | 14/14/25 | 250/50/50 | CVID | Yes | Yes | Lymphadenopathy, splenomegaly, AIHA |
| 8 | M | <1 | 28/30/30 | <500/‡ /‡ | CVID | Yes | Yes | Lymphadenopathy, splenomegaly, granulomas, autoimmunity |
| 9 | M | <1 | 16/29/29 | <500/‡/‡ | CVID | Yes | Yes | Lymphadenopathy, splenomegaly, granulomas, bronchiectasis, autoimmunity, enteropathy |
| 10 | F | ‡ | 33/34/34 | 320/5/25 | CVID | Yes | Yes | Splenomegaly, bronchiectasis |
| 11 | F | 55 | 68/68/72 | 438/146/33 | CVID | Yes | No | Lymphoma, bronchiectasis, ANCA associated vasculitis |
| 12 | F | 110 | 25/26/35 | 223/6/48 | LOCID | Yes | Yes | Splenomegaly, GLILD, lymphoid hyperplasia, AIHA, AIN, allergies, basal cell carcinoma, enteropathy, PSVD |
| 13 | M | 188 | ‡/32/47 | <500/‡/‡ | CVID | No | No | AIHA |
| 14 | F | 167 | 46/31/44 | 486/40/46 | CTLA4 | Yes | No | Lymphadenopathy, psoriasis, AIHA, allergies, HPV infections and related cancer, celiac disease |
| 15 | M | 64 | 20/19/25 | 450/42/47 | CVID | Yes | No | Lymphadenopathy, splenomegaly, autoimmunity |
| 16 | M | 62 | 26/26/31 | 450/47/‡ | CVID | Yes | No | Lymphoid hyperplasia, granulomas, AIHA, enteropathy |
| 17 | F | 27 | 32/31/34 | 430/26/45 | CVID | Yes | No | Lymphadenopathy, granulomas, psoriasis, AIHA, B12 deficiency |
| 18 | F | 191 | 50/50/66 | 426/104/109 | CVID | Yes | Yes | Lymphadenopathy, GLILD, LGL leukemia, bronchiectasis, AIHA and AIN |
| 19 | M | 21 | 43/43/45 | 350/20/45 | CVID | Yes | Yes | Allergies, AIN |
| 20 | M | 1 | 27/34/35 | 130/0/37 | CVID | Yes | No | Lymphadenopathy, splenomegaly, AIHA |
| 21 | M | 249 | 51/33/54 | 187/32/126 | PI3KD | No | No | AIHA and AIN |
| 22 | F | 1 | 18/18/18 | 396/25/33 | CVID | No | No | Lymphadenopathy, splenomegaly |
| 23 | F | 48 | ‡/55/59 | 197/28/17 | CVID | Yes | Yes | - |
| 24 | M | 4 | 42/42/42 | 452/32/40 | CVID | No | No | - |
| 25 | M | 54 | 52/53/57 | 302/6/17 | CVID | No | No | Splenomegaly, autoimmunity |
| 26 | M | 47 | 36/39/43 | <500/‡/‡ | CVID | No | No | - |
| 27 | M | 13 | 56/58/59 | 330/69/30 | CVID | Yes | No | - |
| 28 | M | 111 | 15/13/22 | 398/17/60 | Kabuki | No | Yes | AIHA and AIN, cholesteatoma |
AIHA, autoimmune hemolytic anemia; AIN, autoimmune neutropenia; ANCA, anti-neutrophil cytoplasmic antibodies; F, female; GLILD, granulomatous-lymphocytic interstitial lung disease; HPV, human papillomavirus; IRT, immunoglobulin replacement therapy; LGL, large granular lymphocytic; M, male; MALT, mucosa-associated lymphoid tissue.
Normal values (ranges): IgG, 650 to 1400 mg/dL; IgA, 70 to 320 mg/dL; IgM, 50 to 350 mg/dL.
Recurrent sino-pulmonary infections.
Missing data.
Characteristics of PID and associated conditions
A genetic assessment was conducted in 19 patients (67.9%) and showed abnormalities in 7 patients (25%). Patient 14 was diagnosed with CTLA4 deficiency, patient 21 with a PI3KCD mutation, and patient 28 with Kabuki syndrome (heterozygous germ line mutations in the KMT2D gene). Four patients had genetic abnormalities of unknown significance, including telomere biology disorders (patient 1), missense mutations or variant of IKZF1 (patient 7 and 22), and variant of TNFRSF11A (patient 17).
Twenty patients (71.4%) had a known history of recurrent sino-pulmonary infections. Three patients (10.7%) experienced recurrent infections related to human papillomavirus, and 1 of them developed cervical cancer that was categorized as an opportunistic infection.
Autoimmune manifestations other than ITP occurred in 19 patients (67.9%) with autoimmune hemolytic anemia in 11 patients (39.3%) and autoimmune neutropenia in 5 patients (17.9%). Other described autoimmune manifestations included psoriasis, celiac disease, and antineutrophil cytoplasmic antibody–associated vasculitis. Noninfectious complications in our population in comparison with those of the largest reported series of patients with CVID are summarized in Table 3.
Noninfectious complications associated with predominantly antibody deficiencies
| Comorbidities . | Number . | % (N = 28) . | DEFI study4 . | USIDNET5 . | Ho H en, Cunningham-Rundles C6 (N = 623) . | ||
|---|---|---|---|---|---|---|---|
| With autoimmune cytopenia (n = 55) . | Total (N = 311) . | With autoimmune cytopenia (n = 99) . | Total (N = 990) . | ||||
| Immune thrombocytopenia | 28 | 100% | 81.8% | 13.2% | 73.7% | 7.4% | 16.2% |
| AIHA | 11 | 39.3% | 30.1% | 5.5% | 11.1% | 1.1% | 7.7% |
| AIN | 5 | 17.9% | 18.2% | 3.2% | 10.1% | 1% | NS |
| Bronchechiasties | 6 | 21.4% | NS | NS | 12.9% | 11.2% | 6% |
| Gastrointestinal disease | 5 | 17.9% | NS | NS | 12.9% | 10.4% | 17.3% |
| Liver disease | 3 | 10.7% | NS | NS | 12.9% | 6.2% | 12.7% |
| Splenomegaly | 14 | 50% | 65.4% | 34.1% | 27.7% | 10.5% | 15.6% |
| Lymphadenopathy | 13 | 46.4% | NS | NS | 35.6% | 13.7% | 9% |
| Granulomas | 10 | 35.7%∗ | NS | NS | 14.9% | 6.8% | 9.3% |
| Lymphoma | 3 | 10.7% | NS | NS | 6.9% | 3.1% | 6.7% |
| Comorbidities . | Number . | % (N = 28) . | DEFI study4 . | USIDNET5 . | Ho H en, Cunningham-Rundles C6 (N = 623) . | ||
|---|---|---|---|---|---|---|---|
| With autoimmune cytopenia (n = 55) . | Total (N = 311) . | With autoimmune cytopenia (n = 99) . | Total (N = 990) . | ||||
| Immune thrombocytopenia | 28 | 100% | 81.8% | 13.2% | 73.7% | 7.4% | 16.2% |
| AIHA | 11 | 39.3% | 30.1% | 5.5% | 11.1% | 1.1% | 7.7% |
| AIN | 5 | 17.9% | 18.2% | 3.2% | 10.1% | 1% | NS |
| Bronchechiasties | 6 | 21.4% | NS | NS | 12.9% | 11.2% | 6% |
| Gastrointestinal disease | 5 | 17.9% | NS | NS | 12.9% | 10.4% | 17.3% |
| Liver disease | 3 | 10.7% | NS | NS | 12.9% | 6.2% | 12.7% |
| Splenomegaly | 14 | 50% | 65.4% | 34.1% | 27.7% | 10.5% | 15.6% |
| Lymphadenopathy | 13 | 46.4% | NS | NS | 35.6% | 13.7% | 9% |
| Granulomas | 10 | 35.7%∗ | NS | NS | 14.9% | 6.8% | 9.3% |
| Lymphoma | 3 | 10.7% | NS | NS | 6.9% | 3.1% | 6.7% |
Quantitative variables are presented as median (range, minimum to maximum); and qualitative variables as frequency (percentage).
Median age at PID diagnosis was similar across these 4 populations.
NS, not specified.
Overestimation of granulomas is possible because GLILD was classified as granulomas in our population.
Characteristics of ITP
Table 4 describes the characteristics of ITP at the time of TPO-RA introduction and at the end of study.
Characteristics of ITP
| Characteristics . | N = 28 . | |||
|---|---|---|---|---|
| At the time of TPO-RA introduction . | At the end of study . | |||
| ITP duration (y) | 4 | (0-24) | ||
| Chronic or persistent ITP | 22 | (78.6%) | 28 | (100%) |
| Indication of antiplatelet drugs or anticoagulant | 2 | (7.1%) | 4 | (14.3%) |
| Immunoglobulin replacement therapy | 11 | (39.3%) | 22 | (78.6%) |
| Second-line therapies for ITP | 10 | (35.7%) | 7 | (25.0%) |
| Immunosuppressants | 5 | (17.9%) | 5 | (17.9%) |
| Splenectomy | 2 | (7.1%) | 4 | (14.3%) |
| Rituximab received at least once | 5 | (17.9%) | 14 | (50.0%) |
| Characteristics . | N = 28 . | |||
|---|---|---|---|---|
| At the time of TPO-RA introduction . | At the end of study . | |||
| ITP duration (y) | 4 | (0-24) | ||
| Chronic or persistent ITP | 22 | (78.6%) | 28 | (100%) |
| Indication of antiplatelet drugs or anticoagulant | 2 | (7.1%) | 4 | (14.3%) |
| Immunoglobulin replacement therapy | 11 | (39.3%) | 22 | (78.6%) |
| Second-line therapies for ITP | 10 | (35.7%) | 7 | (25.0%) |
| Immunosuppressants | 5 | (17.9%) | 5 | (17.9%) |
| Splenectomy | 2 | (7.1%) | 4 | (14.3%) |
| Rituximab received at least once | 5 | (17.9%) | 14 | (50.0%) |
| Characteristics . | In the month before TPO-RA introduction . | During the treatment with TPO-RA . | ||
|---|---|---|---|---|
| Patients reporting bleeding events | 16 | (57.1%) | 10 | (35.7%) |
| Patients reporting bleeding events with Khellaf score >8 | 6 | (21.4%) | 2 | (7.1%) |
| Characteristics . | In the month before TPO-RA introduction . | During the treatment with TPO-RA . | ||
|---|---|---|---|---|
| Patients reporting bleeding events | 16 | (57.1%) | 10 | (35.7%) |
| Patients reporting bleeding events with Khellaf score >8 | 6 | (21.4%) | 2 | (7.1%) |
Quantitative variables are presented as median (range, minimum to maximum); and qualitative variables as frequency (percentage).
Median age at PID diagnosis was similar across these 4 populations.
NS, not specified.
Nine patients (32.1%) were diagnosed with ITP before they were diagnosed with PID with a median delay of 11 years (maximum delay of 21 years). Nine patients (32.1%) were diagnosed with ITP and PID concomitantly. Nine patients (32.1%) were diagnosed with PID before the onset of ITP with a median delay of 2 years (maximum delay of 13 years).
Bone marrow examination (BME) data were available for 16 patients (57.1%) with abnormalities detected in 5 patients (17.9%), including bone marrow fibrosis grade 1 (patient 4), rare megakaryocytes (patients 19 and 20 for which the BME was hemodiluted), large granular lymphocytic population (patient 18), and dysgranulopoiesis and positive Epstein-Barr virus polymerase chain reaction (patient 28).
All patients previously received treatment with corticosteroids, a high dose of IVIG, or both (Table 5).
Management and outcome of ITP in adults diagnosed with predominantly antibody deficiencies
| Patient . | Previous treatments (y before the introduction of TPO-RA) . | Concomitant therapies at the introduction of TPO-RAs . | TPO-RAs . | Treatments introduced after baseline . | At the end of study . | Follow-up after baseline/duration of treatment . | |
|---|---|---|---|---|---|---|---|
| ITP treatments/TPO-RAs/IRT . | Response . | ||||||
| 1 | CST | Rituximab (for Evan syndrome), CST (10 mg) | E | - | CST (10 mg)/E/yes | R | 2 wk/2 wk (death) |
| 2 | - | - | Ro/E | Rituximab (3 mo later), vinblastine (4 mo later) | No/no/yes | IR | 24 mo/8 mo |
| 3 | Splenectomy, rituximab | Rituximab | Ro | Azathioprine, MMF pursued for 2 y, rituximab (4 y later for lymphoma) | No/no/yes | NR | 127 mo/less than a year |
| 4 | CST, danazol | Danazol | E/Ro | Sirolimus | Sirolimus/no/yes | NR | 36 mo/less than a year |
| 5 | - | - | E/Ro | Splenectomy (2 mo later), rituximab (4 mo later), CST | CST/Ro/yes | IR | 8 mo/8 mo |
| 6 | - | - | Ro | Rituximab (10 mo later) | No/no/yes | IR | 70 mo/26 mo |
| 7 | Sirolimus, dapsone, CST, cyclosporin | Cyclosporin | Ro | Rituximab (5 mo later) | No/no/yes | IR | 39 mo/11 mo |
| 8 | - | - | E | Rituximab (4 y later) | CST/Ro/yes | IR | 65 mo/65 mo |
| 9 | - | - | E | Splenectomy (9 mo later) | No/no/yes | CR | 65 mo/8 mo |
| 10 | - | - | E | - | No/no/yes | CR | 42 mo/20 mo |
| 11 | - | - | E | Rituximab (7 mo later for ANCA associated vasculitis) | No/E/no | CR | 7 mo/7 mo |
| 12 | Dapsone, sirolimus | - | E/Ro | Rituximab and CST (28 months later for AIHA) | CST (for AIHA)/Ro/yes | R | 43 mo/43 mo |
| 13 | CST | CST | E | - | CST (5 mg)/no/no | CR | 9 mo/3 wk |
| 14 | Splenectomy, rituximab, azathioprine, CST | - | Ro/E | HCQ | No/no/yes | CR | 67 mo/7mo |
| 15 | Dapsone | - | E | - | No/E/no | R | 21 mo/21mo |
| 16 | Vinblastine, dapsone | E/Ro | Rituximab (8 y later for AIHA) | No/no/yes | CR | 106 mo/5 wk | |
| 17 | HCQ | HCQ | E | CST, rituximab (3 y later for AIHA) | HCQ/no/yes | CR | 50 mo/45 mo |
| 18 | Cyclosporin | Cyclosporin | E | No/E/yes | CR | 19 mo/19 mo | |
| 19 | - | - | E | - | No/E/yes | CR | 15 mo/15 mo |
| 20 | - | Vinblastine | Ro | - | No/no/yes | CR | 12 mo/5 mo |
| 21 | - | Vinblastine | E/Ro | - | No/Ro/no | CR | 61 mo/61 mo |
| 22 | - | - | E/Ro | - | No/Ro/yes | R | 15 mo/15 mo |
| 23 | Rituximab | - | Ro | Relay for E (3 y later) | No/E/yes | CR | 76 mo / 76 mo |
| 24 | - | - | E/Ro | Dapsone (10 mo later) | Dapsone/Ro/yes | CR | 18 mo/18 mo |
| 25 | Dapsone | - | E/Ro | - | No/Ro/no | CR | 9 mo/9 mo |
| 26 | - | Rituximab | Ro/E | - | No/no/yes | CR | 33 mo/6 mo |
| 27 | - | - | E | - | No/E/no | CR | 16 mo/16 mo |
| 28 | - | - | E | Relay for Ro (2 y later) | No/Ro/yes | CR | 29 mo/29 mo |
| Patient . | Previous treatments (y before the introduction of TPO-RA) . | Concomitant therapies at the introduction of TPO-RAs . | TPO-RAs . | Treatments introduced after baseline . | At the end of study . | Follow-up after baseline/duration of treatment . | |
|---|---|---|---|---|---|---|---|
| ITP treatments/TPO-RAs/IRT . | Response . | ||||||
| 1 | CST | Rituximab (for Evan syndrome), CST (10 mg) | E | - | CST (10 mg)/E/yes | R | 2 wk/2 wk (death) |
| 2 | - | - | Ro/E | Rituximab (3 mo later), vinblastine (4 mo later) | No/no/yes | IR | 24 mo/8 mo |
| 3 | Splenectomy, rituximab | Rituximab | Ro | Azathioprine, MMF pursued for 2 y, rituximab (4 y later for lymphoma) | No/no/yes | NR | 127 mo/less than a year |
| 4 | CST, danazol | Danazol | E/Ro | Sirolimus | Sirolimus/no/yes | NR | 36 mo/less than a year |
| 5 | - | - | E/Ro | Splenectomy (2 mo later), rituximab (4 mo later), CST | CST/Ro/yes | IR | 8 mo/8 mo |
| 6 | - | - | Ro | Rituximab (10 mo later) | No/no/yes | IR | 70 mo/26 mo |
| 7 | Sirolimus, dapsone, CST, cyclosporin | Cyclosporin | Ro | Rituximab (5 mo later) | No/no/yes | IR | 39 mo/11 mo |
| 8 | - | - | E | Rituximab (4 y later) | CST/Ro/yes | IR | 65 mo/65 mo |
| 9 | - | - | E | Splenectomy (9 mo later) | No/no/yes | CR | 65 mo/8 mo |
| 10 | - | - | E | - | No/no/yes | CR | 42 mo/20 mo |
| 11 | - | - | E | Rituximab (7 mo later for ANCA associated vasculitis) | No/E/no | CR | 7 mo/7 mo |
| 12 | Dapsone, sirolimus | - | E/Ro | Rituximab and CST (28 months later for AIHA) | CST (for AIHA)/Ro/yes | R | 43 mo/43 mo |
| 13 | CST | CST | E | - | CST (5 mg)/no/no | CR | 9 mo/3 wk |
| 14 | Splenectomy, rituximab, azathioprine, CST | - | Ro/E | HCQ | No/no/yes | CR | 67 mo/7mo |
| 15 | Dapsone | - | E | - | No/E/no | R | 21 mo/21mo |
| 16 | Vinblastine, dapsone | E/Ro | Rituximab (8 y later for AIHA) | No/no/yes | CR | 106 mo/5 wk | |
| 17 | HCQ | HCQ | E | CST, rituximab (3 y later for AIHA) | HCQ/no/yes | CR | 50 mo/45 mo |
| 18 | Cyclosporin | Cyclosporin | E | No/E/yes | CR | 19 mo/19 mo | |
| 19 | - | - | E | - | No/E/yes | CR | 15 mo/15 mo |
| 20 | - | Vinblastine | Ro | - | No/no/yes | CR | 12 mo/5 mo |
| 21 | - | Vinblastine | E/Ro | - | No/Ro/no | CR | 61 mo/61 mo |
| 22 | - | - | E/Ro | - | No/Ro/yes | R | 15 mo/15 mo |
| 23 | Rituximab | - | Ro | Relay for E (3 y later) | No/E/yes | CR | 76 mo / 76 mo |
| 24 | - | - | E/Ro | Dapsone (10 mo later) | Dapsone/Ro/yes | CR | 18 mo/18 mo |
| 25 | Dapsone | - | E/Ro | - | No/Ro/no | CR | 9 mo/9 mo |
| 26 | - | Rituximab | Ro/E | - | No/no/yes | CR | 33 mo/6 mo |
| 27 | - | - | E | - | No/E/no | CR | 16 mo/16 mo |
| 28 | - | - | E | Relay for Ro (2 y later) | No/Ro/yes | CR | 29 mo/29 mo |
-, no treatment was introduced; CR, complete responder (platelet count >100 × 109/L); CS, corticosteroids in short courses; CST, corticosteroids in long courses (dosage, mg per day); E, eltrombopag; E/R, eltrombopag switched by romiplostim; HCQ, hydroxychloroquine; IR, insufficient responder (required immunosuppressors upon an ITP relapse); MMF, mycophenolate mofetil; NR, nonresponder; R, responder (platelet count 30 × 109 to 100 × 109/L); Ro, romiplostim; Ro/E, romiplostim switched by eltrombopag.
Characteristics at the time of TPO-RA introduction
In the months before TPO-RA introduction, short courses of corticosteroids were reportedly administered in 21 patients (75%) and high-dose IVIG was reportedly administered in 15 patients (53.6%). Unfortunately, there are no data available on the timing between these therapies and the introduction of TPO-RAs.
In the month before the introduction of TPO-RA, the lowest platelet count was strictly below 30 × 109/L for 23 patients (82.1%) and between 30 and 50 × 109/L for 1 patient (3.6%); data were missing for patients 3, 6, and 8. Patient 19 had a platelet nadir of 50 × 109/L; the introduction of TPO-RAs was justified by the need for neurosurgery, and in this particular case response to treatment was defined as reaching a platelet count >80 × 109/L.
In the month before TPO-RA introduction, 16 patients (57.1%) reported bleeding events, 6 (21.4%) of whom had a Khellaf score >8.
Treatment with TPO-RAs
The management of ITP in the 28 patients is detailed in Table 5. Eltrombopag was administered to 24 patients (85.7%), romiplostim to 18 patients (64.3%), and 14 patients (50%) received both sequentially. The median duration of exposure was 218 days (range, 17 days to 4 years) for eltrombopag; 325 days (range, 7 days to 5 years) for romiplostim, and 485 days (range, 17 days to 6.6 years) for the cumulative exposure to any TPO-RA. The median follow-up time after introduction of the first TPO-RAs was 33 months (range, 2 weeks to 10.6 years).
Of the 24 patients treated with eltrombopag, 5 (20.8%) did not reach the recommended maximum dosage of 75 mg/d. Among them, 2 patients (40.0%) experienced ITP relapses that led to the introduction of romiplostim, which led to persistent complete responses. Of the 18 patients treated with romiplostim, 3 (16.7%) never received the recommended maximum dosage of 10 μg/kg per week; all of them maintained a treatment response. Patients 6 and 20 received repeated on-demand therapy with intermittent dosing of TPO-RAs. Dose adjustments were numerous with ITP relapses for 7 of 24 patients (29.2%) treated with eltrombopag and 8 of 18 patients (44.4%) treated with romiplostim. None of these cases led to bleeding events with a Khellaf score >8.
Effectiveness of TPO-RAs
Initial and long-term treatment responses
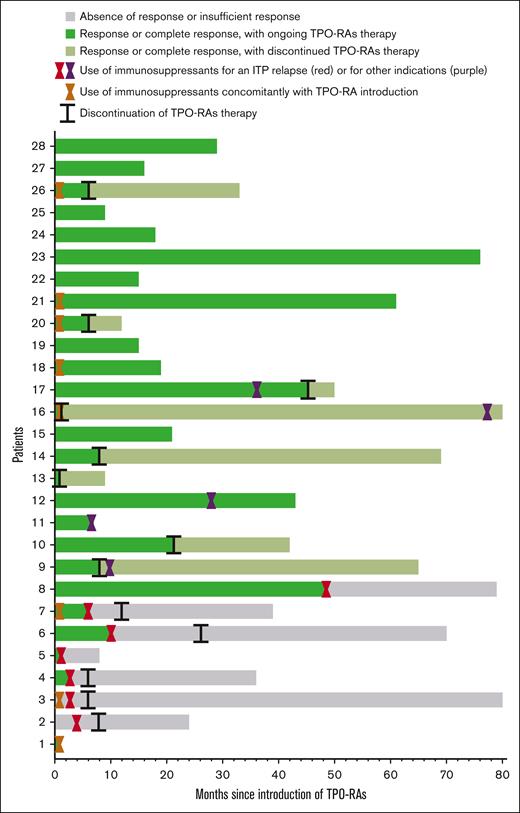
Figure 2 illustrates treatment responses.
Swimmer plot. The x-axis represents time since the introduction of TPO-RAs, measured in months and the y-axis shows each of the 28 patient’s individual treatment courses. Colors are used to indicate different responses: the gray color denotes absence of response or insufficient response (with the use of immunosuppressants for an ITP relapse); the green color indicates a response or complete response to TPO-RAs, with the treatment ongoing; and the green/grey color represents a response or complete response to TPO-RAs, after discontinuation of treatment. Hourglass symbols: orange, immunosuppressants used concomitantly to TPO-RAs; red, time at which immunosuppressants were introduced for an ITP relapse; and purple, time at which immunosuppressants were introduced for other medical reasons. Lines represent time when TPO-RAs were discontinued.
Swimmer plot. The x-axis represents time since the introduction of TPO-RAs, measured in months and the y-axis shows each of the 28 patient’s individual treatment courses. Colors are used to indicate different responses: the gray color denotes absence of response or insufficient response (with the use of immunosuppressants for an ITP relapse); the green color indicates a response or complete response to TPO-RAs, with the treatment ongoing; and the green/grey color represents a response or complete response to TPO-RAs, after discontinuation of treatment. Hourglass symbols: orange, immunosuppressants used concomitantly to TPO-RAs; red, time at which immunosuppressants were introduced for an ITP relapse; and purple, time at which immunosuppressants were introduced for other medical reasons. Lines represent time when TPO-RAs were discontinued.
In total, an overall response was achieved at least once for 26 of 28 patients (92.9%) among which 23 patients (82.1%) showed at least 1 complete response. Patients 12, 15, and 22 never reached a platelet count greater than 100 × 109/L; they exhibited a respective spleen size of 30 cm, 18 cm, and 20 cm.
After 6 weeks of follow-up, response was achieved in 24 of the 28 patients (85.7%) among which 21 patients (75%) displayed a complete response.
At the end of the study, after a median follow-up of 33 months, response was maintained in 20 of the 28 patients (71.4%) among which 17 patients (60.7%) displayed a complete response.
Patient 1 died of severe infection 2 weeks after eltrombopag introduction, despite response to treatment. Patient 2 exhibited an initial absence of response to eltrombopag, potentially linked to nonadherence to dietary restrictions, and after adjusting such restrictions, there was complete response. Patients 3 and 4 were nonresponders. Patients 5, 6, 7, and 8 showed incomplete responses. Patients 9 to 28 experienced treatment responses without any ITP relapses that required immunosuppressants.
Discontinuation of TPO-RAs
Among the 20 patients for whom response was persistent at the end of follow-up, TPO-RAs were ultimately discontinued in 10 patients (50.0%). Eight of them (80.0%) exhibited no ITP relapses during the follow-up period, and data were missing for patients 11 and 27. The reasons for discontinuation included the potential efficacy of another ITP treatment (patients 9, 11, 16, 17, 20, and 26), persistent efficacy of TPO-RAs (patients 10, 13, 16, 26, and 27), thrombocytosis (patients 14 and 20), pregnancy (patient 17), and intolerance of TPO-RAs (patients 20 and 27).
Bleeding events
During ongoing TPO-RA therapy, bleeding events with a Khellaf score <8 were reported in 8 patients (28.6%) and bleeding events with a Khellaf score ≥8 were reported for 2 patients (7.1%). Most events were related to dose adjustments or the initial inefficacy of 1 TPO-RA, justifying a switch to another TPO-RA. Patient 2 experienced an ITP relapse after 3 months of treatment with romiplostim and presented with visible hematuria. Patient 14 experienced menorrhagia and moderate hemodynamic instability 16 days after initiating romiplostim therapy and required IVIG and blood and platelet transfusions. The usual time period for onset of response in adult patients with ITP who were treated with romiplostim falls within 1 to 3 weeks.27 Thus, this event was not considered to be failure of TPO-RAs treatment. Successful romiplostim treatment was continued for 17 months without any subsequent bleeding events.
Other ITP treatments
ITP treatments at the last visit are reported in Table 4.
At the last assessment, among responders who had not required immunosuppressors upon an ITP relapse, 10 of the 18 patients (55.6%) who were concurrently receiving steroids successfully tolerated a complete tapering off of the steroids. Continuous or intermittent regimens of corticosteroids were necessary to sustain a response in 8 patients (28.6%), and occasional IVIG was required in 4 patients (14.3%).
Two patients (7.1%) underwent splenectomy after introduction of TPO-RAs, both because of a suspicion of lymphoma (although none confirmed after histological examination of the spleen). Patient 5 underwent a splenectomy while romiplostim treatment was ongoing and patient 9 underwent a splenectomy a month after discontinuation of eltrombopag; they did not experience any thrombotic event.
Ten patients (35.7%) received rituximab after introduction of TPO-RAs. Patients 2, 5, 6, 7, and 8 received rituximab for an ITP relapse. Five patients (17.9%) received rituximab for indications other than ITP, such as mucosa-associated lymphoid tissue lymphoma, myeloperoxidase (MPO) antineutrophil cytoplasmic antibody–associated vasculitis, and autoimmune hemolytic anemia for 3 patients (10.7%).
Safety
Thrombotic events
Thrombotic events were reported in 3 patients (10.7%).
For patient 19, a deep vein thrombosis was diagnosed 6 months after the introduction of eltrombopag, shortly after the dosage was increased to 75 mg per day, with a platelet count of 150 × 109/L at that time. Therapeutic anticoagulation was initiated, and eltrombopag was continued.
Patient 27, a 60-year-old male with a history of arterial hypertension and dyslipidemia, was diagnosed with triple vessel coronary artery disease during an acute coronary syndrome 16 months after eltrombopag introduction. The exact platelet count was unknown but was >250 × 109/L. Eltrombopag treatment was continued and combined with dual antiplatelet therapy.
Patient 28 was diagnosed with sigmoid sinus venous thrombosis 30 months after TPO-RAs introduction, in the postoperative context of cholesteatoma. The platelet count at that time was 280 × 109/L. Romiplostim treatment was continued, and therapeutic anticoagulation was initiated.
These 3 patients exhibited a complete response to TPO-RAs and no bleeding events were reported. None of them presented with antiphospholipid antibodies or lupus anticoagulant. No other risk factors for arterial or venous thrombosis were identified in these patients.
HBLAs
Hepatobiliary adverse events and laboratory abnormalities (HBLAs) were reported in 3 patients (10.7%). Patient 2 had a known history of hepatomegaly; she presented with transient alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels up to 2 times the upper limit of normal (ULN) less than a month after romiplostim introduction, which resolved rapidly and spontaneously. TPO-RAs treatment was continued for a total of 24 months. Screening for PSVD through a liver biopsy was initially considered but ultimately not carried out. Patient 5 exhibited HBLAs before TPO-RAs introduction, which persisted during TPO-RAs treatment. These abnormalities included a maximum alkaline phosphatase (ALP) and gamma-glutamyl transferase (GGT) level of up to 10 times the ULN, ALT and AST levels up to 2 times the ULN, and total bilirubin levels up to 4 times the ULN. Hyperbilirubinemia and cytolysis resolved spontaneously, although cholestasis gradually worsened. An abdominal ultrasound revealed isolated splenomegaly, and no further tests were conducted during the 7 month follow-up period. Romiplostim treatment was continued. Patient 12 had a documented history of PSVD and portal hypertension (esophageal varices and splenomegaly). She presented with ALT and AST levels up to 2 times the ULN and with ALP and GGT levels up to 2 times the ULN. However, given the challenges in definitively attributing these biochemical abnormalities to romiplostim, the treatment was continued. With a follow-up of >40 months, no serious hepatobiliary and liver-associated adverse events were reported, especially no sign of portal thrombosis or hepatocellular insufficiency.
Other observations
There was no evidence that bone marrow fibrosis occurred during TPO-RAs therapy. Patient 4 had bone marrow fibrosis before TPO-RAs initiation, no subsequent BME was conducted, and he maintained a platelet count of 130 × 109/L in the latest report.
Two patients (7.1%) reported other nonspecific adverse events. Patient 20 exhibited a range of symptoms, including fatigue, nausea, abdominal pain, headaches, and injection site reactions. He had been receiving an on-demand therapeutic regimen for 5 months. This intolerance led to the discontinuation of the treatment and there was no subsequent evidence of ITP relapse. Patient 27 displayed similar symptoms, including fatigue, headaches, difficulty concentrating, tremors, sweating, nausea, sleep disturbances, and irritability. Treatment was discontinued at the last available visit.
Pregnancy
Patient 17 ceased eltrombopag treatment because of pregnancy given the absence of data on the safety of TPO-RAs in pregnant women. Hydroxychloroquine treatment was pursued, and the patient experienced no further ITP relapse.
Mortality
Patient 1 died during the course of therapy. The cause of death was not considered to be related to TPO-RAs. This patient, aged 29, had multiple associated comorbidities (Table 2). He presented with worsening liver disease and a suspected hepatic carcinoma that was pending confirmation through liver biopsy. Concomitantly, he encountered a flare-up of Evan syndrome, which led to a treatment regimen comprising rituximab, corticosteroids, IVIG, and eltrombopag. This combination allowed the patient’s platelet count to reach 100 × 109/L. However, 2 weeks later, he presented with a severe infection caused by influenza A virus with a suspected undocumented bacterial superinfection and died.
Discussion
The second-line treatment for secondary ITP associated with PIDs relies on limited scientific data. The benefit to risk ratio and the place of each treatment have not been assessed in light of the approvals of TPO-RAs, despite their efficacy in primary ITP and their promising safety profile in terms of the absence of immunosuppression. This multicenter retrospective study based on real-life data presents the largest population to date and describes the outcomes and safety of TPO-RAs in this specific population.
Regarding the study population, to achieve a more homogeneous group, only patients with PID with hypogammaglobulinemia (IgG <5 g/dL) were included. Our study population seemed to be comparable with published cohorts of patients with CVIDs in terms of comorbidities (Table 3).
TPO-RAs have been used successfully in secondary ITP with various etiologies, including Wiskott-Aldrich syndrome and DiGeorge syndrome.19,28,29 The outcomes observed in our population suggest that TPO-RAs may exhibit similar effectiveness in patients with ITP linked to primary hypogammaglobulinemia. Indeed, after 6 weeks of treatment, response was achieved in 24 of 28 patients (85.7%), and after a median follow-up of 33 months, response was persistent in 20 of 28 patients (71.4%). Bleeding events were reported less frequently after TPO-RAs introduction.
Previous studies have suggested that romiplostim should be used preferentially over eltrombopag in patients at risk for malabsorption (including patients diagnosed with CVID-associated enteropathy or recurrent gastrointestinal infections),30 but our sample size did not allow us to provide insights on this question.
Considering the retrospective design of our study, HBLAs may have been underreported. The open-label EXTEND study (Safety and Efficacy of Eltrombopag in Adults with Chronic ITP), which evaluated the long-term safety of eltrombopag, reported HBLAs events in 15% of patients and found that the majority occurred within the first year after treatment introduction.13 Although mild HBLAs have little impact on primary ITP management, they could impact PSVD screening in patients with PIDs. In addition, PSVD is more frequently described in patients with PIDs who were diagnosed with autoimmune cytopenias.3 Physicians should therefore be aware of the potential interference of TPO-RAs in PSVD screening and consider the need for histologic examination in cases of progressive liver function abnormalities, particularly elevated alkaline phosphatase levels.31
Three patients (10.7%) from our study experienced thrombotic events. In comparison, during the treatment phase of the EXTEND study, 19 patients (6%) had thromboembolic events.13 Two of these 3 patients presented with thrombotic risk factors; this prompts caution in prescribing TPO-RAs to such patients. An anticipated side effect of TPO-RAs was the occurrence of portal thrombosis, especially in patients diagnosed with PSVD. Interestingly, patient 12, who showed a history of severe liver disease and symptomatic portal hypertension, received TPO-RAs for >40 months without any sign of portal thrombosis. However, close monitoring of liver function should be considered in these situations.
The 2019 American Society of Hematology recommendations suggest choosing between rituximab, splenectomy, and TPO-RAs as second-line treatments for primary ITP in adults.10Table 6 summarizes the data extracted from our study and from major studies on second-line therapies used in the treatment of PID-associated ITP in adults.16,17 However, comparing these 3 studies may be limited by the dates of their execution, because current management practices might differ.
Second-line treatments of secondary ITP associated with predominantly antibody deficiencies
| Section . | Rituximab (Gobert et al16) . | Splenectomy (Wong et al17) . | TPO-RAs . |
|---|---|---|---|
| Population | Adults with secondary ITP N = 25 CVID, criteria from Conley et al 199932 | Adults with secondary ITP N = 14 CVID, criteria of ESID in 2013. | Adults with secondary ITP N = 28 Predominantly antibody deficiencies |
| Concurrent treatments | At baseline, concurrent treatments (IVIG, steroids, romiplostim) were ongoing in 27 of 33 cases. | No data available apart from that a number of patients still required long-term oral corticosteroids (prednisolone 4-15 mg). | At baseline, second-line treatments were ongoing in 10 of 28 cases. |
| Criteria for response | Platelet count >30 × 10/L with at least a twofold increase, without any other medication but substitutive IVIG and/or corticosteroids at stable or decreasing doses | Complete or partial responses according to treating physician’s judgment, without any other medication but low-dose maintenance corticosteroid | Platelet count >30 × 10/L without bleeding events with a Khellaf score >8 without any other medication but substitutive IVIG and/or corticosteroids at stable or decreasing doses or low-dose maintenance corticosteroid |
| Initial outcome | After a median delay of 4 wk (range 2-8): initial response in 21 of 25 (84%). | After 6 wk of treatment: response in 24 of 28 patients (85.7%). | |
| End of study outcome | After a mean follow-up of 38 ± 26 mo: response in 13 of 25 patients (52%); 4 were successfully retreated with rituximab. | After a median follow-up of >100 mo, 11 patients (78.6%) were considered responders. | After a median follow-up of 33 mo: response in 20 of 28 patients (71.4%). |
| Safety | (Among adult ITP cases)
| (Among the 45 patients with PID)
|
|
| Other indications in PIDs | AIHA, autoimmune diseases Lymphoproliferative diseases Granulomatous diseases | AIHA Suspicion of lymphoma | None |
| Section . | Rituximab (Gobert et al16) . | Splenectomy (Wong et al17) . | TPO-RAs . |
|---|---|---|---|
| Population | Adults with secondary ITP N = 25 CVID, criteria from Conley et al 199932 | Adults with secondary ITP N = 14 CVID, criteria of ESID in 2013. | Adults with secondary ITP N = 28 Predominantly antibody deficiencies |
| Concurrent treatments | At baseline, concurrent treatments (IVIG, steroids, romiplostim) were ongoing in 27 of 33 cases. | No data available apart from that a number of patients still required long-term oral corticosteroids (prednisolone 4-15 mg). | At baseline, second-line treatments were ongoing in 10 of 28 cases. |
| Criteria for response | Platelet count >30 × 10/L with at least a twofold increase, without any other medication but substitutive IVIG and/or corticosteroids at stable or decreasing doses | Complete or partial responses according to treating physician’s judgment, without any other medication but low-dose maintenance corticosteroid | Platelet count >30 × 10/L without bleeding events with a Khellaf score >8 without any other medication but substitutive IVIG and/or corticosteroids at stable or decreasing doses or low-dose maintenance corticosteroid |
| Initial outcome | After a median delay of 4 wk (range 2-8): initial response in 21 of 25 (84%). | After 6 wk of treatment: response in 24 of 28 patients (85.7%). | |
| End of study outcome | After a mean follow-up of 38 ± 26 mo: response in 13 of 25 patients (52%); 4 were successfully retreated with rituximab. | After a median follow-up of >100 mo, 11 patients (78.6%) were considered responders. | After a median follow-up of 33 mo: response in 20 of 28 patients (71.4%). |
| Safety | (Among adult ITP cases)
| (Among the 45 patients with PID)
|
|
| Other indications in PIDs | AIHA, autoimmune diseases Lymphoproliferative diseases Granulomatous diseases | AIHA Suspicion of lymphoma | None |
Comorbidities associated with PIDs should be carefully considered in the treatment strategy for ITP in patients with PIDs. Rituximab, for example, was initially preferred in 5 patients (17.8%) because of autoimmune or lymphoproliferative comorbidities. Splenectomy may be considered in patients with a strong suspicion of lymphoma associated with significant splenomegaly. In our population, sirolimus was reported as treatment for ITP in 3 patients (10.7%) and was successfully maintained at last available visit in 1 case (3.6%). Sirolimus and other targeted therapies, such as abatacept, belatacept, hydroxychloroquine, or idelalisib, may represent effective and safe treatment options for autoimmune and lymphoproliferative complications of certain PIDs, although clinical trials are still needed.33
Eight patients presented with a milder phenotype (characterized by autoimmune cytopenias without organomegaly or enteropathy), and all 8 (100%) responded to TPO-RA by the end of the study. Among the other 20 patients with a more severe phenotype, 12 patients (60.0%) responded to TPO-RA by the end of the study. This may suggest that more severe phenotypes are associated with more resistant ITP. It is also possible that in the presence of more severe CVID phenotypes, ITP relapses are preferentially treated with immunosuppressants to achieve efficacy against both autoimmune cytopenia and the associated comorbidities.
Interestingly 8 patients (28.6%) presented with a persistent response to TPO-RAs despite its discontinuation. For 3 cases (10.7%), no other ITP treatments were used concomitantly. Further research is currently ongoing regarding prolonged response after TPO-RA discontinuation in primary ITP.34
Our study comes with limitations. The retrospective nature of this study presents an inherent limitation. There is likely missing data because of the nature of file-based data collection with potential underreported events. In addition, selection bias may have occurred because the results primarily rely on declared cases and not on a systematic screening. Nonetheless, the use of several sources, including a nationwide database, led to a reduction in the risk of such bias. It should also be noted that several patients received multiple treatments concurrently; therefore, the observed efficacy cannot be attributed solely to the use of TPO-RAs.
Our study is the largest case series based on real-life data and assessed the safety and effectiveness of TPO-RAs in patients with ITP associated with PIDs. Together, our results suggest that eltrombopag or romiplostim should be considered as second-line therapy of ITP associated with PIDs, although clinical trials are still needed. TPO-RAs may also be combined with adapted targeted therapies whenever genetic research identifies an appropriate target.
Acknowledgments
The authors thank all the collaborators who played an important role in collecting the data. Notably, the authors acknowledge the Department of Clinical Immunology, and Immunodeficiencies Center, Center of Reference for Hereditary Immunodeficiency Disorders, and Center of Reference for Autoimmune Cytopenias in Adults networks that were crucial in identifying potential cases.
Authorship
Contribution: M.S. and A.P. designed the research; M.S., A.P., L.G., N.M., C.F., B.G., C.L., C.G., S.H., J.F.V., S.D., M.M., D.G., R.L.C., M.-l.P.-j., and H.H. contributed to the acquisition of the data; M.S., A.P., and A.L. wrote the manuscript; and all authors provided critical review of the manuscript and approval of the final version of the manuscript.
Conflict-of-interest disclosure: B.G. reports serving as an expert for Amgen, Grifols, and Novartis. H.H. reports serving as an expert for Amgen and Novartis. L.G. reports serving as an expert for Amgen, GlaxoSmithKline, Novartis, and Sanofi. M.M. reports serving as a consultant (advisory boards) for and receiving speaker fees from Grifols, Novartis, Sanofi, and Sobi. The remaining authors declare no competing financial interests.
Correspondence: Margaux Soulard, Department of Internal Medicine, University Hospital of Rennes,16 Bd de Bulgarie, 35200 Rennes, France; email: margaux.soulard@chu-rennes.fr.
References
Author notes
Original data are available on request from the corresponding author, Margaux Soulard (margaux.soulard@chu-rennes.fr).