Key Points
The proposed model-informed remedial approach is superior to existing guidelines, minimizing the time spent outside the therapeutic window.
A web-based dashboard has been devised to offer remedial dosing regimen for managing delayed or missed dose of DOACs.
Visual Abstract
Nonadherence to direct oral anticoagulant (DOAC) pharmacotherapy may increase the risk of thromboembolism or bleeding, and delayed or missed doses are the most common types of nonadherence. Current recommendations from regulatory agencies or guidelines regarding this issue lack evidence and fail to consider individual differences. This study aimed to develop individual remedial dosing strategies when the dose was delayed or missed for DOACs, including rivaroxaban, apixaban, edoxaban, and dabigatran etexilate. Remedial dosing regimens based on population pharmacokinetic (PK)-pharmacodynamic (PD) modeling and simulation strategies were developed to expeditiously restore drug concentration or PD biomarkers within the therapeutic range. Population PK-PD characteristics of DOACs were retrieved from previously published literature. The effects of factors that influence PK and PD parameters were assessed for their impact on remedial dosing regimens. A web-based dashboard was established with R-shiny to recommend remedial dosing regimens based on patient traits, dosing schedules, and delay duration. Addressing delayed or missed doses relies on the delay time and specific DOACs involved. Additionally, age, body weight, renal function, and polypharmacy may marginally affect remedial strategies. The proposed remedial dosing strategies surpass current recommendations, with less deviation time beyond the therapeutic range. The online dashboard offers quick and convenient solutions for addressing missed or delayed DOACs, enabling individualized remedial dosing strategies based on patient characteristics to mitigate the risks of bleeding and thrombosis.
Introduction
Direct oral anticoagulants (DOACs), including rivaroxaban, apixaban, edoxaban, and dabigatran etexilate, have rapidly emerged as appealing substitutes for vitamin K antagonists in anticoagulation.1 They are widely used nowadays for the prevention of stroke in patients with nonvalvular atrial fibrillation (NVAF) and for the treatment and prevention of deep vein thrombosis and pulmonary embolism in patients after orthopedic surgery.1,2
For patients receiving DOACs for long-term treatment, the medication adherence was ∼60% to 71% and decreased over time.3,4 Poor adherence is related to high risk of thromboembolic or bleeding events and an increased risk of all-cause death.5,6 The pattern of nonadherence is diverse and complicated in real-world patients, with delayed or missed dose being the pattern of most frequent occurrence.7
To address this issue, the package inserts approved by the US Food and Drug Administration suggested that “the patient take the missed dose immediately, and do not double the dose within the same day to make up for a missed dose.”8 The Medicines and Healthcare Products Regulatory Agency of the United Kingdom,9 New Zealand Medicines and Medical Devices Safety Authority,10 and European Medicines Agency11 provide similar recommendations. Moreover, practical guidelines from the European Heart Rhythm Association (EHRA) recommends that “the forgotten dose may be taken until half of the dosing interval has passed, and after this time point, the dose should be skipped, and the next scheduled dose should be taken.”12
However, these recommendations lack supporting evidence and do not consider individual patient characteristics. It is well-known that the longer a medication is delayed, the less drug remains in the body, strengthening the necessity for a remedial dose, which is contrary to the aforementioned recommendations to skip the missed dose. Moreover, the recommendations do not take the characteristics and risks of thrombosis and bleeding in patients into consideration, which may also have a large impact on the remedial dose regimen. An excessive remedial dose could result in bleeding, whereas an insufficient remedial dose may increase the risk of thromboembolism. Hence, optimal remedial dosing guidelines for delayed or missed DOAC doses are urgently needed.
Pharmacokinetic (PK) and pharmacodynamic (PD) modeling and simulations are powerful tools for resolving this issue. These approaches have been applied to the development of DOACs, supporting the rational DOACs dosing regimens for both adult13-17 and pediatric patients.18-20 This approach was applied to handle delayed or missed doses of rivaroxaban21 and edoxaban22 in our previous studies. However, only common nonadherence scenarios in typical adult patients with NVAF were investigated because of the time-consuming calculation procedure involved in using the gold standard software NONMEM. A comprehensive evaluation to establish remedial regimens for various nonadherent scenarios across all DOACs and treated populations is needed. Moreover, rapid and easy approaches for retrieving these remedial regimens are also necessary.
Therefore, our study aimed to investigate the appropriate remedial dosing regimens for patients who delay or miss their DOACs dose in diverse scenarios. Moreover, a dashboard providing a remedial regimen with a rapid algorithm was developed to manage delayed or missed DOAC doses.
Methods
Rationale
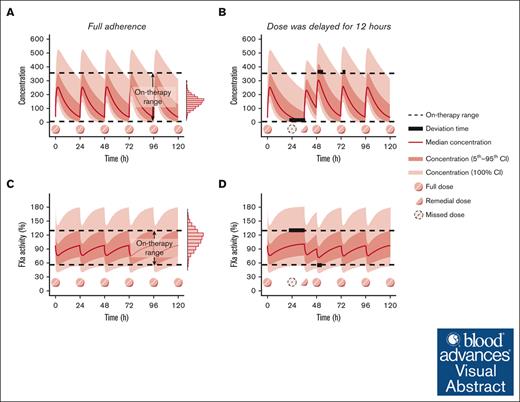
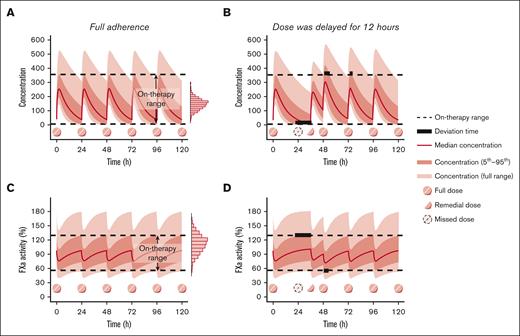
When the patient fully adheres to medication therapy and achieves desired therapeutic effect, the concentration or PD biomarker would fluctuate within a certain range. If a dose is delayed or missed, drug concentration or PD biomarker levels would fluctuate beyond the range. Although DOACs are extensively used in current medical practice, their therapeutic ranges have not been well defined.23 Therefore, the on-therapy range, defined as “the interval delineated by the 5th percentile trough concentration and the 95th percentile peak concentration at steady state” after multiple DOAC doses,24 was applied in this study based on expert consensus, accounting for the variability in therapeutic efficacy between patients.25,26
The aim of a remedial dosing regimen is to restore the concentration and level of PD biomarker back to on-therapy range and minimize the deviation time, which was defined as drug concentrations or levels of PD biomarkers outside the on-therapy range,27 as much as possible. Figure 1 illustrates a schematic diagram outlining the selection of optimal remedial dosing regimens, in which “deviation time” is presented by bold black lines to represent the duration when drug concentration or level of PD biomarker was out of the on-therapy range.
To estimate individualized on-therapy ranges and deviation times for various nonadherence scenarios and remedial regimens, a Monte Carlo simulation based on the established population PK/PD models was used.28 This approach has also been successfully applied to determine the remedial dosing regimen for various medications, including immunosuppressive agents,29 antiseizure medications,27 antitubercular agents,30 antipsychotics,31 and antisense oligonucleotides.32
Remedial dosing regimen
Population PK and PK/PD characteristics of DOACs
Four DOACs were investigated in our study, namely rivaroxaban, apixaban, edoxaban, and dabigatran. The population PK and PK/PD characteristics of these 4 DOACs were retrieved from previous studies identified in PubMed, Embase, and Web of Science databases until 31 March 2024.
Remedial dosing strategy
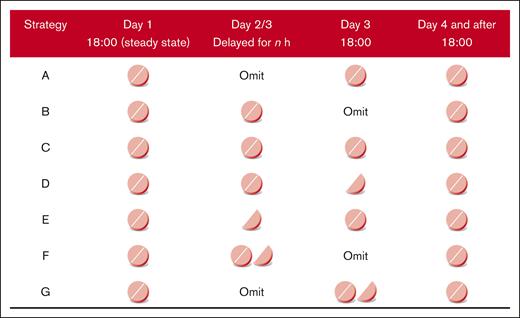
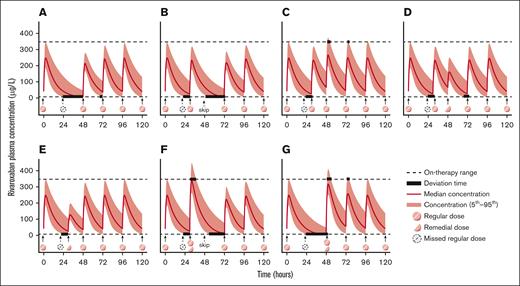
Considering clinical feasibility, 7 remedial strategies were formulated, encompassing dose adjustment at the delayed time and the time for the next appointed dose.27 The remedial strategies are listed below, and a graphic presentation is illustrated in Figure 2.
Graphic representation of remedial strategies after delayed or missed dose of DOACs administered once daily. Strategy A, omit the missed dose and consume the next regular dose at the appointed time; strategy B, consume the regular dose immediately and omit the missed dose at the next appointed time; strategy C, consume the regular dose immediately and take a regular dose at the next appointed time; strategy D, consume the regular dose immediately and take a remedial dose at the next appointed time; strategy E, consume a remedial dose immediately and take a regular dose at the next appointed time; strategy F, consume the regular dose plus a remedial dose immediately and omit the next appointed dose; and strategy G, omit the missed dose and consume the regular dose plus a remedial dose at the next appointed time.
Graphic representation of remedial strategies after delayed or missed dose of DOACs administered once daily. Strategy A, omit the missed dose and consume the next regular dose at the appointed time; strategy B, consume the regular dose immediately and omit the missed dose at the next appointed time; strategy C, consume the regular dose immediately and take a regular dose at the next appointed time; strategy D, consume the regular dose immediately and take a remedial dose at the next appointed time; strategy E, consume a remedial dose immediately and take a regular dose at the next appointed time; strategy F, consume the regular dose plus a remedial dose immediately and omit the next appointed dose; and strategy G, omit the missed dose and consume the regular dose plus a remedial dose at the next appointed time.
Strategy A was to omit the missed dose and consume the next regular dose at the appointed time.
Strategy B was to consume the regular dose immediately and omit the missed dose at the next appointed time.
Strategy C was to consume the regular dose immediately and take a regular dose at the next appointed time.
Strategy D was to consume the regular dose immediately and take a remedial dose at the next appointed time.
Strategy E was to consume a remedial dose immediately and take a regular dose at the next appointed time.
Strategy F was to consume the regular dose plus a remedial dose immediately and omit the next appointed dose.
Strategy G was to omit the missed dose and consume the regular dose plus a remedial dose at the next appointed time.
When the scheduled dose was missed, 3 strategies were evaluated according to previous studies,21,22,27 including taking 1 regular dose, taking a dose 1.5-times the regular dose, or taking a dose double the regular dose at the time of the next scheduled dose. Half of the regular dose could be obtained by breaking the tablet into 2 halves for rivaroxaban, edoxaban, and apixaban. However, dabigatran etexilate cannot be broken into halves due to its capsule formulation. However, there are 3 dose specifications for dabigatran etexilate (75, 110, and 150 mg), which could support its remedial dose selection.
The strategies with the least deviation times were considered optimal. For those strategies with approximate deviation time (<0.2 hour and 0.1 hour for once every 24-hour and 12-hour dosing regimens, respectively), they were considered as equivalent. Moreover, the deviation time for each remedial dosing strategy was estimated and compared with that recommended by the EHRA guidelines.
It was assumed that patients who were administered DOACs with complete adherence reached a steady state before the occurrence of missing or delayed doses. The recommendation is evaluated for a typical older patient with normal renal function, who was not taking any concomitant medications that might potentially cause drug-drug interactions. The dosing regimens are detailed in supplemental Appendix 1.
For full adherence and various nonadherence scenarios, the concentration-time and PD biomarker-time profiles of 1000 virtual patients were simulated based on the identified population PK/PD models. Because the behaviors of exact complete adherence and delayed dosing were simulated without any noticeable “noise,” the model parameters were set to the reported values, with the exception of the residual unexplained variability that was set to 0.1% for the proportional error and 0.1 mg/L for the additive error.27,33 Simulations and plots were performed using rxode2 (version 2.1.2) and ggplot2 (version 3.5.1) in R (version 4.2.2).
Impact of clinical characteristics on remedial dosing regimens
The factors reported to significantly influence the PK and PD behaviors of DOACs were investigated for their impact on remedial regimens by sensitivity analysis. For continuous variables, the common range within the study population was examined. For categorical variables, the corresponding influence was evaluated. On each occasion, 2000 Monte Carlo simulations were performed in R using rxode2 (version 2.1.2).
Online dashboard
To facilitate the design of remedial strategies for delayed or missed doses, an online dashboard was developed in R using rxode2 (version 2.1.2), ggplot2 (version 4.2.3), and Shiny (version 1.7.5.1). The dashboard could estimate the deviation times for all candidate remedial strategies and provide the corresponding PK/PD profiles, further facilitating the selection of the most appropriate individualized remedial regimens. In addition, to adapt the dashboard to diverse clinical settings, an advanced module was devised, allowing users to adjust all model parameters according to their requirements.
Results
Population PK and PK/PD characteristics of DOACs
A total of 48 population PK and PK/PD models of DOACs were identified. Details of the literature review and publication identification are described in supplemental Appendices 2-6. The apparent clearance (CL/F) of rivaroxaban, apixaban, and dabigatran in Asians has been reported to be 20% to 43%,34,35 40% to 48%,36 and 20%37 lower than that in Caucasians, respectively. Moreover, the apparent volume of distribution (V/F) of edoxaban in Asians was reported to be 23% higher than that of Caucasians.38 Therefore, population PK and PK/PD models of 4 DOACs in Asians and non-Asians were investigated separately. The clinical characteristics and demographics of patients in each study and parameter estimates of the identified models are outlined in supplemental Appendices 7 and 8.
Remedial dosing regimen
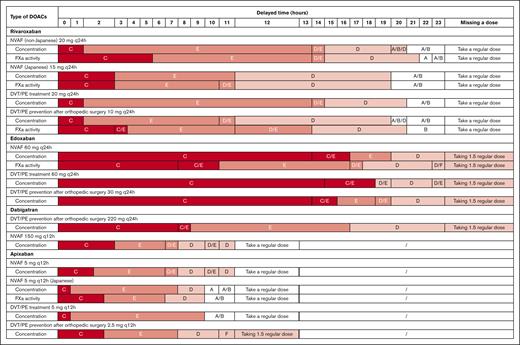
The remedial dosing regimen was first investigated based on drug concentration in a typical patient (supplemental Appendix 9). Then all PD biomarkers applied in reported population PK-PD models were evaluated, including factor Xa (FXa) activity, prothrombin time, activated partial thromboplastin time, prothrombinase-induced clotting time, HEPTEST for FXa inhibitors (rivaroxaban, apixaban, and edoxaban), and activated partial thromboplastin time for dabigatran. Remedial dosing regimens based on PD biomarkers other than FXa activity were consistent with those based on drug concentration due to their high linearity, as illustrated in supplemental Appendix 10. Therefore, only remedial dosing regimens based on drug concentration and FXa activity were used in the further analysis and presented in the dashboard. Figure 3 summarizes the recommended remedial dosing regimens for typical patients in various nonadherence scenarios.
Recommended remedial regimens when the DOAC dose was delayed for typical patient. The typical patient characteristics included the following: age 60 years, body weight 70 kg, height 170 cm, serum creatinine 1 mg/dL, blood urea nitrogen 16 mg/dL, and without any potential comedication. Strategy A, omit the missed dose and consume the next regular dose at the appointed time; strategy B, consume the regular dose immediately and omit the missed dose at the next appointed time; strategy C, consume the regular dose immediately and take a regular dose at the next appointed time; strategy D, consume the regular dose immediately and take a remedial dose at the next appointed time; strategy E, consume a remedial dose immediately and take a regular dose at the next appointed time; strategy F, consume the regular dose plus a remedial dose immediately and omit the next appointed dose; and strategy G, omit the missed dose and consume the regular dose plus a remedial dose at the next appointed time. DVT, deep vein thrombosis; PE, pulmonary embolism.
Recommended remedial regimens when the DOAC dose was delayed for typical patient. The typical patient characteristics included the following: age 60 years, body weight 70 kg, height 170 cm, serum creatinine 1 mg/dL, blood urea nitrogen 16 mg/dL, and without any potential comedication. Strategy A, omit the missed dose and consume the next regular dose at the appointed time; strategy B, consume the regular dose immediately and omit the missed dose at the next appointed time; strategy C, consume the regular dose immediately and take a regular dose at the next appointed time; strategy D, consume the regular dose immediately and take a remedial dose at the next appointed time; strategy E, consume a remedial dose immediately and take a regular dose at the next appointed time; strategy F, consume the regular dose plus a remedial dose immediately and omit the next appointed dose; and strategy G, omit the missed dose and consume the regular dose plus a remedial dose at the next appointed time. DVT, deep vein thrombosis; PE, pulmonary embolism.
The remedial dosing regimens largely depend on the delay time and specific type(s) of DOACs used. For delays <2 hours, an immediate remedial dose followed by regular dosing regimens (strategy C) was recommended for most DOACs; for edoxaban, an immediate remedial dose followed by regular dosing could be administered for delays up to 7 hours. When the dose was delayed for up to 2 hours before the next scheduled dose, the recommended remedial dose was half of the regular dose (strategy D or E), depending on the type of DOACs and delay time. When the delayed dose was close or equal to the next appointed time, a regular dose or 1.5-times the regular dose was recommended, which is also dependent on the type of DOACs. It is not recommended to omit the missed dose and take the regular dose plus a remedial dose at the next appointed time (strategy G), regardless of how long the medication has been delayed.
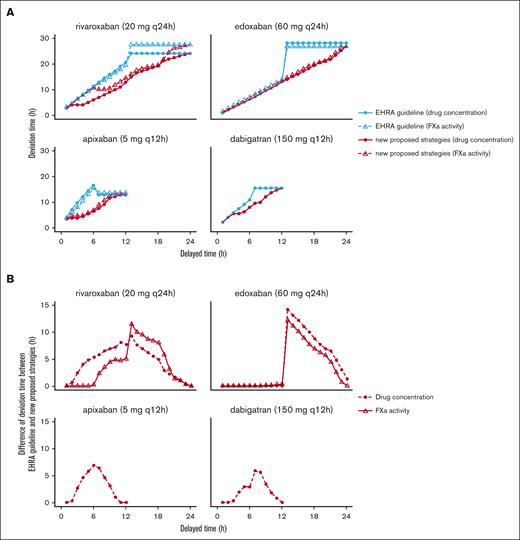
Compared with the recommendations of the EHRA, our proposed remedial strategy has less or equal deviation time for all DOACs from the perspective of both drug concentration and PD biomarkers. An example of this comparison regarding stroke prevention in patients with NVAF is shown in Figure 4A. In general, for DOACs administered every 24 hours, the adoption of our recommendation would lead to a decrease in the deviation time by up to 14.4 hours. For DOAC administered every 12 hours, our proposal would lead to a decrease in the deviation time by up to 12.5 hours, as shown in Figure 4B.
Comparison of deviation times from the recommendations of the EHRA guidelines and proposed remedial dosing regimens. (A) Deviation time from the recommendations of the EHRA guidelines and proposed remedial dosing regimens. (B) Difference in the deviation time between the recommendations of the EHRA guidelines and proposed remedial dosing regimens. The typical patient: age 60 years, body weight 70 kg, height 170 cm, serum creatinine 1 mg/dL, blood urea nitrogen 16 mg/dL, and without any potential comedication. Dosing regimens for each DOAC: rivaroxaban 20 mg q24h; edoxaban 60 mg q24h; apixaban 5 mg q12h; dabigatran 150 mg q12h. q12h, every 12 hours; q24h, every 24 hours.
Comparison of deviation times from the recommendations of the EHRA guidelines and proposed remedial dosing regimens. (A) Deviation time from the recommendations of the EHRA guidelines and proposed remedial dosing regimens. (B) Difference in the deviation time between the recommendations of the EHRA guidelines and proposed remedial dosing regimens. The typical patient: age 60 years, body weight 70 kg, height 170 cm, serum creatinine 1 mg/dL, blood urea nitrogen 16 mg/dL, and without any potential comedication. Dosing regimens for each DOAC: rivaroxaban 20 mg q24h; edoxaban 60 mg q24h; apixaban 5 mg q12h; dabigatran 150 mg q12h. q12h, every 12 hours; q24h, every 24 hours.
Impact of clinical characteristics on remedial dosing regimens
Based on previous studies, the effects of the most commonly identified factors on remedial regimens were investigated, including age (30-90 years), body weight (BW; 40-120 kg), serum creatinine (SCr; 0.4-1.8 mg/dL), and creatinine clearance (CrCl; 50-120 mL/min). In addition, the effect of concomitant medication was also investigated.
Renal function significantly influences the apparent clearance (CL/F) of all 4 DOACs, and dose adjustment is required accordingly. Compared with patients with normal renal function (SCr, 1 mg/dL; CrCl, 90 mL/min), patients with moderately impaired renal function (SCr, 1.5 mg/dL; CrCl, 50 mL/min) had DOACs CL/F decreased by 14% to 32%. Therefore, along with the decrease in renal function, the duration for which the regular dose can be taken immediately (strategy C) is slightly extended. Moreover, when the delayed dose was close to the next scheduled dose, the duration for strategies A or B was reduced.
Age has also been reported to affect the CL/F of DOACs. The CL/F of the older population (80-90 years) would decrease by >20% compared with that of the 60-year-old population. The changes in the recommended remedial strategies were consistent with the impaired renal function demonstrated above due to decreased CL/F in older patients.
Amiodarone, verapamil, and strong/moderate CYP3A4/P-gp inhibitors would decrease the CL/F of apixaban and dabigatran by 12% to 23%,37-39 and concomitant proton pump inhibitors increased the CL/F of dabigatran by 12% to 15%.38,40 The changes in the recommended remedial strategies were similar to those for renal impairment. Although previous studies have not reported the effects of strong inducers of CYP enzymes and P-glycoprotein, these inducers should be avoided in combination with DOACs. In cases in which a combination of inducers is necessary, changes in remedial strategies can be inferred from changes in the CL/F.
BW may affect the Vd/F of DOACs. Compared with individuals weighing 70 kg, Vd/FVd in those weighing 100 to 120 kg would increase by 14% to 64%, and in those weighing 40 to 50 kg, it would decrease by 10% to 58%. Along with the increase in BW, the duration for strategy C is slightly extended. The duration for strategies A or B when it was close to next scheduled dose was reduced.
Online dashboard
An accessible online dashboard was developed, comprising general and advanced modules. Both modules allow for input parameters such as patient clinical characteristics, concomitant medication, dosing regimens, delayed time, and minimum divided dose amount. The output includes the on-therapy range, total deviation time, deviation time above and below the on-therapy range, graphic presentation of the concentration-time or PD marker-time profiles of each candidate remedial strategy, and the optimal remedial strategy. For advanced modules, all PK and PD parameters and on-therapy range could be modified as required.
General module
All PK and PD parameters in the general module were fixed at the values reported in the original study (supplemental Appendix 7). Because of the different population PK and PK/PD models for rivaroxaban and apixaban used for Asian and non-Asian patients, corresponding sets of model parameters have been used for the respective patient groups. The general module could be visited via https://remedial-doac-dose.shinyapps.io/General_module/. An example is illustrated below, which demonstrates the remedial dosing strategies for a 70-year-old patient weighing 70 kg with NVAF and an CrCl of 90 mL/min. The patient was administered 20 mg of rivaroxaban every 24 hours with food to prevent stroke. Typical nonadherent scenarios were addressed: dose delay for 8 hours (less than half of the dosing interval) and 20 hours (more than half of the dosing interval) and missed dose. The minimum divided dose of rivaroxaban was 10 mg.
Example 1: dose delayed by 8 hours
In the scenario of dose delayed for 8 hours, the recommendation of taking 10 mg immediately, followed by 20 mg at the next appointed time (strategy E), is supported. The total deviation time was 8.0 hours and was much less than the 14.5 hours estimated following the recommendations by EHRA guidelines. The simulated concentration-time profiles are illustrated in Figure 5. Considering FXa activity, the total deviation time following strategy E is also less than that of the EHRA guideline (10.4 vs 13.6 hours).
Example of rivaroxaban concentration-time profiles for each remedial strategy in patients remembering the missed dose after delaying for 8 hours. During the development of general module, the values of demographic covariates other than age, body weight, height, sex, renal function, and concomitant medications were fixed as the median values reported in the original literatures and could not be adjusted. The following parameters were set in this case: non-Asian male patient, age 60 years, body weight 70 kg, height 170 cm, serum creatinine 1 mg/dL, blood urea nitrogen 16 mg/dL, and without any potential comedication. The patient was prescribed with rivaroxaban 20 mg q24h. The minimum divided dose was set as 10 mg.
Example of rivaroxaban concentration-time profiles for each remedial strategy in patients remembering the missed dose after delaying for 8 hours. During the development of general module, the values of demographic covariates other than age, body weight, height, sex, renal function, and concomitant medications were fixed as the median values reported in the original literatures and could not be adjusted. The following parameters were set in this case: non-Asian male patient, age 60 years, body weight 70 kg, height 170 cm, serum creatinine 1 mg/dL, blood urea nitrogen 16 mg/dL, and without any potential comedication. The patient was prescribed with rivaroxaban 20 mg q24h. The minimum divided dose was set as 10 mg.
Example 2: dose delayed by 20 hours
The screenshots of the dashboard and the PK profiles when the dose was delayed by 20 hours are shown in supplemental Appendix 11. From the perspective of rivaroxaban concentration, strategy D, which involves administering 10 mg immediately followed by 20 mg at the next scheduled time, resulted in the least total deviation time of 23.6 hours, with 20.1 hours below the on-therapy range and 3.5 hours above it. Strategies A (skipping the missed dose and taking 20 mg at the next appointed time) and B (taking 20 mg immediately and skipping the next dose) would result in total deviation times of 24.1 and 24.2 hours, respectively, both of which exceed 24 hours due to the delay caused by drug absorption, and the concentrations are below the on-therapy range. The difference in deviation times among strategies A, B, and D was <1 hour, indicating that the 3 remedial strategies were similar.
For patients with a higher risk of bleeding, strategies A and B, with less deviation times above the on-therapy range, would be preferable to strategy D (0 vs 3.5 hours). On the contrary, patients with a higher risk of thrombosis may consider strategy D, with less deviation time below the on-therapy range (20.1 vs 24.1 hours).
Example 3: missed dose
Regular dosing is recommended when the dose is missing. Taking an additional half of the regular dose results in a longer total deviation time (24.1 vs 32.1 hours), which is consistent with the results obtained from the FXa activity (27.5 vs 31.3 hours). The simulated profiles of the remedial dosing regimens are shown in supplemental Appendix 12.
Advanced module
All parameters and the on-therapy range could be modified as required in the advanced module. The advanced module could be visited via https://remedial-doac-dose.shinyapps.io/Advanced_module/. If the clinical characteristics of the patient change or if the influence of other potential factors needs to be examined, individualized remedial dosing can be achieved accordingly by inputting the PK or PD parameters directly. For parameters that were not obtained or were inaccessible, the values listed in supplemental Appendix 8 could be used. For example, if baseline FXa activity for rivaroxaban was not available, 104% can be used as a reference to proceed with the analysis. An illustration of the user-defined module is provided in supplemental Appendix 13.
Discussion
Long-term medication adherence is important for DOACs to achieve their expected anticoagulation effects. It is still crucial to use appropriate remedial regimens after episodes of nonadherence to preserve a favorable treatment outcome. In this study, we developed and evaluated several remedial dosing regimens for 4 DOACs commonly used in various clinical settings using population PK/PD modeling and simulation. Furthermore, an online dashboard was created to facilitate individualized remedial dosing regimens.
The analysis of delayed or missed doses is often challenging due to the diverse patterns of drug-taking behaviors in real patients. Ethical restriction in clinical trials and the variation of abundant parameters during investigation may further increase the complexity of the analysis.41 Therefore, various statistical models of adherence have been developed for computational analyses.41-43 Previous studies had contributed to quantifying the PK effects of taking a late dose using mathematical formulas.43,44 For DOACs, previous studies mainly assessed the impact of nonadherence to DOACs,45,46 but few studies focused on assessing what to do when missed or delayed dose occurs. This study is an extension and improvement of previous studies, which not only quantify the effect on PK exposure but also on PD biomarkers.
The therapeutic range of DOACs varies significantly among patients and remains unclear. Hence, we adopted the well-recognized concept of “on-therapy range,”25,26 instead of the fixed reference range. The “on-therapy range” was based on the exposure-response relationship of DOACs demonstrated by PK/PD modeling during clinical development.47 The on-therapy range was estimated using pooled data from well-designed clinical studies, most of which were phase 2/3 clinical trials supporting the dose selection or approved dosing regimen.14,37-39,48 This rationale has also been used in other therapeutic areas27,49 to characterize a patient-based scenario in which the desired therapeutic effects are attained without intolerable adverse reactions.
The primary determinant of optimal remedial dosing strategies for delayed or missed doses of DOACs is the delay time. The finding is broadly consistent with our previous reports in patients with NVAF.21,22 All approved DOACs and other targeted populations were also investigated, such as patients requiring deep vein thrombosis/pulmonary embolism treatment or prophylaxis after surgery. Moreover, this study evaluated multiple factors and found that age, BW, renal function, concomitant medication, and other factors could also influence the optimal remedial strategy by influencing exposure or response, which was not achieved in our previous studies. Moreover, compared with the instructions of regulatory agencies and EHRA guidelines, our model provides superior recommendations for addressing delayed or missed doses, leading to a noteworthy reduction in the deviation time outside the on-therapy range.
Furthermore, in the newly developed advanced module of the online dashboard, users have the ability to adjust individual parameters as needed. For example, considering that the individual therapeutic range may vary among patients, users can specify the lower and upper limits of their therapeutic ranges as necessary in this module. By adjusting the parameters according to the needs of users, this dashboard can offer more accurate remedial regimens, tailored to the specific circumstances of individual patients.
Individualized remedial dosing for patients with different bleeding and thrombosis risks can also be achieved using our dashboard. The risk of thrombosis and bleeding in individual patients can be evaluated using the CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 [doubled], diabetes mellitus, prior stroke or transient ischemic attack [doubled], vascular disease, age 65–74, female) and HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly) scores.50,51 In general, a drug concentration above the on-therapy range is considered a risk factor for bleeding. Therefore, patients with higher HAS-BLED scores could choose remedial strategies with shorter deviation times above the on-therapy range. In contrast, patients with higher CHA2DS2-VASc scores could choose remedial strategies with a less deviation time below the on-therapy range. Considering that it requires professional knowledge to assess the risk of bleeding and thrombosis in patients, the dashboard should be used by medical professionals rather than patients to avoid misuse.
Our study had some limitations. First, we only investigated remedial dosing regimens after a single delayed or missed dose of DOACs. In reality, nonadherence patterns to anticoagulant therapy are more complicated, involving other patterns such as missing multiple doses and irregular dosing. Efforts to investigate these patterns of nonadherent behaviors quantitatively are needed before attempting to address missed or delayed dose in these cases. Secondly, we did not evaluate the findings of our study in real patients. However, ethnical constraints make it impractical to evaluate these results in real patients. Our modeling and simulation strategy can serve as a helpful tool to evaluate remedial strategies in the early stages before clinical implementation.
Conclusion
For patients who delay or miss their DOAC dose, remedial dosing strategies should be selected based on the type of DOACs and delay time. A model-informed online dashboard was developed to facilitate quick and convenient selection of optimal remedial dosing regimens.
Acknowledgments
The authors thank Yuan-Zhe Wang and Fu-Qing Gu for their critical comments on this manuscript. The authors thank Chen Xie for his great help in the development of Rshiny dashboard.
X.-Q.L. was funded by the National Natural Science Foundation of China (82304620) and the Shanghai Chest Hospital, Shanghai Jiao Tong University Young Researcher Development Support Program.
Authorship
Contribution: X.-Q.L. contributed to conceptualization, methodology, software, formal analysis, data curation, writing the original draft of the manuscript, and reviewing and editing the manuscript, visualization, and funding acquisition; Z.-R.L. contributed to methodology, software, formal analysis, data curation, reviewing and editing the manuscript; C.-Y.W. contributed to methodology, formal analysis, and reviewing and editing the manuscript; and Z.J. contributed to conceptualization, methodology, resources, writing the original draft of the manuscript, reviewing and editing the manuscript, visualization, supervision, and project administration.
Conflict-of-interest disclosure: Z.J. received consulting fees from Takeda, AstraZeneca, and Pharmaron. The remaining authors declare no competing financial interests.
Correspondence: Zheng Jiao, Department of Pharmacy, Shanghai Chest Hospital, Shanghai Jiao Tong University School of Medicine, 241 Huaihai West Rd, Shanghai 200030, P.R. China; email: jiaozhen@online.sh.cn.
References
Author notes
All data generated or analyzed during this study are included in this published article and its supplemental information files. The underlying code for this study is not publicly available but may be made available to qualified researchers on reasonable request from the corresponding author, Zheng Jiao (jiaozhen@online.sh.cn).
The full-text version of this article contains a data supplement.