In this issue of Blood Advances, Carpenter et al1 present their single-center analysis demonstrating a significant and progressively declining incidence of National Institutes of Health (NIH)–defined chronic graft-versus-host disease (cGVHD) requiring systemic immune suppression for both malignant and nonmalignant diseases (NMDs) over a 15-year period since 2005. In their study of 3066 pediatric and adult survivors, adjusting for known patient, donor, and transplant factors, including advances in T-cell depletion strategies for GVHD prophylaxis, did not fully account for the contemporary decline in cGVHD incidence.
Prevention and treatment of cGVHD are arguably some of the greatest advancements in the field of hematopoietic cell transplantation (HCT) over the past decade.2-4 Collaborative efforts lead by the NIH consensus development project to standardize cGVHD diagnosis and criteria for clinical trials lead to bench-to-bedside progress that advanced the field since the first consensus conference in 2005. Despite significant advancements in the treatment of cGVHD, including 4 US Food and Drug Administration–approved therapies for steroid-refractory cases, the prevention of moderate to severe cGVHD without compromising the graft-versus-leukemia (GVL) effect remains a prudent goal, with the potential for the greatest impact on long-term HCT outcomes and quality of life.
Through their current observation, Carpenter et al provide a testament to being a step closer to a sought-after goal in the field: achieving a progressive decline in cGVHD without compromising GVL or increasing the risk of relapse. Adjusting for historically identified cause-associated factors of cGVHD, such as recipient age, race, donor type/stem cell source, patient/donor sex, cytomegalovirus serostatus, disease risk, conditioning intensity, total body irradiation, and GVHD prophylaxis strategies such as T-cell depletion and posttransplant cyclophosphamide, did not fully explain the observed decline in the incidence of cGVHD, although the latter was associated with lower cGVHD rates. The incidence of cGVHD declined in both pediatric and adult populations, and among those who received transplant for NMD, even in patients receiving uniform methotrexate/calcineurin inhibitor–based prophylaxis.
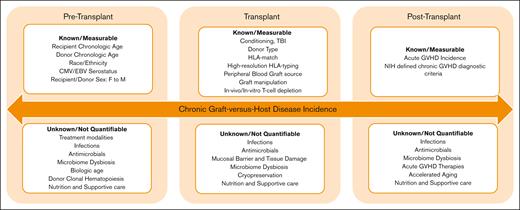
The findings of this analysis need to be validated by reviewing trends in NIH-defined cGVHD across institutions through multicenter studies and large registries. However, there are 2 important overarching lessons to learn from this report. First, contemporary controls should be used as benchmarks in cGVHD studies and reports instead of historic controls. Second, despite monumental efforts to improve our understanding of cGVHD biology, risk factors, and prevention, we still have not uncovered all the pieces of the puzzle. As described by the authors, although many cGVHD risk factors are well known and can be easily identified and measured, this report also clearly indicates that much work remains to identify previously unknown factors that may not yet be as easy to measure or associate with cGVHD outcome (see figure).
Chronic graft-versus-host disease risk factors. CMV, cytomegalovirus; EBV, Epstein-Barr virus.
Chronic graft-versus-host disease risk factors. CMV, cytomegalovirus; EBV, Epstein-Barr virus.
The immune modulatory role of microbial dysbiosis in increasing predisposition to immune-mediated post-HCT complications such as acute GVHD (aGVHD), infections, and transplant-related mortality is well described. Gut microbiome–derived metabolites, including short-chain fatty acids, secondary bile acids, and aryl hydrocarbon receptor ligands, have been shown to influence immune responses in the intestines as well as the integrity of the gut epithelial barrier. Not surprisingly, early reports indicate that the pre-HCT5 and post-HCT6 microbiome profiles could affect the risk of subsequent cGVHD development. A multitude of factors influence the microbial composition and its recovery throughout the transplant course, many of which are not easily captured or quantifiable. The list includes pre-HCT treatment modalities, conditioning regimen used, infections, antimicrobial exposure, aGVHD treatment, nutrition, lifestyle, and supportive care, among others. Additionally, it is plausible that these factors may have nonmicrobiome-related impacts on both cGVHD risk and outcomes. It is likely that improved antimicrobial stewardship, applying anaerobic-sparing approaches, reduced toxicity treatment and transplant modalities, and the encouragement of enteral nutrition and exercise after HCT all had systematic, additive beneficial effects, especially when implemented in a specialized transplant center with multidisciplinary expertise. It is conceivable that novel approaches to preventing and treating aGVHD, particularly those with overlapping mechanisms of action, may have an additional positive impact on reducing the risk of cGVHD as well as its severity and outcomes. The authors indicate a decline in aGVHD for children but not for the total population, a strong predictor of cGVHD. However, improved donor selection, advancements in graft manipulations, better treatment outcomes for aGVHD, and enhanced supportive care approaches could, at least partially and theoretically, explain the decline in cGVHD risk and importantly cGVHD-related mortality.
Donor selection is well known to influence post-HCT outcomes, including cGVHD. Several donor-related factors such as HLA matching and younger donor chronologic age are now well described in relation to improved outcomes.7 Additional donor-related factors, such as donor-derived clonal hematopoiesis (CH) and donor biological or cellular age, are currently under investigation. Longer donor leukocyte telomere length has been associated with improved survival and reduced infection-related death after HCT for severe aplastic anemia.8 However, the findings were not validated in patients with acute leukemia,9 nor was there an indication of an association with GVHD risk. Epigenetic clocks are DNA methylation–based biomarkers of aging, currently being investigated in various fields as novel tools for predicting cellular age and correlating with health outcomes. Early reports highlight the potential role of cellular aging in HCT outcomes, with accelerated DNA methylation age after HCT, compared with chronologic age, being associated with higher risk of cGVHD and reduced survival, particularly for NMD.10 Taken together, these data suggest that donor’s biologic age and other age-related factors, such as CH, might play a role in donor selection in the future. Additionally, reassessing the impact of the recipient’s chronological age in relation to their biological age on cGVHD risk would be of interest, especially as the field continues expanding to include older adult recipients.
The report by Carpenter et al serves as a refreshing reminder of the advances in our field aimed at preventing cGVHD and improving outcomes. It underscores the importance of the cumulative, additive effects of changes in transplant approaches and supportive care. Additionally, it highlights the urgent need to enhance GVL effects and reduce relapse risk, which remains the leading cause of treatment failure after HCT for malignant conditions.
Conflict-of-interest disclosure: The authors declare no competing financial interests.