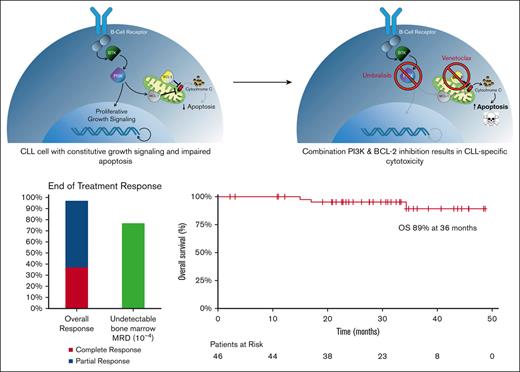
Time limited, response-adapted venetoclax, umbralisib, and ublituximab are safe and yield high rates of undetectable minimal residual disease for relapsed CLL.
Visual Abstract
Many patients with chronic lymphocytic leukemia (CLL) will develop treatment resistance to Bruton tyrosine kinase (BTK) inhibitors. Phosphatidylinositol-3-kinase (PI3K) inhibitors, including umbralisib, have significant clinical activity in relapsed/refractory CLL, but prolonged exposure is associated with potential toxicities. Owing to the synergistic antitumor effects of combined PI3K and BCL-2 inhibition, we sought to explore the feasibility of response-adapted, time-limited therapy to optimize disease control while mitigating the risks of prolonged treatment. We conducted a phase 1/2 clinical trial to determine the safety and efficacy of venetoclax in combination with umbralisib and the anti-CD20 monoclonal antibody, ublituximab, (U2-VeN) in patients with relapsed/refractory CLL (N = 46) and Richter transformation (N = 5). After 12 cycles, treatment was stopped for patients with CLL who achieved undetectable minimal residual disease (uMRD). Adverse events of special interest included diarrhea in 50% of patients (11% grade 3/4), and aspartate aminotransferase and/or alanine aminotransferase elevation in 15 patients (33%), with 3 (7%) grade 3/4. There were no cases of tumor lysis syndrome related to venetoclax, with outpatient initiation in 96% of patients. The intent-to-treat overall response rate for CLL was 98% with best response of 100% in evaluable patients (42% complete responses). The end-of-treatment rate of uMRD at 10−4 in bone marrow was 77% (30/39), including a 71% uMRD rate among 14 patients refractory to prior BTK inhibitor. Time-limited venetoclax and U2 is safe and highly effective combination therapy for patients with relapsed/refractory CLL including those who have been previously treated with covalent BTK inhibitors. This trial was registered on www.clinicaltrials.gov as #NCT03379051.
Introduction
The management of chronic lymphocytic leukemia (CLL) has dramatically improved in recent years, but the disease remains incurable with a typical pattern of relapse or development of therapy resistance (ie, refractory disease) requiring subsequent treatment. The advent of highly-effective orally bioavailable inhibitors of Bruton tyrosine kinase (BTK) has significantly altered the treatment approach for most patients with CLL.1-3 Despite these advances, covalent BTK inhibitors rarely produce deep remissions and/or undetectable minimal residual disease (uMRD) status, resulting in the need for continuous drug exposure which may be associated with long-term toxicities, including cardiovascular events, excessive health care costs, and the possibility of acquired resistance. The most commonly described mechanism of treatment resistance to oral covalent BTK inhibitors is through acquisition of a point mutation in BTK, leading to a single amino acid change in C481 in the catalytic domain of the enzyme that renders covalent BTK inhibitors ineffective.4 In addition, mutations in the downstream intermediary, phosphoinositide phospholipase C (PLC-γ) can also confer treatment resistance to BTK inhibitors.5 Although the noncovalent BTK inhibitor pirtobrutinib has gained regulatory approval for CLL with potential for therapeutic benefit in patients with C481 mutations, treatment resistance to this monotherapy has already been described, as well as a limited duration of disease control.6-8
Bcl-2 overexpression is a hallmark of CLL, because the malignant cells depend on this antiapoptotic protein for survival and resulting in exquisite sensitivity to BH3 mimetics, such as venetoclax.9 This orally available BCL-2 inhibitor has significant clinical activity in patients with CLL when used as a single agent or in combination with anti-CD20 monoclonal antibody therapy.10,11 With proper mitigation against tumor lysis syndrome (TLS), venetoclax-based treatment is safe and produces high rates of treatment response, frequently resulting in uMRD, thereby allowing for time-limited therapy and prolonged treatment-free intervals. Prospective trials and real-world data have shown that venetoclax is active in patients with prior exposure to BTK inhibitors, but the durability of this response is suboptimal.12-14
Anti-CD20 monoclonal antibodies have an active role in the management of B-cell malignancies and specifically, when added to traditional chemotherapy treatment of CLL.15 Head-to-head comparative studies suggest that obinutuzumab, which has been glycoengineered to enhance natural killer cell engagement and improve antibody-directed cellular cytotoxicity, is a more effective partner than rituximab for CLL treatment with chemotherapy as well as with venetoclax.15,16 Ublituximab is a next-generation anti-CD20 humanized monoclonal antibody with significant clinical activity in CLL and related conditions which also has enhanced antibody-directed cellular cytotoxicity and a novel CD20 binding epitope relative to rituximab.17
Phosphatidylinositol-3-kinase (PI3K) is a downstream intermediary, distal to BTK in the B-cell receptor (BCR) pathway, which perpetuates growth signals in B-cell malignancies including CLL. Abrogation of constitutive activation of the BCR prosurvival program may be accomplished by inhibitors of BTK as well as the δ isoform of PI3K. Clinical activity of the PI3K-δ inhibitors, idelalisib, and umbralisib in patients with CLL and/or indolent lymphoma led to rapid regulatory approval of these agents as well as duvelisib and copanlisib which inhibit a broader repertoire of PI3K isoforms.18-21 Although umbralisib was found to have relatively low rates of immune-mediated toxicities, because additional experience was garnered with this and other PI3K inhibitors, unique and sometimes serious toxicities including infection, colitis, and pneumonitis were observed with prolonged and continuous administration, thereby curtailing widespread clinical use and prompting interest in intermittent dosing strategies as well as the possibility of time-limited therapy.22 Specifically, achievement of very low levels of disease burden with uMRD at the conclusion of time-limited therapy is associated with improved progression free survival (PFS).11
Dual blockage of both BCR prosurvival and antiapoptotic signaling by PI3K-δ and BCL-2 inhibitors has synergistic antineoplastic effects in preclinical studies, likely mediated by downregulation of MCL-1.23-25 To explore the safety profile and antitumor effect of PI3K-δ and BCL-2 inhibitors when combined with highly active anti-CD20 monoclonal antibody therapy, we conducted a phase 1/2 clinical trial to determine the safety and efficacy of venetoclax, umbralisib, and ublituximab (U2-Ven) in previously-treated patients with CLL and Richter transformation (RT).
Methods
Patient eligibility
We conducted a multicenter phase 1/2 dose-escalation study to assess the safety and efficacy of venetoclax with umbralisib and ublituximab (U2-Ven) in patients with relapsed/refractory CLL or RT in need of treatment according to standard criteria.26 Patients were enrolled at the Wilmot Cancer Institute, University of Rochester, Rochester, New York; the Taussig Cancer Institute, Cleveland Clinic, Cleveland, Ohio; and the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, Illinois.
To be eligible for the study, patients were required to have a diagnosis of CLL, small lymphocytic lymphoma (SLL), or RT and to have progressed after at least 1 prior therapy and require treatment. In order to enrich the study population for patients previously treated with BTK inhibitors, the protocol was later amended to require patients to be intolerant or refractory to BTK-inhibitor treatment (ibrutinib or acalabrutinib), in which refractory disease was defined as progressive disease during or within 6 months of discontinuing BTK-inhibitor treatment. A 21-day washout period from prior therapy was required except for prior BTK inhibitor for which 3 days or 5 half-lives (whichever was longer) was required. A minimum of both an absolute neutrophil count of 750/μL and platelet count of 40 x 109/L were required. Serum creatinine clearance >50 mL/min was required for the phase 1 part of the study and >30 mL/min for phase 2.
Prior exposure to BCL-2 and/or PI3K inhibitor(s) was allowed. Prestudy testing for BTK or PLCγ mutations was not mandated, but these data were collected from subjects for whom such testing had been conducted.
The trial was approved by the institutional review board at each trial site and conducted in accordance with the principles of the declaration of Helsinki, and all applicable regulatory requirements.
Statistical design
Because of small sample sizes and dose heterogeneity, no prespecified statistical tests were planned for the phase 1 portion of the study, but in the phase 2 portion, the proposed regimen was to be considered promising in the initial CLL/SLL cohort if a true complete response (CR) rate of ≥45% was observed, and not worthy of pursuing if a CR rate <20% was observed. Simon 2-stage design was used. The null hypothesis of a true CR rate of 20% was tested against a 1-sided alternative. In the first stage, 13 subjects were accrued. If there were ≤2 CR rates in these 13 subjects, enrollment into this cohort would be stopped. Otherwise, up to 8 additional subjects could be accrued for a goal of at least 21. Enrollment could continue while response assessments were conducted. The null hypothesis would be rejected if ≥8 CR rates were observed in 21 subjects. This design yielded a type 1 error rate of 0.0427 and power of 80% when the true CR rate is 45%. The study tested whether the CR rate was ≤20% (null hypothesis) and was designed to have 80% power under the alternative rate of 45%, using a 1-sided binomial test. The assumptions for sample size calculation were based on the CR rate reported for venetoclax in subjects with relapsed refractory CLL (overall response rate [ORR], 79%; CR rate, 20%).11
The regimen was also specifically evaluated in the cohort of subjects with CLL or SLL who relapsed after prior exposure to, or were refractory to, BTK inhibitor therapy. Simon 2-stage design was used. The study was designed to test whether the CR rate was ≤9% (null hypothesis) and was designed to have 80% power under the alternative rate of 34%. The significance level was given an upper bound of 5%. In the first stage, 5 subjects were to be accrued. If there were 0 CRs in these 5 subjects, enrollment into this cohort would be stopped. Otherwise, up to 12 additional subjects would be accrued for a target of at least 17. Enrollment could continue while response assessments were conducted. The null hypothesis would be rejected if ≥4 CR rates were observed in 17 subjects. If the true CR rate was 9%, there would be a 62% chance of stopping the trial after enrolling only 5 subjects (ie, at stage 1). The assumptions for this sample size calculation in this cohort were based on the CR rate reported for subjects with CLL/SLL who relapsed after or were refractory to ibrutinib and then received venetoclax as their subsequent regimen (CR rate 9%).12
The RT cohort was designed to include up to 10 subjects. The efficacy evaluation in the RT cohort was exploratory in nature. The CR rate will be estimated, but no formal statistics were to be applied.
Study design
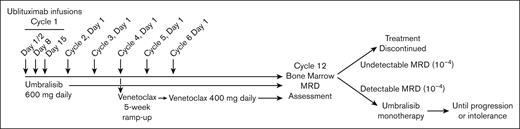
A schematic of the clinical trial treatment is shown in Figure 1. Ublituximab was administered IV with split first dose of 150 mg on day 1 and 750 mg on day 2, and then fixed doses of 900 mg on day 8 and 15 of cycle 1, and day 1 of subsequent cycles. The original protocol included 3 cycles of ublituximab. Once safety had been demonstrated in combination with umbralisib and venetoclax, ublituximab dosing was expanded to 6 cycles after the first 9 patients. Umbralisib was administered starting cycle 1, day 1 at a dose of 600 mg by mouth daily for dosing cohort 1 (first 3 patients) and 800 mg daily for dosing cohort 2 patients in phase 1 and all patients in phase 2. The standard method of phase 1 design for dose escalation was used to determine the maximum tolerated dose, enrolling 3 subjects per dose level cohort, and a minimum of 6 subjects at the maximum tolerated dose.
Treatment schematic for patients with CLL at phase 2 dose. Patients received ublituximab infusions on day 1, 2, 8, and 15 of cycle 1, followed by a single infusion every 28 days on day 1 of cycles 2 to 6. Umbralisib was administered daily for cycles 1 to 12. Venetoclax was initiated on day 1 of cycle 4 with 5-week ramp-up and continued at target dose of 400 mg daily until the conclusion of cycle 12. Patients with undetectable MRD after cycle 12 discontinued treatment. Patients with detectable MRD continued umbralisib monotherapy until intolerance or progression.
Treatment schematic for patients with CLL at phase 2 dose. Patients received ublituximab infusions on day 1, 2, 8, and 15 of cycle 1, followed by a single infusion every 28 days on day 1 of cycles 2 to 6. Umbralisib was administered daily for cycles 1 to 12. Venetoclax was initiated on day 1 of cycle 4 with 5-week ramp-up and continued at target dose of 400 mg daily until the conclusion of cycle 12. Patients with undetectable MRD after cycle 12 discontinued treatment. Patients with detectable MRD continued umbralisib monotherapy until intolerance or progression.
After response assessment and TLS risk assessment, venetoclax was administered beginning on cycle 4, day 1 for CLL (and cycle 2 day 1 for RT) and escalated over a standard 5-week ramp-up to a target dose of 400 mg, with TLS monitoring and management per the approved venetoclax package insert.27 At the conclusion of 12 cycles of therapy, MRD was assessed centrally in both peripheral blood (PB) and bone marrow aspirate by 8-color flow cytometry as previously described with a sensitivity of 10−4.28 After 12 cycles, all treatments were stopped for patients with CLL who achieved uMRD in the bone marrow, regardless of traditional response assessment. Umbralisib monotherapy was continued for those who still had residual disease, per treating physician preference until clinical progression or intolerance. For RT patients, extended treatments with umbralisib and/or venetoclax could continue after 12 cycles per investigator’s discretion until clinical progression or intolerance, regardless of response. The study was terminated by the sponsor in April 2022 because of a shift in priorities and changing regulatory landscape for PI3K inhibitors in patients with CLL.
Objectives
The primary objective of phase 1 was to evaluate the safety of venetoclax addition to umbralisib and ublituximab (U2). The primary objectives of phase 2 was to define the CR rate and the undetectable MRD rate after 12 cycles of therapy. Secondarily, PFS (per 2018 International Workshop on Chronic Lymphocytic Leukemia [iwCLL] criteria) and ORRs were evaluated.26
Safety
TLS risk was assessed at the initiation of protocol treatment and reassessed after 3 cycles of combined U2 treatment using standard criteria based on Food and Drug Administration approval of venetoclax as detailed in the package insert.27 Adverse events (AEs) were collected and graded by the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0. AE attributions to study drug(s) and/or disease or other causes were determined by the investigators.
Efficacy
Disease response was assessed by investigator by iwCLL 2008 criteria for CLL, and the International Working Group Revised Response Criteria for Malignant Lymphoma criteria for RT after cycle 3, cycle 7 and cycle 12.26 PFS and overall survival (OS) were calculated using standard methods.
Results
Patient characteristics
Forty-six patients with CLL were enrolled with 45 evaluable for efficacy (1 patient was removed because of noncompliance). The baseline patient characteristics are shown in Table 1. The median age of patients with CLL was 64 (range, 43-83) years. Seventy-six percent had at least 1 high risk molecular feature including 17p deletion and/or TP53 mutation, unmutated immunoglobulin variable heavy chain, 11q deletion, and NOTCH1 or SF3B1 mutation. The median number of prior therapies was 2 (range, 1-6). Among the 26 patients previously treated with BTK inhibitors, 18 (69%) patients were refractory to this treatment and 10 out of 14 (71%) of those tested had either a detectable BTK and/or phospholipase Cγ mutation. One patient had a prior venetoclax treatment and 3 patients had prior PI3K inhibitor treatment. One received idelalisib with rituximab as their most recent treatment and was refractory to this therapy. Two other patients received prior treatment with idelalisib. One was discontinued for reasons other than refractory disease and 1 discontinued for unknown reasons.
CLL patient characteristics
| Evaluable for safety, n | 46 |
| Evaluable for efficacy, n | 45∗ |
| Median age, y (range) | 64 (43-83) |
| Male/female, n | 33/13 |
| ECOG, 0/1/2, n | 6/38/2 |
| Prior therapy regimens, median (range) | 2 (1-6) |
| Refractory to immediate prior therapy, n (%) | 18 (39%) |
| Prior anti-CD20, n (%) | 39 (85%) |
| Prior chemoimmunotherapy, n (%) | 35 (76%) |
| Prior targeted therapy, n (%) | 30 (65%) |
| Prior BTKi (ibrutinib/acalabrutinib), n (%) | 26 (57%) |
| Refractory to prior BTK inhibitor, % (n/N) | 69% (18/26) |
| BTK or PLCγ mutation detected, % (n/N) | 71% (10/14) |
| Prior PI3K inhibitor, n (%) | 3 (7%) |
| Prior venetoclax, n (%) | 1 (2%) |
| High risk features | |
| 11q deletion | 10/46 (22%) |
| 17p deletion | 10/46 (22%) |
| TP53 mutation | 10/33 (30%) |
| NOTCH1 mutation | 8/26 (31%) |
| SF3B1 mutation | 5/26 (19%) |
| IGHV unmutated | 28/38 (74%) |
| Evaluable for safety, n | 46 |
| Evaluable for efficacy, n | 45∗ |
| Median age, y (range) | 64 (43-83) |
| Male/female, n | 33/13 |
| ECOG, 0/1/2, n | 6/38/2 |
| Prior therapy regimens, median (range) | 2 (1-6) |
| Refractory to immediate prior therapy, n (%) | 18 (39%) |
| Prior anti-CD20, n (%) | 39 (85%) |
| Prior chemoimmunotherapy, n (%) | 35 (76%) |
| Prior targeted therapy, n (%) | 30 (65%) |
| Prior BTKi (ibrutinib/acalabrutinib), n (%) | 26 (57%) |
| Refractory to prior BTK inhibitor, % (n/N) | 69% (18/26) |
| BTK or PLCγ mutation detected, % (n/N) | 71% (10/14) |
| Prior PI3K inhibitor, n (%) | 3 (7%) |
| Prior venetoclax, n (%) | 1 (2%) |
| High risk features | |
| 11q deletion | 10/46 (22%) |
| 17p deletion | 10/46 (22%) |
| TP53 mutation | 10/33 (30%) |
| NOTCH1 mutation | 8/26 (31%) |
| SF3B1 mutation | 5/26 (19%) |
| IGHV unmutated | 28/38 (74%) |
ECOG, Eastern Cooperative Oncology Group performance status; IGHV, immunoglobulin heavy-chain variable region gene.
One patient was removed from study before response assessment due to noncompliance.
Five patients (all male) were enrolled in the RT cohort. The median age was 64 (range, 47-76) years. The median number of prior therapies for RT was 1 (range, 1-2), with 3 having prior chemoimmunotherapy and 3 having prior BTK-inhibitor treatment.
Safety
During the phase 1 portion of the study, there were 3 patients treated at the 600 mg dose-level and 6 patients treated at the 800 mg dose-level. All 9 patients (100%) experienced at least 1 AE of any grade. All patients (100%) treated at the 600 mg dose-level experienced at least 1 grade 3 or greater toxicity, whereas 5 out of 6 (83%) patients treated at the 800 dose-level experienced a grade 3 or greater toxicity. At 600 mg dose-level 2 (67%) patients experienced serious AEs, whereas 2 (33%) patients treated at the 800 mg dose-level experienced serious AEs. Because the incidence and severity of AEs was similar between the 2 dose levels explored, the maximum tolerated dose was not reached, and the recommended phase 2 dose was established at 800 mg of umbralisib.
With a median follow-up of 35 months, 14 of 46 patients with CLL (30%) discontinued treatment because of reasons other than protocol-stipulated achievement of undetectable MRD. The reasons for discontinuation included treatment-related AEs (n = 6), study closure (n = 3), clinical progression (n = 2), noncompliance (n = 1), patient choice before formal response assessment (n = 1), and death (n = 1, owing to COVID-19). Treatment-related AEs leading to discontinuation were diarrhea (n = 4), rash (n = 1), COVID-19−associated pneumonia (n = 1). Of the 6 patients who discontinued because of AEs, 4 of the discontinuations occurred after cycle 12. In addition to the death that occurred on therapy (COVID-19) there were 2 additional deaths that occurred off-therapy: 1 owing to COVID-19 and 1 owing to progressive disease. One patient was removed for noncompliance, and 1 patient was removed because of patient choice before formal response assessment. Three patients who completed 12 cycles of combination therapy continued from ongoing study treatment on umbralisib monotherapy until cycles 39, 43, and 53 at which treatment was terminated because of study closure.
The common AEs are shown in Table 2. The most common AEs of any grade (>20%) include infusion reaction, cytopenias, diarrhea, and nausea. Grade 3/4 AEs were primarily hematologic with neutropenia occurring most frequently in 35% of patients with CLL. Twenty-two percent of patients received hematopoietic growth factor, which was allowed to be given at investigator discretion. Ublituximab-associated infusion reaction occurred in 83% of patients (all grade) with 9% grade 3/4, and mostly on cycle 1 day 1. AEs of special interest included diarrhea which occurred in 50% of patients (11% grade 3/4), and aspartate aminotransferase and/or alanine aminotransferase elevation in 15 patients (33%), with 3 (7%) grade 3/4. There were 3 cases of any grade colitis (7%). The median time (in months) to first onset for the following AE’s: any grade diarrhea was 3.0 (range, 0-16.6); colitis was 8.0 (range 3.5-9.8), and transaminitis was 0.1 (0-10.1). Seven percent of patients developed grade 3/4 pneumonia; there was 1 case of grade 4 myocarditis that resolved to grade zero after discontinuing umbralisib, and there were no cases of pneumonitis. There was 1 case of laboratory TLS attributed to the first dose of ublituximab, before venetoclax exposure. The pooled rate of all grade infectious complications among all 51 patients with CLL and RT treated was 49% (14% grade 3) with a single grade 5 event owing to COVID-19 pneumonia. Notably, there was a second patient with CLL who died of COVID-19 ∼4 months after completing study treatment.
AEs in greater than or equal to 20% of CLL and RT cohorts, listed in order of frequency
| AE (CLL cohort, N = 46) . | All grades . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|---|
| N (%) . | N (%) . | N (%) . | N (%) . | N (%) . | |
| Infusion reaction | 38 (83) | 3 (6.5) | 31 (67) | 4 (8.7) | — |
| Neutropenia | 27 (59) | 4 (8.7) | 7 (15) | 10 (22) | 6 (13) |
| Leukopenia | 26 (57) | 7 (15) | 10 (22) | 8 (17) | 1 (2.2) |
| Diarrhea | 23 (50) | 13 (28) | 5 (11) | 5 (11) | — |
| Infection∗ | 23 (50)∗ | 2 (4.3) | 14 (30) | 6 (13) | — |
| Nausea | 21 (46) | 16 (35) | 5 (11) | — | — |
| Lymphopenia | 20 (43) | 4 (8.7) | 9 (20) | 6 (13) | 1 (2.2) |
| Thrombocytopenia | 20 (43) | 15 (33) | 4 (9) | — | 1 (2.2) |
| Anemia | 18 (39) | 13 (28) | 2 (4) | 3 (7) | — |
| Creatinine increase | 18 (39) | 17 (37) | 1 (2) | — | — |
| Fatigue | 13 (28) | 10 (22) | 3 (6) | ||
| AST increase | 13 (28) | 9 (20) | 2 (4) | 2 (4) | — |
| ALT increase | 11 (24) | 5 (11) | 4 (9) | 2 (4) | — |
| Alkaline phosphate increase | 10 (22) | 9 (20) | 1 (2) | — | |
| Hypocalcemia | 10 (22) | 7 (15) | 3 (7) | ||
| Headache | 10 (22) | 7 (15) | 3 (7) | ||
| Chills | 9 (20) | 9 (20) | — | ||
| Hyperkalemia | 9 (20) | 8 (17) | 1 (2) | ||
| Richter cohort (N = 5) | |||||
| Diarrhea | 3 (60%) | 1 (20%) | |||
| Fatigue | 3 (60%) | 2 (40%) | |||
| Nausea | 3 (60%) | 2 (40%) | |||
| Infusion reaction | 2 (40%) | 1 (20%) | 1 (20%) | ||
| Infection∗ | 2 (40%) | 1 (20%) | 1 (20%) | ||
| Headache | 2 (40%) | 1 (20%) | |||
| Abdominal pain | 2 (40%) | 1 (20%) | |||
| Decreased appetite | 2 (40%) | 1 (20%) | |||
| Neutropenia | 1 (20%) | ||||
| Hypotension | 1 (20%) | ||||
| Rash maculopapular | 1 (20%) | ||||
| AE (CLL cohort, N = 46) . | All grades . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|---|
| N (%) . | N (%) . | N (%) . | N (%) . | N (%) . | |
| Infusion reaction | 38 (83) | 3 (6.5) | 31 (67) | 4 (8.7) | — |
| Neutropenia | 27 (59) | 4 (8.7) | 7 (15) | 10 (22) | 6 (13) |
| Leukopenia | 26 (57) | 7 (15) | 10 (22) | 8 (17) | 1 (2.2) |
| Diarrhea | 23 (50) | 13 (28) | 5 (11) | 5 (11) | — |
| Infection∗ | 23 (50)∗ | 2 (4.3) | 14 (30) | 6 (13) | — |
| Nausea | 21 (46) | 16 (35) | 5 (11) | — | — |
| Lymphopenia | 20 (43) | 4 (8.7) | 9 (20) | 6 (13) | 1 (2.2) |
| Thrombocytopenia | 20 (43) | 15 (33) | 4 (9) | — | 1 (2.2) |
| Anemia | 18 (39) | 13 (28) | 2 (4) | 3 (7) | — |
| Creatinine increase | 18 (39) | 17 (37) | 1 (2) | — | — |
| Fatigue | 13 (28) | 10 (22) | 3 (6) | ||
| AST increase | 13 (28) | 9 (20) | 2 (4) | 2 (4) | — |
| ALT increase | 11 (24) | 5 (11) | 4 (9) | 2 (4) | — |
| Alkaline phosphate increase | 10 (22) | 9 (20) | 1 (2) | — | |
| Hypocalcemia | 10 (22) | 7 (15) | 3 (7) | ||
| Headache | 10 (22) | 7 (15) | 3 (7) | ||
| Chills | 9 (20) | 9 (20) | — | ||
| Hyperkalemia | 9 (20) | 8 (17) | 1 (2) | ||
| Richter cohort (N = 5) | |||||
| Diarrhea | 3 (60%) | 1 (20%) | |||
| Fatigue | 3 (60%) | 2 (40%) | |||
| Nausea | 3 (60%) | 2 (40%) | |||
| Infusion reaction | 2 (40%) | 1 (20%) | 1 (20%) | ||
| Infection∗ | 2 (40%) | 1 (20%) | 1 (20%) | ||
| Headache | 2 (40%) | 1 (20%) | |||
| Abdominal pain | 2 (40%) | 1 (20%) | |||
| Decreased appetite | 2 (40%) | 1 (20%) | |||
| Neutropenia | 1 (20%) | ||||
| Hypotension | 1 (20%) | ||||
| Rash maculopapular | 1 (20%) | ||||
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
There was 1 case of grade 5 infection owing to COVID in CLL cohort and 1 case of grade 3/4 COVID-1 in the Richter cohort.
Thirty-five patients with CLL and 2 RT patients received steroids specifically for the management of AEs. The most common reasons were infusion related reactions (29 patients), rash (3 patients), diarrhea (2 patients), COVID-19 (2 patients), and myocarditis (1 patient).
TLS risk assessment
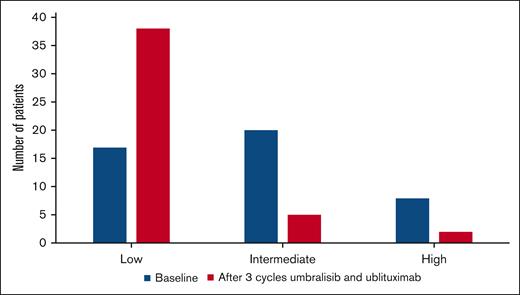
At study entry, patients’ risk categories for TLS were categorized as low (n = 17), intermediate (n = 20), and high risk (n = 8) based on largest lymph node size and absolute lymphocyte counts. As shown in Figure 2, after 3 cycles of treatment with umbralisib and ublituximab, there were 38, 5, and 2 patients with low, intermediate, or high risk for TLS respectively, with 26 (58%) patients being able to down-grade the TLS risk category. All but 2 patients received venetoclax ramp-up treatment as an outpatient, and there were no cases of TLS during venetoclax ramp-up.
TLS risk category at baseline and after 3 cycles of treatment with umbralisib and ublituximab.
TLS risk category at baseline and after 3 cycles of treatment with umbralisib and ublituximab.
Efficacy
In the CLL cohort, the median duration of treatment was 12 cycles (range 2-53), and 7 (15%) patients continued umbralisib after cycle 12. One patient was noncompliant and taken off study after 3 months for progressive disease and was not evaluable for response. Of the remaining 45 evaluable patients, the best ORR was 100%, with CR of 42%. Treatment responses improved over time during treatment. After cycle 3, there were 2% unconfirmed CRs (because of absence of confirmatory bone marrow biopsy), 76% partial responses (PR), and 22% patients with stable disease; after cycle 7, the unconfirmed CRs rate was 6 out of 41 (15%), and the PR rate was 35 of 41 (85%) in evaluable cases. At 12 months, 39 out of 40 evaluable cases responded (ORR 98%) with 15 (38%) and 24 (60%) patients achieving CR/Cri and PR, respectively, and there was 1 case of progressive disease (3%). These results met all prespecified end points for efficacy. This patient, who had progressive disease at cycle 12, initially had stable disease after cycle 3 and PR after cycle 7, which later progressed. This patient had prior therapies including bendamustine-rituximab, ibrutinib, and idelalisib plus rituximab and was refractory to the latter 2 treatments.
As shown in Table 3, there were 40 patients who had either PB or bone marrow uMRD. Twenty-eight out of 42 tested in the PB had uMRD at 10−4 (67%), which represents 61% of the intent-to treat population. Thirty out of 39 (77%) patients tested in the bone marrow had uMRD (65% intent-to treat population). Out of 28 patients with uMRD in the PB, 18 also had uMRD in the bone marrow (64%). Out of 30 patients with uMRD in the bone marrow, 18 also had uMRD by PB (60%). Thirty-two patients discontinued owing to achievement of uMRD. The median time off therapy was 16.6 months. Six patients converted to detectable MRD during treatment-free follow-up. There were 5 events of clinical progression. At 2 years of treatment-free follow-up, 75% maintained uMRD status.
Response and minimal residual disease status during the 12-cycle treatment and follow-up
| Clinical response . | Cycle 3 N = 45 . | Cycle 7 N = 41 . | Cycle 12 N = 40∗ . | Best response N = 45 . |
|---|---|---|---|---|
| Overall response, % | 78 | 100 | 98† | 100 |
| CR(u)/CRi‡, % | 2 | 15‡ | 38 | 42 |
| PR, % | 76 | 85 | 60 | 58 |
| Clinical response . | Cycle 3 N = 45 . | Cycle 7 N = 41 . | Cycle 12 N = 40∗ . | Best response N = 45 . |
|---|---|---|---|---|
| Overall response, % | 78 | 100 | 98† | 100 |
| CR(u)/CRi‡, % | 2 | 15‡ | 38 | 42 |
| PR, % | 76 | 85 | 60 | 58 |
| MRD response . | Cycle 12∗ . | Cycle 18 . | Cycle 24 . | |
|---|---|---|---|---|
| Peripheral Blood N = 42 . | Bone marrow N = 39 . | Peripheral Blood . | ||
| N = 29 . | N = 25 . | |||
| Detectable >10−2, % | 2 | 2 | ||
| Detectable 10−4 to 10−2, % | 31 | 21 | 10 | 4 |
| Undetectable <10−4, % | 67 | 77 | 90 | 96 |
| MRD response . | Cycle 12∗ . | Cycle 18 . | Cycle 24 . | |
|---|---|---|---|---|
| Peripheral Blood N = 42 . | Bone marrow N = 39 . | Peripheral Blood . | ||
| N = 29 . | N = 25 . | |||
| Detectable >10−2, % | 2 | 2 | ||
| Detectable 10−4 to 10−2, % | 31 | 21 | 10 | 4 |
| Undetectable <10−4, % | 67 | 77 | 90 | 96 |
CR, complete repsonse; CRu, unconfirmed CR because of absence of confirmatory bone marrow biopsy; CRi, CR with incomplete hematologic recovery; PR, partial response.
Forty patients had clinical response available at cycle 12 and 42 had MRD assessments at cycle 12.
One patient had PR at cycle 7 and progressive disease at cycle 12.
Cycle 3 and cycle 7 CRs are CRu.
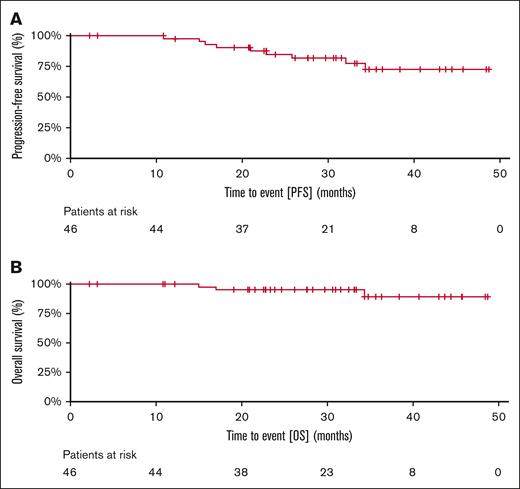
Of the 14 patients who were refractory to prior BTK inhibitor and evaluable for MRD, 9 (64%) and 10 (71%) had uMRD (10−4) in the PB and bone marrow, respectively, at the conclusion of 12 cycles of therapy. Among patients who had longer follow-up, the rate of PB uMRD rate at 18 and 24 months was 90% (n = 29) and 96% (n = 25), respectively. At a median follow-up of 35 (range 20-51) months, the median PFS, and OS have not been reached. Of the 5 patients who progressed during the entire duration of study follow-up, 2 had prior BTKi. The Kaplan-Meier estimates of PFS and OS at 36 months were 74% and 89%, respectively (Figure 3).
Kaplan-Meir estimates of treatment outcomes of U2-Ven treated patients. (A) PFS and (B) OS of 46 patients with CLL treated with venetoclax, umbralisib, and ublituximab.
Kaplan-Meir estimates of treatment outcomes of U2-Ven treated patients. (A) PFS and (B) OS of 46 patients with CLL treated with venetoclax, umbralisib, and ublituximab.
Among the 5 patients in the RT cohort, 3 of whom had prior treatment with ibrutinib for CLL before transformation, 2 patients had CR and 3 had progressive disease. The duration of treatment was ongoing at 11 and 16 months for the 2 responding patients.
Discussion
In this phase 1/2 trial of U2-Ven, we tested a lower dose of umbralisib, limited the cycles incorporating ublituximab, and focused on time-limited therapy with the 3 agents in relapsed/refractory CLL. The U2-Ven combination was tolerable and met all prespecified end points for efficacy. The phase 1 results demonstrated relatively similar tolerance over the 2 umbralisib dose levels of 600 and 800 mg daily, albeit in a limited number of patients. There was no discernable difference in the safety profile for patients who received 3 vs 6 cycles of ublituximab. Furthermore, most patients with CLL (70%) stopped therapy after 12 cycles owing to uMRD, per protocol prespecified stopping criteria. With these measures, grade 3/4 AEs were primarily hematologic with neutropenia (35%) being the most common, and the rate of grade ≥3 infection similar to that observed with other targeted treatments for r/r CLL. Regarding potential immune-mediated AEs, 50% of patient had diarrhea. Because of the potential impact on quality of life, this relatively high incidence further underscores the interest in time-limited therapy with PI3K inhibitors. There were 5 cases of grade 3/4 diarrhea, and colitis occurred in 3 cases. Transaminitis (elevation of aspartate aminotransferase and/or alanine aminotransferase) occurred in 33% of cases, but only 7% were grade 3/4 events. There was no case of pneumonitis of any grade. This resulted in 93% of patients (43/46) completing the intended 12 cycles of protocol directed therapy. At last follow-up, only 1 patient had died of progressive disease, whereas the other 2 deaths were because of COVID-19 infection.
Venetoclax-based therapy is now an established therapeutic option for patients with CLL and is commonly used after discontinuation of BTK-inhibitor treatment.29 The achievement of remission depth below 10−4 has allowed for the development of time-limited therapy without the need for cytotoxic agents. In the Murano trial, which enrolled a cohort of patients naïve to BTK inhibitors, venetoclax and rituximab were administered over 2 years and resulted in uMRD in 83% of patients and a 2-year PFS rate of 85%.11 The similar rates of MRD clearance and identical 2-year PFS with U2-Ven suggest that this treatment can achieve comparable results in ∼1 year of therapy. Moreover, among patients with CLL who were refractory to prior BTK-inhibitor treatment, treatment with 12 cycles of U2-Ven achieved uMRD rates of 64% and 71% in the PB and bone marrow, respectively. It is worth noting that there was some discrepancy between bone marrow and PB uMRD rates, which may have been because of a small number of patients who had intermediate MRD in the PB (0.01%-1%) which was undetectable (<0.01%) in the bone marrow, possibly owing to impaired sensitivity of bone marrow aspirate sample size collection. Overall, the fixed duration of U2-Ven combination is efficacious in achieving deep response in relapsed CLL, even after prior failure of BTK inhibitor. The responses were durable with 36-month estimated PFS and OS of 74% and 89%, respectively.
In April 2022, the Oncologic Drugs Advisory Committee of the US Food and Drug Administration reviewed the data supporting the approvals of 4 PI3K inhibitors for indolent non-Hodgkin lymphoma and CLL.30 Despite an OS advantage observed in the phase 3 trial by addition of idelalisib to chemoimmunotherapy with bendamustine with rituximab for r/r CLL, outcomes of the randomized UNITY trial showed a trend toward worse OS with U2 when compared with the control arm or chlorambucil with obinutuzumab, suggesting that prolonged administration was unsafe in the COVID-19 era.31,32 Immune-mediated side effects have been well described with PI3K inhibitors.33,34 The concern for excessive infectious complications was recognized in 2016 when 6 randomized studies with idelalisib were halted after a decrease in OS was identified. Despite this knowledge, PI3K inhibitor development largely continued with limited dose exploration and administration until disease progression.
With the long-term safety concerns of PI3K inhibitors, it is apparent that continuous and indefinite administration until disease progression or intolerance is not a viable use of these agents. Nonetheless, they still play a role in the management of R/R patients with CLL. For patients with refractory disease after prior BTK-inhibitor treatment, PI3K inhibition may provide added disease control to treatment with venetoclax, allowing for subsequent cellular therapy administration in select cases. Indeed, approximately all patients in the CLL cohort responded to the U2-Ven treatment, with response deepening over time during treatment. The addition of venetoclax for 9 cycles in this study resulted in a uMRD response in most patients, allowing for treatment cessation and the avoidance of late complications. Although the development of umbralisib has stopped, these results remain of interest as similar combinations continue to be explored (NCT03534323).
Tonic signaling through the PI3K-AKT-mTOR axis is a characteristic feature in RT and may play a role in the disease pathogenesis through activation of NOTCH.35 The combination of PI3K inhibition with duvelisib and venetoclax produced a synergistic effect in inducing apoptosis and prolonged survival in RT patient-derived xenograft models.36 This combination is being explored in a phase 1/2 clinical trial.37 Two of 5 patients with RT achieved a CR with U2-Ven showing the value of the combination for these difficult to treat patients and supporting other PI3Ki and venetoclax studies.38
In summary, time-limited U2-Ven is safe and highly effective combination therapy for patients with R/R CLL including those who have been previously treated with covalent BTK inhibitors. Although the indications for PI3K inhibitor use have been narrowed, these results support the ongoing development of similar combinations to safely maximize the significant therapeutic potential of this class of targeted therapy.
Authorship
Contribution: P.M.B. and H.P.M. designed the trial; B.T.H., S.M., C.S.Z., A.M.B., D.S.W., A.A., A.W., J.W., L.G., R.K., J.L.L., D.A.M., J.W.F., and P.M.B. collected and interpreted the data; C.R., A.B., P.S., and H.P.M. analyzed and interpreted the data; B.T.H. wrote the first draft and all subsequent versions of the manuscript; and all authors approved the manuscript.
Conflict-of-interest disclosure: B.T.H., S.M., C.S.Z., A.M.B., D.S.W., A.A., A.W., J.W., L.G., R.K., J.L.L., D.A.M., J.W.F., and P.M.B. received research funding from TG Therapeutics. B.T.H., S.M., and P.M.B. received consulting fees from AbbVie. C.R., A.B., P.S., H.P.M., and M.S.W. are employees of and have equity ownership in TG Therapeutics.
Correspondence: Brian T. Hill, Taussig Cancer Institute, Cleveland Clinic, 9500 Euclid Ave CA6, Cleveland, OH 44195; email: hillb2@ccf.org.
References
Author notes
∗B.T.H. and S.M. contributed equally to this study.
Data are available upon reasonable request from to the corresponding author, Brian T. Hill (hillb2@ccf.org).