NICE CD19 CAR T cells have increased markers of stemness and decreased markers of exhaustion.
NICE CD19 CAR T cells have improved proliferative potential and mediate a superior therapeutic response.
Visual Abstract
CD19-specific chimeric antigen receptor (CAR) T cells have demonstrated impressive responses in patients with relapsed and refractory B cell malignancies. However, many patients relapse or fail to respond to CD19 CAR T cells, demonstrating the need to improve its efficacy and durability. Current protocols for generating CAR T cells involve T cell activation through CD3 stimulation to facilitate efficient CAR transfer followed by ex vivo expansion with exogenous cytokines to obtain adequate cell numbers for treatment. Both T cell activation and expansion inevitably lead to terminal differentiation and replicative senescence, which are suboptimal for therapy. Interleukin-7 (IL-7) was previously shown to allow for lentiviral transduction of T cells in the absence of activation. In these studies, we used IL-7 to generate CD19 CAR T cells without stimulating CD3. Nonactivated and IL-7 cultured (NICE) CD19 CAR T cells were enriched with the T memory stem cell population, retained novel markers of stemness, had lower expression of exhaustion markers, and increased proliferative potential. Furthermore, our findings are consistent with engraftment of NICE CD19 CAR T cells and demonstrate a superior therapeutic response in both intraperitoneal and subcutaneous in vivo B cell lymphoma models. These results suggest that NICE CD19 CAR T cells may improve outcomes for B cell malignancies and warrant clinical evaluation.
Introduction
Adoptive cellular therapy with CD19-specific chimeric antigen receptor (CAR) T cells has revolutionized the treatment of B cell malignancies. Clinical trials using CD19 CAR T cells for relapsed or refractory B cell acute lymphoblastic leukemia demonstrated complete response (CR) rates of 70% to 94%.1-4 Similarly, CD19 CAR T cells have demonstrated responses in B cell lymphomas with CR rates of 40% to 58%.5-9 Despite these successes, 30% to 60% of patients with B cell acute lymphoblastic leukemia in remission relapse within 1 year.3,4,10,11 Moreover, a significant number of patients with B cell lymphomas or chronic lymphocytic leukemia fail to enter remission.5,6,12 Thus, further studies are needed to improve the efficacy and durability of CD19 CAR T cells.
There is growing evidence that durable and CRs are associated with the expansion and engraftment of CD19 CAR T cells.12-15 Recently, there has been focus on T memory stem cells (TSCM) because they are the least differentiated memory subset and have demonstrated the highest potential for in vivo expansion and self-renewal.16,17 Indeed, clinically observed expansion and persistence of detectable CD19 CAR T cells have been associated with the TSCM population.18 However, current manufacturing protocols for CAR T cells involve T cell activation to allow for efficient gene transfer through viral or electroporation methods, resulting in differentiation, replicative senescence, and a suboptimal infusion product.19,20
Recently, Ghassemi et al demonstrated the feasibility of generating CD19 CAR T cells without CD3 stimulation using interleukin-7 (IL-7) and IL-15. These cells had improved in vivo responses compared with activated cells in mouse xenograph models.21 Although IL-7 is a homeostatic regulator that promotes T cell survival, IL-15 is known to activate T cells, inducing proliferation and differentiation.22,23 We previously demonstrated that culturing with IL-7 allowed for efficient transduction of T cells with a lentiviral vector containing a melanoma reactive T cell receptor.24 In these studies, we use IL-7 alone to generate CD19 CAR T cells in the complete absence of activation and preserve maximal immunocompetence. Furthermore, to the best of our knowledge, we evaluate these nonactivated, IL-7 cultured (NICE) CD19 CAR T cells for differentiation phenotypes, markers of stemness, exhaustion, and proliferative potential for the very first time while showing superior in vivo responses compared with activated cells.
Materials and methods
Cells lines
T2 and HEK 293T cell lines were described previously and obtained from ATCC (Rockford, MD).24 Raji/firefly luciferase (Raji), a cell line derived from human Burkitt lymphoma modified to express firefly luciferase, was obtained from Cellomics Technology (Halethorpe, MD). GPRTG was described previously and obtained from the National Gene Vector Biorepository (Indiana University, Indianapolis, IN).24,25 T2 and Raji cells were maintained in RPMI (Thermo Fisher, Walther, MA) with 10% heat-inactivated fetal bovine serum (Tissue Culture Biologics, Long Beach, CA). All other cell lines were maintained in Dulbecco modified Eagle medium (DMEM) (Thermo Fisher) with 10% heat-inactivated fetal bovine serum. A total of 1 ng/mL doxycycline (Clontech Laboratories, Palo Alto, CA) was added for GPRTG cells. Cells were maintained at 37°C in a humidified 5% CO2 incubator.
T cells
Peripheral blood lymphocytes (PBLs) were isolated from apheresis products (Key Biologics, Memphis, TN) using Ficoll-Hypaque (Sigma-Aldrich, St Louis, MO), as previously described.24 T cells were activated by stimulating PBLs with 50 ng/mL anti-CD3 monoclonal antibody (Miltenyi Biotec, Bergisch Gladbach, Germany), 300 IU/mL recombinant human IL-2 (rhIL-2; Novartis Pharmaceuticals, East Hanover, NJ), and 100 ng/mL rhIL-15 (Biological Resources Branch, National Cancer Institute, Bethesda, MD) for 3 days before transduction. Alternatively, PBLs were cultured with 20 ng/mL rhIL-7 (Biological Resources Branch, National Cancer Institute) for 7 days before transduction.24
Lentivirus production
The pLVX-E1a-N1 (Clontech, Mountain View, CA) vector was modified to include the CD19-specific single-chain variable fragment, CD8 hinge domain, CD28, T cell receptor–ζ chain signaling domain, T2A linker, and a truncated CD34 molecule (CD34t) as a transgene expression marker as previously described (CD19CAR).26 This modified vector was used to generate a stable GPRTG lentiviral producer cell line as previously described.24 Briefly, viral supernatants were produced by transfecting 293T cells with the pLVX-CD19CAR plasmid using Lenti-X single shots (Takara, Mountain View, CA), spinoculated with viral supernatants containing 8 μg/mL of polybrene (Sigma-Alrich) and resuspended in DMEM with doxycycline after 72 hours. CD19CAR lentiviral supernatants for T cell transductions were produced as previously described.24 Briefly, transduced GPRTG cells were seeded in doxycycline-free DMEM and replaced with Freestyle 293 expression media (Gibco, Gaithersburg, MD) 24 hours later. CD19CAR lentiviral supernatant was then collected after 48 hours.
Lentiviral transduction of T cells
PBLs cultured with IL-7 or activated with anti-CD3, IL-2, and IL-15 were resuspended in CD19CAR lentiviral supernatant containing 8 μg/mL of polybrene, plated in 24-well plates, and spinoculated at 2000g for 2 hours at 32°C. CD19CAR-transduced cells were harvested and resuspended in RPMI supplemented with either IL-7 or IL-2 and IL-15.
T cell phenotypic analysis
Evaluation of T cell surface markers was performed via immunofluorescence staining and analyzed via flow cytometry, as previously described.24 Additional monoclonal antibodies used to characterize T cells include: anti–TIM-3-BV650, anti–CTLA-4-PE Cy7, and anti–LAG-3-FITC (BioLegend, San Diego, CA) as well as anti–PD-1-BV711, anti–CD69-AF700, and anti–CD39-PE (BD Biosciences). Flow cytometry was performed using the LSR Fortessa flow cytometer (BD Biosciences). Data were analyzed with FlowJo software.
Proliferation assay
Cell proliferation was determined by carboxyfluorescein succinimidyl ester (CFSE) dilution. A total of 1 × 106 CD19-specific CAR T cells were labeled with CFSE (ThermoFisher) per manufacturer’s protocol. Labeled cells were then stimulated for 5 days with 25 μL anti-CD3/CD28–coated beads (Thermo Fisher) at a 1:1 beads-to-T cell ratio in RPMI media supplemented with either IL-7 or IL-2 and IL-15. Fluorescence was measured using the Canto flow cytometer. Percentage of cells divided and proliferation index were determined with FlowJo software.
Telomere analysis
Flow–fluorescence in situ hybridization method using the telomere peptide nucleic acid Kit/FITC for Flow Cytometry Kit (Dako, Glostrup, Denmark) was used to analyze telomere length per manufacturer’s protocol. Raji cells were used as the control. Fluorescence was measured using the Canto flow cytometer. Data were analyzed with FlowJo software.
Cytokine release assay
Raji cells were used as target cells, whereas T2 cells were used as negative control targets. A total of 1 × 105 CD19-specific CAR T cells were cocultured with targets at a 1:1 ratio in a 96-well U-bottom tissue culture plate in 200 μL of media. After overnight incubation, supernatants were harvested and evaluated for interferon gamma and tumor necrosis factor α by enzyme-linked immunosorbent assay (BioLegend).
Mouse xenograft studies
NOD/SCID/γ−/− (NSG) male and female mice (Jackson Laboratory, Bar Harbor, ME) aged 8 to 12 weeks were treated under an approved Institutional Animal Care and Use Committee protocol and Animal Research Reporting of In vivo Experiments (ARRIVE) guidelines. To evaluate persistence, 1 × 106 CD19-specific CAR T cells were injected IV in 100 μL of phosphate-buffered saline. Samples from submandibular blood collections were analyzed using flow cytometry. To evaluate antitumor activity in an intraperitoneal model, 1 × 105 Raji cells were injected into the lower abdomen of mice. At 7 days after Raji injection, 1 × 106 CD19-specific CAR T cells were administered IV as above. Control mice received Raji injections but no treatment. Tumors were monitored by bioluminescence imaging using the IVIS Spectrum and Living Image Software (PerkinElmer, Waltham, MA). Kaplan-Meier survival analysis and log-rank test to compare the groups were performed using Prism (GraphPad Software, San Diego, CA). Alternatively, 5 × 106 Raji cells were injected subcutaneously into flanks, forming palpable tumors by day 7. At 7 days after Raji injection, 1 × 106 CD19-specific CAR T cells were administered IV in mice with palpable tumors. Control mice received Raji injections but no treatment. Tumor size was measured in 3 perpendicular dimensions twice a week using calipers.
Results
Generating NICE CD19-specific CAR T cells
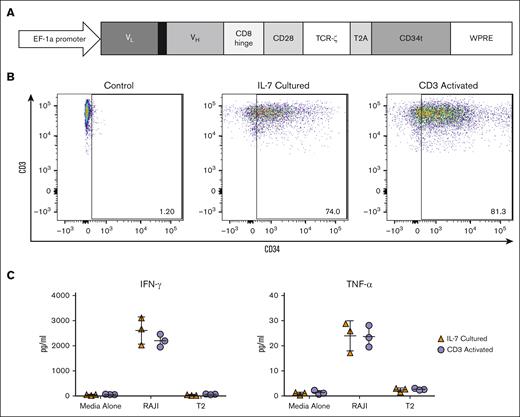
As we previously demonstrated that IL-7 culture allows for efficient lentiviral transduction of T cells without activation and yields a product with improved therapeutic potential, we aimed to engineer NICE CD19 CAR T cells.24 A lentiviral vector was constructed to include a CD19-specific CAR and the CD34t expression marker (Figure 1A). We used this vector to transduce T cells from 10 separate donors either after no treatment, IL-7 culture, or a standard CD3 activation protocol. Viability of all cells was consistently >90% because cells were either transduced immediately after thawing, cultured in the presence of the survival-promoting IL-7, or stimulated to proliferate (data not shown). Flow cytometry demonstrated significant CD34 expression in both CD3+ cells transduced after IL-7 culture (range, 59.9%-74.0%) and CD3 activation (range, 37.1%-81.3%). Untreated CD3+ cells demonstrated minimal CD34 expression as expected (Figure 1B). We next evaluated the CD19-reactivity of the NICE CD19 CAR T cells by quantifying cytokine release after stimulation with the CD19+ cell line, Raji. CD3+CD34+ cells that were generated after either IL-7 culture or CD3 activation were incubated with Raji cells or a control cell line. Enzyme-linked immunosorbent assays performed on supernatants from 3 separate donors demonstrated significant presence above background of interferon gamma and tumor necrosis factor α from both CD3+CD34+ cells transduced after IL-7 culture and CD3 activation (Figure 1C). Although flow cytometry may not be a true quantitative measure of transduction, these results suggest that IL-7 culture allows for efficient generation of functional, NICE CD19 CAR T cells.
Transduction of PBLs with a lentiviral vector containing a CD19 CAR. (A) A diagram of the CD19 CAR, consisting of the variable region light chain (VL) and heavy chain (VH) of the single chain variable fragment (scFv), CD8 hinge domain, CD28 costimulatory molecule, and TCR-ζ signaling domain attached to a truncated CD34 molecule (CD34t) with a T2A self-cleaving peptide inserted into the pLVX-E1a-N1 lentiviral vector. (B) Flow cytometry analysis of CD3+ cells transduced with the CD19 CAR-containing lentiviral vector either after no treatment, IL-7 culture, or CD3 activation. These results are representative of 10 experiments using cells from 10 different donors. (C) CD19 CAR T cells transduced either after IL-7 treatment or CD3 activation were cocultured with CD19+ Raji cells in a 1:1 ratio. CD19 CAR T cells cultured with T2 or media alone were used as negative controls. IFN-γ and TNF-α release in supernatants were measured by ELISA. Graphs depict 3 experiments using 3 different donors with each data point representing the triplicate mean and error bars indicating standard deviation. ELISA, enzyme-linked immunosorbent assay; IFN-γ, interferon gamma; TCR, T cell receptor; TNF-α, tumor necrosis factor α; WPRE, woodchuck hepatitis virus posttranscriptional response element.
Transduction of PBLs with a lentiviral vector containing a CD19 CAR. (A) A diagram of the CD19 CAR, consisting of the variable region light chain (VL) and heavy chain (VH) of the single chain variable fragment (scFv), CD8 hinge domain, CD28 costimulatory molecule, and TCR-ζ signaling domain attached to a truncated CD34 molecule (CD34t) with a T2A self-cleaving peptide inserted into the pLVX-E1a-N1 lentiviral vector. (B) Flow cytometry analysis of CD3+ cells transduced with the CD19 CAR-containing lentiviral vector either after no treatment, IL-7 culture, or CD3 activation. These results are representative of 10 experiments using cells from 10 different donors. (C) CD19 CAR T cells transduced either after IL-7 treatment or CD3 activation were cocultured with CD19+ Raji cells in a 1:1 ratio. CD19 CAR T cells cultured with T2 or media alone were used as negative controls. IFN-γ and TNF-α release in supernatants were measured by ELISA. Graphs depict 3 experiments using 3 different donors with each data point representing the triplicate mean and error bars indicating standard deviation. ELISA, enzyme-linked immunosorbent assay; IFN-γ, interferon gamma; TCR, T cell receptor; TNF-α, tumor necrosis factor α; WPRE, woodchuck hepatitis virus posttranscriptional response element.
Characterizing the phenotype of NICE CD19-specific CAR T cells
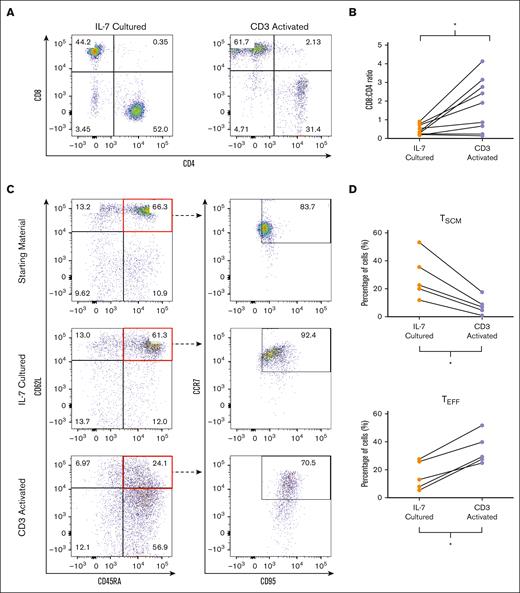
We next evaluated the phenotype of NICE CD19 CAR T cells. Although studies suggest that CD4+ cells are important for both cytotoxicity and maintaining persistence, current products have inconsistent CD8+:CD4+ ratios, often with a predominantly CD8+ population.12,27-30 We therefore assessed the percentage of the CD8+ and CD4+ populations in the CD34+ cells transduced either after IL-7 culture or CD3 activation. Flow cytometry analysis on 10 donors demonstrated a relatively balanced CD8+:CD4+ ratio in CD3+CD34+ cells transduced after IL-7 culture (Figure 2A). In contrast, there was an increase in the CD8+ population in cells transduced after CD3 activation in most donors, resulting in a significantly higher CD8+:CD4+ ratio (means, 0.46 vs 2.94; P = .0355; Figure 2B).
Phenotypic characterization of CD19 CAR T cells. (A) Flow cytometry analysis depicting CD4+ and CD8+ populations of CD3+CD34+ cells transduced after IL-7 culture or CD3 activation. (B) CD8:CD4 ratio of CD3+CD34+ cells transduced after IL-7 culture or CD3 activation. The graph depicts each data point from 10 donors with lines connecting data points from an individual donor. Statistics were calculated by 2-tailed Wilcoxon rank sum test. ∗P = .0355. (C) Flow cytometry analysis depicting the TSCM (CD62L+CD45RA+CCR7+CD95+), TCM (CD62L+CD45RA−), TEM (CD62L−CD45RA−), and TEFF (CD62L−CD45RA+) populations of starting material and CD3+CD34+ cells transduced after IL-7 culture or CD3 activation. (D) Percentage of TSCM and TEFF populations in CD3+CD34+ cells transduced after IL-7 culture or CD3 activation. The graphs depict each data point from 5 donors with lines connecting data points from an individual donor. Statistics were calculated by 2-tailed Wilcoxon rank sum test. TSCM ∗P = .0159; TEFF ∗P = .0465. Graphs in panel A are representative of 10 experiments using cells from 10 different donors, whereas graphs in panel C are representative of 5 experiments using cells from 5 different donors.
Phenotypic characterization of CD19 CAR T cells. (A) Flow cytometry analysis depicting CD4+ and CD8+ populations of CD3+CD34+ cells transduced after IL-7 culture or CD3 activation. (B) CD8:CD4 ratio of CD3+CD34+ cells transduced after IL-7 culture or CD3 activation. The graph depicts each data point from 10 donors with lines connecting data points from an individual donor. Statistics were calculated by 2-tailed Wilcoxon rank sum test. ∗P = .0355. (C) Flow cytometry analysis depicting the TSCM (CD62L+CD45RA+CCR7+CD95+), TCM (CD62L+CD45RA−), TEM (CD62L−CD45RA−), and TEFF (CD62L−CD45RA+) populations of starting material and CD3+CD34+ cells transduced after IL-7 culture or CD3 activation. (D) Percentage of TSCM and TEFF populations in CD3+CD34+ cells transduced after IL-7 culture or CD3 activation. The graphs depict each data point from 5 donors with lines connecting data points from an individual donor. Statistics were calculated by 2-tailed Wilcoxon rank sum test. TSCM ∗P = .0159; TEFF ∗P = .0465. Graphs in panel A are representative of 10 experiments using cells from 10 different donors, whereas graphs in panel C are representative of 5 experiments using cells from 5 different donors.
As it is believed that TSCM have properties that make them ideal for adoptive cell therapy, there has been great interest in increasing TSCM in infusion products.16,17 Thus, we assessed the differentiation markers CD45RA, CD62L, CCR7, and CD95 in CD3+CD34+ cells transduced either after IL-7 culture or CD3 activation. Analysis on 5 donors demonstrated increased percentage of TSCM (CD45RA+CD62L+CCR7+CD95+) in CD3+CD34+ cells transduced after IL-7 culture when compared with CD3 activated cells (means, 28.7% vs 7.9%; P = .0159; Figure 2C-D). Furthermore, we found a significantly lower percentage of differentiated effector T cells (TEFF; CD62L−CD45RA+) in CD3+CD34+ cells generated after IL-7 culture than with CD3 activation (means, 16.0% vs 34.7%; P = .0465; Figure 2C-D). There were no significant differences in the percentages of TN (CD45RA+CD62L+CCR7+CD95−), TCM (CD45RA+CD62L−), or TEM (CD45RA−CD62L−) populations. Altogether, these findings suggest that NICE CD19 CAR T cells consist of a balanced CD8+:CD4+ ratio and are enriched in the TSCM population.
Evaluating NICE CD19-specific CAR T cells for stem-like tumor–infiltrating lymphocyte markers
Recently, a novel stem-like population characterized by the absence of the inhibitory marker CD39 and activation marker CD69 was identified in tumor–infiltrating lymphocyte (TIL) products from patients with melanoma who achieved a CR.31 This CD39–CD69– double negative population was absent in nonresponders, suggesting importance in cellular therapy.31 We therefore assessed CD3+CD34+ cells generated after IL-7 culture or CD3 activation for CD39 and CD69. Results from 5 different donors demonstrated that CD3+CD34+ cells transduced after IL-7 culture maintain a significantly higher CD39–CD69– population than activated cells (means, 49.8% vs 9.5%; P = .0079; Figure 3A). Interestingly, CD3+CD34+ cells generated using IL-7 maintained a significant TSCM population regardless of CD39 or CD69 presence. Conversely, most activated cells demonstrated differentiated T cell phenotypes, even in the CD39−CD69− population (Figure 3B). Taken together, these data suggest that NICE CD19 CAR T cells maintain a stem-like CD39−CD69− population and may be a more effective therapy. In addition, these results suggest that the stem-like CD39−CD69− phenotype is independent from T cell differentiation.
Presence of stem-like TIL markers on CD19 CAR T cells. (A) Percentage of stem-like CD39−CD69− population in CD3+CD34+ cells transduced either after IL-7 culture or CD3 activation. The graph depicts each data point from 5 donors with lines connecting data points from an individual donor. Statistics were calculated by 2-tailed Wilcoxon rank sum test. ∗∗P = .0079. (B) Flow cytometry analysis depicting TN/TSCM (CD62L+CD45RA+), TCM (CD62L+CD45RA−), TEM (CD62L−CD45RA−), and TEFF (CD62L−CD45RA−) populations in CD3+CD34+CD39−CD69− and CD3+CD34+CD39+CD69+ cells transduced either after IL-7 culture or CD3 activation. Graphs are representative of 5 experiments using cells from 5 different donors.
Presence of stem-like TIL markers on CD19 CAR T cells. (A) Percentage of stem-like CD39−CD69− population in CD3+CD34+ cells transduced either after IL-7 culture or CD3 activation. The graph depicts each data point from 5 donors with lines connecting data points from an individual donor. Statistics were calculated by 2-tailed Wilcoxon rank sum test. ∗∗P = .0079. (B) Flow cytometry analysis depicting TN/TSCM (CD62L+CD45RA+), TCM (CD62L+CD45RA−), TEM (CD62L−CD45RA−), and TEFF (CD62L−CD45RA−) populations in CD3+CD34+CD39−CD69− and CD3+CD34+CD39+CD69+ cells transduced either after IL-7 culture or CD3 activation. Graphs are representative of 5 experiments using cells from 5 different donors.
Assessing for exhaustion in NICE CD19-specific CAR T cells
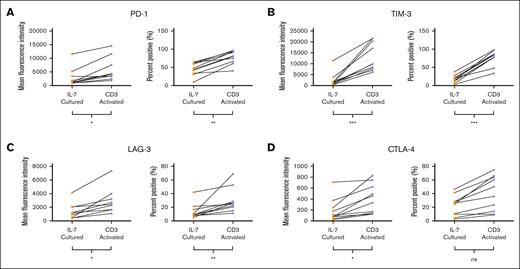
To assess the therapeutic potential of NICE CD19 CAR T cells, we evaluated 10 donors for expression of T cell exhaustion markers, PD-1, TIM-3, LAG-3, and CTLA-4. Although there was expected heterogeneity between donors, we found that CD3+CD34+ cells transduced after IL-7 culture had an overall significantly lower mean fluorescence and percent positive PD-1 expression than that of cells transduced after CD3 activation (mean fluorescence intensities, 2827 vs 5728; P = .0185; means, 46.2% vs 77.8%; P = .0015; Figure 4A). We also found that CD3+CD34+ cells generated after IL-7 culture had a lower expression of TIM-3 than that of activated cells (mean fluorescence intensities, 2370 vs 12 930; P = .0003; means, 17.6% vs 75.9%; P ≤ .0001; Figure 4B). Similarly, there was an overall significantly lower expression of LAG-3 in CD3+CD34+ cells transduced after IL-7 culture than that of cells transduced after CD3 activation (mean fluorescence intensities, 1336 vs 2729; P = .0288; means, 13.7% vs 29.4%; P = .0039; Figure 4C). Analysis on CTLA-4 demonstrated that CD3+CD34+ cells generated using IL-7 had a lower mean fluorescence than activated cells (mean fluorescence intensities, 123 vs 407; P = .0288; Figure 4C). Although not reaching statistical significance, CD3+CD34+ cells transduced after IL-7 culture trended to a lower percentage of cells positive for CTLA-4 than did activated cells (means, 22.1% vs 40.5%; P = .1230; Figure 4D). Overall, these results suggest that NICE CD19 CAR T cells have less exhaustion and may prove to be optimal for therapy.
Presence of exhaustion markers on CD19 CAR T cells. (A) Mean fluorescence and percentage positive of PD-1 on CD3+CD34+ cells transduced either after IL-7 culture or CD3 activation. MFI ∗P = .0185; percent positive ∗∗P = .0015. (B) Mean fluorescence and percentage positive of TIM-3 on CD3+CD34+ cells transduced either after IL-7 culture or CD3 activation. MFI ∗∗∗P = .0003; percent positive ∗∗∗P < .0001. (C) Mean fluorescence and percentage positive of LAG-3 on CD3+CD34+ cells transduced either after IL-7 culture or CD3 activation. MFI ∗P = .0288; percent positive ∗∗P = .0039. (D) Mean fluorescence and percentage positive of CTLA-4 on CD3+CD34+ cells transduced either after IL-7 culture or CD3 activation. MFI ∗P = .0288; percent positive P = .1230. Graphs depict each data point from 10 donors with lines connecting data points from an individual donor. Statistics were calculated by 2-tailed Wilcoxon rank sum test. MFI, mean fluorescence intensity.
Presence of exhaustion markers on CD19 CAR T cells. (A) Mean fluorescence and percentage positive of PD-1 on CD3+CD34+ cells transduced either after IL-7 culture or CD3 activation. MFI ∗P = .0185; percent positive ∗∗P = .0015. (B) Mean fluorescence and percentage positive of TIM-3 on CD3+CD34+ cells transduced either after IL-7 culture or CD3 activation. MFI ∗∗∗P = .0003; percent positive ∗∗∗P < .0001. (C) Mean fluorescence and percentage positive of LAG-3 on CD3+CD34+ cells transduced either after IL-7 culture or CD3 activation. MFI ∗P = .0288; percent positive ∗∗P = .0039. (D) Mean fluorescence and percentage positive of CTLA-4 on CD3+CD34+ cells transduced either after IL-7 culture or CD3 activation. MFI ∗P = .0288; percent positive P = .1230. Graphs depict each data point from 10 donors with lines connecting data points from an individual donor. Statistics were calculated by 2-tailed Wilcoxon rank sum test. MFI, mean fluorescence intensity.
Assessing the proliferative potential of NICE CD19-specific CAR T cells
As described above, therapeutic efficacy correlates with CAR T cell expansion after infusion. We therefore evaluated the proliferative potential of CD19 CAR T cells generated with IL-7 culture or CD3 activation through CFSE dilution on 5 donors after stimulation with T cell activation beads. Although both CD3+CD34+ cells transduced after IL-7 culture and those after CD3 activation demonstrated a similar percent divided, the IL-7 cultured cells had a significantly higher proliferation index (means, 4.1 vs 2.9; P = .0317; Figure 5A-B). Because telomere length is associated with proliferative potential of lymphocytes and correlates with clinical response to cellular therapies, we compared the telomere lengths of CD19 CAR T cells transduced after IL-7 culture or CD3 activation.20,32 Using fluorescence in situ hybridization with a FITC-labeled peptide nucleic acid probe recognizing the 6-nucleotide telomere sequence, we found higher fluorescence in CD3+CD34+ cells transduced after IL-7 culture than in activated cells (Figure 5C). We then calculated the relative telomere length of CD3+CD34+ cells generated in each condition using Raji as a control. Analysis on 5 donors demonstrated a significantly higher relative telomere length in CD3+CD34+ cells transduced with IL-7 culture than in cells generated with CD3 activation (means, 78.2% vs 36.4%; P = .0014; Figure 5D). These data suggest that NICE CD19 CAR T cells have an increased proliferative potential and may be more efficacious.
Proliferation potential of CD19 CAR T cells. (A) Flow cytometry analysis of CFSE-labeled cells transduced either after IL-7 culture or CD3 activation after stimulation with anti-CD3/CD28–coated beads for 5 days. (B) Proliferation index of CFSE-labeled cells transduced either after IL-7 culture or CD3 activation after stimulation above. Graph depicts each data point from 5 donors with lines connecting data points from an individual donor. Statistics were calculated by 2-tailed Wilcoxon rank sum test. ∗P = .0317. (C) Flow cytometry analysis of depicting fluorescence of cells transduced either after IL-7 culture or CD3 activation after in situ hybridization with a FITC–labeled PNA telomere probe. (D) Mean relative telomere length calculated using Raji cells as the control population. Graph depicts each data point from 5 donors with lines connecting data points from an individual donor. Statistics were calculated by 2-tailed Wilcoxon rank sum test. ∗∗P = .0079. PNA, peptide nucleic acid.
Proliferation potential of CD19 CAR T cells. (A) Flow cytometry analysis of CFSE-labeled cells transduced either after IL-7 culture or CD3 activation after stimulation with anti-CD3/CD28–coated beads for 5 days. (B) Proliferation index of CFSE-labeled cells transduced either after IL-7 culture or CD3 activation after stimulation above. Graph depicts each data point from 5 donors with lines connecting data points from an individual donor. Statistics were calculated by 2-tailed Wilcoxon rank sum test. ∗P = .0317. (C) Flow cytometry analysis of depicting fluorescence of cells transduced either after IL-7 culture or CD3 activation after in situ hybridization with a FITC–labeled PNA telomere probe. (D) Mean relative telomere length calculated using Raji cells as the control population. Graph depicts each data point from 5 donors with lines connecting data points from an individual donor. Statistics were calculated by 2-tailed Wilcoxon rank sum test. ∗∗P = .0079. PNA, peptide nucleic acid.
Evaluating the in vivo function of NICE CD19-specific CAR T cells
To evaluate NICE CD19 CAR T cells in vivo, we performed experiments using NSG mice. Because durable clinical responses of CD19 CAR T cells are associated with successful engraftment, we first sought to assess the persistence of NICE CD19 CAR T cells. A total of 1 × 106 CD3+CD34+ cells transduced either after IL-7 culture or standard activation were transferred IV into NSG mice. As we and others have observed a reversible loss of transgene expression through epigenetic mechanisms, we used human CD3 as a marker for transferred cells.33,34 Flow cytometry was performed on peripheral blood of mice to assess for the presence of CD3. Analysis on samples from day 7 demonstrated detectable CD3+ populations in both mice infused with cells generated through IL-7 culture and mice infused with activated cells. However, CD3 was no longer detectable by day 21 in samples from mice that were infused with activated cells. In contrast, the percentage of CD3 cells increased in the peripheral blood of mice infused with cells transduced after IL-7 culture, consistent with successful engraftment (Figure 6A).
In vivo functions of CD19 CAR T cells. (A) NSG mice were injected IV with 1 × 106 CD19 CAR T cells transduced either after IL-7 culture or CD3 activation (n = 6 per group). Flow cytometry analysis for CD3+ human T cells was performed on peripheral blood samples obtained every 14 days. Graph depicts the mean of 3 at each time point per group, with error bars representing standard deviation. (B) NSG mice were injected intraperitoneally with 1 × 105 luciferase expressing Raji cells. On day 7 after injection, mice were treated IV with 1 × 106 CD19 CAR T cells transduced either after IL-7 culture or CD3 activation (n > 8 per treatment group). Mice injected with Raji cells that received no treatment were used as controls. The graph depicts in vivo bioluminescence of tumors from 2 representative mice from each group at each time point. (C) Kaplan-Meier survival curve of Raji-injected mice. Statistically significant differences in survival were determined by log-rank test. CD3 activated compared to control ∗P = .0102; IL-7 cultured compared to control ∗∗P = .0013; IL-7 cultured compared with CD3 activated ∗P = .0211. The graph depicts 2 experiments with a total of 18 mice per group. (D) NSG mice were injected subcutaneously with 5 × 106 Raji cells. Mice with palpable tumors were treated IV on day 7 with 1 × 106 CD19 CAR T cells transduced either after IL-7 culture or CD3 activation. Tumor-bearing mice receiving no treatment were used as controls (n = 5 mice per each group). Data represent the mean at each time point with error bars indicating standard deviation. Statistically significant differences in tumor size were determined by 2-tailed Wilcoxon rank sum test. CD3 activated compared with control ∗P = .0317; IL-7 cultured compared with control ∗∗P = .0079; IL-7 cultured compared with CD3 activated ∗∗P = .0079.
In vivo functions of CD19 CAR T cells. (A) NSG mice were injected IV with 1 × 106 CD19 CAR T cells transduced either after IL-7 culture or CD3 activation (n = 6 per group). Flow cytometry analysis for CD3+ human T cells was performed on peripheral blood samples obtained every 14 days. Graph depicts the mean of 3 at each time point per group, with error bars representing standard deviation. (B) NSG mice were injected intraperitoneally with 1 × 105 luciferase expressing Raji cells. On day 7 after injection, mice were treated IV with 1 × 106 CD19 CAR T cells transduced either after IL-7 culture or CD3 activation (n > 8 per treatment group). Mice injected with Raji cells that received no treatment were used as controls. The graph depicts in vivo bioluminescence of tumors from 2 representative mice from each group at each time point. (C) Kaplan-Meier survival curve of Raji-injected mice. Statistically significant differences in survival were determined by log-rank test. CD3 activated compared to control ∗P = .0102; IL-7 cultured compared to control ∗∗P = .0013; IL-7 cultured compared with CD3 activated ∗P = .0211. The graph depicts 2 experiments with a total of 18 mice per group. (D) NSG mice were injected subcutaneously with 5 × 106 Raji cells. Mice with palpable tumors were treated IV on day 7 with 1 × 106 CD19 CAR T cells transduced either after IL-7 culture or CD3 activation. Tumor-bearing mice receiving no treatment were used as controls (n = 5 mice per each group). Data represent the mean at each time point with error bars indicating standard deviation. Statistically significant differences in tumor size were determined by 2-tailed Wilcoxon rank sum test. CD3 activated compared with control ∗P = .0317; IL-7 cultured compared with control ∗∗P = .0079; IL-7 cultured compared with CD3 activated ∗∗P = .0079.
We next assessed the antitumor activity of NICE CD19 CAR T cells by injecting 1 × 105 luciferase expressing Raji cells intraperitoneally into NSG mice. Mice were then treated IV 7 days after tumor injection with 1 × 106 CD3+CD34+ cells generated either after IL-7 culture or CD3 activation. Raji-injected mice that were untreated all developed tumors as demonstrated by an increased bioluminescent signal (Figure 6B). These control mice all died by day 39 after Raji injection (Figure 6C). Mice treated with CD3+CD34+ cells generated through CD3 activation demonstrated a delay in tumor development and significant improvement in survival compared with untreated mice (Figure 6B-C; P = .0102). However, all mice treated with activated CD3+CD34+ cells eventually died by day 67 after Raji infection (Figure 6C). In contrast, a significant group of mice treated with CD3+CD34+ cells transduced after IL-7 culture did not develop detectable tumors. Furthermore, there was significantly improved survival compared with both untreated mice (Figure 6C; P = .0013) and mice treated with activated CD3+CD34+ cells (Figure 6C; P = .0211). To confirm antitumor response, ensure tumor formation before treatment, and accurately measure tumors over time, we established subcutaneous tumors by injecting 5 × 106 Raji cells into flanks of NSG mice. At 7 days after tumor injection, mice with palpable tumors were treated IV with 1 × 106 CD3+CD34+ cells generated either after IL-7 culture or CD3 activation. Untreated tumor-bearing mice were used as controls. Both CD3+CD34+ cells generated through IL-7 culture or CD3 activation significantly delayed tumor growth in mice compared with no treatment (Figure 6D; P = .0079 and P = .0317), However, mice treated with CD3+CD34+ cells generated after IL-7 culture had significantly lower tumor volumes than mice treated with activated cells (Figure 6D; P = .0079). Altogether, these results suggest that NICE CD19 CAR T cells have more persistence after infusion and have superior antitumor activity in vivo compared with activated cells.
Discussion
Reports of durable remissions demonstrate the potential for CD19 CAR T cells to cure B cell malignancies. The intrinsic T cell features of the final cellular product appear to play an important role in achieving a durable remission and are most certainly affected by the activation and expansion during manufacturing.19 Hence, there have been great efforts to generate CAR T cells in the absence of activation. Ghassemi et al first demonstrated successful production of CD19 CAR T cells without CD3 stimulation through a protocol involving serum starvation, deoxynucleosides, IL-7, and IL-15.21 Here, we show that culturing with IL-7 alone allows for efficient generation of NICE CD19 CAR T cells in the complete absence of activation.
Expression of our transduction marker may be affected at some level by pseudotransduction, in which surface proteins are transferred from the lentiviral vector envelop by viral fusion.35 This phenomenon was demonstrated in CAR T cells when Ghassemi et al exhibited transduction marker expression even in the presence of integrase or reverse transcriptase inhibitors.21 They further show that cells expressing transduction markers without vector integration have no antigen-specific activity.21 Because NICE CD19 CAR T cells demonstrate robust antigen-specific cytokine release (Figure 1C), generation most definitely involves true vector integration. This is expected because IL-7 is known to induce lymphocyte progression from G0 to G1, a cell cycle phase associated with nuclear pores.36
Similar to CD3 stimulation, cytokines play a crucial role in CAR T cell production. Current products rely on IL-2 to promote CAR T cell expansion during production.19 Although effective in driving proliferation, IL-2 is well-known to induce differentiation, lead to activation-induced cell death, and facilitate regulatory T cell expansion.37,38 Interestingly, CD8+ cells appear to be more susceptible to the mitogenic effects of IL-2 because it reduces the threshold for receptor signaling-induced proliferation.39 This may cause the increased CD8+:CD4+ ratio we frequently obtain after activation (Figure 2A). To avoid the deleterious effects of IL-2, ongoing trials are evaluating CAR T cells expanded with IL-7 and IL-15.18,40 However, these studies depend on CD3 activation for efficient transduction, and most cells were consistent with the TEM phenotype.18 As described above, Ghassemi et al developed a protocol using IL-7 and IL-15 to produce CD19 CAR T cells without CD3 stimulation.21 However, IL-15 shares similarities with IL-2 in which it activates T cells and induces proliferation through the JAK and STAT pathway.23 In contrast, IL-7 does not commit lymphocytes to S phase or affect T cell phenotype.22 Here, we demonstrate that transduction after IL-7 culture alone results in a product consisting of mostly TN/TSCM cells while retaining a physiologic CD8+/CD4+ ratio (Figure 2). Because data suggest optimal CAR T cell activity with a 1:1 CD8+:CD4+ ratio, a product was developed involving sequential infusions of separately transduced CD8+ and CD4+ cells.8,29,30 Although therapeutic advantages to this labor-intensive process remain to be seen, generating CD19 CAR T cells using IL-7 culture appears to be a practical way to obtain a less-differentiated product with a similar CD8+:CD4+ ratio.
Recently, a novel stem-like CD39−CD69− T cell population was identified in melanoma TIL products and associated with CR to therapy. Further analysis demonstrated expression of traditional memory markers, CD62L and CD27, and markers of stem-like “progenitor exhausted” cells, SLAMF6 and TCF1.31 In our studies, we found that NICE CD19 CAR T cells had a higher percentage of the CD39–CD69– phenotype than that of cells generated with standard activation. Although it remains unclear how the various “stem-like” T cell markers relate to each other, we demonstrate for the first time that CD39 and CD69 expression occurs independently of traditional T cell differentiation status (Figure 3). Interestingly, the TIL studies above found that only a minority of CD39–CD69– TIL were reactive to tumor antigen.31 Our data demonstrate that using IL-7 culture to generate CAR T cells without activation is a feasible way to transfer tumor-reactivity while maintaining a significant CD39−CD69− phenotype (Figure 3).
Studies have demonstrated an inverse correlation between clinical response and exhaustion markers in infusion products.6,13,14 Efforts to prevent CAR T cell exhaustion include altering the framework regions of the single-chain variable fragment, the variable region of heavy chain domain, and the hinge portions of the CAR to limit excessive receptor signaling.41 Interestingly, Feucht et al demonstrated that mutations to the immunoreceptor tyrosine-based activation motifs on the CD3ζ chain can decrease exhaustion and improve antitumor activity.42 Although all these modifications may result in a less exhausting receptor, CD3 stimulation alone during manufacturing is sufficient to drive cells toward activation-induced cell death.20 Our studies show that CD19-specific CAR T cells generated without CD3 activation have lower expression levels of PD-1, TIM-3, LAG-3, and CTLA-4 (Figure 4). This suggests that using IL-7 to engineer modified cells alone is a straightforward way to prevent exhaustion and may potentially be used with future generation CARs to improve therapy.
To improve the replicative ability of infusion products, there are efforts to enhance telomerase activity.43 One group found that transiently overexpressing telomerase reverse transcriptase in CD19 CAR T cells through messenger RNA electroporation improved proliferation and persistence in a murine xenograft lymphoma model.44 In our studies, we demonstrate that CD19 CAR T cells generated using IL-7 without activation have increased proliferation after stimulation and longer relative telomere lengths without additional modification (Figure 5). In addition, ex vivo expansion inevitably causes shortening of telomeres and replicative senescence.43 Although it may not be feasible to achieve the same absolute cell number without activation or expansion, the increased proliferative potential of nonactivated CAR T cells will likely result in a lower dose required for response. Even in case of variable transduction efficiencies, our CD34t transduction marker allows for clinical-grade magnetic selection to enhance product yield.26 Furthermore, elimination of the expansion phase would shorten production time and facilitate timelier treatment. Altogether, a 7-day IL-7 culture followed by transduction and magnetic selection has the potential to generate a potent and pure cellular product within 10 days, which is significantly shorter than the industry standard of several weeks.
Improving the duration of response to CD19 CAR T cells is critical because even patients who achieve a CR often relapse.3,4,10,11 It is becoming clear that durable clinical responses are correlated with the persistence of a functional CAR T cell population.12-15 Although our in vivo experiments are consistent with successful engraftment of CD19-specific CAR T cells generated after IL-7 culture (Figure 6A), molecular studies are needed to confirm the persistence of transduced cells. Nevertheless, our in vivo experiments confirm the superior antitumor activity and improved survival of NICE CD19-specific CAR T cells compared with the activated cells (Figure 6B-D).
In conclusion, we demonstrate that CD19-specific CAR T cells can be efficiently generated in the absence of activation using a lentiviral vector and IL-7 culture. NICE CD19 CAR T cells are enriched with the TSCM phenotype, retain novel markers for stemness, are less exhausted, and have increased proliferative potential in vitro. Furthermore, our findings are consistent with the engraftment of NICE CD19 CAR T cells and demonstrate superior antitumor activity in vivo. Overall, these studies should be taken together with previous work and provide rationale for evaluating NICE CD19 CAR T cells in a clinical trial.
Acknowledgment
This research was funded by the Hollis Brownstein Research Grant from the Leukemia Research Foundation.
Authorship
Contribution: S.Y.W. and M.I.N. designed the study; S.Y.W., G.M.S., A.V.D., and S.Q. performed the experiments; S.Y.W., P.J.S., and M.I.N. analyzed the data; S.Y.W., P.J.S., and M.I.N. wrote the manuscript; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Siao-Yi Wang, Cardinal Bernardin Cancer Center, 2160 S 1st Ave, Maywood, IL 60153; email: wang.siaoyi@gmail.com.
References
Author notes
Data and materials that support this study are available upon reasonable request from the author Michael I. Nishimura (mnisihimura@luc.edu).