Key Points
Copanlisib plus R-B did not demonstrate improved PFS or overall survival in patients with relapsed iNHL.
Copanlisib plus R-B was associated with more serious TEAEs, TEAE-related deaths, and discontinuations than placebo plus R-B.
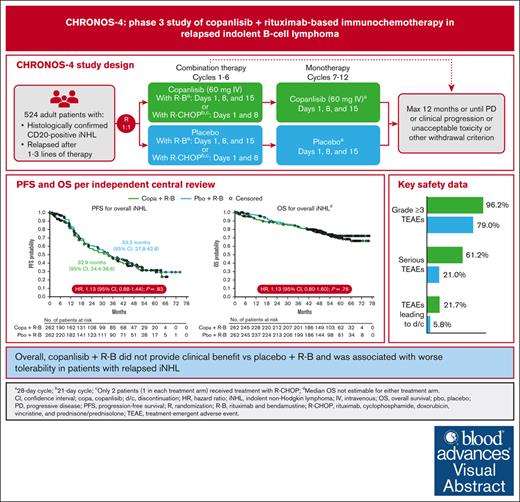
Visual Abstract
Copanlisib, a pan-class I phosphatidylinositol 3-kinase inhibitor with predominant activity against the α and δ isoforms, previously demonstrated durable responses as monotherapy and improved progression-free survival (PFS) in combination with rituximab in patients with relapsed indolent non-Hodgkin lymphoma (iNHL). CHRONOS-4 was a phase 3, randomized, double-blind, placebo-controlled study to investigate the efficacy and safety of copanlisib in combination with standard immunochemotherapy in patients with relapsed iNHL. Patients (n = 524) were randomized (1:1) to copanlisib (60 mg IV) plus immunochemotherapy (rituximab and bendamustine [R-B] or placebo plus R-B). Copanlisib/placebo were administered with R-B (days 1, 8, and 15 of each 28-day cycle) for ≤6 cycles and as monotherapy from cycle 7 up to 12 months. The primary study end point was PFS. Median exposure was 8.5 months (0.2-12.9) for copanlisib plus R-B and 11.4 months (0.1-12.6) for placebo plus R-B. Median PFS was 32.9 months (95% confidence interval [CI], 24.4-38.6) for copanlisib plus R-B and 33.3 months (95% CI, 27.8-42.8) for placebo plus R-B (hazard ratio, 1.13; 95% CI, 0.88-1.44; P = .83). No differences between treatment arms were observed in overall survival (data not yet mature), objective response rate, and duration of response for the overall population or individual histology types. Overall, copanlisib plus R-B was associated with higher rates of serious treatment-emergent adverse events (TEAEs), grade 4 and 5 TEAEs, and treatment discontinuation. A number of serious TEAEs were infections. Overall, copanlisib plus R-B did not provide clinical benefit vs placebo plus R-B and was associated with worse tolerability in patients with relapsed iNHL. This trial was registered at www.ClinicalTrials.gov as #NCT02626455.
Introduction
Rituximab-based treatment as monotherapy or in combination with chemotherapy is a standard treatment for B-cell malignancies, including indolent non-Hodgkin lymphoma (iNHL) subtypes.1 Many patients become refractory to treatment and relapse, requiring multiple lines of treatment.2 Because dysregulation of pathways associated with phosphatidylinositol 3-kinase (PI3K) overactivation is common in B- and T-cell lymphoid malignancies,3 several PI3K inhibitors have been investigated and have shown preclinical and clinical activity in the context of relapsed and refractory iNHL, including the IV-administered PI3K inhibitor copanlisib.3 In a phase 2, single-arm study (CHRONOS-1),4 copanlisib monotherapy resulted in significant and durable responses with a manageable safety profile in heavily pretreated patients with relapsed iNHL, which led in 2017 to the accelerated approval of copanlisib in the United States and other countries, for the treatment of patients with relapsed follicular lymphoma (FL) after ≥2 systemic lines of therapy.4,5 After CHRONOS-1, a phase 3 study (CHRONOS-3) was conducted to compare the combination of copanlisib plus rituximab with standard rituximab monotherapy in patients with relapsed iNHL who were ineligible or unwilling to receive chemotherapy. CHRONOS-3 met the primary end point of improved progression-free survival (PFS) for copanlisib plus rituximab over placebo plus rituximab, without improvement in interim overall survival (OS) during a limited follow-up for OS assessment.6 In line with US Food and Drug Administration (FDA) postmarketing requirements to further demonstrate the clinical benefit of copanlisib,7 a phase 3 study was conducted to investigate copanlisib plus standard immunochemotherapy in patients with relapsed iNHL. Here, we report efficacy and safety results from that additional confirmatory phase 3 study, CHRONOS-4.
Methods
Study design and treatments
CHRONOS-4 (NCT02626455) was a phase 3, randomized, double-blind, placebo-controlled, multicenter, 2-arm study in patients with relapsed rituximab-sensitive iNHL to evaluate the efficacy and safety of copanlisib in combination with standard immunochemotherapy (rituximab and bendamustine [R-B] or rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone/prednisolone [R-CHOP]) compared with R-B or R-CHOP. Patients who met the eligibility criteria of histologically confirmed CD20+ iNHL that had relapsed or progressed after 1 to 3 lines of therapy (supplemental Materials) were randomized (1:1) to receive copanlisib in combination with R-B or R-CHOP or placebo in combination with R-B or R-CHOP.
R-B treatment was administered every 4 weeks and included rituximab 375 mg/m2 IV on day 1 and bendamustine 90 mg/m2 IV on days 1 and 2. R-CHOP treatment was administered every 3 weeks and included rituximab 375 mg/m2 IV, cyclophosphamide 750 mg/m2 IV, doxorubicin 50 mg/m2 IV, and vincristine 1.4 mg/m2 IV (maximum dose 2 mg), all on day 2, plus prednisone/prednisolone 100 mg daily orally from days 2 to 6. Based on findings from the safety run-in part,8 copanlisib was administered at 60 mg as a 1-hour IV infusion in combination with R-B on days 1, 8, and 15 of each 28-day cycle or in combination with R-CHOP on days 1 and 8 of each 21-day cycle. Owing to slow enrollment after initiation of the phase 3 trial, only 2 patients (1 in each treatment arm) received treatment with R-CHOP.
Combination therapy (copanlisib/placebo with R-B) was administered for a maximum of 6 cycles; then copanlisib or placebo monotherapy was started on cycle 7 with the same copanlisib dose used during immunochemotherapy. Monotherapy and combination therapy were continued until completion of the study treatment period (up to 12 months) or the occurrence of progressive disease (PD; per central independent blinded radiology review defined by the Lugano classification or Owen criteria for patients with Waldenström macroglobulinemia9,10), clinical progression (eg, Eastern Cooperative Oncology Group performance status of ≥3), or unacceptable toxicity or until a withdrawal criterion was met, whichever came first. The maximum duration of treatment including combination therapy and monotherapy was 12 months. Prophylactic therapy for opportunistic infections was not mandated for all patients but could be initiated based on the local standard of care per investigator discretion for any patient, irrespective of whether a high-risk feature was present (ie, ≥2 lines of myelosuppressive cytotoxic therapy, history of cytomegalovirus or herpes, history of lower respiratory tract infection, history of immunodeficiency in the last 12 months [excluding lymphoma], or lymphocyte count < 0.5 x 109/L while on treatment).
An end-of-treatment visit was performed within 7 days of treatment discontinuation, and a safety follow-up visit was conducted 30 days after the last study treatment. Patients who completed a 12-month study treatment period without PD or discontinued study treatment for reasons other than PD entered the active follow-up period for further tumor assessments until radiologic PD.
Study end points and assessments
The primary study end point was PFS defined as the time (days) from randomization to the occurrence of PD as assessed by independent central review or death from any cause (if no progression occurred). Efficacy assessments were based on radiologic tumor evaluations via enhanced computed tomography/magnetic resonance imaging and positron emission tomography–computed tomography. Radiologic assessments were conducted every 12 weeks from cycle 1, day 1 during years 1 and 2, and every 24 weeks during year 3 and beyond. Secondary end points included objective response rate (ORR), duration of response (DoR), complete response rate (CRR), OS, time to improvement and time to deterioration in disease-related symptoms–physical (DRS-P) of ≥3 points, and safety and tolerability. ORR was reported according to the Lugano classification or Owen criteria.9,10
Exploratory objectives included pharmacokinetics of copanlisib and biomarkers of efficacy, mode of action-related effect, safety, and/or the pathomechanism of the disease (supplemental Materials).
Safety evaluations were conducted at screening, during treatment, and during the safety follow-up visit. The intensity and frequency of adverse events (AEs) and the occurrence of new and unexpected AEs were monitored and graded using Common Terminology Criteria for Adverse Events version 4.03. Doses were delayed or reduced following predefined rules if clinically significant hematologic or other toxicities that were possibly, probably, or definitely related to study treatment were observed.
The study protocol was approved by the relevant institutional review board/independent ethics committee at each study site in accordance with the updated Declaration of Helsinki. All participants provided a written informed consent before initiating the study.
Statistical analysis
The efficacy analysis was conducted using the full analysis set. The primary analysis of PFS was based on tumor assessments of the central independent blinded review and was performed using a log-rank test, using the same stratification factors as in randomization. Similar analyses were conducted for OS, DoR, time to deterioration, and time to improvement in DRS-P of ≥3 points. ORR and CRR were compared between the 2 treatment arms using a Cochran-Mantel-Haenszel test with the randomization factors as strata. Descriptive statistics were used for the analysis of safety data using the safety analysis set, which included all randomized patients in the phase 3 part who received ≥1 dose of the study drug.
Results
Patients
Of 524 randomized patients, an equal distribution of 262 patients were assigned to receive copanlisib or placebo in combination with R-B (supplemental Figure 1). One patient in each treatment arm (of the 520 patients who received ≥1 dose of treatment) received R-CHOP in place of R-B. In the copanlisib arm vs the placebo arm, a smaller number of patients completed treatment (108 vs 153) and a higher number of patients discontinued treatment (153 vs 106). The primary cause of treatment discontinuation among patients in the copanlisib arm was the occurrence of AEs not associated with clinical PD in 65 patients (24.8%); in the placebo arm, treatment discontinuation was mainly caused by patient decision or radiologic PD in 22 patients each (8.4%).
Baseline characteristics and demographics were relatively well balanced between treatment arms (Table 1). Most patients were male (55.9%) and the median patient age was 61 years (range, 26-96). FL was the predominant histologic subtype (67.4%), followed by marginal zone lymphoma (17.6%), small lymphocytic lymphoma (7.4%), lymphoplasmacytic lymphoma/Waldenström macroglobulinemia (7.4%), and other (0.2%). Among the 524 patients, 334 (63.7%) had received only 1 line of anticancer therapy.
Patient demographics and baseline characteristics
| . | Copanlisib + R-B (n = 262) . | Placebo + R-B (n = 262) . |
|---|---|---|
| Sex, n (%) | ||
| Male | 159 (60.7) | 134 (51.1) |
| Female | 103 (39.3) | 128 (48.9) |
| Median age, y (range) | 61.0 (29-96) | 61.0 (26-85) |
| ECOG performance status, n (%) | ||
| 0 | 155 (59.2) | 156 (59.5) |
| 1 | 105 (40.1) | 96 (36.6) |
| 2 | 2 (0.8) | 10 (3.8) |
| Histology of lymphoma, n (%) | ||
| FL | 175 (66.8) | 178 (67.9) |
| MZL | 43 (16.4) | 49 (18.7) |
| SLL | 19 (7.3) | 20 (7.6) |
| LPL/WM | 25 (9.5) | 14 (5.3) |
| Other | 0 | 1 (0.4) |
| Previous systemic anticancer therapy lines, median (range) | 1.0 (1-3) | 1.0 (1-7) |
| Previous systemic anticancer therapy lines, n (%) | 262 (100) | 262 (100) |
| 1 | 170 (64.9) | 164 (62.6) |
| 2 | 73 (27.9) | 73 (27.9) |
| ≥3 | 19 (7.3) | 25 (9.5) |
| Median time since last systemic anticancer therapy, mo (range) | 28.3 (0.5-155.5) | 24.8 (0.3-171.9) |
| Previous systemic anticancer therapy∗, n (%) | ||
| Rituximab | 260 (99.2) | 262 (100) |
| Bendamustine | 12 (4.6) | 15 (5.7) |
| Cyclophosphamide | 254 (96.9) | 256 (97.7) |
| Doxorubicin | 131 (50.0) | 146 (55.7) |
| Vincristine | 206 (78.6) | 225 (85.9) |
| . | Copanlisib + R-B (n = 262) . | Placebo + R-B (n = 262) . |
|---|---|---|
| Sex, n (%) | ||
| Male | 159 (60.7) | 134 (51.1) |
| Female | 103 (39.3) | 128 (48.9) |
| Median age, y (range) | 61.0 (29-96) | 61.0 (26-85) |
| ECOG performance status, n (%) | ||
| 0 | 155 (59.2) | 156 (59.5) |
| 1 | 105 (40.1) | 96 (36.6) |
| 2 | 2 (0.8) | 10 (3.8) |
| Histology of lymphoma, n (%) | ||
| FL | 175 (66.8) | 178 (67.9) |
| MZL | 43 (16.4) | 49 (18.7) |
| SLL | 19 (7.3) | 20 (7.6) |
| LPL/WM | 25 (9.5) | 14 (5.3) |
| Other | 0 | 1 (0.4) |
| Previous systemic anticancer therapy lines, median (range) | 1.0 (1-3) | 1.0 (1-7) |
| Previous systemic anticancer therapy lines, n (%) | 262 (100) | 262 (100) |
| 1 | 170 (64.9) | 164 (62.6) |
| 2 | 73 (27.9) | 73 (27.9) |
| ≥3 | 19 (7.3) | 25 (9.5) |
| Median time since last systemic anticancer therapy, mo (range) | 28.3 (0.5-155.5) | 24.8 (0.3-171.9) |
| Previous systemic anticancer therapy∗, n (%) | ||
| Rituximab | 260 (99.2) | 262 (100) |
| Bendamustine | 12 (4.6) | 15 (5.7) |
| Cyclophosphamide | 254 (96.9) | 256 (97.7) |
| Doxorubicin | 131 (50.0) | 146 (55.7) |
| Vincristine | 206 (78.6) | 225 (85.9) |
ECOG, Eastern Cooperative Oncology Group; LPL, lymphoplasmacytic lymphoma; MZL, marginal zone lymphoma; SLL, small lymphocytic lymphoma; WM, Waldenström macroglobulinemia.
Patients may have had >1 entry.
Study treatment
Median duration of exposure to copanlisib was shorter than exposure to placebo at 8.5 months (range, 0.2-12.9) vs 11.4 months (range, 0.1-12.6), respectively (Table 2). This difference was greater in the FL subgroup, who had a median copanlisib exposure of 6.8 months (range, 0.2-12.6) vs 11.3 months (range, 0.1-12.6) of placebo exposure. Overall, R-B exposure was comparable between treatment arms, with 5.6 months (range, 0.2-12.4) in the copanlisib arm vs 5.5 months (range, 0.1-7.2) in the placebo arm. Dose modifications (ie, delays, interruptions, reductions) occurred in 83.3% and 66.9% of patients in the copanlisib and placebo arms, respectively. A greater proportion of dose reductions occurred in the copanlisib arm than the placebo arm (Table 2). Dose reductions to 45 mg were reported in 30.8% of patients receiving copanlisib vs 10.9% of patients receiving placebo, and reductions to 30 mg were reported in 11.4% vs 1.6% of patients, respectively. Dose interruptions or delays were observed in 81.7% of patients in the copanlisib arm vs 65.8% of patients in the placebo arm, and overall median duration of delay was 7 days (range, 1-173). R-B dose interruptions or delays were also more frequent among patients in the copanlisib arm vs the placebo arm (51.7% and 52.1% vs 37.0% and 36.2%, respectively).
Summary of treatment duration and exposure
| . | Copanlisib + R-B (n = 263) . | Placebo + R-B (n = 257) . |
|---|---|---|
| Median extent of copanlisib/placebo exposure for patients receiving R-B, mo (range)∗,† | ||
| Overall iNHL | 8.5 (0.2-12.9) | 11.4 (0.1-12.6) |
| FL | 6.8 (0.2-12.6) | 11.3 (0.1-12.6) |
| Median extent of R/B exposure, mo (range)∗,† | ||
| Overall iNHL | 5.6 (0.2-12.4) | 5.5 (0.1-7.2) |
| FL | 5.5 (0.9-8.7) | 5.5 (0.1-7.2) |
| Copanlisib/placebo dose modifications (any), n (%) | 219 (83.3) | 172 (66.9) |
| Copanlisib/placebo dose reductions, n (%) | ||
| Reduction to 45 mg | 81 (30.8) | 28 (10.9) |
| Reduction to 30 mg | 30 (11.4) | 4 (1.6) |
| Copanlisib/placebo dose interruptions/delays, n (%) | 215 (81.7) | 169 (65.8) |
| Median duration of delay from copanlisib/placebo, d (range) | 7 (1-173) | 7 (1-50) |
| Rituximab dose interruptions/delays‡, n (%) | 136 (51.7) | 95 (37.0) |
| Bendamustine dose interruptions/delays, n (%) | 137 (52.1) | 93 (36.2) |
| . | Copanlisib + R-B (n = 263) . | Placebo + R-B (n = 257) . |
|---|---|---|
| Median extent of copanlisib/placebo exposure for patients receiving R-B, mo (range)∗,† | ||
| Overall iNHL | 8.5 (0.2-12.9) | 11.4 (0.1-12.6) |
| FL | 6.8 (0.2-12.6) | 11.3 (0.1-12.6) |
| Median extent of R/B exposure, mo (range)∗,† | ||
| Overall iNHL | 5.6 (0.2-12.4) | 5.5 (0.1-7.2) |
| FL | 5.5 (0.9-8.7) | 5.5 (0.1-7.2) |
| Copanlisib/placebo dose modifications (any), n (%) | 219 (83.3) | 172 (66.9) |
| Copanlisib/placebo dose reductions, n (%) | ||
| Reduction to 45 mg | 81 (30.8) | 28 (10.9) |
| Reduction to 30 mg | 30 (11.4) | 4 (1.6) |
| Copanlisib/placebo dose interruptions/delays, n (%) | 215 (81.7) | 169 (65.8) |
| Median duration of delay from copanlisib/placebo, d (range) | 7 (1-173) | 7 (1-50) |
| Rituximab dose interruptions/delays‡, n (%) | 136 (51.7) | 95 (37.0) |
| Bendamustine dose interruptions/delays, n (%) | 137 (52.1) | 93 (36.2) |
An interruption is when an infusion is either stopped earlier than specified in the protocol or stopped and restarted. A delay is when an infusion is not given as expected and 0 dose is administered during this period. Multiple interruptions with the same start date were counted as 1 interruption.
Includes interruptions/delays and drug holidays. If the treatment ended with interruptions, the day of the last actual dose was considered the last day.
One patient with FL received R-CHOP in each treatment arm.
Per protocol, there are no dose reductions or re-escalations for rituximab in any cycle.
PFS
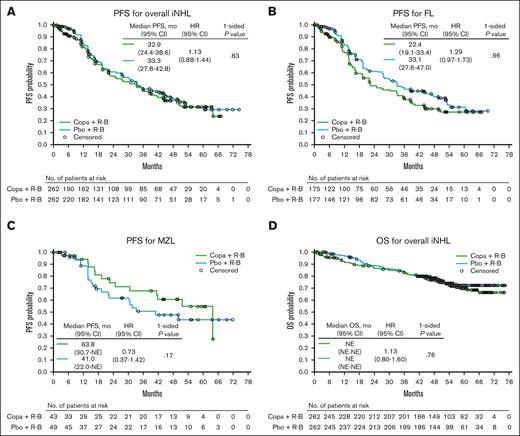
The primary end point of improved PFS for copanlisib plus R-B vs placebo plus R-B (including 1 patient in each treatment arm treated with R-CHOP) was not met for CHRONOS-4. After a median follow-up of 47.1 months, median PFS in the overall iNHL population was 32.9 months (95% confidence interval [CI], 24.4-38.6) in the copanlisib arm vs 33.3 months (95% CI, 27.8-42.8) in the placebo arm (hazard ratio [HR], 1.13; 95% CI, 0.88-1.44; P = .83; Table 3; Figure 1A). In patients with FL, the largest histology subgroup, the copanlisib arm had a median PFS of 22.4 months (95% CI, 19.1-33.4) vs 33.1 months (95% CI, 27.6-47.0) in the placebo arm (HR, 1.29; 95% CI, 0.97-1.73; P = .96; Table 3; Figure 1B). In the second largest histology subgroup (marginal zone lymphoma), median PFS was 63.8 months (95% CI, 30.7-not estimable) for the copanlisib arm vs 41.0 months (95% CI, 22.0-not estimable) for the placebo arm (HR, 0.73; 95% CI, 0.37-1.42; P = .17; Table 3; Figure 1C). Similarly, numerical improvements in PFS were noted in the copanlisib arm vs the placebo arm across all the other iNHL subtypes, including for pooled PFS values for patients with “other/non-FL” subtype (supplemental Table 1; supplemental Figure 2).
Efficacy results in the overall iNHL population and FL and MZL subgroups (full analysis set)
| . | Overall iNHL . | HR (95% CI) . | P value . | |
|---|---|---|---|---|
| Copanlisib + R-B (n = 262) . | Placebo + R-B (n = 262) . | |||
| PFS per independent radiologic review | ||||
| Median PFS (95% CI), mo | 32.9 (24.4-38.6) | 33.3 (27.8-42.8) | 1.13 (0.88-1.44) | .83 |
| Objective tumor responses per independent radiologic review | ||||
| ORR, n (%) (95% CI) | 224 (85.5) (80.6-89.5) | 227 (86.6) (81.9-90.5) | — | — |
| DoR per independent radiologic review∗ | ||||
| Median DoR (95% CI), mo | 32.2 (22.8-38.8) | 32.4 (27.7-42.0) | 1.15 (0.88-1.48) | .85 |
| OS | ||||
| Median OS (95% CI), mo | NE (NE-NE) | NE (NE-NE) | 1.13 (0.80-1.60) | .76 |
| . | Overall iNHL . | HR (95% CI) . | P value . | |
|---|---|---|---|---|
| Copanlisib + R-B (n = 262) . | Placebo + R-B (n = 262) . | |||
| PFS per independent radiologic review | ||||
| Median PFS (95% CI), mo | 32.9 (24.4-38.6) | 33.3 (27.8-42.8) | 1.13 (0.88-1.44) | .83 |
| Objective tumor responses per independent radiologic review | ||||
| ORR, n (%) (95% CI) | 224 (85.5) (80.6-89.5) | 227 (86.6) (81.9-90.5) | — | — |
| DoR per independent radiologic review∗ | ||||
| Median DoR (95% CI), mo | 32.2 (22.8-38.8) | 32.4 (27.7-42.0) | 1.15 (0.88-1.48) | .85 |
| OS | ||||
| Median OS (95% CI), mo | NE (NE-NE) | NE (NE-NE) | 1.13 (0.80-1.60) | .76 |
| . | FL . | HR (95% CI) . | P value . | |
|---|---|---|---|---|
| Copanlisib + R-B (n = 175) . | Placebo + R-B (n = 177) . | |||
| PFS per independent radiologic review | ||||
| Median PFS (95% CI), mo | 22.4 (19.1-33.4) | 33.1 (27.6-47.0) | 1.29 (0.97-1.73) | .96 |
| Objective tumor responses per independent radiologic review | ||||
| ORR, n (%) (95% CI) | 147 (84.0) (77.7-89.1) | 155 (87.1) (81.2-91.6) | — | — |
| DoR per independent radiologic review∗ | ||||
| Median DoR (95% CI), mo | 22.8 (16.8-33.3) | 32.3 (25.5-45.0) | 1.31 (0.96-1.77) | .96 |
| OS | ||||
| Median OS (95% CI), mo | NE (NE-NE) | NE (NE-NE) | 1.18 (0.77-1.82) | .78 |
| . | FL . | HR (95% CI) . | P value . | |
|---|---|---|---|---|
| Copanlisib + R-B (n = 175) . | Placebo + R-B (n = 177) . | |||
| PFS per independent radiologic review | ||||
| Median PFS (95% CI), mo | 22.4 (19.1-33.4) | 33.1 (27.6-47.0) | 1.29 (0.97-1.73) | .96 |
| Objective tumor responses per independent radiologic review | ||||
| ORR, n (%) (95% CI) | 147 (84.0) (77.7-89.1) | 155 (87.1) (81.2-91.6) | — | — |
| DoR per independent radiologic review∗ | ||||
| Median DoR (95% CI), mo | 22.8 (16.8-33.3) | 32.3 (25.5-45.0) | 1.31 (0.96-1.77) | .96 |
| OS | ||||
| Median OS (95% CI), mo | NE (NE-NE) | NE (NE-NE) | 1.18 (0.77-1.82) | .78 |
| . | MZL . | HR (95% CI) . | P value . | |
|---|---|---|---|---|
| Copanlisib + R-B (n = 43) . | Placebo + R-B (n = 49) . | |||
| PFS per independent radiologic review | ||||
| Median PFS (95% CI), mo | 63.8 (30.7-NE) | 41.0 (22.0-NE) | 0.73 (0.37-1.42) | .17 |
| Objective tumor responses per independent radiologic review | ||||
| ORR, n (%) (95% CI) | 39 (90.7) (77.9-97.4) | 43 (87.8) (75.2-95.4) | — | — |
| DoR per independent radiologic review∗ | ||||
| Median DoR (95% CI), mo | 60.9 (21.9-NE) | 47.3 (19.8-NE) | 0.80 (0.39-1.65) | .28 |
| OS | ||||
| Median OS (95% CI), mo | NE (NE-NE) | NE (NE-NE) | 0.92 (0.42-2.04) | .42 |
| . | MZL . | HR (95% CI) . | P value . | |
|---|---|---|---|---|
| Copanlisib + R-B (n = 43) . | Placebo + R-B (n = 49) . | |||
| PFS per independent radiologic review | ||||
| Median PFS (95% CI), mo | 63.8 (30.7-NE) | 41.0 (22.0-NE) | 0.73 (0.37-1.42) | .17 |
| Objective tumor responses per independent radiologic review | ||||
| ORR, n (%) (95% CI) | 39 (90.7) (77.9-97.4) | 43 (87.8) (75.2-95.4) | — | — |
| DoR per independent radiologic review∗ | ||||
| Median DoR (95% CI), mo | 60.9 (21.9-NE) | 47.3 (19.8-NE) | 0.80 (0.39-1.65) | .28 |
| OS | ||||
| Median OS (95% CI), mo | NE (NE-NE) | NE (NE-NE) | 0.92 (0.42-2.04) | .42 |
NE, not estimable.
Includes patients with responses in the full analysis set.
Kaplan-Meier curves of PFS and OS. (A-D) PFS in patients with iNHL (A), FL (B), and MZL (C) and OS in patients with iNHL (D) (full analysis set). At-risk patient counts were calculated as at the start of the time point. Patients evaluated by Owen criteria may include investigator assessments in the absence of independent central review. For DoR, only patients with responses in the full analysis set were included. Copa, copanlisib; pbo, placebo.
Kaplan-Meier curves of PFS and OS. (A-D) PFS in patients with iNHL (A), FL (B), and MZL (C) and OS in patients with iNHL (D) (full analysis set). At-risk patient counts were calculated as at the start of the time point. Patients evaluated by Owen criteria may include investigator assessments in the absence of independent central review. For DoR, only patients with responses in the full analysis set were included. Copa, copanlisib; pbo, placebo.
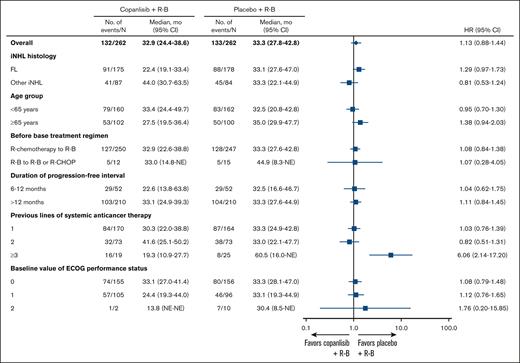
Analysis of PFS across several stratification factors showed no improvement in PFS for the copanlisib arm vs the placebo arm (Figure 2; supplemental Figures 3 and 4). However, although the placebo arm had fewer patients, it showed a longer median PFS of 60.5 months vs 19.3 months in the copanlisib arm for patients who had received ≥3 lines of systemic anticancer therapy (HR, 6.06; 95% CI, 2.14-17.20; P = .20; supplemental Figure 3). In addition, for patients aged ≥65 years, median PFS was higher in the placebo arm at 35.0 months vs 27.5 months in the copanlisib arm (HR, 1.38; 95% CI, 0.94-2.03; P = .95; supplemental Figure 4).
Forest plot of PFS by stratification factors. HRs and 95% CIs are based on unstratified Cox regression except for the overall group. “Before base treatment regimen” categories R-B to R-CHOP and R-B to R-B are combined in this analysis owing to low patient numbers. ECOG, Eastern Cooperative Oncology Group; NE, not estimable.
Forest plot of PFS by stratification factors. HRs and 95% CIs are based on unstratified Cox regression except for the overall group. “Before base treatment regimen” categories R-B to R-CHOP and R-B to R-B are combined in this analysis owing to low patient numbers. ECOG, Eastern Cooperative Oncology Group; NE, not estimable.
Overall response
In the overall iNHL population, ORR was 85.5% (224/262) in the copanlisib arm vs 86.6% (227/262) in the placebo arm (Table 3; supplemental Table 2). CRR was 38.5% (101/262) in the copanlisib arm vs 41.2% (108/262) in the placebo arm. No difference in ORR was observed between copanlisib and placebo across the different histologic subtypes (Table 3; supplemental Tables 1 and 2).
DoR
In the overall iNHL population, median DoR was 32.2 months (95% CI, 22.8-38.8) in the copanlisib arm vs 32.4 months (95% CI, 27.7-42.0) in the placebo arm (HR, 1.15; 95% CI, 0.88-1.48; P = .85; Table 3; supplemental Figure 5A). In the FL subgroup, median DoR was 22.8 months (95% CI, 16.8-33.3) in the copanlisib arm vs 32.3 months (95% CI, 25.5-45.0) in the placebo arm (HR, 1.31; 95% CI, 0.96-1.77; P = .96; Table 3; supplemental Figure 5B). In patients with marginal zone lymphoma, median DoR was 60.9 months (95% CI, 21.9-not estimable) in the copanlisib arm vs 47.3 months (95% CI, 19.8-not estimable) in the placebo arm (HR, 0.80; 95% CI, 0.39-1.65; P = .28; Table 3; supplemental Figure 5C).
OS
With a median follow-up of 54.6 months, median OS in the iNHL population was not reliably estimated and there was no difference in OS between the copanlisib and placebo arms (HR, 1.13; 95% CI, 0.80-1.60; P = .76; Table 3; Figure 1D). OS between treatment arms also showed no difference in the FL subtype of patients or across any other histologic subtypes (Table 3; supplemental Table 1; supplemental Figure 6).
Safety
The safety analysis set included 263 patients in the copanlisib arm and 257 patients in the placebo arm. The most common treatment-emergent AEs (TEAEs) of any grade were hyperglycemia (57.8%), hypertension (44.1%), nausea (42.6%), and decreased neutrophil count (41.8%) for copanlisib-treated patients and nausea (35.8%), neutropenia (34.2%), and decreased neutrophil count (32.3%) for placebo-treated patients (Table 4). Grade ≥3 TEAEs were reported in 96.2% of patients in the copanlisib arm and 79.0% of patients in the placebo arm. The most common copanlisib-related TEAEs of any grade were hyperglycemia (54.4%), hypertension (40.7%), and decreased neutrophil count (37.6%). Serious TEAEs were higher in the copanlisib arm (61.2%) than the placebo arm (21.0%). The most common serious TEAEs in the copanlisib arm were pneumonia (12.9%), pyrexia (8.4%), and febrile neutropenia (6.8%) compared with febrile neutropenia (3.1%), neutropenia (1.9%), and pyrexia (1.6%) in the placebo arm. Grade ≥3 serious TEAEs were reported in 51.7% and 14.8% of patients in the copanlisib and placebo arms, respectively. Safety profiles were largely consistent across histologic subgroups (data not shown).
Summary of most common TEAEs (safety analysis set)
| . | Copanlisib + R-B∗ (n = 263) . | Placebo + R-B (n = 257) . | ||||
|---|---|---|---|---|---|---|
| All grade . | Grade 3 . | Grade 4 . | All grade . | Grade 3 . | Grade 4 . | |
| Any TEAE, n (%) | 263 (100) | 83 (31.6) | 162 (61.6) | 250 (97.3) | 89 (34.6) | 114 (44.4) |
| Most common TEAEs occurring in ≥20% of patients in either treatment arm, n (%) | ||||||
| Hyperglycemia | 152 (57.8) | 99 (37.6) | 13 (4.9) | 53 (20.6) | 13 (5.1) | 0 |
| Hypertension | 116 (44.1) | 90 (34.2) | 0 | 42 (16.3) | 21 (8.2) | 1 (0.4) |
| Nausea | 112 (42.6) | 2 (0.8) | 0 | 92 (35.8) | 3 (1.2) | 0 |
| Decreased neutrophil count | 110 (41.8) | 39 (14.8) | 60 (22.8) | 83 (32.3) | 34 (13.2) | 32 (12.5) |
| Pyrexia | 103 (39.2) | 6 (2.3) | 0 | 36 (14.0) | 1 (0.4) | 0 |
| Diarrhea | 89 (33.8) | 10 (3.8) | 1 (0.4) | 38 (14.8) | 3 (1.2) | 0 |
| Neutropenia | 87 (33.1) | 36 (13.7) | 43 (16.3) | 88 (34.2) | 36 (14.0) | 39 (15.2) |
| Anemia | 86 (32.7) | 18 (6.8) | 0 | 64 (24.9) | 10 (3.9) | 1 (0.4) |
| Decreased white blood cell count | 79 (30.0) | 44 (16.7) | 16 (6.1) | 65 (25.3) | 37 (14.4) | 9 (3.5) |
| Decreased lymphocyte count | 73 (27.8) | 11 (4.2) | 61 (23.2) | 50 (19.5) | 12 (4.7) | 38 (14.8) |
| Decreased platelet count | 72 (27.4) | 13 (4.9) | 6 (2.3) | 52 (20.2) | 9 (3.5) | 1 (0.4) |
| Decreased weight | 64 (24.3) | 4 (1.5) | 0 | 21 (8.2) | 0 | 0 |
| Vomiting | 59 (22.4) | 1 (0.4) | 0 | 31 (12.1) | 0 | 0 |
| Rash | 59 (22.4) | 3 (1.1) | 0 | 26 (10.1) | 2 (0.8) | 0 |
| Pneumonia | 56 (21.3) | 26 (9.9) | 2 (0.8) | 6 (2.3) | 3 (1.2) | 0 |
| Most common serious TEAEs occurring in ≥2% of patients in either treatment arm, n (%) | ||||||
| Pneumonia | 34 (12.9) | 21 (8.0) | 2 (0.8) | 3 (1.2) | 2 (0.8) | 0 |
| Pyrexia | 22 (8.4) | 5 (1.9) | 0 | 4 (1.6) | 1 (0.4) | 0 |
| Febrile neutropenia | 18 (6.8) | 11 (4.2) | 5 (1.9) | 8 (3.1) | 5 (1.9) | 2 (0.8) |
| Cytomegalovirus infection | 12 (4.6) | 8 (3.0) | 1 (0.4) | 2 (0.8) | 2 (0.8) | 0 |
| Hyperglycemia | 10 (3.8) | 7 (2.7) | 3 (1.1) | 1 (0.4) | 1 (0.4) | 0 |
| Decreased neutrophil count | 7 (2.7) | 1 (0.4) | 5 (1.9) | 3 (1.2) | 1 (0.4) | 2 (0.8) |
| Interstitial lung disease | 7 (2.7) | 4 (1.5) | 0 | 0 | 0 | 0 |
| Diarrhea | 6 (2.3) | 5 (1.9) | 1 (0.4) | 0 | 0 | 0 |
| TEAEs leading to discontinuation of copanlisib/placebo, n (%) | 57 (21.7)† | 33 (12.5) | 11 (4.2) | 15 (5.8) | 10 (3.9) | 4 (1.6) |
| TEAEs of interest, n (%) | ||||||
| Pneumonitis | 8 (3.0) | 1 (0.4) | 1 (0.4) | 2 (0.8) | 0 | 0 |
| Colitis | 3 (1.1) | 1 (0.4) | 0 | 2 (0.8) | 0 | 0 |
| . | Copanlisib + R-B∗ (n = 263) . | Placebo + R-B (n = 257) . | ||||
|---|---|---|---|---|---|---|
| All grade . | Grade 3 . | Grade 4 . | All grade . | Grade 3 . | Grade 4 . | |
| Any TEAE, n (%) | 263 (100) | 83 (31.6) | 162 (61.6) | 250 (97.3) | 89 (34.6) | 114 (44.4) |
| Most common TEAEs occurring in ≥20% of patients in either treatment arm, n (%) | ||||||
| Hyperglycemia | 152 (57.8) | 99 (37.6) | 13 (4.9) | 53 (20.6) | 13 (5.1) | 0 |
| Hypertension | 116 (44.1) | 90 (34.2) | 0 | 42 (16.3) | 21 (8.2) | 1 (0.4) |
| Nausea | 112 (42.6) | 2 (0.8) | 0 | 92 (35.8) | 3 (1.2) | 0 |
| Decreased neutrophil count | 110 (41.8) | 39 (14.8) | 60 (22.8) | 83 (32.3) | 34 (13.2) | 32 (12.5) |
| Pyrexia | 103 (39.2) | 6 (2.3) | 0 | 36 (14.0) | 1 (0.4) | 0 |
| Diarrhea | 89 (33.8) | 10 (3.8) | 1 (0.4) | 38 (14.8) | 3 (1.2) | 0 |
| Neutropenia | 87 (33.1) | 36 (13.7) | 43 (16.3) | 88 (34.2) | 36 (14.0) | 39 (15.2) |
| Anemia | 86 (32.7) | 18 (6.8) | 0 | 64 (24.9) | 10 (3.9) | 1 (0.4) |
| Decreased white blood cell count | 79 (30.0) | 44 (16.7) | 16 (6.1) | 65 (25.3) | 37 (14.4) | 9 (3.5) |
| Decreased lymphocyte count | 73 (27.8) | 11 (4.2) | 61 (23.2) | 50 (19.5) | 12 (4.7) | 38 (14.8) |
| Decreased platelet count | 72 (27.4) | 13 (4.9) | 6 (2.3) | 52 (20.2) | 9 (3.5) | 1 (0.4) |
| Decreased weight | 64 (24.3) | 4 (1.5) | 0 | 21 (8.2) | 0 | 0 |
| Vomiting | 59 (22.4) | 1 (0.4) | 0 | 31 (12.1) | 0 | 0 |
| Rash | 59 (22.4) | 3 (1.1) | 0 | 26 (10.1) | 2 (0.8) | 0 |
| Pneumonia | 56 (21.3) | 26 (9.9) | 2 (0.8) | 6 (2.3) | 3 (1.2) | 0 |
| Most common serious TEAEs occurring in ≥2% of patients in either treatment arm, n (%) | ||||||
| Pneumonia | 34 (12.9) | 21 (8.0) | 2 (0.8) | 3 (1.2) | 2 (0.8) | 0 |
| Pyrexia | 22 (8.4) | 5 (1.9) | 0 | 4 (1.6) | 1 (0.4) | 0 |
| Febrile neutropenia | 18 (6.8) | 11 (4.2) | 5 (1.9) | 8 (3.1) | 5 (1.9) | 2 (0.8) |
| Cytomegalovirus infection | 12 (4.6) | 8 (3.0) | 1 (0.4) | 2 (0.8) | 2 (0.8) | 0 |
| Hyperglycemia | 10 (3.8) | 7 (2.7) | 3 (1.1) | 1 (0.4) | 1 (0.4) | 0 |
| Decreased neutrophil count | 7 (2.7) | 1 (0.4) | 5 (1.9) | 3 (1.2) | 1 (0.4) | 2 (0.8) |
| Interstitial lung disease | 7 (2.7) | 4 (1.5) | 0 | 0 | 0 | 0 |
| Diarrhea | 6 (2.3) | 5 (1.9) | 1 (0.4) | 0 | 0 | 0 |
| TEAEs leading to discontinuation of copanlisib/placebo, n (%) | 57 (21.7)† | 33 (12.5) | 11 (4.2) | 15 (5.8) | 10 (3.9) | 4 (1.6) |
| TEAEs of interest, n (%) | ||||||
| Pneumonitis | 8 (3.0) | 1 (0.4) | 1 (0.4) | 2 (0.8) | 0 | 0 |
| Colitis | 3 (1.1) | 1 (0.4) | 0 | 2 (0.8) | 0 | 0 |
Medical Dictionary for Regulatory Activities version 26.0. Common Terminology Criteria for Adverse Events version 4.03.
Two patients were randomized to placebo plus R-B but received ≥1 dose of copanlisib by mistake and are thus included in the copanlisib plus R-B arm.
Includes 5 grade 5 TEAEs leading to discontinuation.
Grade 5 TEAEs occurred in 8 patients (3.0%) in the copanlisib arm, including 3 with related death. Among them, 1 patient had 3 serious TEAEs of febrile neutropenia, left ventricular dysfunction, and sepsis that were deemed to be related to copanlisib plus R-B, and 2 patients had a serious TEAE of febrile neutropenia or acute respiratory distress syndrome, which were both considered to be related to R-B. No grade 5 TEAEs and no treatment-related deaths were reported in the placebo arm.
TEAEs leading to discontinuation were reported in 21.7% of patients in the copanlisib arm vs 5.8% in the placebo arm (Table 4).
TEAEs related to copanlisib/placebo treatment led to treatment discontinuation in 15.6% of patients in the copanlisib arm and 3.9% in the placebo arm (supplemental Table 3).
Infection TEAEs were reported in 74.5% of patients in the copanlisib treatment arm; of these, 28.5% were grade 3, 3.4% were grade 4, and 0.8% were grade 5. The most common infection TEAEs in the copanlisib arm were pneumonia (21.3%), cytomegalovirus infection (19.8%), and upper respiratory tract infection (16.7%). In comparison, 55.3% of patients in the placebo treatment arm experienced infection TEAEs, 8.6% of which were grade 3 and none of which were grade 4 or 5. For the placebo treatment arm, the most common infection TEAEs were upper respiratory tract infection (14.8%), urinary tract infection (7.0%), and herpes zoster (6.2%). Concurrent use of systemic anti-infective therapy (before or during treatment) was higher for patients in the copanlisib arm than the placebo arm at 93.9% vs 75.6%, respectively (supplemental Table 4). Nineteen patients (7.2%) in the copanlisib treatment arm experienced infection TEAEs leading to discontinuation of treatment, comprising nearly one-third of the 57 reported TEAEs leading to discontinuation within that arm. The most common infection TEAE leading to discontinuation within the copanlisib treatment arm was pneumonia, which was reported in 7 patients (2.7%).
Deaths during study treatment were reported in 8.4% of patients in the copanlisib arm and 3.5% of patients in the placebo arm. The primary cause of death was AEs not associated with PD in both treatment arms and included 6.1% of patients in the copanlisib arm and 1.9% of patients in the placebo arm.
Quality of life
Median time to deterioration in DRS-P of ≥3 points in the copanlisib arm was lower than the placebo arm (2.7 vs 6.3 months, respectively; HR, 1.39; 95% CI, 1.15-1.69; P = .99). Moreover, median time to improvement in DRS-P of ≥3 points was higher in the copanlisib arm vs the placebo arm (12.8 vs 5.1 months, respectively; HR, 0.81; 95% CI, 0.64-1.02; P = .97).
Pharmacokinetics and ER analyses
Of the 263 patients in the copanlisib arm, 256 (97.3%) provided ≥1 valid pharmacokinetic observation and were included in the pharmacokinetic analyses and exposure-response (ER) analyses for safety. ER analyses for PFS included the subset of patients completing 3 cycles and full copanlisib treatment.
The previously established population pharmacokinetic model was able to reasonably capture copanlisib pharmacokinetics in this study to derive individual copanlisib exposure metrics for comparison across studies and for use in ER analyses, based on visual predictive checks (supplemental Figure 7). Copanlisib clearance in patients enrolled in this study was similar to the historical CHRONOS-1 study and slightly higher than a previously identified effect seen in patients enrolled in CHRONOS-3 (supplemental Figure 8).11
When accounting for dose modifications, the range of average daily copanlisib exposure was lower in CHRONOS-4 than in CHRONOS-1 and CHRONOS-3, reflecting the high dose modifications and low dose intensity in this study (supplemental Figure 9).12
Exposure-efficacy analyses confirmed no overall treatment effect for patients enrolled in the copanlisib arm vs the placebo arm and showed no apparent PFS relationship between tertiles of copanlisib exposure during the first 3 treatment cycles or until the end of treatment in patients reaching those time points, respectively (supplemental Figure 10).
Exposure-safety analyses confirmed the clear treatment effect for patients in the copanlisib arm vs the placebo arm; however, ER analyses demonstrated no ER relationship for time to first serious TEAE or grade ≥3 TEAE, beyond cycle 1 (supplemental Figure 11). An early exposure-dependent effect was identified during cycle 1, which dissipated in patients who continued copanlisib.
Biomarkers
A benefit in PFS was observed for patients with FL with low or undetectable (≤0.356 pg/mL) baseline levels of interleukin-2 vs those with high baseline levels in the copanlisib arm (HR, 0.45; 95% CI, 0.26-0.78; unadjusted P = .0048; supplemental Figure 12A). OS was also higher for patients with FL with low vs high baseline levels of interleukin-2 who received copanlisib (HR, 0.31; 95% CI, 0.12-0.80; P = .0154; supplemental Figure 12B). However, in the placebo arm, no difference in OS was observed between patients with FL and low and high baseline levels of interleukin-2 (HR, 1.10; 95% CI, 0.54-2.24; unadjusted P = .7922; supplemental Figure 12B).
Discussion
In the CHRONOS-4 study, no difference was observed in median PFS between the copanlisib arm (32.9 months) and the placebo arm (33.3 months) in the overall iNHL population; therefore, the study did not meet its primary end point. Similarly, there were no differences in the secondary end points of OS, ORR, and DoR in the overall population. In the FL subgroup, median PFS and median DoR were lower in the copanlisib arm than the placebo arm, whereas in all the other histologic subgroups, PFS increased in the copanlisib arm. No differences were observed in OS and ORR for any of the histologic subgroups. Failure of CHRONOS-4 to meet its primary end point of improved PFS for the combination of copanlisib with standard immunochemotherapy led to study termination by the sponsor.13
Comparisons between CHRONOS-4 and previous copanlisib trials yield valuable insights but are complicated by key differences in trial design, most notably the use of a 12-month treatment period limit for CHRONOS-4 compared with treatment until progression for CHRONOS-3 and CHRONOS-1.4,6 Although not improved compared with placebo plus R-B, median PFS for copanlisib plus R-B in CHRONOS-4 was longer at 32.9 months than median PFS for copanlisib with rituximab alone in CHRONOS-3 at 21.5 months.6 In addition, PFS observed in the placebo arm of CHRONOS-3 (rituximab only) was considerably lower than that observed in this study for patients treated with rituximab plus chemotherapy (13.8 vs 33.3 months), suggesting a beneficial impact from the addition of chemotherapy to rituximab. However, the PFS and DoR observed in the placebo arm in CHRONOS-4 were greater than previously reported data for the combination of rituximab with chemotherapy.14 In a phase 2, open-label study, the combination of the chemotherapy agent bendamustine plus rituximab in patients with relapsed indolent B-cell or mantle cell lymphoma resulted in PFS of 23 months, which was substantially shorter than the 33 months of PFS observed in this study for the placebo plus R-B arm.14 Similarly, DoR for the placebo plus R-B arm in CHRONOS-4 was higher than previous reports of rituximab plus bendamustine (32 vs 21 months).14
The lack of clinical benefit seen in the copanlisib arm vs the placebo arm of CHRONOS-4 may have been caused by shorter treatment duration (8.5 vs 11.4 months, respectively) and greater discontinuations owing to TEAEs (21.7% vs 5.8%, respectively). Infusion-related TEAEs of hyperglycemia (57.8% vs 20.6%) and hypertension (44.1% vs 16.3%) were higher in the copanlisib arm as expected,4,6 but serious TEAEs of pneumonia (12.9% vs 1.2%), pyrexia (8.4% vs 1.6%), and febrile neutropenia (6.8% vs 3.1%) were also higher. Indeed, grade ≥3 TEAEs occurred more frequently in the copanlisib arm than the placebo arm, mostly accounted for by grade 4 (61.6% vs 44.4%) and grade 5 (3.0% vs 0%) TEAEs. Consistent with the overall lack of tolerability seen in the copanlisib arm, measures of quality of life using the DRS-P subscale of the Functional Assessment of Cancer Therapy–Lymphoma Symptom Index-18 questionnaire favored the longer time to deterioration and shorter time to improvement in the placebo arm than the copanlisib arm.
Pharmacokinetic and ER analyses in this study may support the notion of the lack of combinability of copanlisib with chemotherapy and potentially suggest that the lower copanlisib dose intensity led to lower copanlisib exposures during treatment, which may have limited the potential clinical benefit of copanlisib and may have contributed to the lack of treatment benefit compared to control. Indeed, copanlisib exposure, when accounting for dose modifications, revealed a lower range of copanlisib average concentrations than previous copanlisib studies, suggesting limited ability to deliver copanlisib and derive benefit in this combination. A lack of copanlisib ER is seen for PFS in this study, which differs from previous analyses in CHRONOS-3 that justified the recommended copanlisib dosing regimen based on CHRONOS-3 study results.11,12 These differences may be caused by the differing treatment combination used in CHRONOS-4, the smaller number of patients completing copanlisib treatment for investigation of ER relationships and/or deriving benefit from copanlisib treatment, the fixed treatment duration used in this study, and/or the lower copanlisib exposure achieved in this study, reflecting the overall intolerability of the combination regimen used in CHRONOS-4. ER analyses for safety confirmed the clear treatment effect for copanlisib vs placebo but no clear ER relationship for the 2 investigated safety end points. The ER analyses are limited by evaluation of only 1 dosing regimen in CHRONOS-4. Lower copanlisib doses or less frequent schedules may have improved the tolerability in this combination; however, the resulting impact on efficacy would need to be considered given that previous CHRONOS-3 analyses demonstrated a significant ER relationship for efficacy using an identical dose and schedule.11,12
The safety profile for the copanlisib plus R-B arm in CHRONOS-4 demonstrated higher toxicity than the copanlisib plus rituximab arm in CHRONOS-3.8 In CHRONOS-4, any-grade TEAEs leading to discontinuation of copanlisib were reported at a similar rate (∼22%) to CHRONOS-3 and CHRONOS-1 (25% for both). However, grade 4 TEAEs were markedly higher for copanlisib plus R-B (62%) than for the primary disclosures of copanlisib plus rituximab (36%) and copanlisib as monotherapy (27%).4,6 In addition, rates of cytomegalovirus infection were markedly higher for the copanlisib plus R-B arm in CHRONOS-4 (31.9% for TEAEs; 7.6% for serious TEAEs) than the copanlisib plus rituximab arm in CHRONOS-3 (7.2% for TEAEs; 0.3% for serious TEAEs; Bayer data on file), suggesting an increase in infections after combination with chemotherapy.6
Recent studies have highlighted the immunomodulatory activity of copanlisib, showing that it promotes immune activity and enhances antitumor responses by overcoming immune suppression driven by regulatory T-cell and M2 phenotype tumor-associated macrophages.15 Therefore, interleukin-2, a well-studied cytokine that influences maintenance of regulatory T-cells and differentiation of T-cell subsets, was assessed. Biomarker analyses in CHRONOS-4, although limited by the exploratory nature of the analyses and the small number of patients across biomarker subgroups, supported recent findings from CHRONOS-3.16 In both CHRONOS-3 and CHRONOS-4, OS in patients with FL was improved for those with low/undetectable baseline interleukin-2 vs high baseline interleukin-2 in patients in the copanlisib arm, but not the placebo arm, suggesting a potential enhancement of patient survival for copanlisib in patients with low/undetectable baseline interleukin-2.16
The lack of tolerability observed for copanlisib plus R-B vs placebo plus R-B mirrors similar tolerability issues for other PI3K inhibitors evaluated for iNHL.3 In addition to copanlisib, 4 PI3K inhibitors were previously approved for patients with advanced hematologic malignancies after demonstrating efficacy as monotherapy in phase 2 trials.17,18 However, multiple randomized trials of PI3K inhibitors (eg, idelalisib, duvelisib, and umbralisib) in combination with other therapies have resulted in safety concerns owing to the occurrence of infections, other toxicities, high rates of fatal events, and concerning OS results indicating possible harm to patients.7 These observations resulted in the withdrawal of FDA-approved PI3K inhibitors and led to an FDA recommendation that future PI3K inhibitor approvals should be supported by randomized trials3,19,20 (the sponsor has decided to withdraw the FDA New Drug Application for copanlisib for adult patients with relapsed FL who have received ≥2 therapies13).
In conclusion, the results of this study suggest that combination of copanlisib with immunochemotherapy does not improve survival or patient responses vs immunochemotherapy and is associated with decreased tolerability in patients with iNHL who had previously received anticancer therapy. Together, these data suggest an unfavorable benefit-risk profile for copanlisib in combination with standard immunochemotherapy and support the recent sponsor decision to terminate the CHRONOS-4 trial and the copanlisib program.
Acknowledgments
The authors thank the patients and their families, coinvestigators, and referring physicians who participated in this study.
The study was supported by Bayer AG. Rosalba Satta, Ana Alafarga Mafe, and Rachel Fairbanks, on behalf of Complete HealthVizion, IPG Health Medical Communications, provided medical writing and editing assistance in the development of this manuscript, funded by Bayer AG.
Authorship
Contribution: All authors had access to primary trial data and were involved in the collection and analysis of data; and all authors were involved in the writing, reviewing, and approval of the manuscript.
Conflict-of-interest disclosure: P.L.Z. reports consulting fees from EUSA Pharma, Merck, Sharp & Dohme, and Novartis; speaker bureau involvement for AstraZeneca, BeiGene, Bristol Myers Squibb (BMS), Celltrion, EUSA Pharma, Gilead, Incyte, Janssen-Cilag, Kyowa Kirin, Merck, Sharp & Dohme, Novartis, Roche, Servier, and Takeda; and advisory board participation for ADC Therapeutics, AstraZeneca, BeiGene, BMS, Celltrion, EUSA Pharma, Gilead, Incyte, Janssen-Cilag, Kyowa Kirin, Merck, Sharp & Dohme, Novartis, Roche, Sandoz, Secura Bio, Servier, and Takeda, outside the submitted work. H.W. reports travel grants from Amgen, Bayer, Pfizer, and Takeda; and research grants from Amgen, Bayer, and Pfizer, outside the submitted work. T.M.K. reports institutional support from AbbVie, AstraZeneca, Bayer, Black Diamond Therapeutics, Blueprint Medicines, BMS, Boryung, Celgene, F. Hoffmann-La Roche/Genentech, Hanmi, Janssen, Novartis, Regeneron, Sanofi, Takeda, and Yuhan; consulting fees from AstraZeneca, IMBDx, Janssen, Regeneron, Samsung Bioepis, Takeda, and Yuhan; and advisory board participation for AstraZeneca, Janssen, Regeneron, and Takeda, outside the submitted work. M.Ö. reports research grants from AbbVie, Acerta, Bayer, Janssen, Merck, Sharp & Dohme, Pfizer, PSI, Roche, and Takeda; and travel support from AbbVie, Merck, Sharp & Dohme, and Sandoz, outside the submitted work. I.K. reports involvement in clinical trials for AbbVie, Acerta, Bayer, Cromos Pharma, GlaxoSmithKline, InnoCare Pharma, Merck, MorphoSys, Pharmacyclics, and Takeda; and speaker bureau involvement for AbbVie, AstraZeneca, Biopharma, Janssen, Merck, Roche, and Takeda, outside the submitted work. C.P. is an employee of Bayer HealthCare Pharmaceuticals, Inc and may hold stock or stock options in the company. S.C. is an employee of Bayer HealthCare Pharmaceuticals, Inc. A.W. is an employee of Bayer AG and may hold stock or stock options in the company. P.N.M. is an employee of Bayer HealthCare Pharmaceuticals, Inc and may hold stock or stock options in the company. F.O. is an employee of Bayer SA. V.B. is an employee of Bayer HealthCare Pharmaceuticals, Inc and may hold stock or stock options in the company. B.H.C. is an employee of Bayer HealthCare Pharmaceuticals, Inc and may hold stock or stock options in the company. M.D. reports research grants from AbbVie, Bayer, BMS/Celgene, Gilead/Kite, Janssen, Lilly, and Roche; honoraria from AstraZeneca, BeiGene, Gilead/Kite, Janssen, Lilly, Novartis, and Roche; and advisory board participation for AbbVie, AstraZeneca, BeiGene, BMS/Celgene, Gilead/Kite, Janssen, Lilly/Loxo Oncology, Novartis, and Roche, outside the submitted work. M.M. reports research grants from Bayer, Genentech, GM Biosciences, ImmunoVaccine Technologies, Janssen, Pharmacyclics, Roche, and Seattle Genetics; honoraria from ADC Therapeutics, AstraZeneca, Bayer, BMS, Celgene, Epizyme, ImmunoVaccine Technologies, Janssen, Kite, Pharmacyclics, Regeneron, Roche, Seagen, Seattle Genetics, and Takeda; stipends from ADC Therapeutics, AstraZeneca, BMS, Celgene, Epizyme, ImmunoVaccine Technologies, Kite, Regeneron, and Seagen; consultancy for Bayer, Genentech, Juno Therapeutics, Roche, Seattle Genetics, Takeda, and Teva; membership on board/advisory committee for Genentech and Merck; and equity ownership in Merck, outside the submitted work. P.G. reports consulting fees from AbbVie, AstraZeneca, and Daiichi Sankyo; and research grants from Kite, outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Paola Ghione, Memorial Sloan Kettering Cancer Center, 530 E 74th St, New York, NY 10021; email: ghionep@mskcc.org.
References
Author notes
P.L.Z. and H.W. are joint first authors.
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the European Federation of Pharmaceutical Industries and Associations/Pharmaceutical Research and Manufacturers of America “Principles for responsible clinical trial data sharing.” This pertains to scope, time point, and process of data access. As such, Bayer commits to sharing, upon request from qualified scientific and medical researchers, patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States and European Union (EU), as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the US and EU regulatory agencies on or after 1 January 2014. Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the study sponsor’s section of the portal. Data access will be granted to anonymized patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
The full-text version of this article contains a data supplement.