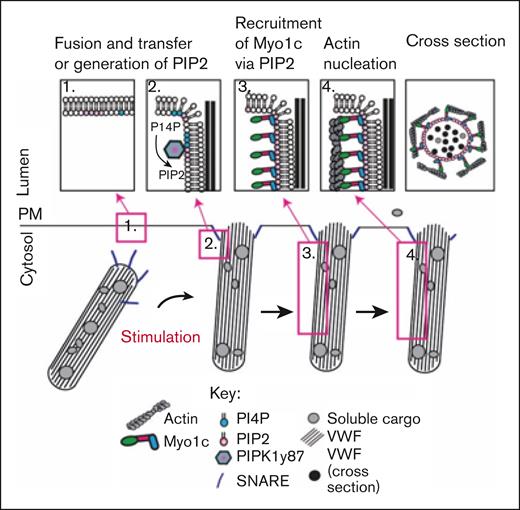
In this issue of Blood Advances, El-Mansi et al1 communicate a novel mechanism important in the context of hemostasis and thrombosis related to the role of myosin 1C (Myo1c) in von Willebrand factor (VWF) expulsion from endothelial cell (EC) Weibel-Palade bodies (WPBs). Their research demonstrates that Myo1c is recruited to WPBs after fusion with the plasma membrane (PM) by its interaction with phosphoinositide 4,5 bisphosphate (PI(4,5)P2), a highly negatively charged phospholipid that promotes membrane dynamics by altering membrane fluidity and curvature known to play key roles in exocytosis. Myo1c is necessary for maximally evoked secretion of VWF, and the study clarifies the active processes associated with actin framework reorganization that implicate the ATPase activity of Myo1c in the reorganization and initiation of actin nucleation, ring assembly, and contractile processes surrounding WPBs (see figure). To our knowledge, the study provides the first evidence for the role of a class I myosin in the exocytosis of WPB contents from ECs and thus significantly contributes to our understanding of the intricate mechanisms regulating VWF secretion and resultant hemostasis or thrombosis.
Schematic representation of working model of Myo1c-mediated membrane actin nucleation required for EC WPB exocytosis.1
Schematic representation of working model of Myo1c-mediated membrane actin nucleation required for EC WPB exocytosis.1
The study extends El-Mansi et al2 recent observations published in the February 2023 issue of Blood, which provided a novel inventory of potential regulators of WPB fusion and exocytosis, among which Myo1c was found by proximity biotinylation proteomics in stimulated cultured human ECs to be in close association with Rab27, a Ras-related small guanosine triphosphate hydrolase found on the cytoplasmic face of the WPB membrane. Importantly, Myo1c was enriched only after stimulation with phorbol-myristoyl acetate (PMA), thrombin, or a cocktail of histamine/adrenaline/3-isobutyl-1-methylxanthine, and thus they hypothesized that Myo1c may be involved in regulated WPB exocytosis.
Myo1c has also been implicated in the trafficking of receptors such as protease-activated receptor-1 and vascular endothelial growth factor receptor-2 (VEGFR2) to the PM, in which its inhibition was shown to reduce receptor expression and stability in the membrane. Interestingly, these experiments revealed that Myo1c inhibition and the resultant decrease in PM VEGFR2 correlated with a decrease in VWF secretion, suggesting Myo1c-dependent exocytosis is essential for both receptor recycling and VWF secretion. To avoid effects on receptor expression, subsequent studies primarily used PMA to study the mechanism of Myo1c activation and its role in VWF secretion from human umbilical vein endothelial cells (HUVEC).
PM homeostasis requires the maintenance of phospholipids under normal resting conditions. Upon stimulation and Ca2+ influx, the amount of PI(4,5)P2 rises temporarily during exocytosis.3 The presence of PIP2 in excess at the inner leaflet of the PM initiates spontaneous negative membrane curvature (inward bending, concave structure) because of the inverted conical shape of PIP2. The process is continued by membrane-curving proteins, such as BAR domain–containing proteins.4 Independent pools of PIP2 regulate distinct steps during exocytosis.3 During regulated WPB exocytosis from HUVEC, a positive-feed-forward loop elevates PIP2 and phosphatidic acid levels at the site of vesicle fusion with the PM in a PIP5K1γ87 and phospholipase D1–dependent manner, respectively.3 Clustering PIP2 into larger domains1,3 is a recruitment signal for bringing in proteins required for tethering WPBs to the PM, priming, fusion, actin ring contraction, and VWF expulsion.3,5,6 During regulated WPB exocytosis, spatial and temporal constraints at the PM and phospholipid-binding affinities orchestrate the sequence of protein-lipid and protein-protein interactions.3 These factors have been extensively studied, but the motor link between PIP2 and the actin ring responsible for VWF extrusion had been unclear3,7 until now.
The study by El-Mansi et al defines the dynamics of Myo1c interaction with PIP2 during regulated VWF secretion. Myo1c constructs with point mutations K892A and R903A in the phosphoinositide-binding domain4 disrupted the interaction with PIP2 and resulted in their accumulation in the cytosol, confirming the critical role of these residues in Myo1c recruitment to the membrane at sites of WPB fusion. PIP2 sensors and generating enzymes, such as phospholipase C δ1 (PLCδ1) and PIP5K1γ87, were observed at sites of membrane fusion in the context of WPB exocytosis, confirming PIP2 generation in the inner leaflet of the PM is a key driver of protein recruitment required for exocytosis of VWF.
The involvement of Myo1c in regulated WPB secretion aligns with previous observations of Myo1c-dependent activity in other secretory cell types. For instance, surfactant exocytosis from alveolar type 2 cells8 and glucose transporter type 4 transport to the membrane upon insulin activation of pancreatic beta cells9 revealed Myo1c localized near secretory vesicle membranes after fusion. Similarly, Miklavc et al showed in pneumocytes overexpressing actin-GFP that lamellar bodies merging with the cell membrane were accompanied by the actin coating of fused lamellar bodies, which could be inhibited by phalloidin.10 Here, they advance these findings by demonstrating the novel role of Myo1c in the expulsion of VWF from HUVEC WPBs. Their use of live-cell imaging of fluorescent Myo1c at sites of WPB fusion provides visual confirmation of its functional involvement in secretion. The use of soluble fluorophore-tagged P-selectin as a fusion marker to determine when myosin1C is recruited was ingenious and revealed it to be postfusion as P-selectin was already visible when Myo1c-GFP began to accumulate. Another well-designed experiment was the use of fluorophore-tagged anti-VWF antibody in the culture medium to capture endogenous VWF unwinding from fused WPBs while concomitantly observing the sequential recruitment of Myo1c-GFP or LifeAct-Ruby inside the cell via super resolution spinning disk confocal microscopy. To refine our understanding of the mechanism of Myo1c recruitment to the PM, the investigators used truncated and single amino acid substitution mutants of Myo1c coexpressed with LifeAct-Ruby, which demonstrated that it is the Myo1c N-terminus that interacts with the leading edge of WPBs. In addition, in support of the hypothesis that Myo1c also interacts with the WPB membrane via its C-terminus, these studies further revealed a role for the Myo1c C-terminal plekstrin homology domain, as shown by the colocalization of expressed Myo1c-tail + 3IQ-GFP with fused WPBs after stimulation.
In summary, WPB exocytosis requires Myo1c domain-specific11 electrostatic interactions with negatively charged PIP2 at the PM and Myo1c ATPase activity for the contractile actin ring to efficiently promote VWF release. However, lingering questions remain. Are there specific membrane domains essential for WPB fusion? Does Myo1c and actin engulf the entire WPB and contract in a manner that promotes the expulsion of all WPB contents? Would inhibition of Myo1c in vivo lead to unwanted effects? One can anticipate that these new insights into WPB secretion dynamics will bring about new therapeutic approaches for inhibition of prothrombotic high-molecular weight VWF secretion at its primary source, EC WPBs. This could be applied through a local delivery system such as drug-eluting stents, whereby targeting WPB exocytosis would have the advantage of fine-tuning thrombotic propensity such that plasma VWF levels are sufficient to support hemostasis but not so high as to drive pathological clot formation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.