Key Points
In severe HLA-alloimmune platelet transfusion refractoriness, DSA-MFI sums >10 000 and major-ABO mismatch actionably predict poor 2-hour CCI.
Computer-aided histocompatibility ranking to lower DSA-MFI sums may streamline donor selection and improve CCIs.
Visual Abstract
Up to a third of patients with hemato-oncologic conditions who have received multiply transfusions develop immune-mediated platelet transfusion refractoriness. Yet factors that influence posttransfusion platelet corrected count increments (CCI) in patients with HLA-alloimmune platelet transfusion refractoriness remain less well elucidated. Recent advances in HLA antibody characterization using fluorescent bead-based platforms enable the study of donor-specific antibody (DSA) avidity (as measured by mean fluorescence intensity [MFI]) and its impact on HLA-alloimmune platelet transfusion refractoriness. In this large retrospective study of 2012 platelet transfusions among 73 HLA-alloimmunized patients, we evaluated the impact of cumulative HLA DSA-MFI alongside other donor, platelet component, and patient characteristics on CCI at 2 and 24 hours after transfusion. As part of a quality improvement initiative, we also developed and tested a computerized algorithm to optimize donor–recipient histocompatibility based on cumulative DSA-MFI and sought other actionable predictors of CCI. In multivariate analyses, cumulative HLA DSA-MFI of ≥10 000, major/bidirectional ABO-mismatch, splenomegaly, transfusion reactions, and platelet storage in additive solution negatively affected 2-hour but not 24-hour posttransfusion CCI. The DSA-MFI threshold of 10 000 was corroborated by greater antibody-mediated complement activation and significantly more CCI failures above this threshold, suggesting the usefulness of this value to inform “permissive platelet mismatching” and to optimize CCI. Furthermore, DSA-MFI decreases were deemed feasible by the computer-based algorithm for HLA-platelet selection in a pilot cohort of 8 patients (122 transfusions) evaluated before and after algorithm implementation. When HLA-selected platelets are unavailable, ABO-identical/minor-mismatched platelet concentrates may enhance 2-hour CCI in heavily HLA-alloimmunized patients with platelet transfusion refractoriness.
Introduction
HLA-alloimmunization–mediated platelet transfusion refractoriness and the associated bleeding risks remain therapy-limiting and potentially life-threatening complications in patients with hematologic malignancies and those undergoing hematopoietic stem cell transplantation.1 Platelet transfusion refractoriness due to HLA alloimmunization may be mitigated by transfusing platelets that are matched at HLA-A and -B antigens between the platelet donor and transfusion recipient,2 by avoiding donors with antigens to which the recipient has formed antibodies,3 and/or by platelet crossmatching donor serum with platelets to be transfused.4 When HLA-matched donors are unavailable, platelet components with lesser degrees of donor–recipient histocompatibility mismatch may be transfused. These “permissively mismatched” platelet components may express HLA antigens in cross-reactive groups5 or HLAMatchmaker-defined antigen epitope mismatches6 to which the transfusion recipients are less likely to be alloimmunized. Blood centers currently use these methods alone or in combination. However, cross-reactive group–based HLA platelet selection has demonstrated inconsistent benefit,5 and epitope matching requires high-resolution HLA typing (not routinely performed in platelet donors) or a need to impute HLA allele subtype based on the probability of occurrence in local donor pools.6,7
Antigen-conjugated fluorescent bead–based platforms currently used for HLA antibody testing can measure antibody specificity and avidity to specific antigens by mean fluorescence intensity (MFI). These MFI measurements appear to correlate loosely with the strength of clinically significant HLA antibodies and the effectiveness of platelet transfusions in small studies.8,9 We hypothesized that when the summation of patient antibody MFI directed against the platelet donor’s HLA antigens was low, a platelet transfusion would more likely be successful. We tested whether “permissive platelet mismatching” below a certain cumulative donor specific antibody (DSA)–MFI threshold results in sufficient posttransfusion platelet count increments in HLA alloimmunized patients with platelet transfusion refractoriness.
Because other immune and nonimmune factors10 including an enlarged spleen, ABO blood group antigen mismatching, infection, and bleeding may concomitantly complicate platelet transfusion refractoriness, we evaluated the relative influence of other recipient, donor, and component factors11 on platelet increments in patients with HLA antibody–associated platelet transfusion refractoriness. Finally, via collaborative interinstitutional quality improvement efforts, we developed and tested a computerized algorithm to streamline donor selection and mitigate the impact of cumulative DSA-MFI on platelet transfusion responsiveness in these patients.
Methods
Patient selection and data abstraction
In a subset of prestored patient serum samples, we performed complement (C1q) testing (supplemental Laboratory Assays) to examine the relationship between DSA-MFI, complement binding, and CCI.
Statistical methods
Primary outcomes were CCI at 2 and 24 hours after each platelet transfusion. Statistical analysis included univariate and multivariable modeling.
For the univariate analysis, graphics and standard data analysis were performed via R statistical software (version 4.00: R Core Team 2020). Significance tests for comparisons between 2 groups were conducted with 2-tailed, nonpaired t tests. Values between >2 groups were compared using analysis of variance. Proportions between 2 groups were compared using a 2-tailed Fisher exact test, and comparisons of proportions between multiple groups were made using χ2 analysis. Results are provided as the mean ± standard deviation unless otherwise stated.
Multivariable analyses were performed using linear mixed-effects models using R statistical software (version 4.13: R Core Team 2021). Autocorrelation function plots were used to assess autocorrelation among transfusions from the same patient over time and accounted for using autoregressive lag-1 correlation structure when present. To account for the possibility that the relationship between the sum of MFI and the 2 CCI outcomes may change when the sum of MFI exceeds 10 000, we compared a model containing a separate slope and intercept for the sum of MFI >10 000 to reduced models assuming a constant linear relationship for each outcome using a likelihood ratio test, selecting the more complicated model only if it resulted in significantly better prediction. Models were estimated using maximum likelihood for the purpose of the above comparison, and then refit using restricted maximum likelihood for subsequent estimation and inference.
Regarding dependent variables, we tested the effects of 16 separate patient, donor, or platelet component characteristics on CCI outcomes (supplemental Table 2). Using the Holm-Šídák method, we tested the effect of each characteristic at a 2-tailed α = 1 − (0.95ˆ(1/16)) = 0.0032 to maintain a familywise α of .05 for each outcome; 95% confidence intervals are not adjusted for multiple comparisons.
Interinstitutional quality improvement algorithm development
To operationalize the findings of this study, we collaborated with the HLA laboratory and medical data science specialists at the National Institutes of Health Clinical Center, Department of Transfusion Medicine, to develop a computerized algorithm designed to optimize HLA-selection by prioritizing lower cumulative DSA-MFI (version 3.10.10). Using 122 consecutive HLA-selected transfusions at the National Institutes of Health (NIH) Clinical Center, we tested the algorithm on a small scale before and after implementation (61 transfusions before, and 61 transfusions after) under a quality improvement–exempt NIH Clinical Center protocol.
Results
Demographics
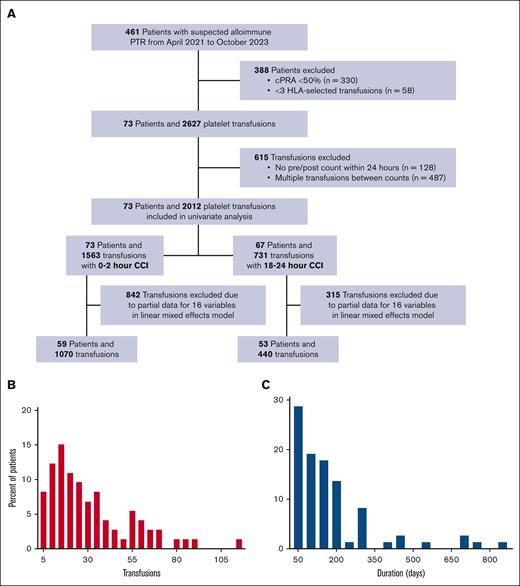
Of 2012 platelet transfusions (73 patients) analyzed, 1070 and 440 transfusions had complete data at 2 and 24 hours after transfusion (for inclusion in the multivariable model), respectively (Figure 1A). On average, each patient received 29 transfusions (Figure 1B) over a mean duration of 45 days (Figure 1C). Each patient received a mean of 1 randomly selected apheresis platelet for every 4 HLA-selected units. Recipient demographics by the 2-outcome measures are summarized in Table 1. Of note, significantly fewer transfusions had CCI data at 24 hours (n = 731) than at 2 hours (n = 1563).
Patient selection and inclusion/exclusion criteria. (A) Patients with a cPRA of <50% were excluded because our institutional practice is to give random donor platelets in this setting. Generally, alloimmunization is suspected as a cause of PTR after 2 sequential platelet transfusions with poor count increment, however, this is not a strict criterion. Histograms of the total number (B) of platelet transfusions per patient among 2140 total platelet transfusions and the duration (C) of platelet transfusion support (encompassing the time between the first and the last documented transfusion), during the study period. The y-axis in each figure represents patient counts. cPRA, calculated panel reactive antibody; PTR, platelet transfusion refractoriness.
Patient selection and inclusion/exclusion criteria. (A) Patients with a cPRA of <50% were excluded because our institutional practice is to give random donor platelets in this setting. Generally, alloimmunization is suspected as a cause of PTR after 2 sequential platelet transfusions with poor count increment, however, this is not a strict criterion. Histograms of the total number (B) of platelet transfusions per patient among 2140 total platelet transfusions and the duration (C) of platelet transfusion support (encompassing the time between the first and the last documented transfusion), during the study period. The y-axis in each figure represents patient counts. cPRA, calculated panel reactive antibody; PTR, platelet transfusion refractoriness.
Patient and platelet component factors
| . | All transfusions . | 2-h CCI . | 24-h CCI . |
|---|---|---|---|
| Number of patients | 73 | 73 | 67 |
| Number of transfusions | 2 140 | 1 563 | 731 |
| Mean recipient age, y (SD) | 57.9 (14.3) | 58 (14.3) | 58.8 (14.1) |
| Male sex, n (%) | 15 (20.5) | 15 (20.5) | 15 (22.4) |
| Caucasian, n (%) | 58 (79.5) | 58 (79.5) | 52 (77.6) |
| AML diagnosis, n (%) | 36 (49.3) | 36 (49.3) | 33 (49.3) |
| Allogeneic HSCT, n (%) | 30 (41.1) | 30 (41.1) | 25 (37.3) |
| Autologous HSCT, n (%) | 2 (2.8) | 2 (2.8) | 2 (3) |
| Median cPRA (IQR) | 88 (60-98) | 89 (68-99) | 88 (58-99) |
| Median cumulative MFI (IQR) | 531 (92-1 736) | 522 (85-1 653) | 531 (66-2 366) |
| Mean CCI (SD) | 12 259 (12 310) | 13 795 (12 196) | 6 237 (10 822) |
| Median spleen size, cm (IQR) | 14 (10.6-17.9) | 14.7 (10.4-18) | 14.7 (11.3-17.9) |
| Major or minor bleeding, n (%) | 166 (7.8) | 114 (7.3) | 101 (13.8) |
| Any transfusion reaction, n (%) | 104 (4.9) | 77 (4.9) | 38 (5.2) |
| Fever/infection/sepsis, n (%) | 247 (12.1) | 143 (9.6) | 100 (13.9) |
| Mean pretransfusion ANC (SD) | 1.38 (3.09) | 1.38 (3.2) | 1.95 (4.23) |
| Major/bidirectional ABO incompatibility, n (%) | 820 (38.3) | 624 (39.9) | 274 (37.5) |
| Pathogen inactivation, n (%) | 284 (13.4) | 208 (13.5) | 92 (12.7) |
| PAS storage solution, n (%) | 978 (46.2) | 729 (47.2) | 317 (43.8) |
| Mean time since first HLA product, days (SD) | 142 (195) | 140 (191) | 118 (195) |
| Proportion HLA-selected to RAP | 0.6 | 0.6 | 0.5 |
| Antibiotic use, n (%) | 744 (34.8) | 510 (32.6) | 319 (43.6) |
| DIC/TMA, n (%) | 86 (4.0) | 66 (4.2) | 34 (4.7) |
| Median number of prior pregnancies (IQR) | 3 (2-4) | 3 (2-4) | 2 (2-4) |
| Prior myeloablative conditioning, n (%) | 189 (33) | 125 (32) | 55 (25.2) |
| History of acute GVHD, n (%) | 312 (14.6) | 219 (14) | 101 (13.8) |
| . | All transfusions . | 2-h CCI . | 24-h CCI . |
|---|---|---|---|
| Number of patients | 73 | 73 | 67 |
| Number of transfusions | 2 140 | 1 563 | 731 |
| Mean recipient age, y (SD) | 57.9 (14.3) | 58 (14.3) | 58.8 (14.1) |
| Male sex, n (%) | 15 (20.5) | 15 (20.5) | 15 (22.4) |
| Caucasian, n (%) | 58 (79.5) | 58 (79.5) | 52 (77.6) |
| AML diagnosis, n (%) | 36 (49.3) | 36 (49.3) | 33 (49.3) |
| Allogeneic HSCT, n (%) | 30 (41.1) | 30 (41.1) | 25 (37.3) |
| Autologous HSCT, n (%) | 2 (2.8) | 2 (2.8) | 2 (3) |
| Median cPRA (IQR) | 88 (60-98) | 89 (68-99) | 88 (58-99) |
| Median cumulative MFI (IQR) | 531 (92-1 736) | 522 (85-1 653) | 531 (66-2 366) |
| Mean CCI (SD) | 12 259 (12 310) | 13 795 (12 196) | 6 237 (10 822) |
| Median spleen size, cm (IQR) | 14 (10.6-17.9) | 14.7 (10.4-18) | 14.7 (11.3-17.9) |
| Major or minor bleeding, n (%) | 166 (7.8) | 114 (7.3) | 101 (13.8) |
| Any transfusion reaction, n (%) | 104 (4.9) | 77 (4.9) | 38 (5.2) |
| Fever/infection/sepsis, n (%) | 247 (12.1) | 143 (9.6) | 100 (13.9) |
| Mean pretransfusion ANC (SD) | 1.38 (3.09) | 1.38 (3.2) | 1.95 (4.23) |
| Major/bidirectional ABO incompatibility, n (%) | 820 (38.3) | 624 (39.9) | 274 (37.5) |
| Pathogen inactivation, n (%) | 284 (13.4) | 208 (13.5) | 92 (12.7) |
| PAS storage solution, n (%) | 978 (46.2) | 729 (47.2) | 317 (43.8) |
| Mean time since first HLA product, days (SD) | 142 (195) | 140 (191) | 118 (195) |
| Proportion HLA-selected to RAP | 0.6 | 0.6 | 0.5 |
| Antibiotic use, n (%) | 744 (34.8) | 510 (32.6) | 319 (43.6) |
| DIC/TMA, n (%) | 86 (4.0) | 66 (4.2) | 34 (4.7) |
| Median number of prior pregnancies (IQR) | 3 (2-4) | 3 (2-4) | 2 (2-4) |
| Prior myeloablative conditioning, n (%) | 189 (33) | 125 (32) | 55 (25.2) |
| History of acute GVHD, n (%) | 312 (14.6) | 219 (14) | 101 (13.8) |
n shown for each variable unless otherwise denoted; 20 of 73 patients had splenomegaly, accounting for 472 transfusions. Antibiotics included vancomycin, linezolid, and amphotericin. Proportion HLA-selected to RAP denotes the proportion of prior transfusions received by each patient in the study period that were HLA-selected products (excluding the first transfusion).
AML, acute myeloid leukemia; ANC, absolute neutrophil count; cPRA, calculated panel reactive antibody; DAT, direct antiglobulin test; DIC, disseminated intravascular coagulation; GVHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplant; IQR, interquartile range; RAP, randomly selected apheresis platelets; SD, standard deviation; TMA, thrombotic microangiopathy.
With regard to platelet donors, the age ranges and sex distribution of the donor pool used for HLA-selected platelet transfusions resembled that of the overall platelet donor pool at the blood collection center (supplemental Figure 1A-B). Transfusion recipients/HLA-alloimmunized patients demonstrated a female predominance across age groups (supplemental Figure 1C). Self-reported racial composition across all 3 groups (blood center platelet donor pool, utilized platelet donors, and transfusion recipients) was predominantly White/Caucasian (supplemental Figure 1D-F). HLA antigen frequencies were also similarly distributed among the 3 groups with HLA-A2, A1, and A3 being common and HLA-A2 antigens present in up to 50% of the donors and transfusion recipients (supplemental Figure 1G-I). Regarding transfusion recipient HLA antibody frequencies above a DSA-MFI of 2500, the most common were anti-HLA B57, B58, B13, B49, B63, followed by others (supplemental Figure 1J). Several of the antigens corresponding to these HLA antibodies shared the HLA Bw4 epitope.
Patient and component factors affecting CCI
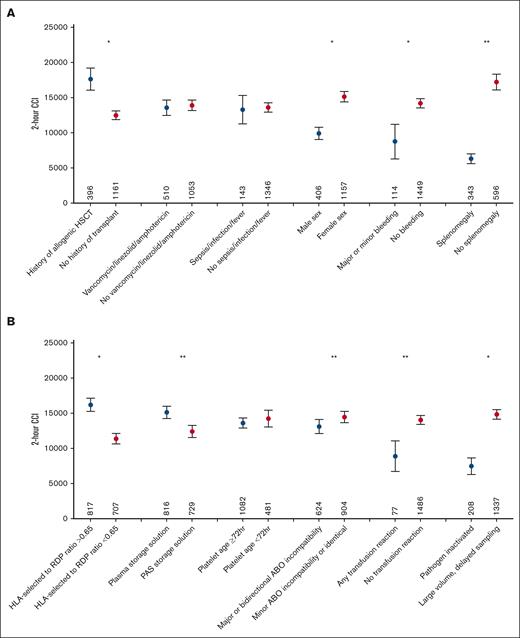
In univariate analyses, the strongest predictors of lower 2-hour CCI were higher cumulative DSA-MFI and splenomegaly, followed by other factors, as shown in Figures 2A-B and 3A and supplemental Figure 2A-B. Other predictors of lower 2-hour CCI included several patient factors: hepatomegaly, male sex, major/minor bleeding, and transfusion reactions. Predictors of higher 2-hour CCI included a history of acute graft-versus-host disease, allogeneic hematopoietic stem cell transplantation, higher pretransfusion absolute neutrophil count, pretransfusion hemoglobin, and pretransfusion platelet counts. Numerous factors had no significant impact on 2-hour CCI in this data set, including recipient diagnosis, disseminated intravascular coagulation/thrombotic microangiopathy, calculated panel reactive antibody, antibiotics (vancomycin, linezolid, and amphotericin), sepsis, fever and/or infection, and myeloablative conditioning. Several component factors were predictors of lower 2-hour CCI: pathogen inactivation, platelet additive solution as storage solution, and major ABO incompatibility. Positive impacts on 2-hour CCI were observed with platelet storage in plasma, use of nonpathogen inactivation strategies including large-volume delayed sampling for bacterial mitigation, and a higher proportion of component transfused (ie, no volume reduction).
Patient and component factors affecting 2-hour CCI. Mean 2-hour posttransfusion CCI with 95% confidence interval (y-axis) by patient (A) or component factors (B) analyzed (x-axis); n denotes the number of transfusions affected by the given variable. For time since first HLA-selected component and ratio of HLA-selected products to randomly selected apheresis platelet transfusions (RAP) in previous transfusions, given thresholds were divided by the median values. ∗∗Indicate variables that were statistically significant in univariate and multivariate analyses. ∗Highlights variables that were statistically significant by univariate analysis alone. DSA-MFI impact on CCI is not included in this figure.
HSCT, hematopoietic stem cell transplant; PAS, platelet additive solution.
Patient and component factors affecting 2-hour CCI. Mean 2-hour posttransfusion CCI with 95% confidence interval (y-axis) by patient (A) or component factors (B) analyzed (x-axis); n denotes the number of transfusions affected by the given variable. For time since first HLA-selected component and ratio of HLA-selected products to randomly selected apheresis platelet transfusions (RAP) in previous transfusions, given thresholds were divided by the median values. ∗∗Indicate variables that were statistically significant in univariate and multivariate analyses. ∗Highlights variables that were statistically significant by univariate analysis alone. DSA-MFI impact on CCI is not included in this figure.
HSCT, hematopoietic stem cell transplant; PAS, platelet additive solution.
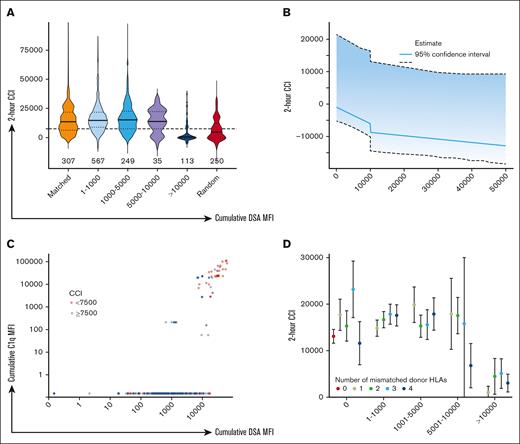
Impact of cumulative DSA-MFI on 2-hour CCI. (A) Violin plots showing 2-hour posttransfusion CCI (x-axis) by ranges of cumulative DSA-MFI, RAP transfusions, or transfusions with no donor HLA mismatches (y-axis). The horizontal dashed line denotes CCI = 7500. Horizontal bars for each violin plot denote the median for each group, and n for each plot denotes the number of transfusions in each group. ∗Denotes P < .05 in 2-tailed t test compared against 4/4 HLA matched or antigen-negative transfusions. (B) Estimated population mean 2-hour CCI at various values for total MFI conditional on all other predictors included at the linear mixed effects model at their median values; ∗95% confidence band calculated using bootstrap resampling of participants. (C) Association between cumulative DSA-MFI (x-axis) and cumulative C1q MFI compared across 282 transfusions (C1q testing on 58 patient samples). Both axes are log10 scaled. Points are colored by adequate (blue, ≥7500) or inadequate (red, <7500) 2-hour posttransfusion CCI. (D) Mean 2-hour posttransfusion CCI with 95% confidence interval (x-axis) across given ranges of cumulative DSA-MFI by number of mismatched donor HLAs for each DSA-MFI stratum (y-axis).
Impact of cumulative DSA-MFI on 2-hour CCI. (A) Violin plots showing 2-hour posttransfusion CCI (x-axis) by ranges of cumulative DSA-MFI, RAP transfusions, or transfusions with no donor HLA mismatches (y-axis). The horizontal dashed line denotes CCI = 7500. Horizontal bars for each violin plot denote the median for each group, and n for each plot denotes the number of transfusions in each group. ∗Denotes P < .05 in 2-tailed t test compared against 4/4 HLA matched or antigen-negative transfusions. (B) Estimated population mean 2-hour CCI at various values for total MFI conditional on all other predictors included at the linear mixed effects model at their median values; ∗95% confidence band calculated using bootstrap resampling of participants. (C) Association between cumulative DSA-MFI (x-axis) and cumulative C1q MFI compared across 282 transfusions (C1q testing on 58 patient samples). Both axes are log10 scaled. Points are colored by adequate (blue, ≥7500) or inadequate (red, <7500) 2-hour posttransfusion CCI. (D) Mean 2-hour posttransfusion CCI with 95% confidence interval (x-axis) across given ranges of cumulative DSA-MFI by number of mismatched donor HLAs for each DSA-MFI stratum (y-axis).
In a multivariable analysis, cumulative DSA-MFI, and spleen size and major/bidirectional ABO-mismatch, storage in platelet additive solution instead of plasma, and transfusion reactions remained significant predictors of lower 2-hour CCI (Figure 2A-B; Table 2). None of the analyzed factors were predictive of 24-hour CCI (Table 2).
Multivariate analysis of factors that affect 2-hour and 24-hour CCI
| Variable . | Estimate (range) . | P value . |
|---|---|---|
| 2 hours | ||
| Sum of MFIs | NA | 2.9E−10 |
| ABO mismatch (bidirectional/major) | −2 900.05 (−4 133.88 to −1 666.23) | 4.6E−06 |
| Platelet additive solution | −2 565.06 (−3 749.63 to −1 380.48) | 2.4E−05 |
| Transfusion reaction | −5 206.62 (−7 889.21 to −2 524.03) | 1.5E−04 |
| Spleen size (cm) | −787.48 (−1 224.35 to −350.61) | 4.3E−04 |
| Time since first HLA-selected transfusion | 0.37 (−0.02 to 0.77) | .07 |
| Baseline platelets >50 × 103/μL | 0.40 (−0.10 to 0.89) | .12 |
| Platelet component age | 14.44 (−4.27 to 33.15) | .13 |
| Patient sex (male) | −3 724.98 (−8 508.92 to 1 058.95) | .13 |
| Sepsis/infection/fever | −1 635.73 (−4 226.91 to 955.45) | .22 |
| cPRA, % | 32.49 (−19.56 to 84.54) | .22 |
| Major or minor bleeding | 1 616.27 (−1 589.92 to 4 822.45) | .32 |
| Antibiotics | −649.88 (−2 869.83 to 1 570.08) | .57 |
| HLA-selected components | 639.37 (−2 797.46 to 4 076.19) | .72 |
| DIC | −538.83 (−9 549.17 to 8 471.52) | .91 |
| Allogeneic HSCT | 94.11 (−2 600.11 to 2 788.33) | .95 |
| 24 hours | ||
| Platelet component age | 53.50 (18.07-88.92) | .003 |
| Sum of MFIs | −0.10 (−0.19 to −0.01) | .03 |
| Spleen size (cm) | −439.64 (−911.59 to 32.30) | .07 |
| Transfusion reaction | −4 429.94 (−10 144.53 to 1 284.65) | .13 |
| Time since first HLA-selected transfusion | 0.22 (−0.21 to 0.65) | .31 |
| Allogeneic HSCT | −1 690.57 (−5 001.89 to 1 620.76) | .32 |
| DIC | 5 582.28 (−5 425.35 to 16 589.90) | .32 |
| cPRA % | −30.13 (−91.80 to 31.54) | .34 |
| Platelet additive solution | −937.43 (−3 021.39 to 1 146.52) | .38 |
| Patient sex (male) | −1 940.74 (−6 286.36 to 2 404.87) | .39 |
| Sepsis/infection/fever | −1 457.98 (−5 194.48 to 2 278.52) | .44 |
| Antibiotics | −1 367.36 (−4 879.56 to 2 144.83) | .45 |
| Baseline platelets >50 × 103/μL | −620.44 (−3 016.83 to 1 775.96) | .61 |
| Major or minor bleeding | −374.23 (−4 694.77 to 3 946.31) | .87 |
| HLA-selected components | −327.94 (−4 760.14 to 4 104.25) | .88 |
| ABO mismatch (bidirectional/major) | −56.24 (−2 206.85 to 2 094.37) | .96 |
| Variable . | Estimate (range) . | P value . |
|---|---|---|
| 2 hours | ||
| Sum of MFIs | NA | 2.9E−10 |
| ABO mismatch (bidirectional/major) | −2 900.05 (−4 133.88 to −1 666.23) | 4.6E−06 |
| Platelet additive solution | −2 565.06 (−3 749.63 to −1 380.48) | 2.4E−05 |
| Transfusion reaction | −5 206.62 (−7 889.21 to −2 524.03) | 1.5E−04 |
| Spleen size (cm) | −787.48 (−1 224.35 to −350.61) | 4.3E−04 |
| Time since first HLA-selected transfusion | 0.37 (−0.02 to 0.77) | .07 |
| Baseline platelets >50 × 103/μL | 0.40 (−0.10 to 0.89) | .12 |
| Platelet component age | 14.44 (−4.27 to 33.15) | .13 |
| Patient sex (male) | −3 724.98 (−8 508.92 to 1 058.95) | .13 |
| Sepsis/infection/fever | −1 635.73 (−4 226.91 to 955.45) | .22 |
| cPRA, % | 32.49 (−19.56 to 84.54) | .22 |
| Major or minor bleeding | 1 616.27 (−1 589.92 to 4 822.45) | .32 |
| Antibiotics | −649.88 (−2 869.83 to 1 570.08) | .57 |
| HLA-selected components | 639.37 (−2 797.46 to 4 076.19) | .72 |
| DIC | −538.83 (−9 549.17 to 8 471.52) | .91 |
| Allogeneic HSCT | 94.11 (−2 600.11 to 2 788.33) | .95 |
| 24 hours | ||
| Platelet component age | 53.50 (18.07-88.92) | .003 |
| Sum of MFIs | −0.10 (−0.19 to −0.01) | .03 |
| Spleen size (cm) | −439.64 (−911.59 to 32.30) | .07 |
| Transfusion reaction | −4 429.94 (−10 144.53 to 1 284.65) | .13 |
| Time since first HLA-selected transfusion | 0.22 (−0.21 to 0.65) | .31 |
| Allogeneic HSCT | −1 690.57 (−5 001.89 to 1 620.76) | .32 |
| DIC | 5 582.28 (−5 425.35 to 16 589.90) | .32 |
| cPRA % | −30.13 (−91.80 to 31.54) | .34 |
| Platelet additive solution | −937.43 (−3 021.39 to 1 146.52) | .38 |
| Patient sex (male) | −1 940.74 (−6 286.36 to 2 404.87) | .39 |
| Sepsis/infection/fever | −1 457.98 (−5 194.48 to 2 278.52) | .44 |
| Antibiotics | −1 367.36 (−4 879.56 to 2 144.83) | .45 |
| Baseline platelets >50 × 103/μL | −620.44 (−3 016.83 to 1 775.96) | .61 |
| Major or minor bleeding | −374.23 (−4 694.77 to 3 946.31) | .87 |
| HLA-selected components | −327.94 (−4 760.14 to 4 104.25) | .88 |
| ABO mismatch (bidirectional/major) | −56.24 (−2 206.85 to 2 094.37) | .96 |
Factors that were significant predictors of CCI at 2 hours are in bold. Estimate and ranges for the sum of MFI are listed as NA because a nonlinear model was the best fit for this variable. P values were significant in the nonlinear model. Estimates are given in relationship to the associated variable. For continuous variables, the estimate is per 1 unit of the associated variable.
cPRA, calculated panel reactive antibody; DIC, disseminated intravascular coagulation; NA, not applicable.
DSA-MFI threshold predictive of successful CCI
Above a cumulative DSA-MFI of ∼10 000, the 2-hour posttransfusion CCI fell and became like those of randomly selected apheresis platelet transfusions (Figure 3A). This was corroborated by the multivariable analysis, wherein the model containing a separate slope and intercept at an MFI threshold of 10 000 predicted the 2-hour CCI (likelihood ratio (2) = 8.04, P = .018) significantly better than the reduced (linear) model (Figure 3B). This trend was also evident in the analysis based on the C1q assay results. Nearly all (87%) platelet transfusions involving antibodies that demonstrated C1q binding (indicative of complement activation) were observed to have cumulative DSA-MFI thresholds of >10 000 (Figure 3C). The MFI threshold of 10 000 demonstrated no association with 24-hour CCI, higher bleeding events, or mortality. When transfused platelet components were stratified by cumulative MFI subgroups of <10 000, the number of donor-recipient HLA-antigenic mismatches did not affect the CCI within each subgroup (Figure 3D). No other demographics differed by DSA-MFI strata (supplemental Table 3).
Computational algorithm to rank platelet donors by MFI/histocompatibility
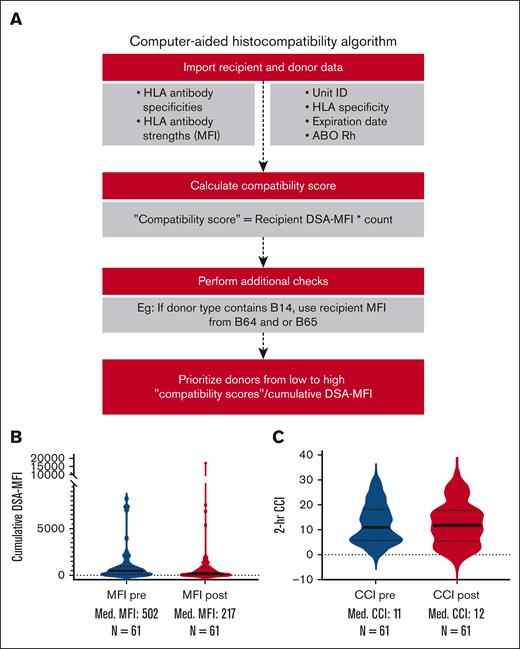
A computational framework developed at the NIH Clinical Center, HLA Laboratory to order HLA-selected platelet components based on MFI is outlined in Figure 4A (Python code in supplemental Table 4). When applied to 122 consecutive HLA-selected platelet transfusions (61 before, and 61 after, implementation), cumulative DSA-MFI values for transfused platelets appeared to decrease after algorithm implementation (Figure 4B). However, the median and mean CCI did not increase in the overall group after the application of the selection algorithm (Figure 4C; supplemental Figure 4). Time required for platelet unit selection was not strictly tracked. However, blood center staff estimated that the time to select a unit decreased from ≥15 minutes under the prior manual process to <2 minutes using the algorithm.
The NIH-HLA laboratory platelet component selection algorithm by cumulative DSA-MFI “scores.” (A) The computational framework developed for the allocation of platelet units (Python version 3.10.0). Data manipulation was facilitated using the “Pandas” package. Recipient information, including HLA antibody specificities and their respective immunogenic strengths, was imported for analysis. Additionally, donor information such as unit number, HLA type, unit expiry date, ABO blood group, and Rh type was also imported. The framework conducts an iterative comparison to evaluate compatibility between recipient antibodies and donor HLA types, from which a “compatibility score” or cumulative DSA-MFI is derived. Special attention is paid to certain HLA types (B14, B15, and B40) because of their serological cross-reactivities, with score adjustments for associated antigens. Donors are then ranked according to their compatibility scores, with the system prioritizing those with lower scores to optimize donor-recipient histocompatibility. Violin plots showing cumulative DSA-MFI changes (B) and 2-hour CCI changes (C) before and after algorithm implementation for 61 transfusions each. Horizontal dark lines for each violin plot denote the median for each group, and n for each plot denotes the number of transfusions in each group. Neither of the comparisons was significant at a P < .05 in 2-tailed t tests.
The NIH-HLA laboratory platelet component selection algorithm by cumulative DSA-MFI “scores.” (A) The computational framework developed for the allocation of platelet units (Python version 3.10.0). Data manipulation was facilitated using the “Pandas” package. Recipient information, including HLA antibody specificities and their respective immunogenic strengths, was imported for analysis. Additionally, donor information such as unit number, HLA type, unit expiry date, ABO blood group, and Rh type was also imported. The framework conducts an iterative comparison to evaluate compatibility between recipient antibodies and donor HLA types, from which a “compatibility score” or cumulative DSA-MFI is derived. Special attention is paid to certain HLA types (B14, B15, and B40) because of their serological cross-reactivities, with score adjustments for associated antigens. Donors are then ranked according to their compatibility scores, with the system prioritizing those with lower scores to optimize donor-recipient histocompatibility. Violin plots showing cumulative DSA-MFI changes (B) and 2-hour CCI changes (C) before and after algorithm implementation for 61 transfusions each. Horizontal dark lines for each violin plot denote the median for each group, and n for each plot denotes the number of transfusions in each group. Neither of the comparisons was significant at a P < .05 in 2-tailed t tests.
Discussion
In this large retrospective study, we confirmed the use of an alternate permissive-platelet-mismatching strategy using cumulative DSA-MFI thresholds when HLA-matched or antigen-negative platelets are not available. To determine patients’ HLA antibody repertoires, current bead-based platforms use arbitrary individual MFI thresholds ranging from >1000 to 3000.9,13,14 Using permissively mismatched platelets with cumulative DSA-MFIs of up to 10 000 may allow for donor pool expansion without compromising CCI. Toward this goal, we also confirmed the feasibility of using a computerized algorithm to reorder and prioritize platelet components with antigens against less-avid HLA-DSA. The open-source NIH algorithm may help platelet component selection even in labor- and resource-limited settings. It may be particularly helpful with avoiding components with very high cumulative DSA-MFI. Although other automated applications have been developed to optimize “virtual HLA-selection and platelet crossmatching,”15-17 this is, to our knowledge, the first algorithm to incorporate cumulative DSA-MFI in HLA-platelet component selection.
HLA antigen matching among patient donors and transfusion recipients when MFI was <10 000 offered no additional CCI benefit across various strata of cumulative DSA-MFI. This suggests that component prioritization via cumulative DSA-MFI may be more useful than currently used HLA antigen match grade systems. Although this study did not assess the impact of MFI-based platelet component selection on the development of new HLA or other platelet antibodies, in a separate analysis, serial calculated panel reactive antibody measurements in our cohort appeared to wane over time regardless of platelet component HLA match grade,18 likely because of waning antibody production and/or the formation of anti-idiotype antibodies in these patients.19 This was also confirmed in an analysis of antibody persistence using lymphocytotoxicity-based antibody testing among patients with leukemia undergoing induction chemotherapy in the Trial to Reduce Alloimmunization to Platelets study. More than half of HLA-alloimmunized patients lost their antibodies within 12 months of initiating platelet transfusion support, within a median of 14 weeks, irrespective of the number and type of platelet components received.20
When transfusing platelets to HLA-alloimmunized patients above a cumulative DSA-MFI threshold of ∼10 000 we identified a “stepdown” in CCI confirmed via multivariable analyses. These findings align with data from solid organ (liver, kidney, heart, and lung) transplants wherein the presence of higher DSA-MFI in organ recipients was associated with a greater risk of antibody-mediated rejection across all organs.21 Notably, in solid organ transplants21 and in allogeneic hematopoietic stem cell transplant recipients receiving grafts from HLA-mismatched donors,22 cumulative DSA-MFI thresholds of >10 000 portend an even worse prognosis and are associated with antibody persistence.
We found that above a cumulative DSA-MFI threshold of 10 000 DSA demonstrated the ability to bind complement in vitro. However, the added prognostic value of the C1q-binding assay in platelet selection algorithms remains equivocal, partly because in vitro complement activity appears to be tightly tied to antibody “titer.”21 Furthermore, in our analysis, 15% of patients demonstrated adequate CCI despite cumulative DSA-MFIs of >10 000 and C1q binding. We speculate that this was because of variable C1q binding affinity across immunoglobulin G (IgG) isotypes23 and/or the level of subtype-specific HLA-antigen expression on platelets.24
Other immune and nonimmune factors negatively affected posttransfusion CCI in platelet transfusion recipients in this retrospective cohort. Concerning ABO-match status, these findings are comparable with the platelet-dose (PLADO) trial data wherein ABO-identical or minor–ABO-mismatched (donor isoagglutinins against recipient antigens) platelet transfusions resulted in small but statistically significantly higher CCI compared with major–ABO-mismatched (recipient isoagglutinins against donor platelet ABO-antigens) transfusions.25 Consequently, when HLA-selected components are unavailable, providing minor–ABO-mismatched or ABO-identical components in this heavily alloimmunized platelet transfusion refractory population may offer more benefit than has been previously reported in nonalloimmunized platelet transfusion refractoriness.26 From a mechanistic standpoint, it is likely that HLA antibody–mediated platelet destruction is potentiated by concurrently bound IgG and/or IgM ABO isoantibodies.
Concerning other factors, splenomegaly also negatively affected posttransfusion CCI. Barring unusual circumstances,27 the impact of this nonmodifiable risk factor may be challenging to overcome. Nonetheless, we did not observe a correlation between increasing spleen size and higher major/minor bleeding rates in this cohort. Transfusion reactions were associated with lower CCI, likely because of discontinuation of infusions and/or inflammatory potentiation of platelet destruction. Platelet storage in additive solution, which is known to mitigate allergic reactions28 (comprising 32% of the total transfusion reactions in this cohort) and result in less complement activation,29 was also associated with a decreased CCI by 10% (compared with storage in plasma) in our adjusted analysis. This is consistent with previously reported data.26 The clinical decision to consider or avoid HLA-selected platelets stored in platelet-additive solution vs plasma may need to be individualized.
One limitation of this study was that the calculation of CCI was based on a presumed platelet count of 3 × 1011 per bag in the absence of consistent platelet yield data for all transfusions. This likely led to mildly overestimating most CCI values because the number of platelets transfused was presumably higher in some cases. Most blood centers use different target median platelet yields for large volume delayed sampling (3.5 × 1011 per bag) vs pathogen-inactivated platelets (3.2 × 1011 per bag). This may have resulted in a disproportionate advantage toward large volume–delayed sampling platelets confounding the overall analysis. This confounding effect may have also affected ∼10% of the platelet units stored in platelet additive solution because these were also pathogen-inactivated at our blood center. These nuances may explain lower CCI among pathogen-inactivated or platelet-additive solution components in the analyses because these components likely contained fewer platelets.
Confounding may also explain the impact of a few other variables in the univariate analysis. Hepatomegaly was confounded by coexisting splenomegaly in several cases. Immunosuppressive therapy likely confounded a history of graft-versus-host disease and allogeneic hematopoietic stem cell transplantation and may explain higher posttransplant CCIs in these 2 groups. Yet other factors that affected CCI in the univariate analyses corroborated findings from larger prospective studies or aligned with clinical observations. The differential impact of sex on CCI (lower CCI in males than females) was previously reported by Hess et al as part of the PLADO post hoc analysis30 and may be because of differences in tolerance to allosensitization across males and females. Major and/or minor bleeding and lower pretransfusion hemoglobin negatively affected 2-hour CCI but would require a study with a larger sample size for further characterization in multivariable analyses. This study was also not powered to evaluate the impact of donor age, race, sex, HLA-antigen specificities, and relative individual HLA-antibody immunogenicity on CCI. Sample size limitations may have resulted in the lack of impact of DSA-MFI on 24-hour CCIs. As previously described, it is also possible that nonimmunologic factors overshadowed the impact on 24-hour CCI compared with DSA-MFI and immunologic factors studied.1
Despite the overall limitations inherent to retrospective studies, we conclude that cumulative HLA DSA-MFI thresholds of ∼10 000 may be used to guide permissive mismatching strategies to enhance 2-hour CCI (indicative of immune-mediated refractoriness) and to potentially optimize HLA-typed platelet donor pools for the treatment of HLA alloimmunized patients. It may be valuable for blood centers/transfusion service laboratories to incorporate component-specific cumulative DSA-MFI assessments in their platelet selection algorithms for alloimmunized patients. The use of a computerized selection algorithm may be feasible in this regard. This approach may improve HLA selection efficacy compared with HLA-match “grades” or resource-intensive HLAMatchmaker epitope-matching strategies. In the absence of HLA-selected platelet availability, ABO-identical or minor-mismatched platelet concentrates may enhance 2-hour CCI in heavily HLA-alloimmunized patients with platelet transfusion refractoriness.
Acknowledgments
The authors thank Gary Schoch, Clinical Research Division, Fred Hutchinson Cancer Center for assistance with data extraction on patients who underwent hematopoietic stem cell transplantation.
This study received support from an institutional training grant from the National Institutes of Health, National Heart, Lung, and Blood Institute (T32 HL007093) and the University of Washington Center for Scholarship and Patient Care Quality and Safety Quality Improvement Project Accelerator service. The NIH Quality Improvement project was supported by the Intramural Research Program (project ID ZIC CL002128) of the NIH Clinical Center at the National Institutes of Health.
Authorship
Contribution: A.B.B. and M.K.T. collected and analyzed the data, prepared figures, and cowrote the manuscript; A.A. developed and implemented the computerized NIH HLA laboratory platelet selection algorithm as part of a Quality Improvement project; D.Y. collected data, cowrote the manuscript, and supervised research; J.B.R., T.B., N.C., and S.M. collected data; T.G., I.G., J.R.H., L.S.-H., W.A.F., D.F.S., and R.R.V. assisted with study interpretation and revised the manuscript; M.N. performed statistical analysis; H.C.T. developed structured query language code for data extraction and cleaning at the University of Washington/Fred Hutchinson Cancer Center and supervised the research, consulted on database management, and cowrote the manuscript; and S.R.P. supervised the research, analyzed data, and cowrote the manuscript.
Conflict-of-interest disclosure: S.R.P. reports consultancy fees from Sanofi Inc and Sobi Inc. L.S.-H. reports personal fees and nonfinancial support from the Institute for Healthcare Improvement, outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Sandhya R. Panch, Division of Hematology/Oncology, University of Washington, 825 Eastlake Ave E, #LG-700, Seattle, WA 98109; email: srpanch@uw.edu.
References
Author notes
A.B.B., M.K.T., and A.A. contributed equally to this study.
Data are available on request from the corresponding author, Sandhya R. Panch (srpanch@uw.edu).
The full-text version of this article contains a data supplement.