Key Points
Efficacy and safety of caplacizumab in pediatric iTTP are demonstrated, including in children as young as 2 years.
Our real-world data collection of caplacizumab use in pediatric iTTP demonstrates its therapeutic potential in this group of patients.
Visual Abstract
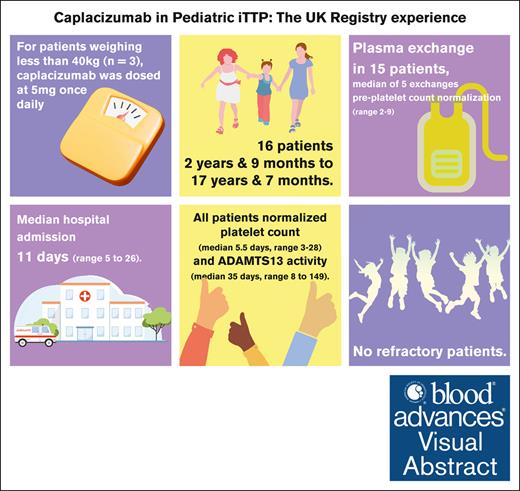
Pediatric thrombotic thrombocytopenic purpura (TTP) is an ultrarare disease. Immune TTP (iTTP) is driven by anti-ADAMTS13 autoantibodies causing an imbalanced von Willebrand factor (VWF):ADAMTS13 axis, and rarer still in children, but potentially life-threatening. Caplacizumab is licensed for iTTP treatment in adults and adolescents aged ≥12 years who weigh ≥40 kg. There is a need to clarify whether caplacizumab can be used in younger children. We retrospectively described caplacizumab use in 16 patients under 18 years of age from the UK TTP Registry, including 4 children aged <12 years. For patients weighing <40 kg (n = 3), caplacizumab was dosed at 5 mg once dailyThe youngest patient was 33 months old at diagnosis. Plasma exchange (PEX) was used in 15 patients, with a median of 5 exchanges required before platelet count normalization (range, 2-9). One patient was managed without PEX. All patients achieved normalization of platelet count (median, 5.5 days; range, 3-28) and ADAMTS13 activity (median, 35 days; range, 8-149), with a median hospital admission of 11 days (range, 5-26). There were no refractory patients. One patient relapsed 9 months after presentation. Bleeding requiring VWF supplementation and reduction of caplacizumab use occurred in 1 patient with severe epistaxis, with no significant intracranial or gastrointestinal bleeding. We demonstrated the efficacy and safety of caplacizumab in the pediatric population, which is synonymous with the adult trial data: primarily, reduction of PEX compared with the precaplacizumab era. This has implications for the intensification and duration of admission, particularly relevant in pediatric care.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a potentially fatal thrombotic microangiopathy, with end-organ compromise frequently causing cardiac, renal, and neurological morbidities. The hallmark of the syndrome is a deficiency in ADAMTS13, the metalloprotease necessary to cleave ultralarge von Willebrand factor (VWF) multimers. ADAMTS13 deficiency is either congenital, caused by biallelic mutations in the ADAMTS13 gene, or acquired via autoantibodies against ADAMTS13, impairing function. Acquired or immune TTP (iTTP) is seen in the vast majority of adult cases.1
Acute treatment of iTTP involves the replacement of ADAMTS13 via plasma exchange (PEX) and immunosuppression to further deplete the autoantibody in the form of steroid and rituximab. Caplacizumab is now recognized as the third therapeutic arm for the first-line treatment of iTTP.2
Trials with caplacizumab have demonstrated a reduced duration of thrombocytopenia, shorter admissions, fewer exacerbations, and a reduction of volume and duration of PEX and refractory disease.3,4
Pediatric TTP is a rare disease that accounts for <10% of all TTP diagnoses. Even rarer is iTTP in young children (<5 years). We retrospectively described the use of caplacizumab, an anti-VWF antibody licensed for the treatment of iTTP, primarily in adults, but including children aged >12 years, registered in the UK TTP Registry. There are few published data regarding the use of caplacizumab in children. The Hercules study allowed the eligibility of pediatric patients at some sites, but none were enrolled during the study period4 (clarification that pediatric patients were eligible for the Hercules study but none enrolled during the study period, as per Reviewer 2). The UK Real World Experience of caplacizumab, published in 2021, described the use of caplacizumab in 4 children.5 We now expand on this by presenting 16 patients from the UK TTP Registry. To our knowledge, this is the largest described cohort of pediatric patients with iTTP treated with caplacizumab.
Method
The UK TTP Registry is a national registry that captures all cases of acute TTP, including yearly follow-ups since 2018. All patients undergo informed consent to be included in the registry, with parents consenting for children aged <16 years. The registry was used as a source for a retrospective review of pediatric patients, classified as <18 years of age, who were treated with caplacizumab for iTTP.
iTTP was classified as patients with ADAMTS13 activity levels <5 IU/dL with the presence of anti-ADAMTS13 autoantibodies above the reference range for the laboratory. For children under 12 years of age, this was off label. Patients weighing ≥40 kg were dosed with 10 mg once daily caplacizumab per product guidelines. A dose of 5 mg once daily was recommended for children weighing <40 kg (n = 3).
The review examined the number of patients achieving clinical remission, exacerbations, disease, time to platelet count response, total length of hospital admission, duration of PEX, time to ADAMTS13 recovery, relapse, and adverse events. Definitions of remission, exacerbation, and relapse followed the International Consensus Report on outcomes in iTTP.6 The review examines the challenges in the management of younger children with iTTP and the altered dosing regimen of caplacizumab in patients weighing <40 kg.
Results
Sixteen patients were included on the UK TTP Registry under the age of 18 years, managed by 7 separate hospitals. These included the 4 pediatric patients previously included in the UK Real World Experience.5 They presented with an initial episode of TTP between December 2018 and January 2023. The median (range) age at the time of iTTP diagnosis was 14 years and 9 months (2 years, 9 months to 17 years, 7 months). Specifically, 4 patients were under 12 years of age.
The cohort included 10 girls and 6 boys, with ethnic backgrounds of Black (n = 6), White (n = 5), Asian (n = 2), and mixed race (n = 3). Body weight ranged from 15.3 kg to 101 kg, median 63.52 kg (Table 1).
Patient characteristics at presentation
| Baseline characteristics . | Caplacizumab cohort . |
|---|---|
| N = 16, 100% | |
| Age, median (range), mo | 14 y and 9 mo (2 y 9 mo to 17 y 17 mo) |
| Body weight, kg, median (range) | 63.2 (15.3-101) |
| Sex | |
| Male | 6 |
| Female | 10 |
| Ethnicity | |
| Black | 6 |
| White | 5 |
| Asian | 2 |
| Mixed race | 3 |
| Clinical features | |
| Neurological symptoms at presentation | 10 |
| Confusion | |
| Headache | 5 (including agitation in 1) 5 (including memory loss in 1) |
| Intensive care | 3 |
| Patients requiring intubation | 1 |
| Baseline characteristics . | Caplacizumab cohort . |
|---|---|
| N = 16, 100% | |
| Age, median (range), mo | 14 y and 9 mo (2 y 9 mo to 17 y 17 mo) |
| Body weight, kg, median (range) | 63.2 (15.3-101) |
| Sex | |
| Male | 6 |
| Female | 10 |
| Ethnicity | |
| Black | 6 |
| White | 5 |
| Asian | 2 |
| Mixed race | 3 |
| Clinical features | |
| Neurological symptoms at presentation | 10 |
| Confusion | |
| Headache | 5 (including agitation in 1) 5 (including memory loss in 1) |
| Intensive care | 3 |
| Patients requiring intubation | 1 |
iTTP was diagnosed with ADAMTS13 activity of <5 IU/dL and an anti-ADAMTS13 antibody or inhibitor measured on presentation.
The laboratory parameters at presentation are tabulated in Table 2.
Laboratory parameters at presentation of iTTP
| Laboratory parameters (NR) . | |
|---|---|
| Hemoglobin: median (range), mean (age-adjusted NR g/L, 105-135 for 2-y-old; up to 120-160 for 17-y-old) | 76 (42-104), 75.6 |
| Reticulocytes: absolute (range), mean (20-100 × 109/L) | 245 (110-347), 223.8 |
| Reticulocytes: % (range), mean | 4.95 (2.3-33.5), 10.3 |
| Platelet count: median (range), mean (×109/L) (150-450 × 109/L) | 10.5 (3-27), 11.6 |
| Cardiac troponin I: median (range), mean, ng/L (<34 ng/L) | 157 (14-164), 111.7 |
| Cardiac troponin T: median (range), mean, ng/L (14 mg/L) | 31.5 (4-1782), 183.6 |
| Urea: median (range), mean (age-adjusted NR mmol/L, 2.5- 6.0 for 2-y-old; up to 2.5-7.5 for 17-y-old) | 5.7 (2.7-11.8), 6.7 |
| Serum creatinine: median (range), mean, (age-adjusted NR umol/L, 15-31 for 2-y-old; up to 48-81 for 17-y-old) | 69 (38-146), 74.8 |
| Bilirubin: median (range), mean, (age-adjusted NR ug/mL, 15-31 for 2-y-old; up to 48-81 for 17-y-old) | 44 (2-85), 44.6 |
| LDH: median (range), mean (IU/L), (age-adjusted NR ug/mL, 500-920 for 2-y-old; up to 130-250 for 17-y-old) | 1344 (249-2353), 1350.8 |
| ADAMTS13 activity: median (range), mean (IU/dL) | 0 (0-10), 1.5 |
| ADAMTS13 inhibitor: median (range), mean (IU/dL) | 55 (11-135), 56.9 |
| Laboratory parameters (NR) . | |
|---|---|
| Hemoglobin: median (range), mean (age-adjusted NR g/L, 105-135 for 2-y-old; up to 120-160 for 17-y-old) | 76 (42-104), 75.6 |
| Reticulocytes: absolute (range), mean (20-100 × 109/L) | 245 (110-347), 223.8 |
| Reticulocytes: % (range), mean | 4.95 (2.3-33.5), 10.3 |
| Platelet count: median (range), mean (×109/L) (150-450 × 109/L) | 10.5 (3-27), 11.6 |
| Cardiac troponin I: median (range), mean, ng/L (<34 ng/L) | 157 (14-164), 111.7 |
| Cardiac troponin T: median (range), mean, ng/L (14 mg/L) | 31.5 (4-1782), 183.6 |
| Urea: median (range), mean (age-adjusted NR mmol/L, 2.5- 6.0 for 2-y-old; up to 2.5-7.5 for 17-y-old) | 5.7 (2.7-11.8), 6.7 |
| Serum creatinine: median (range), mean, (age-adjusted NR umol/L, 15-31 for 2-y-old; up to 48-81 for 17-y-old) | 69 (38-146), 74.8 |
| Bilirubin: median (range), mean, (age-adjusted NR ug/mL, 15-31 for 2-y-old; up to 48-81 for 17-y-old) | 44 (2-85), 44.6 |
| LDH: median (range), mean (IU/L), (age-adjusted NR ug/mL, 500-920 for 2-y-old; up to 130-250 for 17-y-old) | 1344 (249-2353), 1350.8 |
| ADAMTS13 activity: median (range), mean (IU/dL) | 0 (0-10), 1.5 |
| ADAMTS13 inhibitor: median (range), mean (IU/dL) | 55 (11-135), 56.9 |
All patients had red cell fragmentation identified on blood films at presentation.
LDH, lactate dehydrogenase; NR, normal range.
Response
All patients achieved clinical response, defined as normalization of platelet count to >150 × 109/L with no clinical evidence of new or progressive ischemic organ damage. The time to sustained normalization of the platelet count was a median of 5.5 days (range, 3-28). The time to sustained normalization of platelet count after starting caplacizumab was a median of 3 days (range, 0-26).
All patients had normalized ADAMTS13 activity, defined as >60 IU/dL. The time to normalization was 35 days (range, 8-149; mean, 46.9 days).
PEX was used in 15 patients. The remaining patient was managed with plasma infusion because it was clinically well and to avoid the need to anesthetize for central line insertion. The number of exchanges was a median of 6 (range, 3-18). The number of PEXs used before platelet count normalization (>150 × 109/L) was median 5.5 (range, 3-28). Duration of PEX was median 5 days (range, 2-16).
Caplacizumab was used in all patients. Days from admission to initiation of caplacizumab was a median 2 days (range, 0-12). Thirteen patients weighing ≥40 kg were dosed 10 mg once daily of caplacizumab. Three patients weighing <40 kg were dosed 5 mg once daily (Table 3). There were no adverse events recorded in this group.
Characteristics of patients dosed with caplacizumab 5 mg once daily
| Patient . | Date of presentation . | Age . | Sex . | Weight (kg) . |
|---|---|---|---|---|
| 1 | 17 December 2019 | 3 y 1 mo | Male | 15.3 |
| 2 | 9 November 2021 | 2 y 9 mo | Male | 15.4 |
| 3 | 19 September 2022 | 7 y 3 mo | Male | 28.9 |
| Patient . | Date of presentation . | Age . | Sex . | Weight (kg) . |
|---|---|---|---|---|
| 1 | 17 December 2019 | 3 y 1 mo | Male | 15.3 |
| 2 | 9 November 2021 | 2 y 9 mo | Male | 15.4 |
| 3 | 19 September 2022 | 7 y 3 mo | Male | 28.9 |
The duration of caplacizumab treatment was 31 days (range, 15-74). Caplacizumab was discontinued when ADAMTS13 activity level was measured at >20 IU/dL or 30 days after finishing daily PEX therapy.
All of the patients received steroids and anti-CD20 treatment. The initial steroid used was methylprednisolone 1 gm once daily (n = 9), 500 mg (n = 2), or prednisolone 1 mg/kg (n = 5). Prednisolone 1 gm/kg was used in the 2 children aged <4 years, and under 40 kg in weight. Anti-CD20 treatment, rituximab or biosimilar, was administered at a dose of 375 mg/m2, median 4 doses (range, 4-8).
Prophylactic anticoagulation in the form of low molecular weight heparin was used in 7 patients (9 patients not used) according to local guidelines.
Aspirin was used in 1 patient for 10 days. This 14-year-old patient was also reported to have a hematoma at the Vascath insertion site, diagnosed on ultrasound, and possible renal bleeding.
Adverse effects
Bleeding events were identified in 4 patients: 2 were minor, with a petechial rash and minor epistaxis, and 2 were moderate, with 1 developing a hematoma at the Vascath site and possible renal bleeding (in the patient on aspirin). The other patient was a 17-year-old and 7-month-old male, with moderate bleeding and prolonged epistaxis requiring nasal packing and cautery, with VWF replacement given in the form of 1 dose of 1000 units of Wilate (18.9 units/kg body weight). Thereafter, his caplacizumab dose was altered to 5 mg on alternate days. There were no bleeding events recorded in the 13 other patients.
A thrombotic event was identified in 1 patient with jugular vein thrombosis.
Treatment course
The median duration of hospital admission was 11 days (range, 5-26). There were no deaths. There were no refractory patients because all normalized both platelet count and eventually the ADAMTS13 level. Two patients required further immunosuppression: one 16-year-old female patient who subsequently developed systemic lupus erythematosus and lupus nephritis, thereby remaining on a protracted course of steroids, and one 13-year-old male patient, who was subsequently managed with 3 months of mycophenolate mofetil, followed by 3 months of sirolimus on recommendation from an immunology specialist. Both these patients continued in ADAMTS13 remission.
Recurrence was defined as a new decrease in platelet count after the initial normalization of the platelet count. Exacerbation of TTP was defined as recurrence <30 days after the last PEX procedure or anti-VWF therapy dose, as defined in the International Working Group for TTP statement paper.6 Exacerbation was seen in a 17-year-old female. However, she had not yet started caplacizumab after the cessation of the clinical trial and before compassionate use availability. She initially presented on 29 January 2019 and started caplacizumab on 6 February 2019. She stopped treatment on 8 March 2019.
Clinical relapse was defined as a new decrease in the platelet count to <150 × 109/L with severe ADAMTS13 deficiency.6 This was identified in 1 patient 9 months after the initial presentation.
Treatment modalities
| Caplacizumab dosing | 10 mg od n = 13 |
| 5 mg od n = 3 | |
| Immunosuppressive/adjuvant treatment started during admission | |
| Glucocorticoids | 16 (100%) |
| Methylprednisolone 1 gm od (n = 9) | |
| Methylprednisolone 500 mg (n = 2) | |
| Prednisolone 1 mg/kg (n = 5, including the 2 children <40 kg in weight) | |
| Anti-CD20 | 16 (100%) |
| Rituximab or biosimilar | 375 mg/m2, median 4 doses (range, 4-8) |
| MMF | 1 (6%) |
| Caplacizumab dosing | 10 mg od n = 13 |
| 5 mg od n = 3 | |
| Immunosuppressive/adjuvant treatment started during admission | |
| Glucocorticoids | 16 (100%) |
| Methylprednisolone 1 gm od (n = 9) | |
| Methylprednisolone 500 mg (n = 2) | |
| Prednisolone 1 mg/kg (n = 5, including the 2 children <40 kg in weight) | |
| Anti-CD20 | 16 (100%) |
| Rituximab or biosimilar | 375 mg/m2, median 4 doses (range, 4-8) |
| MMF | 1 (6%) |
MMF, mycophenolate mofetil; od, once daily.
Patient outcomes
| Time to normalization of platelet count, median (range), mean, d | 5.5 (3-28), 7.4 |
| No. of patients managed with PEX | 15 |
| No. of patients managed with plasma infusion | 1 |
| No. of exchanges, median (range), d | 6 (3-18) |
| No. of PEXs used before platelet count normalization (>150 × 109/L) | 5 (2-9) |
| Duration of PEX treatment before platelet count normalization (>150 × 109/L) | 5.5 (3-28) |
| Length of caplacizumab treatment, median (range), d | 31 (15-74) |
| Duration of hospital admission, median (range), d | 11 (5-26) |
| VWF levels on samples taken while on caplacizumab (n = 10) 10 mg od | VWF:Ag median 86 (43-194 IU/dL) |
| Normal range according to a laboratory (most 50-150 IU/dL) | VWF:Act median 2 (2-57 IU/dL) |
| Sample date taken after caplacizumab initiation: median, 11 d; mean, 19.5 d; minimum, 7; maximum, 63 d | FVIII median, 60 (6-156 IU/dL) |
| VWF levels on samples taken while on caplacizumab (n = 1) 5 mg od | VWF:Ag 94 |
| Normal range, 50-150 IU/dL | VWF:Act 2 |
| Sample date taken at 2 d after caplacizumab initiation | FVIII, 76 |
| ADAMTS13 at cessation of caplacizumab treatment | 63 (23.9- 89.6) |
| Time to normalization of ADAMTS13 (>64 IU/dL), median (range), d | 35 (8-149) |
| Time to normalization of platelet count, median (range), mean, d | 5.5 (3-28), 7.4 |
| No. of patients managed with PEX | 15 |
| No. of patients managed with plasma infusion | 1 |
| No. of exchanges, median (range), d | 6 (3-18) |
| No. of PEXs used before platelet count normalization (>150 × 109/L) | 5 (2-9) |
| Duration of PEX treatment before platelet count normalization (>150 × 109/L) | 5.5 (3-28) |
| Length of caplacizumab treatment, median (range), d | 31 (15-74) |
| Duration of hospital admission, median (range), d | 11 (5-26) |
| VWF levels on samples taken while on caplacizumab (n = 10) 10 mg od | VWF:Ag median 86 (43-194 IU/dL) |
| Normal range according to a laboratory (most 50-150 IU/dL) | VWF:Act median 2 (2-57 IU/dL) |
| Sample date taken after caplacizumab initiation: median, 11 d; mean, 19.5 d; minimum, 7; maximum, 63 d | FVIII median, 60 (6-156 IU/dL) |
| VWF levels on samples taken while on caplacizumab (n = 1) 5 mg od | VWF:Ag 94 |
| Normal range, 50-150 IU/dL | VWF:Act 2 |
| Sample date taken at 2 d after caplacizumab initiation | FVIII, 76 |
| ADAMTS13 at cessation of caplacizumab treatment | 63 (23.9- 89.6) |
| Time to normalization of ADAMTS13 (>64 IU/dL), median (range), d | 35 (8-149) |
MMF, mycophenolate mofetil; od, once daily.
Discussion
This cohort study from the UK TTP Registry is the largest study on pediatric patients treated with caplacizumab for iTTP. Caplacizumab was demonstrated to be effective and safe in a manner synonymous with that seen in adult trial data, primarily with a reduction in PEX requirement until the platelet count recovery compared with the precaplacizumab era.3,4 Previous clinical studies on caplacizumab have excluded children on the grounds of feasibility. There is very scant pediatric data in TTP, particularly iTTP.
There was no recurrent disease after stopping caplacizumab, albeit with confounding factors of treatment, including steroid and anti-CD20 treatments. Relapse was identified in 1 patient 9 months after presentation in a child who was also an outlier in terms of time to normalize the platelet count after starting caplacizumab (26 days). The child subsequently demonstrated marked immune dysregulation in the form of recurrent autoimmune hemolytic anemia and Rosai-Dorfman syndrome.
One patient was managed without PEX altogether, which suggests a future role for caplacizumab in managing iTTP to platelet and eventual ADAMTS13 recovery while avoiding PEX in children who are well with iTTP. This has significant ramifications for clinical care because pediatric patients with iTTP may avoid intensive care, sedation, ventilation, and central venous access. Avoiding procedures that necessitate general anesthesia and theater list allocation is particularly relevant to pediatric care when such waits can significantly delay the optimal delivery of emergency care.
Safety is evident by how well-tolerated caplacizumab was in this young cohort, with no new safety concerns flagged. Bleeding requiring VWF supplementation and reduction of caplacizumab use occurred in 1 patient, and there was no significant intracranial or gastrointestinal bleeding.
The paucity of clinical trial data on the use of caplacizumab in the pediatric population is particularly relevant to smaller and younger children, in whom assumptions about clinical equivalence are less easy. For patients weighing <40 kg, caplacizumab was dosed at 5 mg once daily. This was based on simulations performed on the pharmacokinetic and pharmacodynamic model developed in adults, to establish a suitable dosing regimen for children >2 years of age and adolescents.7 The model was based on allometric scaling principles, with simulations of caplacizumab and VWF antigen (VWF:Ag) concentrations in an assumed population analysis of 8000 pediatric patients with iTTP, confirmed by comparison to simulations performed for weigh-based dosing of 5 mg for children with body weight <40 kg and 10 mg for children >40 kg. Finally, the suitability of weight-based dosing was confirmed by comparing caplacizumab exposure and VWF:Ag suppression levels simulated in children. It was assumed that children of this age would have similar VWF:Ag levels and disease effects as those of adults. The European Medicines Agency considered the results of this simulation to be adequately convincing to approve an extension of the indication for caplacizumab in children with iTTP.8 The Food and Drug Administration has approved caplacizumab for adult patients with iTTP but not for pediatric patients.9
Our own VWF results support this, with VWF activity in patients treated with caplacizumab rendered synonymous with severe von Willebrand disease. One exception to this in our cohort was the only relative reduction in VWF activity (57 IU/dL) in 1 patient who had caplacizumab dose reduction (from 10 mg once daily to 5 mg alternate day) due to significant epistaxis, necessitating treatment with VWF supplementation, demonstrating reversibility of caplacizumab effect, if necessary. However, the clinical remission was maintained.
The first child under 40 kg in the United Kingdom received caplacizumab in December 2019, aged 3 years and 1 month. This was issued on a compassionate use basis, based on the child being in extremis and with the diagnosis of iTTP clearly established. In our cohort, all 3 pediatric patients who received 5 mg once daily achieved platelet and ADAMTS13 recovery.
Conclusion
This cohort study demonstrated both the efficacy and safety of caplacizumab in a pediatric population, including children as young as 2 years of age. There were no new safety concerns.
Acknowledgment
Patients under 12 years of age received caplacizumab on a compassionate use basis from Sanofi.
Authorship
Contribution: A.T. wrote the manuscript; L.K., E.D., T.D., J.G., R.G., C.M., M.R., S. Stokley, S. Salta, T.T., and M.S. collected registry data; and M.S. reviewed the manuscript.
Conflict-of-interest disclosure: M.S. received honoraria from Sanofi and reports participation in the speakers’ bureau for Sanofi. T.D. has received speaker fees from Sanofi. The remaining authors declare no competing financial interests.
Correspondence: Alice Taylor, Great Ormond Street Hospital for Children NHS Foundation Trust, Haemophilia Centre, 60 Great Ormond St Hospital, London WC1N 3HR, United Kingdom; email: alice.taylor@gosh.nhs.uk.
References
Author notes
Data are available upon request from the corresponding author, Alice Taylor (alice.taylor@gosh.nhs.uk).