Key Points
Consistent with erythrocyte pyruvate kinase activation, adenosine triphosphate increased, and 2,3-DPG decreased with etavopivat treatment.
Clinically, this translated to 73.3% of patients with SCD treated with etavopivat having an Hb increase >1 g/dL at any time during treatment.
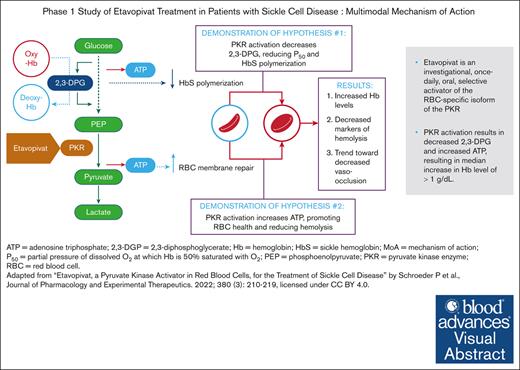
Visual Abstract
Etavopivat is an investigational, once daily, oral, selective erythrocyte pyruvate kinase (PKR) activator. A multicenter, randomized, placebo-controlled, double-blind, 3-part, phase 1 study was conducted to characterize the safety and clinical activity of etavopivat. Thirty-six patients with sickle cell disease (SCD) were enrolled into 4 cohorts: 1 single-dose, 2 multiple ascending doses, and 1 open-label (OL). In the OL cohort, 15 patients (median age 33.0 years [range, 17-55]) received 400 mg etavopivat once daily for 12 weeks; 14 patients completed treatment. Consistent with the mechanism of PKR activation, increases in adenosine triphosphate and decreases in 2,3-diphosphoglycerate were observed and sustained over 12 weeks’ treatment. This translated clinically to an increase in hemoglobin (Hb; mean maximal increase 1.6 g/dL [range, 0.8-2.8]), with >1 g/dL increase in 11 (73%) patients during treatment. In addition, the oxygen tension at which Hb is 50% saturated was reduced (P = .0007) with a concomitant shift in point of sickling (P = .0034) to lower oxygen tension in oxygen-gradient ektacytometry. Hemolysis markers (absolute reticulocyte count, indirect bilirubin, and lactate dehydrogenase) decreased from baseline, along with matrix metalloproteinase-9 and erythropoietin. In the OL cohort, adverse events (AEs) were mostly grade 1/2, consistent with underlying SCD; 5 patients had serious AEs. Vaso-occlusive pain episode was the most common treatment-emergent AE (n = 7) in the OL cohort. In this, to our knowledge, the first study of etavopivat in SCD, 400 mg once daily for 12 weeks was well tolerated, resulting in rapid and sustained increases in Hb, improved red blood cell physiology, and decreased hemolysis. This trial was registered at www.ClinicalTrials.gov as #NCT03815695.
Introduction
Sickle cell disease (SCD) is an inherited hemolytic anemia that affected 300 000 newborns per year globally in 2010 and is expected to affect more than 400 000 newborns per year by 2050.1-4 Impaired red blood cell (RBC) physiology is the hallmark of SCD, which is caused by a single mutation in the β-globin gene, resulting in the formation of hemoglobin S (HbS) rather than hemoglobin A (HbA).1-3 Clinical consequences include vaso-occlusion and hemolytic anemia, causing vaso-occlusive pain episodes (VOEs), acute and progressive end-organ damage, and diminished quality of life.1-3 In high-resource countries, survival has improved because of newborn screening, penicillin prophylaxis, and clinically validated treatment strategies.1,5 However, even there, the average life span of a person with SCD remains 20 to 30 years shorter than peers.1,6,7
Current SCD treatments include supportive care, transfusions, disease-modifying therapies such as hydroxyurea (HU), and hematopoietic stem cell transplantation.1,3,5,8-10 Potentially curative gene therapies have recently been US Food and Drug Administration-approved but are costly.11-13 In addition, disease-modifying therapies are hampered by barriers to access, toxicity profiles, and, for the newer agents, uncertain long-term benefit. Of the available therapies, hematopoietic stem cell transplantation is potentially curative, but poor donor availability, risk, and cost limit its use.1,5,10 There is an unmet need for therapeutic agents that can be initiated early, target underlying SCD pathophysiology, reduce hemolysis and VOEs, limit end-organ damage, and improve quality of life, while having a favorable risk-benefit ratio.1,5,10,14
Several glycolytic enzymes and the Rapoport-Luebering shunt are activated in RBCs under hypoxic conditions, leading to increased 2,3 diphosphoglycerate (2,3-DPG) production.15,16 In sickle RBCs, increased 2,3-DPG reduces the oxygen (O2) affinity of HbS, causing increased dissociation of O2 at a higher partial pressure of dissolved O2 (pO2) compared with normal RBCs.1,15 The increase in deoxygenated HbS induces Hb polymerization and precipitates a cascade of pathologic events, including RBC sickling, hemolysis, endothelial dysfunction, abnormal activation of inflammatory, coagulation, and oxidative pathways.1 This causes oxidative stress, vaso-occlusion, and tissue ischemia-reperfusion injury.1,3,9 Concurrently with increased intracellular 2,3-DPG in sickle RBCs, adenosine triphosphate (ATP) levels are reduced.17 ATP is necessary for normal ion channel function and RBC membrane homeostasis15,18; therefore, RBCs with reduced ATP levels are less flexible than normal RBCs, contributing to premature hemolysis.19
Erythrocyte pyruvate kinase (PKR) catalyzes the last, rate-limiting glycolysis step (phosphoenolpyruvate to pyruvate), generating ATP from adenosine diphosphate. PKR deficiency causes moderate-to-severe hemolytic anemia.20 Etavopivat is an investigational, once-daily pharmacokinetic (PK) activator selective for the RBC isozyme (PKR). PKR activation increases Hb-O2 affinity, decreases HbS polymerization, and improves RBC function and life span by decreasing intracellular 2,3-DPG and increasing intracellular ATP.21,22 Proof of pharmacodynamic (PD) activity for etavopivat was demonstrated in nonhuman primates, healthy humans, and ex vivo–treated RBCs from patients with SCD.21 Etavopivat decreased whole blood 2,3-DPG levels, increased ATP levels, and increased Hb-O2 affinity (decreased P50 [oxygen tension at which Hb is 50% saturated]) in RBCs from healthy participants after a single 700 mg dose. In ex vivo studies involving RBCs from patients with SCD, etavopivat increased Hb-O2 affinity and reduced RBC sickling. Another allosteric activator of PKR has also been recently demonstrated in phase 1 and phase 2 clinical studies that targeting this pathway may lead to clinical benefit in patients with SCD and was also relatively well tolerated and associated with improvements in Hb concentration and markers of hemolysis.23,24
To our knowledge, we report the first study of etavopivat in patients with SCD. The aim of this phase 1 study is to assess the safety and clinical efficacy of etavopivat in single-dose, multiple ascending doses (MADs), followed by open-label (OL) treatment in patients with SCD.
Methods
Clinical trial and human participants
To our knowledge, study 4202-HVS-101 (NCT03815695) was a first-in-human, randomized, placebo-controlled, double-blind, single-dose and MAD phase 1 trial in SCD. Results from healthy volunteers have been reported.25
The protocol and amendments were reviewed and approved by appropriate institutional review boards/independent ethics committees. Patients provided written informed consent before undergoing study-related procedures. The study was conducted in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice, and relevant laws/regulations. Data were analyzed by the study statistician (E.W.) and multiple authors. The authors had access to the data.
Key inclusion criteria were age 12 to 65 years (inclusive) at screening, a minimum weight of 40 kg, and confirmed SCD (HbSS, HbSβ0-thalassemia, HbSβ+-thalassemia, or HbSC). Patients with reproductive potential agreed to use a medically accepted contraceptive during the study and for 90 days after the last dose of study medication.
Key exclusion criteria were >6 episodes of VOEs within the past year requiring a hospital, emergency department, or clinic visit; hospitalization for VOE or other SCD-related event within 14 days of consent or 28 days before study treatment; ≥1 episode of acute chest syndrome requiring hospitalization, intubation, and mechanical ventilatory support within 6 months before screening; pulmonary hypertension; use of HU if started <90 days before study treatment; crizanlizumab if started within 14 days of study treatment; or voxelotor within 7 days of study treatment until the end of the study period. Patients with SCD with >6 VOE were excluded to minimize the risk of including those with chronic pain disorders. Patients were allowed crizanlizumab as scheduled infusions every ≥4 weeks. Stable doses of HU and L-glutamine were permitted.
Additional exclusion criteria included use of moderate or strong inducers/inhibitors of cytochrome P450 3A4/5 within 2 weeks of study treatment; RBC transfusion within 30 days of study treatment; history of deep vein thrombosis (DVT) requiring systemic anticoagulation therapy for ≥6 weeks occurring within 6 months of study treatment; and Hb <7.0 g/dL or >10.5 g/dL during screening.
Study design and treatment
Single-dose segment
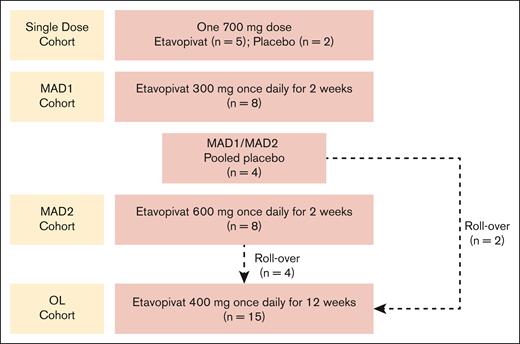
The randomized, placebo-controlled, single-dose portion of the study (Figure 1) was conducted to confirm the safety and PK/PD response to 700 mg etavopivat (previously shown to be safe and tolerable in healthy volunteers25). End of treatment (EOT) was on day 2, 24 hours after dosing (supplemental Appendix 1).
Study design. Patients in the MAD2 cohort could directly rollover into the OL cohort at the time of their EOS visit whether they tolerated the 2-week treatment period and continued to meet eligibility criteria. Patients from other cohorts and the study sites could also enroll in the OL cohort. In the OL cohort, protocol amendment 7.0 allowed etavopivat dosing to extend from 2 days to up to 2 weeks beyond day 84, allowing a stepwise dose decrease in patients with a >2.0 g/dL increase in Hb over baseline or if clinically indicated. MAD, multiple ascending dose; OL, open-label.
Study design. Patients in the MAD2 cohort could directly rollover into the OL cohort at the time of their EOS visit whether they tolerated the 2-week treatment period and continued to meet eligibility criteria. Patients from other cohorts and the study sites could also enroll in the OL cohort. In the OL cohort, protocol amendment 7.0 allowed etavopivat dosing to extend from 2 days to up to 2 weeks beyond day 84, allowing a stepwise dose decrease in patients with a >2.0 g/dL increase in Hb over baseline or if clinically indicated. MAD, multiple ascending dose; OL, open-label.
Seven patients received 1 oral dose of etavopivat 700 mg (n = 5) or placebo (n = 2).
MAD segment
The MAD study had 2 cohorts (MAD1 and MAD2) (Figure 1) with a randomized, placebo-controlled, double-blind design. Patients were randomly assigned (3:1) to receive daily etavopivat 300 mg (MAD1), 600 mg (MAD2), or placebo for 14 days. EOT was on day 14/15, 24 hours after completion of dosing (supplemental Appendix 1). Etavopivat/placebo dosing could extend by 48 hours to enable a 2-day stepwise dose reduction in patients demonstrating Hb increase >2.0 g/dL over baseline.
The MAD segment included 20 patients who received etavopivat 300 mg (MAD1, n = 8), etavopivat 600 mg (MAD2, n = 8), or placebo (n = 4).
OL segment
During OL segment, 15 patients received ≤84 consecutive 400 mg daily oral doses of etavopivat (Figure 1). EOT was on day 84/85, 24 hours after the last dose (supplemental Appendix 1). Patients returned to the clinic on day 84 for the last etavopivat dose and on days 85, 88, 91, 98, and 112 end-of-study (EOS) for follow-up visits to monitor disease parameters post study drug discontinuation.
Protocol amendment 7.0 allowed etavopivat dosing to extend from 2 days to up to 2 weeks beyond day 84, allowing a stepwise dose decrease in patients with >2.0 g/dL Hb increase over baseline, or if clinically indicated.
Safety and tolerability
Adverse events (AEs) were monitored from the time of written consent to the last protocol-defined EOS visit. Safety/tolerability monitoring has been described.25 A treatment-emergent AE (TEAE) was any AE new in onset or aggravated in severity/frequency after the first dose of study medication, up to and including the EOS visit. AE severity was assessed by the investigator using Common Terminology Criteria for Adverse Events v5.0.23,26 The potential relationship of each AE to the study drug (treatment) was categorized by the investigator as yes (possibly, probably, or definitely related) or no (unrelated or unlikely to be related).
PK/PD
Venous blood was collected at prespecified time points for PK/PD, RBC functional assessments, and biomarkers. PK parameters for etavopivat were derived using Phoenix WinNonlin (version 6.4 or higher) software for noncompartmental analysis of plasma concentration data at actual sampling times. Plasma concentrations of etavopivat were determined using liquid chromatography-tandem mass spectrometry.21,25
PD assessments included RBC 2,3-DPG, ATP, pO2 at P50, and exploratory laboratory assessments (RBC functional studies and biomarkers of inflammation and coagulation). ATP and 2,3-DPG concentrations in whole blood were measured using liquid chromatography-tandem mass spectrometry.21,25 The effect of 2,3-DPG reduction on Hb-O2 affinity was assessed before and after dosing using P50 values.21,25
Clinical activity
Indirect bilirubin (iBIL), lactate dehydrogenase (LDH), reticulocyte counts, and Hb were measured at local laboratories. Hb response was defined as >1g/dL change from baseline at any time during treatment.
RBC function
Complete blood counts and hematologic parameters were analyzed by local laboratories. Additional hematology parameters, such as cellular Hb concentration mean and dense RBCs (DRBCs), were centrally analyzed using an ADVIA2120i system (Siemens Healthineers, Hoffman Estates, IL).
Hb-O2 equilibrium curves were collected using a HEMOX Analyzer (TCS Scientific Corp, New Hope, PA).21,25 RBC deformability was measured using O2 gradient ektacytometry (oxygenscan) with the Laser Optical Rotational Red Cell Analyzer (Lorrca; RR Mechatronics, Zwaag, The Netherlands).21 RBC deformability was defined by the elongation index (EI) derived from the laser diffraction pattern in a suspension of RBCs subjected to a cycle of deoxygenation and reoxygenation. pO2 in the RBC suspension was calculated every 20 seconds based on signal quenching using a luminophore O2 sensor. Point of sickling (PoS) was calculated as the pO2 (mmHg) at which the EI dropped below 5% of maximum EI during deoxygenation, thus indicating the O2 pressure at which the polymerization of HbS begins to affect RBC deformability.21
Biomarkers
Biomarkers of inflammation (plasma tumor necrosis factor-α, matrix metalloproteinase-9, white blood cell count), hypercoagulability (prothrombin fragment 1.2; D-dimer), and tissue hypoxia (erythropoietin) were assessed using commercially available kits.
Statistical analyses
Sample size was based on clinical considerations and was not powered for hypothesis testing. Data were analyzed by cohort.
The safety population comprised all patients who received ≥1 dose of study treatment. The PK population included all patients in the safety population with ≥1 evaluable PK profile and no important protocol deviations or other reasons for exclusion from analysis. The PD population included all patients in the safety population with ≥1 postdose PD assessment.
Statistical analyses were performed using SAS software version 9.4. Wilcoxon tests or unadjusted mixed models for repeated measures statistical tests were used as appropriate. A P value <.05 was statistically significant.
Figures were plotted using GraphPad Prism version 9.
The protocol and amendments were reviewed and approved by appropriate institutional review boards/independent ethics committees: Duke University Health System Institutional Review Board; University of California, San Francisco Human Research Protection Program; Advarra Institutional Review Board; University of Illinois at Chicago Office for the Protection of Research Subjects; Children’s Healthcare of Atlanta Institutional Review Board, and Institutional Review Board Office, Augusta University.
Results
Study population
Thirty-six patients were enrolled and treated (supplemental Figure 1). Randomization began in November 2019, with the last patient completing it in December 2021. All patients in the single-dose (n = 7) and MAD cohorts (n = 20) completed the study. Fourteen of the 15 patients in the OL cohort (including 6 patients from the MAD cohorts who elected to roll over) completed the study; 1 withdrew due to an AE. All 15 patients in the OL cohort were included in the analyses.
Table 1 shows baseline patient demographics and clinical characteristics.
Baseline demographic and clinical characteristics
| Characteristic, median (range), unless indicated . | Single dose . | MAD . | OL . | |||
|---|---|---|---|---|---|---|
| Placebo . | Etavopivat single dose . | Pooled placebo . | Etavopivat once daily for 2 wk . | Etavopivat once daily for 12 wk . | ||
| (n = 2) . | 700 mg (n = 5) . | (n = 4) . | MAD1, 300 mg (n = 8) . | MAD2, 600 mg (n = 8) . | 400 mg (n = 15) . | |
| Age, y | 45 (42-48) | 32 (15-42) | 26.5 (17-36) | 24.0 (19-43) | 29.5 (22-64) | 33.0 (17-55) |
| Male sex, n (%) | 1 (50.0) | 1 (20.0) | 3 (75.0) | 2 (25.0) | 1 (12.5) | 5 (33.3) |
| Genotype, n (%) | ||||||
| HbSS | 2 (100) | 5 (100) | 4 (100) | 7 (87.5) | 6 (75.0) | 13 (86.7) |
| HbSC | 0 | 0 | 0 | 0 | 1 (12.5) | 2 (13.3) |
| HbSβ+ thalassemia | 0 | 0 | 0 | 1 (12.5) | 1 (12.5) | 0 |
| Current HU therapy, n (%) | 2 (100) | 5 (100) | 3 (75.0) | 6 (75.0) | 7 (87.5) | 13 (86.7) |
| Hb, g/dL | 7.2 (6.7-7.7) | 9.7 (7.7-10.4) | 7.6 (7.1-8.0) | 9.1 (6.9-10.1) | 8.9 (7.3-10.2) | 8.7 (7.2-10.1) |
| % HbS | 79.7 (70.2-89.1) | 78.8 (70-86.5) | 84.6 (76.6-92.7) | 83.3 (67.0-92.9) | 80.1 (78.2-87.8) | 80.3 (46.2-92.7) |
| % HbF | 17.4 (7.3-27.5) | 11.4 (5.5-20.5) | 10.0 (4.4-16.6) | 9.8 (3.5-20.1) | 15.3 (5.2-19.2) | 11.5 (1.2-23) |
| Advia MCV, fL∗ | 113.3 (101.6-125.0) | 108.7 (96.5-122.8) | 107.4 (100.1-131.5) | 112.9 (75.0-117.6) | 114.7 (68.5-129.6) | 108.1 (77.1-122.7) |
| ARC, ×109/L† | 178.1 (72.9-283.4) | 205.5 (136.0-366.4) | 238.4 (227.0-360.6) | 274.1 (125.6-329.6) | 226.8 (29.4-366.0) | 219.3 (80.5-511.0) |
| iBIL, mg/dL‡ | 3.8 (2.1-5.4) | 2.3 (1.6-5.1) | 2.8 (2.0-5.0) | 1.7 (0.5-10.5) | 1.3 (0.7-4.5) | 1.3 (0.8-5.2) |
| LDH, U/L | 374.5 (348-401) | 405.0 (308-543) | 352.0 (180-683) | 381.5 (207-699) | 368.5 (251-683) | 367.0 (186-683) |
| % F cells§ | 62.6 (33.3-91.8) | 50.6 (34.4-75.5) | 26.2 (22.3-30.1) | 36.1 (16.4-67.2) | 54.4 (13.3-64.8) | 54.4 (6.1-76.9) |
| Characteristic, median (range), unless indicated . | Single dose . | MAD . | OL . | |||
|---|---|---|---|---|---|---|
| Placebo . | Etavopivat single dose . | Pooled placebo . | Etavopivat once daily for 2 wk . | Etavopivat once daily for 12 wk . | ||
| (n = 2) . | 700 mg (n = 5) . | (n = 4) . | MAD1, 300 mg (n = 8) . | MAD2, 600 mg (n = 8) . | 400 mg (n = 15) . | |
| Age, y | 45 (42-48) | 32 (15-42) | 26.5 (17-36) | 24.0 (19-43) | 29.5 (22-64) | 33.0 (17-55) |
| Male sex, n (%) | 1 (50.0) | 1 (20.0) | 3 (75.0) | 2 (25.0) | 1 (12.5) | 5 (33.3) |
| Genotype, n (%) | ||||||
| HbSS | 2 (100) | 5 (100) | 4 (100) | 7 (87.5) | 6 (75.0) | 13 (86.7) |
| HbSC | 0 | 0 | 0 | 0 | 1 (12.5) | 2 (13.3) |
| HbSβ+ thalassemia | 0 | 0 | 0 | 1 (12.5) | 1 (12.5) | 0 |
| Current HU therapy, n (%) | 2 (100) | 5 (100) | 3 (75.0) | 6 (75.0) | 7 (87.5) | 13 (86.7) |
| Hb, g/dL | 7.2 (6.7-7.7) | 9.7 (7.7-10.4) | 7.6 (7.1-8.0) | 9.1 (6.9-10.1) | 8.9 (7.3-10.2) | 8.7 (7.2-10.1) |
| % HbS | 79.7 (70.2-89.1) | 78.8 (70-86.5) | 84.6 (76.6-92.7) | 83.3 (67.0-92.9) | 80.1 (78.2-87.8) | 80.3 (46.2-92.7) |
| % HbF | 17.4 (7.3-27.5) | 11.4 (5.5-20.5) | 10.0 (4.4-16.6) | 9.8 (3.5-20.1) | 15.3 (5.2-19.2) | 11.5 (1.2-23) |
| Advia MCV, fL∗ | 113.3 (101.6-125.0) | 108.7 (96.5-122.8) | 107.4 (100.1-131.5) | 112.9 (75.0-117.6) | 114.7 (68.5-129.6) | 108.1 (77.1-122.7) |
| ARC, ×109/L† | 178.1 (72.9-283.4) | 205.5 (136.0-366.4) | 238.4 (227.0-360.6) | 274.1 (125.6-329.6) | 226.8 (29.4-366.0) | 219.3 (80.5-511.0) |
| iBIL, mg/dL‡ | 3.8 (2.1-5.4) | 2.3 (1.6-5.1) | 2.8 (2.0-5.0) | 1.7 (0.5-10.5) | 1.3 (0.7-4.5) | 1.3 (0.8-5.2) |
| LDH, U/L | 374.5 (348-401) | 405.0 (308-543) | 352.0 (180-683) | 381.5 (207-699) | 368.5 (251-683) | 367.0 (186-683) |
| % F cells§ | 62.6 (33.3-91.8) | 50.6 (34.4-75.5) | 26.2 (22.3-30.1) | 36.1 (16.4-67.2) | 54.4 (13.3-64.8) | 54.4 (6.1-76.9) |
ARC, absolute reticulocyte count; HbF, fetal Hb; HbS, hemoglobin S; HU, hydroxyurea; iBIL, indirect bilirubin, LDH, lactate dehydrogenase; MAD, multiple ascending dose; MCV, mean corpuscular volume; OL, open-label.
n = 3 for MAD pooled placebo; n = 7 for MAD1 (300 mg); n = 14 for the 12-week cohort.
n = 3 for MAD pooled placebo.
n = 4 for single dose 700 mg; n = 3 for MAD pooled placebo.
n = 2 for MAD pooled placebo; n = 7 for MAD1 (300 mg); n = 14 for the 12-week cohort.
Exposure
Patients in the single-dose cohort received 1 dose of etavopivat at 700 mg. Patients in the MAD1 and MAD2 cohorts received etavopivat 300 mg and 600 mg once daily, respectively (median 14 days [range, 14-16 days for 300 mg and 14-14 days for 600 mg]). All patients in the 2 MAD cohorts had ≥ 80% compliance. One patient each in the MAD placebo and 600 mg etavopivat-treated groups had a dose interruption (unspecified nonadherence and other [nausea], respectively).
Patients in the OL cohort had a median exposure of 85 days (range, 14-97). Fourteen patients had ≥80% compliance; 1 had <80% compliance. Median exposure was 33 000 mg (range, 5600-34 400). Two patients experienced dose interruption due to an AE (nausea) and other (self-decreased dose due to headache). Another patient had drug withdrawn because of an AE (DVT).
Safety and tolerability
In the single-dose cohort, 2 (100%) placebo-treated patients and 2 (40%) etavopivat-treated patients had 3 TEAEs each, with 1 treated with etavopivat experiencing treatment-related palpitations. All TEAEs were grade 1 (supplemental Tables 1-3).
In the MAD cohorts, 1 patient (25%) treated with placebo experienced 7 TEAEs, 7 patients (87.5%) in MAD1 had 14 TEAEs, and 6 patients (75%) in MAD2 had 16 TEAEs. Three patients experienced 1 treatment-related TEAE (MAD1, headache and nausea; MAD2, increased total bilirubin). Among patients treated with etavopivat in the MAD cohorts, 10 had grade 1, 9 had grade 2, and 1 had grade 3 TEAEs. One patient in MAD2 experienced a serious unrelated TEAE (VOE). The most frequently reported all-causality TEAEs were VOEs (n = 6), headache (n = 4), and nausea (n = 2; supplemental Tables 1-3).
During the 12-week OL treatment with etavopivat 400 mg daily, all 15 patients (100%) experienced a total of 63 TEAEs. The most frequently reported all-causality TEAEs were VOEs (n = 7), headache (n = 4), nausea (n = 3), upper respiratory tract infection (n = 3), and dizziness, migraine, increased gamma-glutamyl transferase, musculoskeletal chest pain, and noncardiac chest pain (n = 2 each; supplemental Tables 1-3). TEAEs assessed as possibly or probably treatment-related by the investigator were reported in 8 patients; the most common were VOEs (n = 3 patients) occurring on day 89 (last dose day 85 [400 mg], day 89 (stepwise reduction, last dose day 87 [100 mg]), and day 96 (stepwise reduction, last day 87 [100 mg]).
In the OL cohort, 13, 8, and 6 patients had grade 1, 2, and ≥3 TEAEs, respectively. Five patients had serious TEAEs-VOE and COVID-19 infection, acute chest syndrome and VOE, DVT, noncardiac chest pain, and syncope (Table 2). On day 15, 1 patient discontinued treatment because of grade 3 DVT (possibly related), which resolved with mild residual swelling on day 80. No deaths were recorded.
PK parameters (PK population)
| Exposure in patients who received etavopivat . | Tmax, h . | Cmax, ng/mL . | AUC0-24, ng×h/mL . | t½, h∗ . | CL/F, L/h . |
|---|---|---|---|---|---|
| Single dose | |||||
| 700 mg (n = 5) | 2.0 (1.0-4.0) | 2894 (1450); 50.1 | 7552 (3294); 43.6 | 16.9 (7.1); 41.8† | 102.0 (50.8); 49.8† |
| Once-daily multiple doses | |||||
| 300 mg for 2 wk | |||||
| Day 1 (n = 8) | 1.0 (0.9-2.1) | 884 (339); 38.3 | 2508 (995); 39.7‡ | 4.9 (0.9); 18.4‡ | 136.6 (61.5); 45.0‡ |
| Day 14 (n = 7) | -- | 760 (412); 54.2 | 2747 (1047); 38.1 | -- | 123.5 (46.9); 38.0§ |
| 600 mg for 2 wk | |||||
| Day 1 (n = 8) | 1.8 (1.0-4.1) | 1724 (1246); 72.3 | 6177 (2944); 47.7 | 4.0 (0.6); 14.4‖ | 107.2 (45.0); 42.0‖ |
| Day 14 (n = 8) | -- | 3465 (2136); 61.7 | 7728 (4218); 54.6 | -- | 98.8 (50.5); 51.1§ |
| 400 mg for 12 wk | |||||
| Day 1 (n = 15) | 1.8 (1.0-3.9) | 1139 (510); 44.8 | 3474 (1283); 36.9¶ | 4.7 (1.2); 25.6# | 121.8 (31.6); 26.0# |
| Day 84 (n = 13) | -- | 1288 (684); 53.1 | 3105 (901); 29.0∗∗ | -- | 138.2 (37.7); 27.3§,∗∗ |
| Exposure in patients who received etavopivat . | Tmax, h . | Cmax, ng/mL . | AUC0-24, ng×h/mL . | t½, h∗ . | CL/F, L/h . |
|---|---|---|---|---|---|
| Single dose | |||||
| 700 mg (n = 5) | 2.0 (1.0-4.0) | 2894 (1450); 50.1 | 7552 (3294); 43.6 | 16.9 (7.1); 41.8† | 102.0 (50.8); 49.8† |
| Once-daily multiple doses | |||||
| 300 mg for 2 wk | |||||
| Day 1 (n = 8) | 1.0 (0.9-2.1) | 884 (339); 38.3 | 2508 (995); 39.7‡ | 4.9 (0.9); 18.4‡ | 136.6 (61.5); 45.0‡ |
| Day 14 (n = 7) | -- | 760 (412); 54.2 | 2747 (1047); 38.1 | -- | 123.5 (46.9); 38.0§ |
| 600 mg for 2 wk | |||||
| Day 1 (n = 8) | 1.8 (1.0-4.1) | 1724 (1246); 72.3 | 6177 (2944); 47.7 | 4.0 (0.6); 14.4‖ | 107.2 (45.0); 42.0‖ |
| Day 14 (n = 8) | -- | 3465 (2136); 61.7 | 7728 (4218); 54.6 | -- | 98.8 (50.5); 51.1§ |
| 400 mg for 12 wk | |||||
| Day 1 (n = 15) | 1.8 (1.0-3.9) | 1139 (510); 44.8 | 3474 (1283); 36.9¶ | 4.7 (1.2); 25.6# | 121.8 (31.6); 26.0# |
| Day 84 (n = 13) | -- | 1288 (684); 53.1 | 3105 (901); 29.0∗∗ | -- | 138.2 (37.7); 27.3§,∗∗ |
A dash indicates not done. ∗Data are presented as arithmetic mean (± standard deviation) and %CV for Cmax, AUC0-24, t½, and CL/F. Data are presented as median (range) for Tmax.
%CV, percent coefficient of variation; AUC0-24, area under the concentration-time curve from time 0 to 24; CL/F, apparent clearance; Cmax, maximum concentration; PK, pharmacokinetics; t½, terminal elimination half-life; Tmax, time to maximum concentration.
The difference between the 700-mg dose and the 300-mg, 400-mg, and 600-mg doses in estimated t½ is likely due the reduced sampling schedule during the elimination phase of the PK profile in the MAD and OL cohorts.
n = 4.
n = 7.
Steady state.
n = 6.
n = 11.
n = 10.
n = 9.
After etavopivat treatment, there were no clinically meaningful adverse shifts in vital signs, physical examination findings, chemistry, liver function, or hematology laboratory parameters, and no clinically meaningful laboratory abnormalities reported as serious AEs or resulting in study discontinuation. supplemental Appendix 2 has additional details.
The frequency of pain-related TEAEs decreased over time (supplemental Table 4).
No patient received a transfusion during the study.
PK
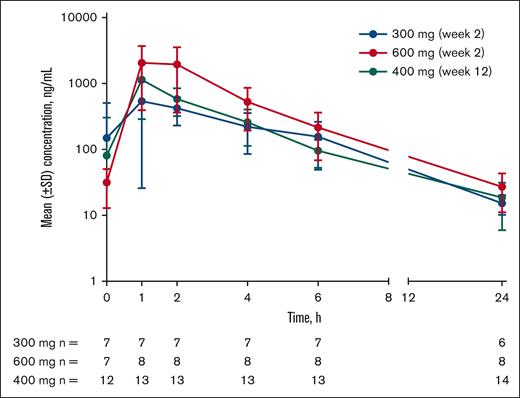
Etavopivat was rapidly absorbed with time to the maximum observed plasma concentration, ranging from 1 to 4 hours after the dose (Table 2; Figure 2). Across cohorts, total exposure (area under the plasma concentration-time curve from 0-24 hours) and maximum observed plasma concentration increased with increasing etavopivat dose. The estimated elimination half-life of etavopivat was 16.9 hours in the 700-mg single-dose cohort and 4 to 4.9 hours in the 300-, 600-, and 400-mg cohorts (Table 2). The apparent etavopivat clearance was similar across cohorts.
Etavopivat concentration vs time after daily dosing in patients with SCD (MAD and OL cohorts). Mean (± SD) etavopivat concentrations after daily dosing on day 14 (MAD) or day 84 (OL) at the indicated time point (hours). MAD, multiple ascending dose; OL, open-label; SCD, sickle cell disease; SD, standard deviation.
Etavopivat concentration vs time after daily dosing in patients with SCD (MAD and OL cohorts). Mean (± SD) etavopivat concentrations after daily dosing on day 14 (MAD) or day 84 (OL) at the indicated time point (hours). MAD, multiple ascending dose; OL, open-label; SCD, sickle cell disease; SD, standard deviation.
PD
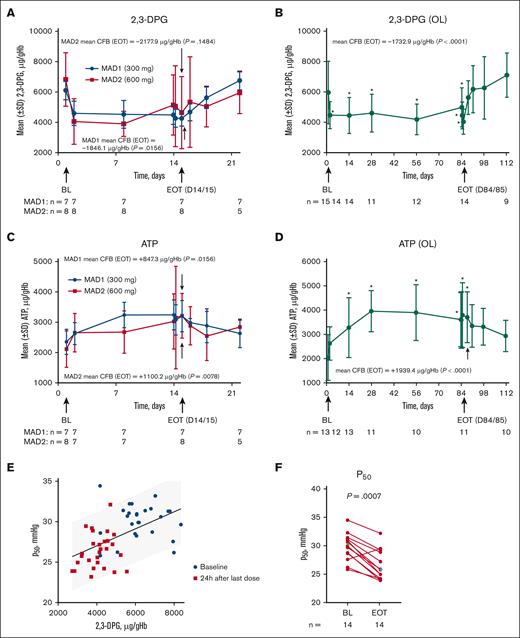
After etavopivat administration, mean whole blood 2,3-DPG levels (microgram/milliliter per gram/milliliter of Hb) declined rapidly from day 1 to day 2 and remained stable throughout the 14-day MAD and 84-day OL periods (Figure 3A-B). At EOT, mean 2,3-DPG levels were significantly lower than baseline in the MAD1 and OL cohorts (Figure 3A-B; Table 3).
PD in patients with SCD. Mean RBC 2,3-DPG and ATP concentrations in the MAD (A,C), and OL (B,D) cohorts. Values were normalized by dividing the Hb value at each time point to adjust for a dilution effect from increased Hb (A-D). The P50 value as a function of intracellular 2,3-DPG concentration in the MAD (excluding placebo patients) and OL cohorts 24 hours after the last dose (E). Scatterplot at baseline (BL) and EOT for P50 in the OL cohort (Median BL and EOT values shown in green and blue diamonds, respectively) (F); each data point corresponds to data from 1 patient. Paired BL and EOT data points from each patient are connected by a line. In the MAD cohorts (A,C), P values were based on Wilcoxon signed rank tests to test the changes at EOT from BL. In the OL cohort (B,D), PD values with statistical significance compared with BL were identified with an asterisk (∗P < .05) at their scheduled visits, based on MMRM, which included PD values as dependent variable, and a fixed effect of scheduled visit during the treatment period with compound symmetry covariance matrix to model the within patient variance-covariance errors; the EOT P values were derived from Wilcoxon signed rank tests. Statistical tests were not performed for the visits after EOT. P values in the scatterplot are from a Wilcoxon matched-pairs signed rank test (F). One MAD1 (300 mg) patient was excluded from 2,3-DPG, ATP, and P50 analyses because the patient only took 1 dose of study drug on day 1. ATP, adenosine triphosphate; CFB, change from baseline; 2,3-DPG, 2,3-diphosphoglycerate; EOT, end of treatment; Hb, hemoglobin; MAD, multiple ascending dose; MMRM, mixed model for repeated measurement; OL, open-label; P50, oxygen tension at which Hb is 50% saturated; PD, pharmacodynamic; SCD, sickle cell disease; SD, standard deviation; RBC, red blood cell.
PD in patients with SCD. Mean RBC 2,3-DPG and ATP concentrations in the MAD (A,C), and OL (B,D) cohorts. Values were normalized by dividing the Hb value at each time point to adjust for a dilution effect from increased Hb (A-D). The P50 value as a function of intracellular 2,3-DPG concentration in the MAD (excluding placebo patients) and OL cohorts 24 hours after the last dose (E). Scatterplot at baseline (BL) and EOT for P50 in the OL cohort (Median BL and EOT values shown in green and blue diamonds, respectively) (F); each data point corresponds to data from 1 patient. Paired BL and EOT data points from each patient are connected by a line. In the MAD cohorts (A,C), P values were based on Wilcoxon signed rank tests to test the changes at EOT from BL. In the OL cohort (B,D), PD values with statistical significance compared with BL were identified with an asterisk (∗P < .05) at their scheduled visits, based on MMRM, which included PD values as dependent variable, and a fixed effect of scheduled visit during the treatment period with compound symmetry covariance matrix to model the within patient variance-covariance errors; the EOT P values were derived from Wilcoxon signed rank tests. Statistical tests were not performed for the visits after EOT. P values in the scatterplot are from a Wilcoxon matched-pairs signed rank test (F). One MAD1 (300 mg) patient was excluded from 2,3-DPG, ATP, and P50 analyses because the patient only took 1 dose of study drug on day 1. ATP, adenosine triphosphate; CFB, change from baseline; 2,3-DPG, 2,3-diphosphoglycerate; EOT, end of treatment; Hb, hemoglobin; MAD, multiple ascending dose; MMRM, mixed model for repeated measurement; OL, open-label; P50, oxygen tension at which Hb is 50% saturated; PD, pharmacodynamic; SCD, sickle cell disease; SD, standard deviation; RBC, red blood cell.
Change from baseline and percentage change from baseline at EOT
| . | OL 400 mg, 12-wk cohort (n = 15)∗,‡ . | MAD pooled placebo (n = 4)∗,†,‡ . | MAD1, 300 mg (n = 8)∗,†,‡,§ . | MAD2, 600 mg (n = 8)∗,†,‡ . | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (± SD) . | Median (min, max) . | Mean (± SD) . | Median (min, max) . | Mean (± SD) . | Median (min, max) . | Mean (± SD) . | Median (min, max) . | |
| 2,3-DPG, μg/mL per g/mL Hb | ||||||||
| Baseline | 5956.5 (2050.8) | 6181.8 (541, 8350) | 4349.7 (2904.8) | 4689.5 (541, 7479) | 6109.7 (621.8) n = 7 | 6053.8 (5368, 7071) n = 7 | 6821.8 (1764.2) | 6103.9 (5067, 10693) |
| EOT | 4099.2 (833.8) n = 14 | 4090.2 (2732.1, 5555.6) n = 14 | 5890.9 (800.4) | 5642.6 (5253, 7025) | 4263.6 (507.3) n = 7 | 4193.5 (3595, 4915) n = 7 | 4643.8 (2374.7) | 3968.8 (2852, 10338) |
| CFB EOT | −1732.9‖ (2213.6) n = 14 | −1988.4‖ (−4511.8, 3595.3) n = 14 | 1541.2 (2430.4) | 873.1 (−454, 4872) | −1846.1‖ (494.2) n = 7 | −1860.2‖ (−2665, –1156) n = 7 | −2177.9 (2919.3) | −2115.2 (−7457, 3310) |
| ATP, μg/mL per g/mL Hb | ||||||||
| Baseline | 2037.7 (947.3) n = 13 | 2250.0 (321, 3111) n = 13 | 1284.5 (1128.5) | 1152.2 (313, 2521) | 2355.5 (418.5) n = 7 | 2149.4 (1989, 3170) n = 7 | 2117.9 (601.6) | 2217.5 (989, 3033) |
| EOT | 3802.0 (1276.1) n = 11 | 4117.0 (1484.9, 5276.8) n = 11 | 1854.7 (1096.4) | 2209.5 (333, 2667) | 3202.9 (516.2) n = 7 | 3265.0 (2577, 4150) n = 7 | 3218.1 (739.2) | 3391.6 (2024, 4255) |
| CFB EOT | 1939.4‖ (1483.7) n = 11 | 2067.5‖ (−802.4, 4112.1) n = 11 | 570.2 (1196.2) | 57.7 (−189, 2354) | 847.3‖ (656.4) n = 7 | 680.1‖ (127, 2101) n = 7 | 1100.2‖ (528.0) | 1282.3‖ (251, 1596) |
| Hb, g/dL | ||||||||
| Baseline | 8.7 (1.0) | 8.7 (7.2, 10.1) | 7.6 (0.4) | 7.6 (7.1, 8.0) | 9.0 (1.1) | 9.1 (6.9, 10.1) | 8.7 (0.9) | 8.9 (7.3, 10.2) |
| EOT | 9.8 (1.1) | 9.5 (8.4, 12.0) | 7.7 (0.2) | 7.7 (7.5, 7.9) | 10.1 (1.6) | 10.8 (6.8, 11.7) | 9.8 (1.5) | 9.8 (7.4, 12.3) |
| CFB EOT | 1.1‖ (0.8) n = 14 | 1.2‖ (−0.2, 2.7) n = 14 | 0.1 (0.5) | 0.0 (−0.4, 0.8) | 1.2‖ (0.9) | 1.2‖ (−0.1, 2.3) | 1.1‖ (1.1) | 1.0‖ (−0.1, 3.5) |
| Absolute reticulocytes, ×109/L | ||||||||
| Baseline | 229.4 (116.9) | 219.3 (80.5, 511.0) | 275.3 (74.1) n = 3 | 238.4 (227.0, 360.6) n = 3 | 252.3 (70.4) | 274.1 (125.6, 329.6) | 227.3 (105.8) | 226.8 (29.4, 366.0) |
| EOT | 163.3 (85.8) n = 14 | 133.1 (48.7, 351.6) n = 14 | 241.9 (115.7) | 261.8 (85.2, 358.9) | 144.0 (121.1) | 99.2 (60.0, 433.9) | 130.1 (64.0) | 135.2 (24.8, 219.1) |
| CFB EOT | −66.8‖ (107.0) n = 14 | −44.6‖ (−305.0, 118.1) n = 14 | 18.8 (98.0) n = 3 | 11.0 (−75.0, 120.5) n = 3 | −108.3‖ (114.7) | −142.0‖ (−233.8, 133.4) | −97.2‖ (66.3) | −87.4‖ (−217.2, −4.6) |
| iBIL, mg/dL | ||||||||
| Baseline | 1.9 (1.4) | 1.3 (0.8, 5.2) | 3.3 (1.6) n = 3 | 2.8 (2.0, 5.0) n = 3 | 3.1 (3.4) | 1.7 (0.5, 10.5) | 1.9 (1.4) | 1.3 (0.7, 4.5) |
| EOT | 1.2 (0.7) n = 13 | 0.9 (0.5, 2.9) n = 13) | 2.6 (1.5) | 2.6 (0.8, 4.4) | 1.8 (1.5) | 0.9 (0.3, 3.8) | 1.1 (0.8) | 0.7 (0.5, 2.6) |
| CFB EOT | −0.5‖ (0.8) n = 13 | −0.5‖ (−2.3, 0.5) n = 13 | −0.03 (0.5) n = 3 | 0.21 (−0.6, 0.3) n = 3 | −1.3 (2.5) | −0.5 (−7.0, 0.9) | −0.8‖ (0.7) | −0.6‖ (−1.9, −0.1) |
| LDH, U/L | ||||||||
| Baseline | 375.2 (142.9) | 367.0 (186, 683) | 391.8 (210.5) | 352.0 (180, 683) | 430.4 (159.0) | 381.5 (207, 699) | 391.4 (129.7) | 368.5 (251, 683) |
| EOT | 319.1 (87.7) n = 14 | 323.0 (193, 470) n = 14 | 449.8 (209.5) | 486.5 (192, 634) | 311.4 (155.3) | 280.0 (159, 641) | 315.5 (94.0) | 308.5 (199, 492) |
| CFB EOT | −38.4‖ (77.7) n = 14 | −23.0‖ (−213, 57) n = 14 | 58.0 (151.4) | 17.0 (−77, 275) | −119.0‖ (113.3) | −97.5‖ (−283, 61) | −75.9 (173.2) | −54.5 (−461, 90) |
| P50, mmHg | ||||||||
| Baseline | 29.9 (2.4) n =14 | 30.3 (25.8, 34.4) n = 14 | 29.2 (5.9) n = 3 | 30.5 (22.8, 34.4) n = 3 | 30.4 (1.3) n = 6 | 30.8 (28.3, 31.6) n = 6 | 30.1 (2.2) | 30.4 (26.2, 33.2) |
| EOT | 26.6 (2.5) n = 14 | 25.8 (23.8, 32.1) n = 14 | 31.4 (2.6) | 31.3 (28.5, 34.6) | 26.4 (1.6) n = 7 | 26.6 (23.8, 28.4) n = 7 | 26.3 (1.9) | 26.5 (23.2, 28.9) |
| CFB EOT | −3.3‖ (2.0) n = 13 | −3.8‖ (−5.9, 1.9) n = 13 | 2.0 (3.2) n = 3 | 0.2 (0.2, 5.7) n = 3 | −4.3‖ (1.9) n = 6 | −4.3‖ (−7.2, −1.6) n = 6 | −3.9‖ (1.6) | −3.5‖ (−6.7, −2.3) |
| PoS, mmHg | ||||||||
| Baseline | 43.2 (7.1) n = 14 | 43.1 (22.0, 50.0) n = 14 | 45.8 (4.9) n = 3 | 48.1 (40.1, 49.2) n = 3 | 36.3 (9.2) n = 6 | 39.1 (19.0, 45.0) n = 6 | 38.5 (8.6) | 39.7 (26.2, 49.2) |
| EOT | 35.1 (12.3) n = 14 | 36.5 (9.5, 51.0) n = 14 | 63.2 (34.2) | 52.8 (34.6, 112.7) | 31.0 (9.7) | 28.5 (18.1, 47.3) | 31.3 (3.8) | 31.1 (25.6, 36.2) |
| CFB EOT | −8.6‖ (8.2) n = 13 | −8.6‖ (−22.8, 3.0) n = 13 | 19.8 (38.1) n = 3 | 1.3 (−5.6, 63.5) n = 3 | −8.0‖ (5.7) n = 6 | −9.0‖ (−15.1, −0.9) n = 6 | −7.3‖ (7.0) | −7.2‖ (−15.4, 4.6) |
| Elmin | ||||||||
| Baseline | 0.12 (0.07) n = 14 | 0.11 (0.0, 0.3) n = 14 | 0.13 (0.15) n = 3 | 0.18 (0.0, 0.3) n = 3 | 0.11 (0.10) n = 6 | 0.07 (0.0, 0.3) n = 6 | 0.19 (0.11) | 0.15 (0.1, 0.4) |
| EOT | 0.16 (0.14) n = 14 | 0.12 (0.0, 0.5) n = 14 | 0.06 (0.11) | 0.03 (0.0, 0.2) | 0.18 (0.15) | 0.12 (0.1, 0.5) | 0.23 (0.10) | 0.21 (0.1, 0.5) |
| CFB EOT | 0.05 (0.10) n = 13 | 0.04 (−0.1, 0.2) n = 13 | −0.06 (0.19) n = 3 | 0.04 (−0.3, 0.1) n = 3 | 0.10 (0.11) n = 6 | 0.05 (0.0, 0.2) n = 6 | 0.04 (0.05) | 0.06 (0.0, 0.1) |
| Elmax | ||||||||
| Baseline | 0.45 (0.10) n = 14 | 0.46 (0.2, 0.5) n = 14 | 0.38 (0.19) n = 3 | 0.49 (0.2, 0.5) n = 3 | 0.43 (0.14) n = 6 | 0.43 (0.3, 0.6) n = 6 | 0.48 (0.06) | 0.49 (0.4, 0.6) |
| EOT | 0.47 (0.08) n =14 | 0.49 (0.2, 0.5) n = 14 | 0.40 (0.14) | 0.44 (0.2, 0.5) | 0.49 (0.08) | 0.51 (0.3, 0.6) | 0.51 (0.04) | 0.51 (0.4, 0.6) |
| CFB EOT | 0.02 (0.05) n = 13 | 0.01 (−0.1, 0.1) n = 13 | 0.00 (0.05) n = 3 | 0.02 (−0.1, 0.0) n = 3 | 0.05 (0.08) n = 6 | 0.02 (0.0, 0.2) n = 6 | 0.03 (0.05) | 0.02 (0.0, 0.1) |
| DRBCs (hyper), % | ||||||||
| Baseline | 3.2 (2.5) n = 14 | 2.8 (1.0, 11.1) n = 14 | 5.0 (5.4) n = 3 | 2.7 (1.1, 11.1) n = 3 | 4.0 (3.1) n = 7 | 3.3 (0.9, 8.9) n = 7 | 2.5 (1.1) | 2.6 (1.0, 4.1) |
| EOT | 2.8 (2.1) n = 14 | 2.0 (1.2, 8.9) n = 14 | 4.8 (3.3) | 3.9 (1.9, 9.6) | 2.6 (1.8) | 2.1 (1.1, 6.9) | 1.9 (0.9) | 1.9 (0.7, 3.3) |
| CFB EOT | −0.3 (1.6) n = 13 | −0.4 (−2.5, 2.7) n = 13 | 0.2 (1.5) n = 3 | 0.8 (−1.5, 1.3) n = 3 | −1.3 (1.7) n = 7 | −1.8 (−4.2, 0.7) n = 7 | −0.6 (1.1) | −0.8 (−2.1, 1.4) |
| CHCM, g/dL | ||||||||
| Baseline | 33.0 (1.3) n = 14 | 32.8 (30.6, 35.3) n = 14 | 32.6 (2.3) n = 3 | 32.3 (30.5, 35.1) n = 3 | 33.0 (0.9) n = 7 | 33.0 (31.7, 34.3) n = 7 | 32.3 (1.3) | 32.6 (30.3, 34.3) |
| EOT | 32.5 (1.5) n = 14 | 32.2 (30.8, 36.1) n = 14 | 33.5 (1.0) | 33.4 (32.5, 34.8) | 32.4 (0.9) | 32.2 (31.3, 33.8) | 31.7 (1.3) | 31.6 (29.2, 33.6) |
| CFB EOT | −0.6 (1.1) n = 13 | −0.9 (−1.8, 2.3) n = 13 | 1.0 (1.2) n = 3 | 1.3 (−0.3, 2.0) n = 3 | −0.7‖ (0.6) n = 7 | −0.7‖ (−1.4, 0.3) n = 7 | −0.6 (0.8) | −0.9 (−1.3, 0.6) |
| TNF-α, pg/mL | ||||||||
| Baseline | 1.2 (0.5) n = 14 | 1.1 (0.6, 2.2) n = 14 | 1.7 (0.7) n = 2 | 1.7 (1.3, 2.2) n = 2 | 1.2 (0.5) | 1.1 (0.6, 2.0) | 1.4 (0.5) n = 7 | 1.2 (0.8, 2.0) n = 7 |
| EOT | 0.8 (0.4) n = 13 | 0.9 (0.2, 1.6) n = 13 | 1.3 (0.1) | 1.2 (1.1, 1.5) | 1.4 (0.6) n = 7 | 1.4 (0.7, 2.3) n = 7 | 0.7 (0.4) | 0.8 (0.2, 1.3) |
| CFB EOT | −0.3 (0.7) n = 12 | −0.1 (−1.7, 0.4) n = 12 | −0.5 (0.6) n = 2 | −0.5 (−0.9, −0.1) n = 2 | 0.1 (0.6) n = 7 | 0.0 (−0.6, 1.0) n = 7 | −0.5‖ (0.6) n = 7 | −0.3‖ (−1.7, 0.0) n = 7 |
| MMP-9, ng/mL | ||||||||
| Baseline | 440.1 (282.4) n = 13 | 434.7 (90.3, 929.4) n = 13 | 573.1 n = 1 | 573.1 (573.1, 573.1) n = 1 | ND | ND | 451.2 (313.5) n = 7 | 434.7 (97.2, 929.4) n = 7 |
| EOT | 296.0 (354.0) n = 13 | 175.6 (0.0, 1280.9) n = 13 | 280.9 (96.6) n = 2 | 280.9 (212.6, 349.1) n = 2 | ND | ND | 282.2 (176.2) | 242.4 (69.3, 602.3) |
| CFB EOT | −149.8‖ (259.3) n = 11 | −175.6‖ (−627.8, 351.5) n = 11 | −224.0 n = 1 | −224.0 (−224.0, −224.0) n = 1 | ND | ND | −193.3‖ (215.4) n = 7 | −96.3‖ (−615.5, −18.5) n = 7 |
| Leukocytes, ×109/L | ||||||||
| Baseline | 9.6 (4.9) | 7.9 (5.0, 24.5) | 11.2 (4.6) | 10.4 (6.9, 17.3) | 8.8 (4.4) | 7.5 (4.4, 15.8) | 8.6 (2.9) | 7.9 (6.0, 14.7) |
| EOT | 8.2 (3.3) n = 14 | 8.9 (2.7, 13.1) n = 14 | 11.1 (6.1) | 9.0 (6.3, 20.1) | 7.1 (3.4) | 5.9 (3.9, 14.4) | 6.2 (2.1) | 5.5 (4.1, 9.9) |
| CFB EOT | −1.4 (3.7) n = 14 | −1.1 (−12.1, 3.0) n = 14 | −0.1 (2.3) | −0.3 (−2.8, 2.8) | −1.7‖ (2.1) | −1.5‖ (−6.4, 0.2) | −2.4 (2.7) | −1.7 (−6.9, 1.2) |
| Prothrombin fragment 1.2, pmol/L | ||||||||
| Baseline | 672.4 (1235.1) n = 14 | 350.5 (150, 4900) n = 14 | 377.0 (234.7) n = 3 | 366.0 (148, 617) n = 3 | 2305.7 (4750.1) n = 6 | 380.5 (252, 12000) n = 6 | 1341.2 (1768.7) n = 6 | 665.0 (371, 4900) n = 6 |
| EOT | 297.0 (151.0) n = 13 | 260.0 (106, 659) n = 13 | 352.3 (23.7) | 352.5 (323, 381) | 408.5 (189.3) n = 6 | 394.0 (177, 663) n = 6 | 1923.6 (4078.4) | 543.0 (154, 12000) |
| CFB EOT | −91.1 (249.2) n = 12 | 1.0 (−720, 154) n = 12 | −25.0 (205.7) n = 3 | −14.0 (−236, 175) n = 3 | 25.0 (171.8) n = 5 | 35.0 (−199, 278) n = 5 | −912.3 (1843.3) n = 6 | −323 (−4611, 353) n = 6 |
| D-dimer, μg/mL FEU | ||||||||
| Baseline | 2.4 (1.4) n = 13 | 2.1 (0.6, 5.9) n = 13 | 2.5 (1.2) n = 3 | 2.1 (1.6, 3.8) n = 3 | 2.5 (2.3) n = 5 | 1.9 (0.2, 6.3) n = 5 | 3.4 (1.5) n = 6 | 3.3 (1.6, 5.9) n = 6 |
| EOT | 1.9 (1.0) n = 13 | 1.8 (0.2, 3.7) n=13 | 2.2 (0.6) | 2.2 (1.6, 2.9) | 3.0 (1.7) n = 6 | 3.4 (1.1, 5.1) n = 6 | 2.7 (1.5) | 2.6 (0.9, 5.0) |
| CFB EOT | −0.6 (1.5) n = 12 | −0.4 (−3.2, 1.6) n = 12 | −0.3 (1.7) n = 3 | −0.2 (−2.1, 1.4) n = 3 | 0.3 (1.2) n = 5 | 0.7 (−1.2, 1.8) n = 5 | −0.6 (1.5) n = 6 | −0.6 (−3.0, 1.7) n = 6 |
| Erythropoietin, mIU/mL | ||||||||
| Baseline | 104.7 (63.6) n = 13 | 94.4 (17.0, 244.9) n = 13 | 157.1 n = 1 | 157.1 (157.1, 157.1) n = 1 | 92.1 (50.1) n = 2 | 92.1 (56.6, 127.5) n = 2 | 152.0 (130.4) n = 7 | 106.7 (65.9, 441.6) n = 7 |
| EOT | 88.0 (65.9) n = 13 | 56.7 (17.5, 234.1) n = 13 | 159.0 (94.6) n = 2 | 159.0 (92.1, 225.9) n = 2 | 82.8 (35.6) n = 2 | 82.8 (57.6, 108.0) n = 2 | 149.3 (142.5) | 128.2 (17.8, 473.8) |
| CFB EOT | −18.6‖ (44.8) n = 11 | −29.4‖ (−76.0, 73.9) n = 11 | −65.0 n = 1 | −65.0 (−65.0, −65.0) n = 1 | −69.9 n = 1 | −69.9 (−69.9, −69.0) n = 1 | 6.3 (70.5) n = 7 | 32.2 (−109.6, 81.5) n = 7 |
| . | OL 400 mg, 12-wk cohort (n = 15)∗,‡ . | MAD pooled placebo (n = 4)∗,†,‡ . | MAD1, 300 mg (n = 8)∗,†,‡,§ . | MAD2, 600 mg (n = 8)∗,†,‡ . | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (± SD) . | Median (min, max) . | Mean (± SD) . | Median (min, max) . | Mean (± SD) . | Median (min, max) . | Mean (± SD) . | Median (min, max) . | |
| 2,3-DPG, μg/mL per g/mL Hb | ||||||||
| Baseline | 5956.5 (2050.8) | 6181.8 (541, 8350) | 4349.7 (2904.8) | 4689.5 (541, 7479) | 6109.7 (621.8) n = 7 | 6053.8 (5368, 7071) n = 7 | 6821.8 (1764.2) | 6103.9 (5067, 10693) |
| EOT | 4099.2 (833.8) n = 14 | 4090.2 (2732.1, 5555.6) n = 14 | 5890.9 (800.4) | 5642.6 (5253, 7025) | 4263.6 (507.3) n = 7 | 4193.5 (3595, 4915) n = 7 | 4643.8 (2374.7) | 3968.8 (2852, 10338) |
| CFB EOT | −1732.9‖ (2213.6) n = 14 | −1988.4‖ (−4511.8, 3595.3) n = 14 | 1541.2 (2430.4) | 873.1 (−454, 4872) | −1846.1‖ (494.2) n = 7 | −1860.2‖ (−2665, –1156) n = 7 | −2177.9 (2919.3) | −2115.2 (−7457, 3310) |
| ATP, μg/mL per g/mL Hb | ||||||||
| Baseline | 2037.7 (947.3) n = 13 | 2250.0 (321, 3111) n = 13 | 1284.5 (1128.5) | 1152.2 (313, 2521) | 2355.5 (418.5) n = 7 | 2149.4 (1989, 3170) n = 7 | 2117.9 (601.6) | 2217.5 (989, 3033) |
| EOT | 3802.0 (1276.1) n = 11 | 4117.0 (1484.9, 5276.8) n = 11 | 1854.7 (1096.4) | 2209.5 (333, 2667) | 3202.9 (516.2) n = 7 | 3265.0 (2577, 4150) n = 7 | 3218.1 (739.2) | 3391.6 (2024, 4255) |
| CFB EOT | 1939.4‖ (1483.7) n = 11 | 2067.5‖ (−802.4, 4112.1) n = 11 | 570.2 (1196.2) | 57.7 (−189, 2354) | 847.3‖ (656.4) n = 7 | 680.1‖ (127, 2101) n = 7 | 1100.2‖ (528.0) | 1282.3‖ (251, 1596) |
| Hb, g/dL | ||||||||
| Baseline | 8.7 (1.0) | 8.7 (7.2, 10.1) | 7.6 (0.4) | 7.6 (7.1, 8.0) | 9.0 (1.1) | 9.1 (6.9, 10.1) | 8.7 (0.9) | 8.9 (7.3, 10.2) |
| EOT | 9.8 (1.1) | 9.5 (8.4, 12.0) | 7.7 (0.2) | 7.7 (7.5, 7.9) | 10.1 (1.6) | 10.8 (6.8, 11.7) | 9.8 (1.5) | 9.8 (7.4, 12.3) |
| CFB EOT | 1.1‖ (0.8) n = 14 | 1.2‖ (−0.2, 2.7) n = 14 | 0.1 (0.5) | 0.0 (−0.4, 0.8) | 1.2‖ (0.9) | 1.2‖ (−0.1, 2.3) | 1.1‖ (1.1) | 1.0‖ (−0.1, 3.5) |
| Absolute reticulocytes, ×109/L | ||||||||
| Baseline | 229.4 (116.9) | 219.3 (80.5, 511.0) | 275.3 (74.1) n = 3 | 238.4 (227.0, 360.6) n = 3 | 252.3 (70.4) | 274.1 (125.6, 329.6) | 227.3 (105.8) | 226.8 (29.4, 366.0) |
| EOT | 163.3 (85.8) n = 14 | 133.1 (48.7, 351.6) n = 14 | 241.9 (115.7) | 261.8 (85.2, 358.9) | 144.0 (121.1) | 99.2 (60.0, 433.9) | 130.1 (64.0) | 135.2 (24.8, 219.1) |
| CFB EOT | −66.8‖ (107.0) n = 14 | −44.6‖ (−305.0, 118.1) n = 14 | 18.8 (98.0) n = 3 | 11.0 (−75.0, 120.5) n = 3 | −108.3‖ (114.7) | −142.0‖ (−233.8, 133.4) | −97.2‖ (66.3) | −87.4‖ (−217.2, −4.6) |
| iBIL, mg/dL | ||||||||
| Baseline | 1.9 (1.4) | 1.3 (0.8, 5.2) | 3.3 (1.6) n = 3 | 2.8 (2.0, 5.0) n = 3 | 3.1 (3.4) | 1.7 (0.5, 10.5) | 1.9 (1.4) | 1.3 (0.7, 4.5) |
| EOT | 1.2 (0.7) n = 13 | 0.9 (0.5, 2.9) n = 13) | 2.6 (1.5) | 2.6 (0.8, 4.4) | 1.8 (1.5) | 0.9 (0.3, 3.8) | 1.1 (0.8) | 0.7 (0.5, 2.6) |
| CFB EOT | −0.5‖ (0.8) n = 13 | −0.5‖ (−2.3, 0.5) n = 13 | −0.03 (0.5) n = 3 | 0.21 (−0.6, 0.3) n = 3 | −1.3 (2.5) | −0.5 (−7.0, 0.9) | −0.8‖ (0.7) | −0.6‖ (−1.9, −0.1) |
| LDH, U/L | ||||||||
| Baseline | 375.2 (142.9) | 367.0 (186, 683) | 391.8 (210.5) | 352.0 (180, 683) | 430.4 (159.0) | 381.5 (207, 699) | 391.4 (129.7) | 368.5 (251, 683) |
| EOT | 319.1 (87.7) n = 14 | 323.0 (193, 470) n = 14 | 449.8 (209.5) | 486.5 (192, 634) | 311.4 (155.3) | 280.0 (159, 641) | 315.5 (94.0) | 308.5 (199, 492) |
| CFB EOT | −38.4‖ (77.7) n = 14 | −23.0‖ (−213, 57) n = 14 | 58.0 (151.4) | 17.0 (−77, 275) | −119.0‖ (113.3) | −97.5‖ (−283, 61) | −75.9 (173.2) | −54.5 (−461, 90) |
| P50, mmHg | ||||||||
| Baseline | 29.9 (2.4) n =14 | 30.3 (25.8, 34.4) n = 14 | 29.2 (5.9) n = 3 | 30.5 (22.8, 34.4) n = 3 | 30.4 (1.3) n = 6 | 30.8 (28.3, 31.6) n = 6 | 30.1 (2.2) | 30.4 (26.2, 33.2) |
| EOT | 26.6 (2.5) n = 14 | 25.8 (23.8, 32.1) n = 14 | 31.4 (2.6) | 31.3 (28.5, 34.6) | 26.4 (1.6) n = 7 | 26.6 (23.8, 28.4) n = 7 | 26.3 (1.9) | 26.5 (23.2, 28.9) |
| CFB EOT | −3.3‖ (2.0) n = 13 | −3.8‖ (−5.9, 1.9) n = 13 | 2.0 (3.2) n = 3 | 0.2 (0.2, 5.7) n = 3 | −4.3‖ (1.9) n = 6 | −4.3‖ (−7.2, −1.6) n = 6 | −3.9‖ (1.6) | −3.5‖ (−6.7, −2.3) |
| PoS, mmHg | ||||||||
| Baseline | 43.2 (7.1) n = 14 | 43.1 (22.0, 50.0) n = 14 | 45.8 (4.9) n = 3 | 48.1 (40.1, 49.2) n = 3 | 36.3 (9.2) n = 6 | 39.1 (19.0, 45.0) n = 6 | 38.5 (8.6) | 39.7 (26.2, 49.2) |
| EOT | 35.1 (12.3) n = 14 | 36.5 (9.5, 51.0) n = 14 | 63.2 (34.2) | 52.8 (34.6, 112.7) | 31.0 (9.7) | 28.5 (18.1, 47.3) | 31.3 (3.8) | 31.1 (25.6, 36.2) |
| CFB EOT | −8.6‖ (8.2) n = 13 | −8.6‖ (−22.8, 3.0) n = 13 | 19.8 (38.1) n = 3 | 1.3 (−5.6, 63.5) n = 3 | −8.0‖ (5.7) n = 6 | −9.0‖ (−15.1, −0.9) n = 6 | −7.3‖ (7.0) | −7.2‖ (−15.4, 4.6) |
| Elmin | ||||||||
| Baseline | 0.12 (0.07) n = 14 | 0.11 (0.0, 0.3) n = 14 | 0.13 (0.15) n = 3 | 0.18 (0.0, 0.3) n = 3 | 0.11 (0.10) n = 6 | 0.07 (0.0, 0.3) n = 6 | 0.19 (0.11) | 0.15 (0.1, 0.4) |
| EOT | 0.16 (0.14) n = 14 | 0.12 (0.0, 0.5) n = 14 | 0.06 (0.11) | 0.03 (0.0, 0.2) | 0.18 (0.15) | 0.12 (0.1, 0.5) | 0.23 (0.10) | 0.21 (0.1, 0.5) |
| CFB EOT | 0.05 (0.10) n = 13 | 0.04 (−0.1, 0.2) n = 13 | −0.06 (0.19) n = 3 | 0.04 (−0.3, 0.1) n = 3 | 0.10 (0.11) n = 6 | 0.05 (0.0, 0.2) n = 6 | 0.04 (0.05) | 0.06 (0.0, 0.1) |
| Elmax | ||||||||
| Baseline | 0.45 (0.10) n = 14 | 0.46 (0.2, 0.5) n = 14 | 0.38 (0.19) n = 3 | 0.49 (0.2, 0.5) n = 3 | 0.43 (0.14) n = 6 | 0.43 (0.3, 0.6) n = 6 | 0.48 (0.06) | 0.49 (0.4, 0.6) |
| EOT | 0.47 (0.08) n =14 | 0.49 (0.2, 0.5) n = 14 | 0.40 (0.14) | 0.44 (0.2, 0.5) | 0.49 (0.08) | 0.51 (0.3, 0.6) | 0.51 (0.04) | 0.51 (0.4, 0.6) |
| CFB EOT | 0.02 (0.05) n = 13 | 0.01 (−0.1, 0.1) n = 13 | 0.00 (0.05) n = 3 | 0.02 (−0.1, 0.0) n = 3 | 0.05 (0.08) n = 6 | 0.02 (0.0, 0.2) n = 6 | 0.03 (0.05) | 0.02 (0.0, 0.1) |
| DRBCs (hyper), % | ||||||||
| Baseline | 3.2 (2.5) n = 14 | 2.8 (1.0, 11.1) n = 14 | 5.0 (5.4) n = 3 | 2.7 (1.1, 11.1) n = 3 | 4.0 (3.1) n = 7 | 3.3 (0.9, 8.9) n = 7 | 2.5 (1.1) | 2.6 (1.0, 4.1) |
| EOT | 2.8 (2.1) n = 14 | 2.0 (1.2, 8.9) n = 14 | 4.8 (3.3) | 3.9 (1.9, 9.6) | 2.6 (1.8) | 2.1 (1.1, 6.9) | 1.9 (0.9) | 1.9 (0.7, 3.3) |
| CFB EOT | −0.3 (1.6) n = 13 | −0.4 (−2.5, 2.7) n = 13 | 0.2 (1.5) n = 3 | 0.8 (−1.5, 1.3) n = 3 | −1.3 (1.7) n = 7 | −1.8 (−4.2, 0.7) n = 7 | −0.6 (1.1) | −0.8 (−2.1, 1.4) |
| CHCM, g/dL | ||||||||
| Baseline | 33.0 (1.3) n = 14 | 32.8 (30.6, 35.3) n = 14 | 32.6 (2.3) n = 3 | 32.3 (30.5, 35.1) n = 3 | 33.0 (0.9) n = 7 | 33.0 (31.7, 34.3) n = 7 | 32.3 (1.3) | 32.6 (30.3, 34.3) |
| EOT | 32.5 (1.5) n = 14 | 32.2 (30.8, 36.1) n = 14 | 33.5 (1.0) | 33.4 (32.5, 34.8) | 32.4 (0.9) | 32.2 (31.3, 33.8) | 31.7 (1.3) | 31.6 (29.2, 33.6) |
| CFB EOT | −0.6 (1.1) n = 13 | −0.9 (−1.8, 2.3) n = 13 | 1.0 (1.2) n = 3 | 1.3 (−0.3, 2.0) n = 3 | −0.7‖ (0.6) n = 7 | −0.7‖ (−1.4, 0.3) n = 7 | −0.6 (0.8) | −0.9 (−1.3, 0.6) |
| TNF-α, pg/mL | ||||||||
| Baseline | 1.2 (0.5) n = 14 | 1.1 (0.6, 2.2) n = 14 | 1.7 (0.7) n = 2 | 1.7 (1.3, 2.2) n = 2 | 1.2 (0.5) | 1.1 (0.6, 2.0) | 1.4 (0.5) n = 7 | 1.2 (0.8, 2.0) n = 7 |
| EOT | 0.8 (0.4) n = 13 | 0.9 (0.2, 1.6) n = 13 | 1.3 (0.1) | 1.2 (1.1, 1.5) | 1.4 (0.6) n = 7 | 1.4 (0.7, 2.3) n = 7 | 0.7 (0.4) | 0.8 (0.2, 1.3) |
| CFB EOT | −0.3 (0.7) n = 12 | −0.1 (−1.7, 0.4) n = 12 | −0.5 (0.6) n = 2 | −0.5 (−0.9, −0.1) n = 2 | 0.1 (0.6) n = 7 | 0.0 (−0.6, 1.0) n = 7 | −0.5‖ (0.6) n = 7 | −0.3‖ (−1.7, 0.0) n = 7 |
| MMP-9, ng/mL | ||||||||
| Baseline | 440.1 (282.4) n = 13 | 434.7 (90.3, 929.4) n = 13 | 573.1 n = 1 | 573.1 (573.1, 573.1) n = 1 | ND | ND | 451.2 (313.5) n = 7 | 434.7 (97.2, 929.4) n = 7 |
| EOT | 296.0 (354.0) n = 13 | 175.6 (0.0, 1280.9) n = 13 | 280.9 (96.6) n = 2 | 280.9 (212.6, 349.1) n = 2 | ND | ND | 282.2 (176.2) | 242.4 (69.3, 602.3) |
| CFB EOT | −149.8‖ (259.3) n = 11 | −175.6‖ (−627.8, 351.5) n = 11 | −224.0 n = 1 | −224.0 (−224.0, −224.0) n = 1 | ND | ND | −193.3‖ (215.4) n = 7 | −96.3‖ (−615.5, −18.5) n = 7 |
| Leukocytes, ×109/L | ||||||||
| Baseline | 9.6 (4.9) | 7.9 (5.0, 24.5) | 11.2 (4.6) | 10.4 (6.9, 17.3) | 8.8 (4.4) | 7.5 (4.4, 15.8) | 8.6 (2.9) | 7.9 (6.0, 14.7) |
| EOT | 8.2 (3.3) n = 14 | 8.9 (2.7, 13.1) n = 14 | 11.1 (6.1) | 9.0 (6.3, 20.1) | 7.1 (3.4) | 5.9 (3.9, 14.4) | 6.2 (2.1) | 5.5 (4.1, 9.9) |
| CFB EOT | −1.4 (3.7) n = 14 | −1.1 (−12.1, 3.0) n = 14 | −0.1 (2.3) | −0.3 (−2.8, 2.8) | −1.7‖ (2.1) | −1.5‖ (−6.4, 0.2) | −2.4 (2.7) | −1.7 (−6.9, 1.2) |
| Prothrombin fragment 1.2, pmol/L | ||||||||
| Baseline | 672.4 (1235.1) n = 14 | 350.5 (150, 4900) n = 14 | 377.0 (234.7) n = 3 | 366.0 (148, 617) n = 3 | 2305.7 (4750.1) n = 6 | 380.5 (252, 12000) n = 6 | 1341.2 (1768.7) n = 6 | 665.0 (371, 4900) n = 6 |
| EOT | 297.0 (151.0) n = 13 | 260.0 (106, 659) n = 13 | 352.3 (23.7) | 352.5 (323, 381) | 408.5 (189.3) n = 6 | 394.0 (177, 663) n = 6 | 1923.6 (4078.4) | 543.0 (154, 12000) |
| CFB EOT | −91.1 (249.2) n = 12 | 1.0 (−720, 154) n = 12 | −25.0 (205.7) n = 3 | −14.0 (−236, 175) n = 3 | 25.0 (171.8) n = 5 | 35.0 (−199, 278) n = 5 | −912.3 (1843.3) n = 6 | −323 (−4611, 353) n = 6 |
| D-dimer, μg/mL FEU | ||||||||
| Baseline | 2.4 (1.4) n = 13 | 2.1 (0.6, 5.9) n = 13 | 2.5 (1.2) n = 3 | 2.1 (1.6, 3.8) n = 3 | 2.5 (2.3) n = 5 | 1.9 (0.2, 6.3) n = 5 | 3.4 (1.5) n = 6 | 3.3 (1.6, 5.9) n = 6 |
| EOT | 1.9 (1.0) n = 13 | 1.8 (0.2, 3.7) n=13 | 2.2 (0.6) | 2.2 (1.6, 2.9) | 3.0 (1.7) n = 6 | 3.4 (1.1, 5.1) n = 6 | 2.7 (1.5) | 2.6 (0.9, 5.0) |
| CFB EOT | −0.6 (1.5) n = 12 | −0.4 (−3.2, 1.6) n = 12 | −0.3 (1.7) n = 3 | −0.2 (−2.1, 1.4) n = 3 | 0.3 (1.2) n = 5 | 0.7 (−1.2, 1.8) n = 5 | −0.6 (1.5) n = 6 | −0.6 (−3.0, 1.7) n = 6 |
| Erythropoietin, mIU/mL | ||||||||
| Baseline | 104.7 (63.6) n = 13 | 94.4 (17.0, 244.9) n = 13 | 157.1 n = 1 | 157.1 (157.1, 157.1) n = 1 | 92.1 (50.1) n = 2 | 92.1 (56.6, 127.5) n = 2 | 152.0 (130.4) n = 7 | 106.7 (65.9, 441.6) n = 7 |
| EOT | 88.0 (65.9) n = 13 | 56.7 (17.5, 234.1) n = 13 | 159.0 (94.6) n = 2 | 159.0 (92.1, 225.9) n = 2 | 82.8 (35.6) n = 2 | 82.8 (57.6, 108.0) n = 2 | 149.3 (142.5) | 128.2 (17.8, 473.8) |
| CFB EOT | −18.6‖ (44.8) n = 11 | −29.4‖ (−76.0, 73.9) n = 11 | −65.0 n = 1 | −65.0 (−65.0, −65.0) n = 1 | −69.9 n = 1 | −69.9 (−69.9, −69.0) n = 1 | 6.3 (70.5) n = 7 | 32.2 (−109.6, 81.5) n = 7 |
The “n” values represent the number of patients with nonmissing values.
ATP, adenosine triphosphate; CFB, change from baseline; CHCM, cellular Hb concentration mean; 2,3-DPG, 2,3 diphosphoglycerate; DRBCs, dense red blood cells; EI, elongation index; Elmax, maximum EI; Elmin, minimum EI; EOT, end of treatment; FEU, fibrinogen equivalent unit; Hb is 50% saturated; Hb, hemoglobin, iBIL, indirect bilirubin; LDH, lactate dehydrogenase; LS, least square; max, maximum; min, minimum; MMP-9, matrix metalloproteinase 9; ND, not done; OL, open-label; P50, oxygen tension at which MAD, multiple ascending dose; PoS, point of sickling; SD, standard deviation; TNF-α, tumor necrosis factor alpha.
Sample sizes that deviate from those in the column header are indicated in the appropriate cells.
For the MAD cohorts, baseline was defined as the last measurement obtained before the first dose of study drug. For the 12-week cohort, baseline was defined as average of pretreatment measurements (screening and predose on day 1) for patients who were newly enrolled in the 12-week cohort; for patients who were enrolled in the MAD2 (600 mg) cohort and later rolled over into the 12-week cohort, baseline was defined as the average of prior treatment measurements (screening and predose on day 1) in the MAD2 (600 mg) period.
EOT was day 14/15 (24 hours after last dosing) in the MAD cohorts and day 84/85 (24 hours after last dosing) in the OL cohort.
One MAD1 (300 mg) patient was excluded from 2,3-DPG, ATP, and P50 analyses because the patient only took 1 dose of study drug on day 1.
P <.05 for baseline vs EOT comparison. For the MAD cohorts, P values were obtained from a Wilcoxon signed rank test. For the OL cohort, P values for Hb, LDH, reticulocytes, iBIL, were derived from LS means using a mixed model for repeated measurement, with hematology/hemolysis assessment as dependent variable and scheduled visit during treatment period as a fixed effect. An unstructured covariance was used for Hb, LDH, and reticulocytes. A compound symmetry covariance was used for iBIL, normalized ATP, and normalized 2,3-DPG. In the OL cohort, P values for normalized 2,3-DPG, normalized ATP, P50, PoS, Elmin, Elmax, hyper RBCs, CHCM, TNF-α, MMP-9, leukocytes, prothrombin fragment 1.2, D-dimer, and erythropoietin were derived from a Wilcoxon signed rank test.
Consistent with the mechanism of PKR activation, ATP levels in whole blood rose concomitantly with 2,3-DPG reductions and remained stable throughout treatment. At EOT, mean normalized ATP levels were significantly higher than baseline in the MAD and OL cohorts (Figure 3C-D; Table 3).
After etavopivat discontinuation, 2,3-DPG levels rose to baseline or above over the next 1 to 4 weeks (Figure 3A-B), whereas ATP levels decreased toward baseline (Figure 3C-D). Decreased 2,3-DPG was associated with a lower P50 (Figure 3E).
In the OL cohort, reductions from baseline in P50 occurred by day 14 and persisted through day 84; mean changes from baseline were 3.5, 2.9, 4.5, and 3.3 mmHg on days 14 (earliest time point), 28, 56, and 84, respectively (Table 3). At EOT, the decrease from baseline in P50 was statistically significant in the OL and MAD cohorts (Figure 3F; Table 3; supplemental Figure 3).
Clinical activity
Hb
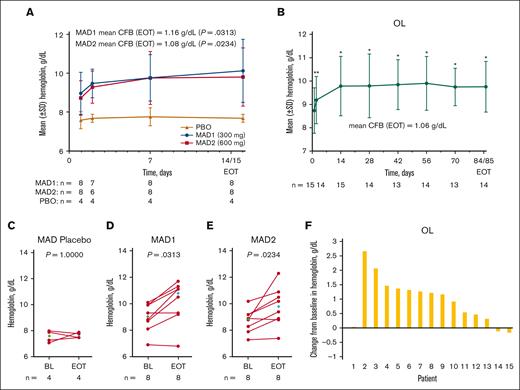
An increase in mean Hb concentration occurred on day 2 of treatment in the MAD and OL cohorts (Figure 4A-B). From baseline to EOT, there were statistically significant increases in mean Hb levels of 1.2 g/ dL (range, –0.1 to 2.3), 1.1 g/dL (range, –0.1 to 3.5), and 1.1 g/dL (range, –0.2 to 2.7) in the MAD1, MAD2, and OL cohorts, respectively (Figure 4A-B; Table 3).
Change in Hb response in patients with SCD (MAD and OL cohorts). Mean (± SE) Hb concentration over time in the MAD (A) and OL (B) cohorts. Values for mean change from BL at EOT are shown on the graphs (A-B). In the MAD cohorts, EOT was equal to the day 15 value, if available, otherwise EOT was equal to day 14 (A). In the OL cohort, EOT was equal to the day 85 value, if available, otherwise EOT was equal to day 84 (B). Scatterplots at BL and EOT for MAD pooled placebo (C), MAD1 (D), MAD2 (E), and OL (F); each data point corresponds to data from 1 patient. Median BL and EOT values shown in green and blue diamonds, respectively (C-F). Paired BL and EOT data points from each patient are connected by a line. In the MAD cohorts (A), P values were based on Wilcoxon signed rank tests to test the changes at EOT from BL. In the OL cohort (B), Hb values with statistical significance as compared with BL were identified using asterisks (∗P ≤ .0001; ∗∗P < .01) at their scheduled visits, based on MMRM, which included Hb values as a dependent variable, and a fixed effect of scheduled visits during the treatment period, with unstructured covariance matrix to model the within-patient variance-covariance errors. Statistical tests were not performed for the visits after EOT. P value in the scatterplots are from a Wilcoxon matched-pairs signed rank test (C-F). BL, baseline; CFB, change from baseline; EOT, end of treatment; Hb, hemoglobin; MAD, multiple ascending dose; MMRM, mixed model for repeated measurement; OL, open-label; PBO, placebo; SE, standard error; SCD, sickle cell disease; SD, standard deviation.
Change in Hb response in patients with SCD (MAD and OL cohorts). Mean (± SE) Hb concentration over time in the MAD (A) and OL (B) cohorts. Values for mean change from BL at EOT are shown on the graphs (A-B). In the MAD cohorts, EOT was equal to the day 15 value, if available, otherwise EOT was equal to day 14 (A). In the OL cohort, EOT was equal to the day 85 value, if available, otherwise EOT was equal to day 84 (B). Scatterplots at BL and EOT for MAD pooled placebo (C), MAD1 (D), MAD2 (E), and OL (F); each data point corresponds to data from 1 patient. Median BL and EOT values shown in green and blue diamonds, respectively (C-F). Paired BL and EOT data points from each patient are connected by a line. In the MAD cohorts (A), P values were based on Wilcoxon signed rank tests to test the changes at EOT from BL. In the OL cohort (B), Hb values with statistical significance as compared with BL were identified using asterisks (∗P ≤ .0001; ∗∗P < .01) at their scheduled visits, based on MMRM, which included Hb values as a dependent variable, and a fixed effect of scheduled visits during the treatment period, with unstructured covariance matrix to model the within-patient variance-covariance errors. Statistical tests were not performed for the visits after EOT. P value in the scatterplots are from a Wilcoxon matched-pairs signed rank test (C-F). BL, baseline; CFB, change from baseline; EOT, end of treatment; Hb, hemoglobin; MAD, multiple ascending dose; MMRM, mixed model for repeated measurement; OL, open-label; PBO, placebo; SE, standard error; SCD, sickle cell disease; SD, standard deviation.
Overall, 87.5%, 50.0%, and 73.3% of patients in the MAD1, MAD2, and OL cohorts, respectively, were Hb responders (>1 g/dL at any time during treatment). In the OL cohort, the mean maximal Hb increase for each patient was 1.6 g/dL (range, 0.8-2.8; Table 4), regardless of responder status during treatment; among Hb responders, the mean maximal Hb increase during treatment was 1.9 g/dL (range, 1.2-2.8).
Hb responders at any time during treatment (all cohorts)
| . | Placebo or etavopivat once daily . | |||
|---|---|---|---|---|
| MAD placebo 0 mg . | MAD1 300 mg . | MAD2 600 mg . | OL 400 mg . | |
| Wk | 2 | 2 | 2 | 12 |
| Number of patients, n | 4 | 8 | 8 | 15 |
| Maximal Hb increase, mean (range), g/dL | 0.4 (0.0-0.6) | 1.4 (0.4-2.4) | 1.4 (0.2-3.5) | 1.6 (0.8-2.8) |
| Hb increase >1 g/dL response on treatment, n (%) | 0 | 7 (87.5) | 4 (50.0) | 11 (73.3) |
| Maximal Hb increase in patients with >1 g/dL response, mean (range), g/dL | NA | 1.5 (1.1-2.4) | 2.2 (1.5-3.5) | 1.9 (1.2-2.8) |
| . | Placebo or etavopivat once daily . | |||
|---|---|---|---|---|
| MAD placebo 0 mg . | MAD1 300 mg . | MAD2 600 mg . | OL 400 mg . | |
| Wk | 2 | 2 | 2 | 12 |
| Number of patients, n | 4 | 8 | 8 | 15 |
| Maximal Hb increase, mean (range), g/dL | 0.4 (0.0-0.6) | 1.4 (0.4-2.4) | 1.4 (0.2-3.5) | 1.6 (0.8-2.8) |
| Hb increase >1 g/dL response on treatment, n (%) | 0 | 7 (87.5) | 4 (50.0) | 11 (73.3) |
| Maximal Hb increase in patients with >1 g/dL response, mean (range), g/dL | NA | 1.5 (1.1-2.4) | 2.2 (1.5-3.5) | 1.9 (1.2-2.8) |
Only measurements up to EOT were included.
Hb, hemoglobin; MAD, multiple ascending dose; NA, not assessed; OL, open-label.
By-patient analyses showed that Hb levels increased in most patients during treatment (Figure 4C-F).
Hemolysis markers
In the MAD and OL cohorts, hemolysis markers (reticulocytes, iBIL, and LDH) decreased over the first 1 to 2 weeks of treatment and remained stable in the OL cohort for the treatment duration (Figure 5). At EOT, mean decreases from baseline in hemolysis markers were statistically significant in the MAD and OL cohorts, except iBIL in MAD1 and LDH in MAD2 (Figure 5; Table 3). Individual patient data at baseline and EOT are shown in supplemental Figure 2.
Hemolysis markers in patients with SCD (MAD and OL cohorts). Mean (± SE) absolute reticulocytes, iBIL, and LDH over time in the MAD cohorts (A,B,C, respectively) and OL cohorts (D,E,F, respectively). In the MAD cohorts (A-C), P values were based on Wilcoxon signed rank tests to test the changes at EOT from BL. In the OL cohort (D-F), hemolysis marker values with statistical significance as compared with BL were identified using an asterisk (∗P ≤ .05) at their scheduled visits, based on MMRM, which included hemolysis marker values as a dependent variable, and a fixed effect of scheduled visits during the treatment period. An unstructured covariance was used for LDH and reticulocytes, and a compound symmetry covariance was used for iBIL. Statistical tests were not performed for the visits after EOT. BL, baseline; CFB, change from baseline; EOT, end of treatment; iBIL, indirect bilirubin; LDH, lactate dehydrogenase; MAD, multiple ascending dose; MMRM, mixed model for repeated measurement; OL, open-label; SCD, sickle cell disease; SD, standard deviation.
Hemolysis markers in patients with SCD (MAD and OL cohorts). Mean (± SE) absolute reticulocytes, iBIL, and LDH over time in the MAD cohorts (A,B,C, respectively) and OL cohorts (D,E,F, respectively). In the MAD cohorts (A-C), P values were based on Wilcoxon signed rank tests to test the changes at EOT from BL. In the OL cohort (D-F), hemolysis marker values with statistical significance as compared with BL were identified using an asterisk (∗P ≤ .05) at their scheduled visits, based on MMRM, which included hemolysis marker values as a dependent variable, and a fixed effect of scheduled visits during the treatment period. An unstructured covariance was used for LDH and reticulocytes, and a compound symmetry covariance was used for iBIL. Statistical tests were not performed for the visits after EOT. BL, baseline; CFB, change from baseline; EOT, end of treatment; iBIL, indirect bilirubin; LDH, lactate dehydrogenase; MAD, multiple ascending dose; MMRM, mixed model for repeated measurement; OL, open-label; SCD, sickle cell disease; SD, standard deviation.
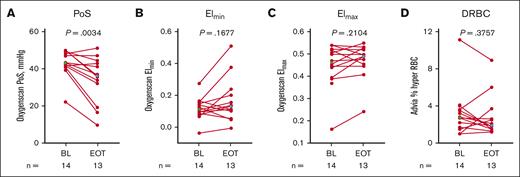
Effect on RBC function
P50 reduction was associated with a shift in mean PoS to lower pO2 values (Table 3). At EOT, decreases in PoS from baseline were statistically significant in the MAD and OL cohorts (Figure 6; Table 3; supplemental Figure 3). Although the mean change from baseline to EOT was not statistically significant for RBC deformability (Elmin [minimum EI] and Elmax [maximum EI]), hydration DRBCs, and cellular Hb concentration mean (MAD2; Table 3), by-patient plots suggest these parameters may have been favorably affected (decreased DRBCs and increased Elmin and Elmax) in many of the patients treated with etavopivat (Figure 6; supplemental Figure 3).
Markers of RBC physiology (OL cohort). Scatterplots for PoS (A), Elmin (B), Elmax (C), and (hyper) DRBCs (D) at BL and EOT. Each data point corresponds to data from 1 patient. Paired BL and EOT data points from each patient are connected by a line. Median BL and EOT values shown in green and blue diamonds, respectively. P values are from a Wilcoxon matched-pairs signed rank test. Percent hyper RBC is defined as the percent of RBCs with >41 g/dL of Hb. BL, baseline; DRBCs, dense RBCs; EI, elongation index; Elmax, maximum EI; Elmin, minimum EI; EOT, end of treatment; OL, open-label; PoS, point of sickling; RBC, red blood cell.
Markers of RBC physiology (OL cohort). Scatterplots for PoS (A), Elmin (B), Elmax (C), and (hyper) DRBCs (D) at BL and EOT. Each data point corresponds to data from 1 patient. Paired BL and EOT data points from each patient are connected by a line. Median BL and EOT values shown in green and blue diamonds, respectively. P values are from a Wilcoxon matched-pairs signed rank test. Percent hyper RBC is defined as the percent of RBCs with >41 g/dL of Hb. BL, baseline; DRBCs, dense RBCs; EI, elongation index; Elmax, maximum EI; Elmin, minimum EI; EOT, end of treatment; OL, open-label; PoS, point of sickling; RBC, red blood cell.
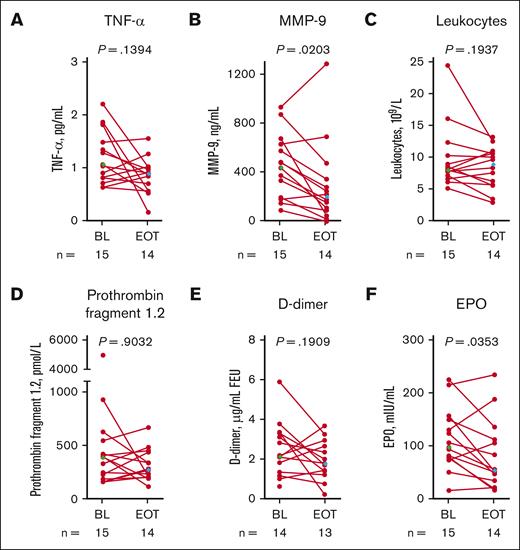
Systemic markers of SCD pathophysiology
At EOT in the OL cohort, there were statistically significant reductions from baseline in matrix metalloproteinase-9 and erythropoietin mean levels (Figure 7; Table 3). Mean changes from baseline in tumor necrosis factor-α, leukocytes, prothrombin fragment 1.2, and D-dimer were not statistically significant (Figure 7; Table 3). By-patient plots for the MAD cohorts are shown in supplemental Figure 4.
Systemic markers of SCD pathophysiology in patients with SCD (OL cohort). Each data point corresponds to data from 1 patient. Paired BL and EOT data points from each patient are connected by a line. Median BL and EOT values shown in green and blue diamonds, respectively. P values are from a Wilcoxon matched-pairs signed rank test. TNF-α (A), MMP-9 (B), leukocytes (C), prothrombin fragment 1.2 (D), D-dimer (E), and erythropoietin (F). BL, baseline; EOT, end of treatment; EPO, erythropoietin; FEU, fibrinogen equivalent unit; MMP-9, matrix metalloproteinase-9; OL, open-label; SCD, sickle cell disease; TNF-α, tumor necrosis factor alpha.
Systemic markers of SCD pathophysiology in patients with SCD (OL cohort). Each data point corresponds to data from 1 patient. Paired BL and EOT data points from each patient are connected by a line. Median BL and EOT values shown in green and blue diamonds, respectively. P values are from a Wilcoxon matched-pairs signed rank test. TNF-α (A), MMP-9 (B), leukocytes (C), prothrombin fragment 1.2 (D), D-dimer (E), and erythropoietin (F). BL, baseline; EOT, end of treatment; EPO, erythropoietin; FEU, fibrinogen equivalent unit; MMP-9, matrix metalloproteinase-9; OL, open-label; SCD, sickle cell disease; TNF-α, tumor necrosis factor alpha.
Discussion
Etavopivat is a novel, selective erythrocyte PKR activator with multimodal PD effects; it decreases 2,3-DPG and increases ATP in whole blood. In this phase 1 study, etavopivat, 300 or 600 mg daily for 2 weeks and 400 mg daily for up to 12 weeks, was well tolerated in patients with SCD. Decreased intracellular 2,3-DPG and increased intracellular ATP at all doses support the proof-of-mechanism of etavopivat, which resulted in rapid and sustained improvement in Hb levels and reduction of hemolysis as demonstrated by improvements in hemolytic biomarkers. Notably, 11 of 15 patients (73%) in the etavopivat 400 mg daily OL cohort achieved a >1.0 g/dL Hb increase from baseline during treatment; improved Hb levels were generally accompanied by decreases in hemolytic markers (reticulocytes, iBIL, and LDH).
Hb-O2 affinity was significantly increased by etavopivat, with a significant reduction in P50 by hemoximetry. Decreased PoS on O2 gradient ektacytometry (oxygenscan), a functional biomarker of sickle RBC pathophysiology,27-29 is associated with a lower risk of acute complications in SCD (eg, cerebral infarction, acute chest syndrome, and VOEs).29-31 Etavopivat improved PoS to lower O2 pressures from study baseline to EOT in the MAD and OL cohorts. The study was not powered to determine if there were fewer VOEs over time.
In this study, 87.5%, 50%, and 73.3% of patients treated with etavopivat in the MAD1, MAD2, and OL cohorts, respectively, were Hb responders (>1 g/dL at any time during treatment). These response rates are consistent with the data reported for another allosteric activator of both wild-type and mutant forms of PKR.32 Although left shifting of the oxygen-dissociation curve may reduce tissue oxygen delivery and raise erythropoietin levels, leading to an increase in Hb concentration with PKR activators, most patients in our study were found to have a decline in serum erythropoietin levels (Figure 7F). The reasons for a lack of Hb response in some patients are not yet completely understood. In exploratory analyses of Hb responders vs nonresponders in our study, we did not observe differences in the change in ATP (+102 μg/mL vs +72.6 μg/mL, respectively; P = .7) or in 2,3-DPG (–113 μg/mL vs –159 μg/mL, respectively; P = .9). Nonresponders in the MAD and OL cohorts had lower baseline reticulocytes and higher baseline erythropoietin (supplemental Table 5). Overall, there was a reduction from baseline in erythropoietin and reticulocyte levels in the OL cohort, but the change from baseline was not significant among Hb nonresponders. Patients with higher baseline hemolysis, as indicated by the higher baseline reticulocyte count, may be more likely to respond to etavopivat because treatment will decrease hemolysis. Further work is needed to determine whether baseline reticulocyte number is a predictor of etavopivat response. Response to etavopivat may also vary by SCD genotype. We included patients with non-Hb SS SCD because they experience varying degrees of hemolytic anemia and SCD-related complications, and effective therapies are needed in this group of patients. However, with only a few non-HbSS patients with SCD enrolled, we are unable to assess the genotype-related effect of etavopivat, and this will be evaluated in the ongoing phase 2/3 study.
Etavopivat was well tolerated, with a safety profile consistent with the data reported with another PKR activator used in SCD. VOEs were the most common TEAEs, occurring in 3 (37.5%), 3 (37.5%), and 7 (46.7%) patients in the MAD1, MAD2, and OL groups, respectively. Three patients in the OL cohort were assessed to have treatment-related VOEs. One patient (12.5%) in the MAD2 group and 2 patients (13.3%) during the 12-week OL period had serious VOEs, all assessed by the investigator as unrelated to treatment. These numbers are comparable with the phase 1 data reported with another PKR activator in SCD, where 4 of 17 patients (23.5%) had serious VOEs.23 Of the 15 patients receiving etavopivat in the OL cohort, 1 had drug withdrawn due to a serious, possibly treatment-related, grade 3 DVT. Despite the increased risk of thrombosis in adults with SCD,33 an association with etavopivat treatment could not be excluded by the investigator because of the temporal relationship with study drug initiation. The number of patients with TEAEs related to SCD pain decreased during the 12-week OL treatment and returned to week 1 to 4 levels after etavopivat was discontinued. Given the small number of patients, an AE withdrawal event cannot be confirmed or refuted; phase 3 data are needed to further inform any causal relationship.
In conclusion, daily etavopivat, up to 600 mg for 2 weeks and 400 mg for up to 12 weeks, was well tolerated in patients with SCD. Consistent with the mechanism of PKR activation, increases in whole blood ATP and decreases in 2,3-DPG levels were sustained over 12 weeks. Improvements in Hb oxygenation, RBC physiology, and biomarkers of SCD pathophysiology translated clinically to 73% of patients in the OL cohort achieving a Hb response (increase from baseline >1 g/dL) during etavopivat treatment. This new, once-a-day PKR activator demonstrated persistent improvement in Hb markers and RBC physiology over a sustained period (12 weeks) in patients with SCD in this multicenter placebo-controlled blinded study for 2 weeks as well as in a multicenter OL study for 12 weeks. We recognize the limitation of a small sample size and relatively short treatment period; in addition, in the OL cohort, patients with Hb <7 g/dL or >10.5 g/dL were excluded during screening, and males and adolescent patients were underrepresented in this study. The safety and efficacy of etavopivat in individuals with SCD aged 12 to 65 years are being further evaluated in HIBISCUS, a registrational, randomized, placebo-controlled, double-blind, multicenter, phase 2/3 trial (NCT04624659).34 These longer-term data (52 weeks double-blind treatment followed by a 52-week OL extension) will further inform the benefit-risk profile of etavopivat and the potential of this PKR activator to modify the course of SCD.
Acknowledgments
The authors thank the patients, their families, investigators, and site personnel for participating in this study. The study sites and key personnel are listed in supplemental Appendix 3. Medical writing assistance was provided by Lori Kornberg and Sue Reinwald, Engage Scientific Solutions, Fairfield, Connecticut, and was funded by Forma Therapeutics, Inc, which was acquired by Novo Nordisk on 14 October 2022.
This study was supported and performed by Forma Therapeutics, Watertown, Massachusetts.
Authorship
Contribution: S.L.S., P.F.K., T.A.K., and M.J.T. conceptualized, performed methodology, validated, analyzed data, conducted investigation, gathered resources, curated data, wrote, prepared, created and/or presented the published work, and supervised research; R.H., M.I., K.C., R.C.B., and M.R. did investigation and writing; P.S., E.W., and S.F. performed data analysis, investigation, and writing; I.O. contributed to investigation, resources, and writing; F.A.K. performed methodology, validation, data analysis, investigation, gathered resources, and wrote the manuscript; J.G. contributed to conceptualization, methodology, validation, data analysis, investigation, resources, data curation, and writing; and all authors approved the final version of the manuscript for submission.
Conflict-of-interest disclosure: E.W., S.F., J.G., I.O., P.S., and M.R. were employees of and held stock in Forma Therapeutics, Inc, which was acquired by Novo Nordisk, at the time of this study. P.F.K was a consultant to Forma Therapeutics, Inc, which was acquired by Novo Nordisk, was an employee when the study was performed, and held stock in Forma Therapeutic, Inc. I.O. is a former principal investigator at Levine Cancer Institute/Atrium health and formerly held consultancies with Forma Therapeutics, Novo Nordisk, Agios, Global Blood Therapeutics (GBT), Novartis, Cheisi, Acceleron, and Emmaus; served on speaker’s bureau for Novartis and GBT; received research funding from the Centers for Disease Control, Health Resources and Service’s Administration, and Patient Centered Outcomes Research Institute; and was an employee of Forma Therapeutics between February and October 2022 and is currently an employee of Novo Nordisk since October 2022. S.L.S. reports consultancies with Forma Therapeutics, Inc, Novo Nordisk, GBT/Pfizer, ORIC Pharmaceuticals, Agios, and Beam Therapeutics; membership on the advisory committees of GBT/Pfizer and Novartis; and research funding from Forma Therapeutics, Inc, Novo Nordisk, GBT, Novartis, and Pfizer. R.H. reports consultancies with Bristol Myers Squib, GBT, Imara, National Institutes of Health (NIH), Novartis and research funding from Chiesi, Forma Therapeutics, Inc, and the University of Pittsburgh. M.I. reports consultancies with GBT; receives research funding from Novartis, Pfizer, GBT, Agios, Alexion, Novo Nordisk, and Forma; serves on the GBT speaker’s bureau; and is a member of the board/advisory committee of GBT. R.C.B. was a principal investigator at Children’s Healthcare of Atlanta at the time of study and formally held consultancies with GBT, Imara, Novartis; received funding from GBT, Novartis, Forma Therapeutics, and Imara; and has been an employee of GBT, a wholly owned subsidiary of Pfizer as of October 2023, since July 2022. F.A.K. received research funding from Forma. T.A.K. reports consultancies, membership on advisory boards, and research funding from Forma Therapeutics, Inc, Novo Nordisk, Agios Pharmaceuticals, Inc; and research funding from the NIH. M.J.T. reports consultancies with GlycoMimetics, Inc; served on a data safety monitoring board of Novartis; and received research funding from Forma Therapeutics, Inc, CSL Behring, Inc, Doris Duke Charitable Foundation, and the NIH. K.C. declares no competing financial interests.
Correspondence: Santosh L. Saraf, Sickle Cell Center, Division of Hematology/Oncology, University of Illinois Hospital & Health Sciences System, 820 South Wood St, MC 712, Chicago, IL 60612; email: ssaraf@uic.edu.
References
Author notes
Preliminary data presented in poster and oral presentation form (does not include encore presentations) at European Hematology Association 2020, 2021 and 2022, Sickle Cell Disease Association of America 2020, Annual Academy for Sickle Cell and Thalassemia Conference 2020, American Society of Hematology 2020 and 2021, British Society for Haematology 2022, and American Society of Pediatric Hematology/Oncology 2022.
Data will be shared with researchers after approval of a research proposal requesting access to data. Data will be shared for use as approved by the institutional review board (IRB) according to the IRB Charter (novonordisk-trials.com). The access request proposal form and access criteria can be found at novonordisk-trials.com. Only de-identified/anonymized individual participant data will be shared on a specialized statistical analysis system data platform.
The full-text version of this article contains a data supplement.