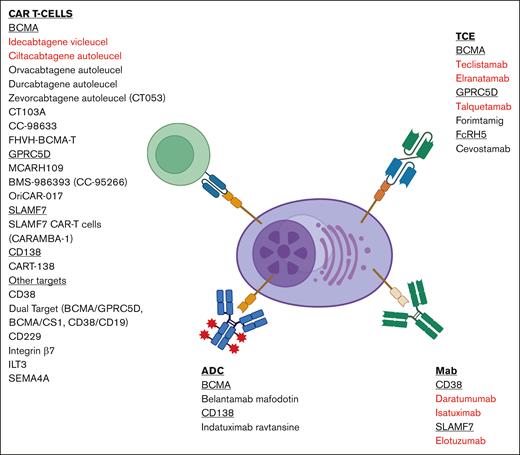
The identification and targeting of B-cell maturation antigen (BCMA) through immunotherapeutic strategies such as antibody-drug conjugates, chimeric antigen receptor T cells, and T-cell engagers have revolutionized the care of patients with multiple myeloma (MM). These treatment modalities have improved the survival outcomes of patients with relapsed and/or refractory MM compared with previously established strategies and are moving into earlier lines of therapy. Despite their efficacy, the majority of patients eventually relapse, necessitating additional therapeutic targets for salvage. G-protein–coupled receptor class 5 member D, Fc receptor-homolog 5, and SLAMF7 are some examples of novel targets in development. This expanding armamentarium of immunotherapeutic agents will be crucial to address the unmet need for relapses after BCMA-targeting therapies, particularly antigen-negative relapses. The utilization of sequential T-cell redirective therapies including agents targeting different tumor-associated antigens and combination therapies appears feasible, paving the way for effective chemotherapy-free regimes. Deliberate consideration of treatment timing, preserving T-cell health, overcoming antigenic loss, and comprehension of the complex tumor microenvironment would be key to maximizing therapeutic benefits and minimizing adverse effects. This review summarizes novel targets in development for myeloma beyond BCMA, presenting pivotal safety and efficacy data derived from clinical trials when available and the considerations vital for navigating this expanding landscape of immunotherapeutic options.
Introduction
In recent years, the concurrent utilization of proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), and monoclonal antibodies (mAbs) has significantly improved overall outcomes in the treatment of multiple myeloma (MM).1-4 However, the increased use of these agents in the frontline or earlier-line setting has led to a higher incidence of refractoriness to PIs, IMiDs, and anti-CD38 mAbs among patients with relapsed/refractory (R/R) MM. Historically, patients with triple-class refractory disease and penta-class refractory diseases have exhibited poor outcomes, with overall response rates (ORR) of 29% and 30% and median overall survival (mOS) of 9.2 months and 5.6 months, respectively.5
The development of B-cell maturation antigen (BCMA)–targeting therapies has significantly improved outcomes for patients with R/R MM. Although the phase 3 DREAMM-3 trial comparing belantamab mafodotin, an antibody-drug conjugate (ADC) targeting BCMA, against pomalidomide, did not demonstrate progression-free survival (PFS) superiority,6 T-cell redirective therapies targeting BCMA in the R/R MM population have shown unprecedented outcomes even as monotherapy. Two chimeric antigen receptor (CAR) T-cell therapies targeting BCMA (idecabtagene vicleucel and ciltacabtagene autoleucel [cilta-cel]) and 2 T-cell engagers (TCEs) targeting BCMA (teclistamab and elranatamab) are now US Food and Drug Administration (FDA)–approved for the treatment of patients with R/R MM based on results from the KarMMa, CARTITUDE-1, MajesTEC-1, and MagnetisMM-3 studies.7-10 The phase 3 KarMMa-3 trial and retrospective real-life studies have provided evidence supporting the significant PFS benefit that triple-class and penta-class refractory patients derive from BCMA-directed therapies.11-13 Similarly, patients who were lenalidomide refractory with 1 to 3 prior lines of therapies (LOTs) and received cilta-cel had improved PFS compared with patients who received standard-of-care therapy in the phase 3 CARTITUDE-4 trial.14 Toxicities associated with immune effector cells (IECs), such as cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS), are linked to T-cell redirective therapies but are predominantly low grade and manageable with supportive measures.15,16
Despite notable responses, post–BCMA-directed CAR T-cell therapy relapses present considerable management challenges. In a retrospective analysis, 79 patients who progressed after BCMA CAR T-cell therapy received a median of 2 salvage regimes (range, 1-10) and 237 salvage treatment lines, highlighting the vast number of available options. These patients received a median of 5 treatment lines before BCMA CAR T-cell therapy, 83.5% being triple-class refractory and 38% penta-class refractory. The ORR was 43.4%, median PFS (mPFS) was 3.5 months for the first salvage regime, and mOS from the time of BCMA-directed CAR T-cell relapse was 17.9 months. Patients who received subsequent T-cell engaging therapies (CAR T-cell therapy and/or TCE) as a salvage regime had an mOS that was not reached after a median follow-up >21 months. The patients who received a TCE as first-line salvage immediately after BCMA CAR T-cell therapy relapse had a median PFS of >9 months, implying a substantive contribution of additional TCE therapies to survival outcomes.17
Strategically sequencing therapies to preserve T-cell health and exploring emerging tumor-associated antigens (TAA) are imperative. This review presents a comprehensive overview of therapeutic targets in MM beyond BCMA, prioritizing studies with clinical data as summarized in Figure 1 and Tables 1 and 2.
Summary of immunotherapies with novel targets and therapeutics. Highlighted in red are FDA-approved therapies. Figure created with BioRender.com.
Summary of immunotherapies with novel targets and therapeutics. Highlighted in red are FDA-approved therapies. Figure created with BioRender.com.
Novel TAA and bispecific BCMA-targeted CAR T-cell therapies and their respective clinical trials
| Target . | GPRC5D . | GPRC5D . | GPRC5D . | CD138 . | BCMA/CD38 bispecific . | BCMA/CD38 bispecific . | BCMA/CS1 bispecific . |
|---|---|---|---|---|---|---|---|
| Drug (study) | MCARH10918 | BMS-98639319 (CC-95266) | OriCAR-01720 (POLARIS NCT05016778) | CART-13821 (NCT01886976) | BM38 CAR22 | BCMA-CD3823 | CS1-BCMA bispecific CAR24 (NCT04662099) |
| Phase | 1 | 1 | 1 | 1/2 | 1 | 1 | 1 |
| Median age (range), y | 60 (38-76) | — | 65 | 57 (48-68) | 59 (49-72) | 58.5 (48-78) | — |
| Med follow-up (range), mo | 10.1 | 5.9 (0.0-24/0) | 7.8 (6-10) | — | 9 | 11.5 | 8.2 |
| Median prior LOTs (range) | 6 (4-14) | — | 6 (3-17) | 8 (5-18) | 4 (2-9) | 3 (2-3) | 4 (2-8) |
| Previous BCMA therapies, % | 59 | 46 | 50 | — | 0 | 0 | 13 |
| Cohort size, n | 17 | 70 | 10 | 5 | 23 | 16 | 16 |
| Efficacy, % | |||||||
| ORR | 71 | 86 | 100 | 0 | 87 | 87.5 | 81 |
| CR/sCR | 35 | 38 | 60 | 0 | 52 | 81.2 | 38 |
| PFS | — | — | — | — | — | 1-y PFS 68.8% | mPFS: 9 mo |
| OS | — | — | — | — | — | 1-y OS: 75% | mOS: NR |
| DOR | mDOR: 7.8 mo | — | — | — | — | — | mDOR: NR |
| Safety, % | |||||||
| CRS all grades/G3+ | 88/6 | 84/6 | 100/0 | 80/0 | 87/17 | 75/31.3 | 38/6 |
| ICANS all grades/G3+ | 6/6 | 11/4 | 0/0 | — | 0/0 | -/- | 0/0 |
| Neutropenia all grades/G3+ | 100/100 | -/69 | 100/100 | — | 96/87 | -/- | 100/94 |
| Anemia all grades/G3+ | 88/41 | -/31 | -/70 | — | 43/13 | -/38 | 25/13 |
| Thrombocytopenia all grades/G3+ | 88/65 | -/30 | -/90 | — | 61/47 | -/25 | 63/31 |
| Other/other G3+ | Infection 18/12 Nail changes 65/0 Dysgeusia 12/0 | Skin TEAE 24/0 Nail changes 16/0 Dysgeusia 3/0 | Lung infection 4/4 | Leukopenia -/94 Infection 38/- Myalgia 50/- Migratory arthralgia 31/- |
| Target . | GPRC5D . | GPRC5D . | GPRC5D . | CD138 . | BCMA/CD38 bispecific . | BCMA/CD38 bispecific . | BCMA/CS1 bispecific . |
|---|---|---|---|---|---|---|---|
| Drug (study) | MCARH10918 | BMS-98639319 (CC-95266) | OriCAR-01720 (POLARIS NCT05016778) | CART-13821 (NCT01886976) | BM38 CAR22 | BCMA-CD3823 | CS1-BCMA bispecific CAR24 (NCT04662099) |
| Phase | 1 | 1 | 1 | 1/2 | 1 | 1 | 1 |
| Median age (range), y | 60 (38-76) | — | 65 | 57 (48-68) | 59 (49-72) | 58.5 (48-78) | — |
| Med follow-up (range), mo | 10.1 | 5.9 (0.0-24/0) | 7.8 (6-10) | — | 9 | 11.5 | 8.2 |
| Median prior LOTs (range) | 6 (4-14) | — | 6 (3-17) | 8 (5-18) | 4 (2-9) | 3 (2-3) | 4 (2-8) |
| Previous BCMA therapies, % | 59 | 46 | 50 | — | 0 | 0 | 13 |
| Cohort size, n | 17 | 70 | 10 | 5 | 23 | 16 | 16 |
| Efficacy, % | |||||||
| ORR | 71 | 86 | 100 | 0 | 87 | 87.5 | 81 |
| CR/sCR | 35 | 38 | 60 | 0 | 52 | 81.2 | 38 |
| PFS | — | — | — | — | — | 1-y PFS 68.8% | mPFS: 9 mo |
| OS | — | — | — | — | — | 1-y OS: 75% | mOS: NR |
| DOR | mDOR: 7.8 mo | — | — | — | — | — | mDOR: NR |
| Safety, % | |||||||
| CRS all grades/G3+ | 88/6 | 84/6 | 100/0 | 80/0 | 87/17 | 75/31.3 | 38/6 |
| ICANS all grades/G3+ | 6/6 | 11/4 | 0/0 | — | 0/0 | -/- | 0/0 |
| Neutropenia all grades/G3+ | 100/100 | -/69 | 100/100 | — | 96/87 | -/- | 100/94 |
| Anemia all grades/G3+ | 88/41 | -/31 | -/70 | — | 43/13 | -/38 | 25/13 |
| Thrombocytopenia all grades/G3+ | 88/65 | -/30 | -/90 | — | 61/47 | -/25 | 63/31 |
| Other/other G3+ | Infection 18/12 Nail changes 65/0 Dysgeusia 12/0 | Skin TEAE 24/0 Nail changes 16/0 Dysgeusia 3/0 | Lung infection 4/4 | Leukopenia -/94 Infection 38/- Myalgia 50/- Migratory arthralgia 31/- |
CR, complete remission; DOR, duration of response; G3, grade 3; mDOR, median duration of response; NR, not reached; Q2W, once every 2 weeks; QW, once weekly; sCR, stringent complete remission; TEAE, treatment-emergent AE.
Non-BCMA–targeted TCE therapies and their respective clinical trials
| Target . | GPRC5D . | GPRC5D + anti-CD38mAb . | GPRC5D + BCMA . | GPRC5D . | FcRH5 . |
|---|---|---|---|---|---|
| Drug/study | Talquetamab MonumenTAL-125 | Talquetamab + daratumumab TRIMM-226 | Talquetamab + teclistamab RedirecTT-127 | Forimtamig (RG6234)28 | Cevostamab29 |
| Phase | 2 | 2 | 1b | 1 | 1 |
| Cohorts | 1: SC 0.4 mg/kg QW 2: SC 0.8 mg/kg Q2W 3. Prior T-cell redirection, either dose | SC 0.4 mg/kg QW or SC 0.8 mg/kg Q2W + SC Dara 1800 mg per schedule | RP2D: SC Tec 3.0 mg/kg Q2W + SC tal 0.8 mg/kg Q2W SC (RP2D, n = 34; All dose levels, n = 93) | 1: IV 6 μg –10 mg 2: SC 30 μg –7.2 mg | 1: C1D1 (IV 0.05-3.6 mg), target dose (0.15-198 mg) C1D8 × 17 cycles 2: C1D1 (IV 0.3-1.2 mg), C1D8 (3.6 mg), target dose (60-160 mg) on C1D15 × 17 cycles |
| Median age (range), y | — | 63 (37-81) | 67 (39-81) | (Cohort 1,2) 62 (27-78), 64 (46-79) | 64 (33-82) |
| Med follow-up (range), mo | 8.6-14.9 | 11.5 | 14.4 | (Cohort 1,2) 7.1, 3.9 | 6.1 |
| Median prior LOTs (range) | 5-6 | 5 (2-16) | 5 (1-11) | (Cohort 1,2) 5 (2-15), 4 (2-14) | 6 (2-18) |
| Previous BCMA therapies, % | 15 (cohort 1) 11 (cohort 2) 12 (cohort 3) | 54 (exposed) 38 (refractory) | — | (Cohort 1,2) 19.6, 20.4 | 33.8 |
| Cohort size (no. of patients) | 143, 145, 51 (Cohort 1,2,3) | 65 | 63 | (Cohort 1,2) 51, 54 | 160 |
| Efficacy, % | (Cohort 1,2,3) | 78 (74/64 if prior BCMA exposed/ refractory) | 84 | (Cohort 1,2) | 54.5 (160 mg dose), 36.7 (90 mg dose), 36.4 (prior anti-BCMA) |
| ORR | 74, 73, 63 | 45 | 34 | 71.4, 60.4 | — |
| CR/sCR (%) | - | 19 | NR | 28.5, 18.8 | — |
| mPFS, mo | 7.5, 11.9, 5.1 | (1-y PFS 76%) | NR | — | — |
| OS | — | 1-y OS: 93% | — | — | |
| DOR | — | — | — | ||
| Safety, % | (Cohort 1,2,3) | 78/0 | (At RP2D) | (Cohort 1,2) | 80/1.3 |
| CRS all grades/G3+ | 79,75,77/- | 5/0 | 81/3 | 82.4,77.8/2,1.9 | 13/- |
| ICANS all grades/G3+ | 11,11,3/- | 38/26 | 2/- | 8.6/1.9 | 18.1/16.3 |
| Neutropenia all grades/G3+ | — | Anemia 52/- | 76/75 | — | 31.9/21.9 |
| Anemia all grades/G3+ | — | — | 60/43 | — | — |
| Thrombocytopenia all grades/G3+ | — | — | — | ||
| Other/other G3+ | Skin AE 56,71,69 Nail-related AE 54,53,61 Dysgeusia 50,48,61 Infections 58,65,71/22,16,26 | Skin exfoliation 45/- Dysgeusia 75/- Infections 63/25 | Dysgeusia 47.1/- Skin toxicity 52.9/- Nail disorders 41.2/- Infections -/38.2 | Skin AE 72.5, 81.5/11.8,14.8 Hair and nail-related AE 17.6, 22.2/0,0 GI/mucosa-related AE 70.6,74.1/0,5.6 | Infections 42.5/18.8 Hemophagocytic lymphohistiocytosis 0/0.6 (G5) |
| Target . | GPRC5D . | GPRC5D + anti-CD38mAb . | GPRC5D + BCMA . | GPRC5D . | FcRH5 . |
|---|---|---|---|---|---|
| Drug/study | Talquetamab MonumenTAL-125 | Talquetamab + daratumumab TRIMM-226 | Talquetamab + teclistamab RedirecTT-127 | Forimtamig (RG6234)28 | Cevostamab29 |
| Phase | 2 | 2 | 1b | 1 | 1 |
| Cohorts | 1: SC 0.4 mg/kg QW 2: SC 0.8 mg/kg Q2W 3. Prior T-cell redirection, either dose | SC 0.4 mg/kg QW or SC 0.8 mg/kg Q2W + SC Dara 1800 mg per schedule | RP2D: SC Tec 3.0 mg/kg Q2W + SC tal 0.8 mg/kg Q2W SC (RP2D, n = 34; All dose levels, n = 93) | 1: IV 6 μg –10 mg 2: SC 30 μg –7.2 mg | 1: C1D1 (IV 0.05-3.6 mg), target dose (0.15-198 mg) C1D8 × 17 cycles 2: C1D1 (IV 0.3-1.2 mg), C1D8 (3.6 mg), target dose (60-160 mg) on C1D15 × 17 cycles |
| Median age (range), y | — | 63 (37-81) | 67 (39-81) | (Cohort 1,2) 62 (27-78), 64 (46-79) | 64 (33-82) |
| Med follow-up (range), mo | 8.6-14.9 | 11.5 | 14.4 | (Cohort 1,2) 7.1, 3.9 | 6.1 |
| Median prior LOTs (range) | 5-6 | 5 (2-16) | 5 (1-11) | (Cohort 1,2) 5 (2-15), 4 (2-14) | 6 (2-18) |
| Previous BCMA therapies, % | 15 (cohort 1) 11 (cohort 2) 12 (cohort 3) | 54 (exposed) 38 (refractory) | — | (Cohort 1,2) 19.6, 20.4 | 33.8 |
| Cohort size (no. of patients) | 143, 145, 51 (Cohort 1,2,3) | 65 | 63 | (Cohort 1,2) 51, 54 | 160 |
| Efficacy, % | (Cohort 1,2,3) | 78 (74/64 if prior BCMA exposed/ refractory) | 84 | (Cohort 1,2) | 54.5 (160 mg dose), 36.7 (90 mg dose), 36.4 (prior anti-BCMA) |
| ORR | 74, 73, 63 | 45 | 34 | 71.4, 60.4 | — |
| CR/sCR (%) | - | 19 | NR | 28.5, 18.8 | — |
| mPFS, mo | 7.5, 11.9, 5.1 | (1-y PFS 76%) | NR | — | — |
| OS | — | 1-y OS: 93% | — | — | |
| DOR | — | — | — | ||
| Safety, % | (Cohort 1,2,3) | 78/0 | (At RP2D) | (Cohort 1,2) | 80/1.3 |
| CRS all grades/G3+ | 79,75,77/- | 5/0 | 81/3 | 82.4,77.8/2,1.9 | 13/- |
| ICANS all grades/G3+ | 11,11,3/- | 38/26 | 2/- | 8.6/1.9 | 18.1/16.3 |
| Neutropenia all grades/G3+ | — | Anemia 52/- | 76/75 | — | 31.9/21.9 |
| Anemia all grades/G3+ | — | — | 60/43 | — | — |
| Thrombocytopenia all grades/G3+ | — | — | — | ||
| Other/other G3+ | Skin AE 56,71,69 Nail-related AE 54,53,61 Dysgeusia 50,48,61 Infections 58,65,71/22,16,26 | Skin exfoliation 45/- Dysgeusia 75/- Infections 63/25 | Dysgeusia 47.1/- Skin toxicity 52.9/- Nail disorders 41.2/- Infections -/38.2 | Skin AE 72.5, 81.5/11.8,14.8 Hair and nail-related AE 17.6, 22.2/0,0 GI/mucosa-related AE 70.6,74.1/0,5.6 | Infections 42.5/18.8 Hemophagocytic lymphohistiocytosis 0/0.6 (G5) |
G3, grade 3; GI, gastrointestinal; NR, not reached; Q2W, once every 2 weeks; QW, once weekly; RP2D, recommended phase 2 dose.
GPRC5D
We identified the G-protein–coupled receptor, class C group 5 member D (GPRC5D), an orphan receptor, as an ideal target for immunotherapeutic strategies due to its increased expression on MM cells compared with normal plasma cells and other immune cells. In normal tissue, GPRC5D is primarily expressed on plasma cells and hard keratinized tissue, exhibiting low expression in other human tissue cells.30,31 In murine and nonhuman primate models, including BCMA escape models, GPRC5D-targeted CAR T cells demonstrated in vivo activity against MM without on-target, off-tumor toxicity. This led to the clinical development of therapeutic agents targeting GPRC5D.
GPRC5D-targeted CAR T-cell therapies
MCARH109
We developed MCARH109 as the first GPRC5D-targeted autologous CAR T-cell therapy. It consists of a human B-cell–derived GPRC5D-targeted single-chain variable fragment (scFv), a 4-1BB costimulatory domain, and a CD3ζ signaling domain. In a phase 1 study at Memorial Sloan Kettering Cancer Center, 17 patients with R/R MM with ≥3 prior LOTs, including a PI, an IMiD, and an anti-CD38 therapy, and prior BCMA therapy exposure received doses of MCARH109 ranging from 25 × 106 to 450 × 106 CAR T cells. The maximum tolerated dose was determined to be 150 × 106 CAR T cells because 1 patient had grade 4 CRS and ICANS, and 2 other patients had grade 3 cerebellar disorder, possibly caused by on-target, off-tumor expression of low-level GPRC5D in the inferior olivary nuclease at the 450 × 106 CAR T-cell dose. Twelve patients received doses of 25 × 106 to 150 × 106 CAR T cells, and no occurrences of cerebellar disorders, ICANS of any grade, or CRS of grade ≥3 were reported in this cohort. The ORR was 71% in all patients and 58% in patients who received doses of up to 150 × 106 CAR T cells. Other on-target, off-tumor toxicities included grade 1 nail changes (65%) and grade 1 dysgeusia or dry mouth (12%). This study prompted a subsequent multicenter study involving BMS-986393 (CC-95266; below).18
BMS-986393 (CC-95266)
BMS-986393 is an autologous CAR T-cell therapy targeting GPRC5D, investigated in the CC-95266-MM-001 (NCT04674813) study. This phase 1, first-in-human, multicenter trial enrolled patients with ≥3 prior LOTs, including a PI, an IMiD, an anti-CD38 therapy, autologous stem cell transplant if eligible, and prior BCMA-directed and CAR T-cell therapies. In the dose expansion cohort of 70 patients, BMS-986393 was administered at doses ranging from 25 × 106 to 450 × 106 CAR T cells. Notably, 46% of these patients had prior BCMA-targeted therapies, and 36% had prior BCMA-directed CAR T-cell therapy. The ORR was 86% (CR 38%) in patients with assessable efficacy and 85% (CR 46%) in patients refractory to prior BCMA-directed therapy.
Grades 3 to 4 treatment-emergent neutropenia, anemia, and thrombocytopenia were observed in 69%, 31%, and 30% of patients, respectively. There were no adverse events (AEs) of grade ≥3 related to skin, nails, or dysgeusia reported. CRS occurred in 84% of patients, the majority being grades 1 to 2; however, 1 patient succumbed to CRS at the highest dose level, and 3 additional patients experienced CRS of grade ≥3. ICANS was noted in 11% of patients, with 3% experiencing grade 3 ICANS. Additionally, there were non-ICANS neurologic treatment-related AEs, including cerebellar toxicity (3%), headache (14%), ataxia (7%), dysarthria (4%), and paresthesia, gait disturbance, and nystagmus (1% each).19 These promising early clinical data provide support for BMS-986393 as a potential treatment option in R/R MM, and further developments are underway.
OriCAR-017
OriCAR-017, another GPRC5D-targeted autologous CAR T-cell therapy, incorporates the proprietary signal activation domain element, Ori, for the enhancement of the expansion efficiency of memory immune cells to increase the antitumor activity and durability of CAR T cells in vivo. In the phase 1 POLARIS trial conducted at the First Affiliated Hospital of Zhejiang University School of Medicine in China, 10 patients with R/R MM received OriCAR-017 at doses ranging from 1 × 106 to 6 × 106 CAR T cells per kg. All patients developed hematological toxicities, including neutropenia (100%), thrombocytopenia (90%), leukopenia (90%), and anemia (70%). Ninety percent of patients developed grade 1 CRS, 10% developed grade 2 CRS, and none developed neurologic toxicities. ORR was 100%, with 60% achieving stringent CR. At a median follow-up of 7.8 months, 2 patients had disease progression.20
GPRC5D TCE
Talquetamab
Talquetamab, a humanized GPRC5DxCD3 TCE, was approved by the FDA in August 2023.31 Talquetamab showed substantial antitumor effects in patients with heavily pretreated R/R MM in the phase 1 MonumenTAL-1 study. Two doses were subsequently recommended for a phase 2 study: 405 μg/kg weekly and 800 μg/kg every other week.32 Patients enrolled in the trial had ≥3 prior LOTs, which included at least a PI, an IMiD, and an anti-CD38 antibody. A large proportion of patients were triple-class refractory (69%-84%), and 11% to 15% had previously received belantamab mafadotin. A total of 143 patients received talquetamab at 0.4 mg/kg QW, and 145 patients received it at 0.8 mg/kg Q2W. An ORR exceeding 70% was observed in these heavily pretreated patients with R/R MM, including patients who had received prior T-cell redirection therapy, with an overall tolerable safety profile. Early evidence suggests that treating patients with TCEs targeting a different TAA in relapses after T-cell redirection may be feasible.25 Promising outcomes were also seen when talquetamab was used in combination with other agents, such as daratumumab in the TRIMM-2 trial and teclistamab in the RedirecTT-1 study. Patients in both trials had 5 median LOTs and were triple-class refractory in 58% and 78%, respectively26,33 (Table 2).
Forimtamig (RG6234)
Forimtamig, a GPRC5DxCD3 TCE with a novel 2:1 GPRC5D:CD3 configuration for increased potency was evaluated in a phase 1 study involving 105 heavily pretreated patients with R/R MM.34 These patients had a median of 5 prior LOTs, with the majority being triple-class refractory, having received at least a PI and an IMiD, as well as prior CAR T cells, ADCs, and TCEs. After a 2-week step-up dose, patients received up to 1 year of forimtamig, either as an IV dose ranging from 6 to 10 mg or a subcuatneous (SC) dose ranging from 30 μg to 7.2 mg, until disease progression or unacceptable toxicity occurred. The ORRs were 71.4% (IV) and 60.4% (SC), with responses also observed in patients who had received prior anti-BCMA therapies. CRS was observed in 82.4% (IV) and 77.8% (SC) of patients, with 2.0% (IV) and 1.9% (SC) experiencing grade ≥3 CRS. ICANS occurred in 8.6%, with 1.9% grade ≥3. As with the other GPRC5D targeting agents, skin and nail-related AEs as well as gastrointestinal AEs were common, as populated in Table 2. Notably, there was a single forimtamig-related fatal AE (1.9%) of acute respiratory failure reported in the SC group.28
Considerations for GPRC5D as a target
GPRC5D-targeted therapies exhibit notable efficacy, even in patients who have relapsed after BCMA therapies. On-target, off-tumor toxicities commonly involve skin, nail, and oral AEs manifesting as rash, skin exfoliation, dry skin, pruritus, palmar-plantar erythrodysesthesia syndrome, onychomadesis, dysgeusia, dry mouth, and dysphagia. These toxicities, typically low grade, can be managed effectively through measures such as dose holds, modifications, and supportive care, although managing dysgeusia has proven challenging in some TCE patients.30,35,36
Notably, the more prevalent on-target, off-tumor toxicities, such as skin rash and dysgeusia, occur less frequently with CAR T-cell therapies compared with TCE therapies. This disparity may arise from variations in the tissue distribution of TCE and living cell therapies and/or differences in dosing schedules (single administration vs repeated dosing). Uncommon toxicities associated with CAR T-cell therapy include dizziness and gait disturbances, observed particularly in patients receiving doses above the maximum tolerated level. Importantly, GPRC5D-directed therapies result in reduced risk of infections compared with BCMA-directed therapies, although comprehensive longer-term data across various therapeutic products are necessary.37 Overall, based on early safety and efficacy data, GPRC5D-targeted therapies will assume a key role in MM treatment.
SLAMF7
SLAMF7, part of the signaling lymphocyte activation molecule (SLAM) family of receptors, also known as CD2 subset-1 (CS1)/CRACC/CD319, promotes myeloma cell proliferation and growth due to its upregulation and high expression in MM cells. Immune cells such as B cells, T cells, dendritic cells, natural killer (NK) cells, and monocytes also express SLAMF7.38
SLAMF7 mAbs
Elotuzumab
Elotuzumab is a humanized immunoglobulin G kappa antibody that binds its Fab portion to SLAMF7 on MM cells and its Fc portion to FcgRIIIa/CD16 on NK cells, activating NK cells through antibody-dependent cellular cytotoxicity or directly via increased interleukin-2 and tumor necrosis factor α levels, leading to downstream granzyme B release.
Elotuzumab has received FDA and European Medicines Agency approval for use in combination with lenalidomide and dexamethasone for MM after at least 1 prior therapy and with pomalidomide and dexamethasone (Pd) for R/R MM after at least 2 prior therapies, including lenalidomide and a PI. These approvals are based on data from ELOQUENT-3, a multicenter, randomized, phase 2 clinical trial that compared elotuzumab, pomalidomide, and dexamethasone (EPd) with Pd in patients with R/R MM refractory to lenalidomide and a PI. Results demonstrated a significantly longer PFS of 10.25 months for EPd than 4.70 months for Pd. The EPd arm also exhibited a higher ORR at 53%, in contrast to 26% in the Pd arm. Importantly, the inclusion of elotuzumab did not lead to a higher occurrence of neutropenia, infections, or grade 3 to 4 AEs.39
SLAMF7 CAR T-cells
The CARAMBA-1 trial (NCT04499339) represents the first-in-human clinical trial evaluating CAR T cells directed against SLAMF7. The CAR T cells are generated by the Sleeping Beauty transposon gene transfer system using a virus-free approach.40 Early findings from the dose-escalation segment of the CARAMBA-1 study were presented at the EBMT-EHA fourth European CAR T-cell Meeting, suggesting that these CAR T cells are safe and have antitumor activity. However, as of now, no formal results have been published.
The MELANI-01 (NCT04142619)41 is phase 1 dose-escalation trial in which UCARTCS1, an allogeneic CAR T-cell therapy directed against the CS1 antigen, was administered to patients with R/R MM. Of the 5 patients who received UCARTCS1A, UCARTCS1A cells were detected in 3 patients, with expansion noted in 1 patient at dose level 1 and another at dose level 2. Although both patients showed clinical responses (1 partial response and 1 very good partial response), significant toxicities were observed. The toxicities observed included grade 2 to 4 CRS and 2 grade 5 events: hemorrhagic pancreatitis in the context of CRS, CAR-related hemophagocytic lymphohistiocytosis, disseminated mucormycosis, and pseudomonal pneumonia in 1 patient and organizing pneumonia during prolonged severe lymphopenia in the second. Although UCARTCS1 is no longer under clinical development as of 2024, this trial underscores the potential of SLAMF7 as a target. Further studies are necessary to optimize safety considerations, and ongoing investigations of novel CAR T-cell products targeting both BCMA and SLAMF7 may provide additional insights into this area.42
Considerations for SLAMF7 as a target
SLAMF7-CAR T cells have been shown to result in fratricide of native SLAMF7+/high normal lymphocytes,43 and drawing insights from the elotuzumab experience, induces a significant reduction in lymphocyte counts.44 The potential implications of a significant and sustained reduction in lymphocytes include increased susceptibility to infectious complications, with further details awaiting disclosure from ongoing clinical trials.
FcRH5
Fc receptor-homolog 5 (FcRH5), also known as FcRL5, CD307e, and IRTA2, is a differentiation antigen homologous to the family of Fc receptors, which is exclusively expressed in the B-cell lineage. Normal plasma cells express FcRH5, with an upregulated expression on malignant plasma cells, especially those with amplification or gain of chromosome 1q21.45
FcRH5 TCE
Cevostamab
Cevostamab is a TCE targeting FcRH5. The phase 1 trial (NCT03275103) was conducted in high-risk patients with a median of 6 prior LOTs (n = 161). Up to 68.1% of patients had penta-refractory disease, and 33.5% had prior BCMA therapy. Responses were dose dependent, with an ORR of 54.4% at the higher dose level of 160 mg, compared with 36.7% at 90 mg. Notably, patients who received prior anti-BCMA therapy showed an ORR of 36.4% at target dose levels >90 mg. CRS and ICANS occurred in 80.7% and 14.3% of patients, respectively, predominantly at grades 1 to 2. Grade 3 CRS and ICANS were observed in 1.2% and 0.6%, respectively, with no instances of grade ≥4 AEs.29 Currently, a phase 1/2 open-label, multicenter trial (CAMMA 2) is underway to evaluate the effectiveness of cevostamab monotherapy in patients with R/R MM who are triple-class refractory and previously received anti-BCMA therapy.
Considerations of FcRH5 as a target
Due to the insufficient clinical data available for FcRH5 T-cell redirecting therapies, it is imperative to await more substantial evidence before making definitive conclusions about their safety profile and therapeutic efficacy.
CD138
CD138 ADC
Indatuximab ravtansine
Indatuximab ravtansine (BT062) is an ADC engineered to target CD138. When administered as a monotherapy, it has limited efficacy in the R/R MM population, as evidenced by a modest ORR of 3.2% in a phase 1/2a study.48 However, preclinical models indicate a synergistic enhancement of antitumor activity when combined with lenalidomide, suggesting that more promising outcomes may be achieved through the combination of indatuximab ravtansine with IMiDs.
In a phase 1/2a study aimed at evaluating the safety, activity, and pharmacokinetics of indatuximab ravtansine in combination with IMiDs, patients received IV indatuximab ravtansine on days 1, 8, and 15 of each 28-day cycle at escalating dose levels (80 mg/m2, 100 mg/m2, and 120 mg/m2) and lenalidomide (25 mg; orally, days 1 to 21 every 28 days) and dexamethasone (20-40 mg; days 1, 8, 15, and 22 every 28 days) during phase 1. In the expanded cohort, indatuximab ravtansine (100 mg/m2) was administered in combination with lenalidomide and dexamethasone or with pomalidomide (4 mg; orally, days 1 to 21 every 28 days) and dexamethasone in phase 2.49
Patients receiving the combination of indatuximab ravtansine with either lenalidomide or pomalidomide had ORRs of 71.7% (33/46 patients) and 70.6% (12/17), respectively. The predominant grade 3 to 4 AEs in both groups included neutropenia (22%), anemia (16%), and thrombocytopenia (11%). Treatment-emergent AEs leading to discontinuation were observed in 35 of 64 patients (55%) and included anemia, cardiac-related AEs (atrial fibrillation, cardiac arrest, and chest pain), gastrointestinal AEs (impaired gastric emptying, nausea, and vomiting), infective complications (pyrexia, hypothyroidism, osteomyelitis, pneumonia, and acute respiratory distress syndrome), skin toxicities (lichenoid keratosis, rash, and skin hyperpigmentation), neurological AEs (gait disturbance, peripheral motor neuropathy, and peripheral sensory neuropathy), fatigue, general physical health deterioration, drug-specific antibody presence, and progression of disease.49
CD138-targeted CAR T-cell therapies
CART-138
A CD138-directed CAR T cell constructed with a 4-1BB domain was studied in 5 patients with R/R MM (prior 5-18 lines) in a dose-escalation phase 1 study (NCT01886976). These patients received escalating doses of CD3+ CART-138 cells. The main AEs were grade 3 fevers, but there was no life-threatening CRS or ICANS reported. Four of the 5 patients achieved stable disease for 3 to 7 months, and the CAR T cells remained detectable by flow cytometry for >3 months.21
Considerations for CD138 as a target
Apart from plasma cells, CD138 is also expressed in the normal squamous epithelium of various organs, goblet and columnar cells of the gastrointestinal tract, and hepatocytes.50 Ongoing clinical trials with CD138-directed CAR T cells have not reported any off-target side effects. Nevertheless, given the limited effectiveness of these constructs compared with other CAR T-cell trials, it remains uncertain whether the safety profile of more potent CD138-targeted CAR T-cell products will be maintained.
Other potential immune targets
CD38
The exploration of CD38-targeted therapies has evolved beyond mAbs such as daratumumab and isatuximab, with studies focusing on CAR T-cell therapy targeting CD38. High expression levels of CD38 are characteristic of both normal and malignant plasma cells. Under physiological conditions, CD38 is also concurrently expressed at comparatively low levels on myeloid and lymphoid cells, as well as in select nonhematopoietic tissues.51 This inherent expression pattern poses challenges regarding the safety profile of T-cell redirective therapies directed at the CD38 antigen due to potential on-target, off-tumor effects.
Although preclinical studies such as Bi38-3, a CD38xCD3 TCE, show promising outcomes,52 ongoing efforts aim to enhance both efficacy and safety through affinity optimization methods. This involves using "light-chain exchange" technology, which merges heavy chains from 2 high-affinity CD38 antibodies with germ-line light chains, generating novel antibodies exhibiting varying affinity ranging from 10-fold to >1000-fold lower to that of CD38. The scFvs of these antibodies are integrated into new CAR T cells. Subsequent selection yielded CD38 CAR T cells displaying ∼1000-fold reduced affinity, demonstrating optimal proliferation, secretion of Th1-like cytokines, and effective lysis of CD38-overexpressing MM cells while preserving healthy CD38-expressing hematopoietic cells.53 These findings present a promising potential for CD38 as a viable and efficacious therapeutic target for the downstream development of T-cell redirective therapies.
CD229
CD229, a SLAM family member, has previously been identified as a target for CAR T-cell–mediated treatment of MM due to its high expression on the surfaces of MM cells. Multiple developments in CD229 targeting CAR T-cell therapy and bispecific CD229/BCMA CAR T cell have shown activity against MM cells both in vitro and in vivo.54
Integrin β7
MMG49 is a mAb that reacts with the integrin β7 protein by expression cloning. Although integrin β7 is expressed in normal lymphocytes, MMG49 does not react with them, because MMG49 recognizes an epitope exposed only when integrin β7 adopts the activated conformation. In MM cells, integrin β7 constitutively adopts the activated conformation, indicating that MMG49 is a mAb that is highly specific for MM cells. CAR T cells using the antigen recognition domain of MMG49 have been generated and successfully eradicated MM cells in mice models.55
ILT3
ILT3 emerged as a promising target for immune-based therapies for R/R MM using a comprehensive computational platform integrating mass spectrometry and RNA-sequencing data from patients with MM. ILT3 is a suppressive immunoreceptor whose expression is highly restricted to monocytes, macrophages, and dendritic cells in healthy individuals. Although ILT3 is not expressed on circulating B cells in healthy individuals, ILT3 expression has been reported on pathogenic, autoantibody-secreting plasmablasts, and plasma cells in patients with untreated systemic lupus erythematosus. A TCE targeting ILT3 has been developed and shows potent killing effects in vitro, leading to decreased tumor burden and prolonged mice survival in vivo, suggesting therapeutic potential.56
SEMA4A
SEMA4A expression on the cell surface in MM cells, as determined by plasma membrane fractionation and mass spectrometry, exceeds that of BCMA and other mooted CAR T-cell targets. Flow cytometry has also shown SEMA4A expression in MM cells that are BCMA negative. However, potential limitations include fracticide of T cells because it is upregulated in a subset of CD8 T cells after activation and off-tumor targets due to the expression of SEMA4A on monocytes. A second-generation CAR construct with a humanized scFv directed against SEMA4A has been produced, and additional information regarding its efficacy and toxicity is anticipated.57
CCR10
CCR10 is a receptor for the chemokine CCL27 and is highly expressed on myeloma plasma cells, with detectable expression on other peripheral blood hematopoietic cells. Increased expression of CCR10 has been detected on relapsed myeloma relative to newly diagnosed samples and high-risk genotypes and is predictive of worse overall survival. Successful demonstration of in vitro killing against MM cells by CCL27-based CAR T cells with CCR10 knockout to limit fratricide serves as a proof of concept for further developments of CCR10 as a promising immunotherapeutic target.58
MUC1
One of the challenges in treating MM revolves around the intricate interactions between tumor cells and the bone marrow microenvironment. Exposure to stromal cells has been shown to promote MM cell resistance to apoptosis and necrosis. One plausible explanation for the mechanism of action of immune-modulatory drugs, such as lenalidomide, is that they disrupt the interactions between stromal and MM cells.59 Mucin1 (MUC1) is an oncoprotein that is aberrantly expressed in MM cell lines and patient samples, and it comprised an N-terminus that is shed and a transmembrane C-terminus. When activated, MUC1 undergoes dimerization and translocation to the nucleus to interact with downstream effectors such as NF-κB and β-catenin/wnt.60 The co-culture of stromal cells with MM cells upregulates MUC1 expression by tumor cells via inducing interleukin-6 and activation of the JAK-STAT3 pathway. Consequently, this leads to MM cell resistance against cytotoxic and biological agents, as seen by decreased rates of apoptosis and cell death after exposure to alkylating agents and the PI bortezomib. Tumor resistance was shown to be partially reversed by silencing MUC1 in MM cells, highlighting the potential role of MUC1 as a therapeutic target for overcoming therapeutic resistance in patients with MM.61
CD46
CD46 is highly expressed in MM cells, especially in patients with a gain of chromosome 1q. FOR46 is an anti-CD46 ADC with monomethyl auristatin E (vc-MMAE) that recognizes a tumor-selective epitope of CD46. The ADC is taken up by MM cells by macropinocytosis, which is a relatively tumor-selective uptake mechanism. The safety data of 25 patients with 6 median LOTs enrolled showed that the main toxicities included grade 4 neutropenia and thrombocythemia in 1 patient each, with 3 of the 6 response-evaluable patients achieving partial responses in the 1.8 mg/kg and 2.4 mg/kg cohorts.62
CD56
Lorvotuzumab mertansine is an ADC targeting CD56, which is expressed at a high frequency on MM cells. In a phase 1 dose-escalation and expansion trial of lorvotuzumab mertansine, 37 patients with R/R MM were enrolled. There were few grade 3/4 AEs, with the highest incidence being fatigue (33%) at the highest dose level. Stable disease was noted in 43% or better. Further studies are required to understand the full potential of this novel target.63
CD74
STRO-001 is an anti-CD74 ADC incorporating a noncleavable linker-maytansinoid warhead with a drug-antibody ratio of 2. Preclinical studies have shown dose-responsive antitumor activity against malignant plasma cells and no evidence of off-target toxicity, making it a promising therapeutic agent for the treatment of MM.64
Immune targets expressed across tumor cell types
The clinical success of T-cell redirection therapies has fueled the search for new, effective therapeutic targets. Presently, T-cell redirective therapies focus on targeting surface TAAs, but there is growing interest in the intracellular proteome as a source of neoantigens. The intracellular-derived targets are peptides presented on the tumor cell surface by major histocompatibility complex (MHC) class 1 molecules, and attempts have been made to create CAR T cells that recognize peptide-MHCs of interest.65,66 One such example is NY-ESO-1 (New York esophageal squamous cell carcinoma 1), a cancer-testis antigen with expression reported in numerous cancer types, including myeloma. A clinical trial investigating autologous NY-ESO-1–specific T cells in patients with MM is ongoing.67 Although the use of intracellular targets in adoptive cell therapies holds promise, further preclinical and clinical studies are imperative to validate safety parameters and evaluate the potential for cross-reactivity with nonmalignant cells expressing peptide-MHC complexes.
Apart from intracellular targets, the natural killer group 2, member D (NKG2D) ligand pathway is also exploited for immune targeting. NKG2D is a cell receptor present on NK cells, NKT cells, cytotoxic T cells, and subsets of γδ T cells. Expression of the NKG2D ligand is induced during oncogenesis. CYAD-01 is an autologous NKG2D-based CAR T-cell therapy being investigated in the THINK study across various hematological malignancies, including MM.68
Future directions
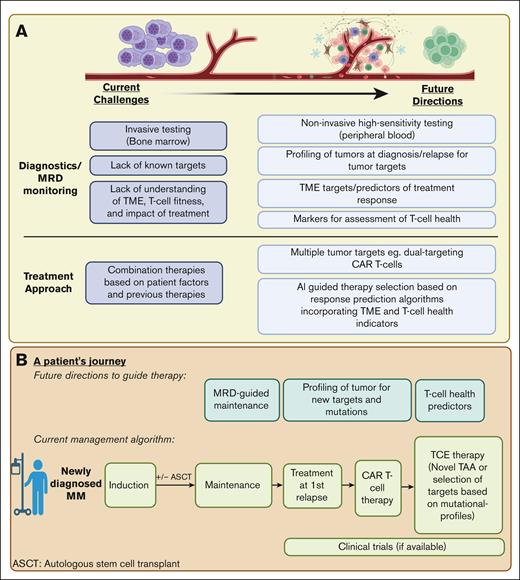
The continued evolution of immune-based therapies is reshaping the landscape of myeloma treatment. The identification of ideal targets and the use of tumor-directed cytotoxicity through therapies such as CAR T cells and TCEs have been instrumental in propelling recent treatment advances. These advances confront the intricacies of relapse and resistance, setting the stage for prospective developments in the treatment paradigm, illustrated in Figure 2.
Prospective developments in the treatment paradigm for R/R MM. (A) The current challenges and future directions in MM diagnostics, MRD monitoring, and treatment approaches. (B) We depict a theoretical roadmap outlining a patient example of treatment options and considerations in the treatment of R/R MM. Figure created with BioRender.com. MRD, minimal residual disease.
Prospective developments in the treatment paradigm for R/R MM. (A) The current challenges and future directions in MM diagnostics, MRD monitoring, and treatment approaches. (B) We depict a theoretical roadmap outlining a patient example of treatment options and considerations in the treatment of R/R MM. Figure created with BioRender.com. MRD, minimal residual disease.
Multitargeted therapies and combination therapies
Due to the clonal heterogeneity in MM cells, single-agent targeted therapies often prove inadequate. Consequently, combination approaches are imperative to effectively address this heterogeneity and prevent clonal escape.69 The pursuit of novel targets, coupled with the simultaneous targeting of multiple antigens, is gaining traction, promising more profound and sustained treatment responses.
One promising avenue is the utilization of dual-targeted CAR T-cell therapy. This can be achieved either through the combination of 2 single-targeted CAR T cells, albeit with increased manufacturing expenses, or by using bispecific CAR T cells that incorporate 2 distinct scFvs in a single CAR structure.70 Clinical trials pairing BCMA with other targets have shown encouraging outcomes, even in patients who have undergone prior BCMA therapies. In a phase 1 clinical trial of the CS1-BCMA bispecific CAR, a 100% ORR was observed.71 Additionally, CAR T cells targeting both CD38 and BCMA demonstrated notable efficacy, with an 87.5% ORR, although it was associated with an elevated incidence of grade 3 CRS in the limited patient cohort studied.23 Several other dual-targeted CAR T-cell therapies for MM, such as BCMA/CD19,72 BCMA/GPRC5D,70 and CD138/CD38 CAR T cells,73 are also under exploration. Safety data from these studies will be crucial in guiding future therapeutic strategies.
Ongoing trials are investigating the use of TCEs, either in combination with a second TCE targeting a different TAA or with other immune therapies. Beyond these approaches, trispecific antibodies such as CDR101 (BCMAxCD3xPD-L1),74 ISB 2001 (BCMAxCD38xCD3),75 and ISB 1442 (CD38xCD47xCD3)76 have been developed to enhance myeloma cell killing and overcome tumor escape mechanisms.
Immune effector cell optimization
Autologous CAR T-cell therapies are the only form of adoptive cell therapy approved for MM. However, there exist constraints with the utilization of autologous T cells, prompting ongoing efforts to improve efficacy, address mechanisms of relapse, and increase access to these cellular therapies by optimizing the CAR construct and manufacturing techniques.
Beyond autologous CAR T cells, other IECs are also explored as the effector vehicle, including allogeneic and induced pluripotent stem cell–derived IECs. Some advantages include their off-the-shelf availability and the potential for superior fitness of healthy donor lymphocytes compared with autologous cells obtained from heavily pretreated patients, which may lead to improved outcomes.77 However, challenges with allogeneic CAR T cells include the risk of graft-versus-host disease and host immune rejection.78 Although ongoing trials such as the UNIVERSAL79 study are evaluating the use of off-the-shelf allogeneic CAR T cells targeting BCMA, initial data on response and efficacy fall short of those observed with autologous therapies, underscoring the need for further research and clinical trials in this domain.
In contrast to conventional CAR T cells, CAR-NK cells are hypothesized to have a lower risk for CRS and no risk for graft-versus-host disease.77 Despite initial developments demonstrating limited antitumor activity and cell proliferation,80 recent progress in NK-cell engineering offers promise for revitalizing this field. The ongoing phase 1 dose-escalation study of FT576 (BCMA CAR-iNK) as monotherapy or in combination with daratumumab appears to demonstrate safety and tolerability.81
Optimal sequencing of immune therapies
Although the expanding array of therapeutics offers more treatment options for patients, sequencing treatment is complex given the wide repertoire of agents and disease heterogeneity. It is foreseeable that optimal treatment selection and sequencing will eventually serve to (1) eradicate residual tumor clones resistant to initial treatment and (2) preserve or enhance immune health such that patients can derive optimal benefit from subsequent LOTs.
In the upfront setting, the sequential use of immunotherapeutic agents targeting different TAA may eradicate residual clones while mitigating additive toxicities associated with combinatorial approaches. For example, the MagnetisMM-7 and MasjecTEC-4 trials evaluate the role of anti-BCMA TCE for maintenance after conventional induction therapy, whereas CARTITUDE-6 examines the role of BCMA-directed CAR T cells as consolidation after induction therapy.
In the relapsed setting, the optimal sequencing of salvage agents takes into consideration disease factors, treatment factors such as prior treatment lines and duration of therapy, and patient factors such as T-cell health. Factors such as antigenic loss, exhausted IECs, and the suppressive tumor microenvironment contribute to relapse, necessitating appropriate intervention for effective salvage therapy.82-84
It is evident that patients who have relapsed after T-cell redirective therapy are most likely to benefit from another TCE or CAR T cells, compared with other treatment options. This includes the possibility of using CAR T cells after TCE or vice versa or targeting a different TAA such as anti-GPRC5D TCE after anti-BCMA TCE or even possibly sequential therapies targeting the same antigen.85 However, ongoing debates surround the optimal sequence of these T-cell redirective therapies. Approved TCEs require continuous administration until disease progression, potentiating T-cell exhaustion and antigen escape.82,86,87 In contrast, CAR T-cell therapy involves a single treatment followed by a treatment-free interval, mitigating challenges associated with endogenous T-cell exhaustion and antigen escape.7,88 In the MonumenTAL-1 trial, among patients with prior exposure to T-cell redirecting therapies, ORR was 73.5% in patients with prior BCMA-targeted CAR T-cell therapy and only 47.1% in those with prior BCMA-directed TCE, further suggesting that the use of CAR T-cell therapy before consecutive use of TCEs may be ideal due to the presence of a treatment-free interval.25 Therefore, when feasible, prioritizing CAR T-cell therapy before TCE may be a rational approach.
Despite the advantages of prioritizing CAR T-cell therapy, practical constraints may limit its feasibility. For instance, in cases in which rapid treatment is imperative due to progressive disease, TCEs, being "off the shelf," offer a more expedited option compared with CAR T cells, which require manufacturing time. Challenges such as manufacturing failure, inadequate T-cell collection, disease progression during manufacturing, high upfront costs associated with CAR T-cell therapy, patient frailty, and individual choice may influence the preference for TCE as the forefront therapy in specific contexts. Additionally, the impact of treatment sequence on the time to second objective disease progression requires further assessment through additional clinical data.
When considering the target TAA for the next therapy, it is crucial to note that patients treated with BCMA-targeting therapies might experience functional epitope loss due to nontruncating mutations and in-frame deletions. These genetic alterations can result in varying sensitivities to different BCMA TCEs. These patients may retain detectable surface BCMA expression, making surface antigenic expression a poor predictive biomarker for treatment responses.82 As such, using an agent targeting a different TAA subsequently will likely be a more effective strategy to salvage patients in a setting in which there is a lack of an ideal biomarker to predict preserved antigen expression. For example, the response rates of patients who have previously been exposed to BCMA-targeting therapies and subsequently treated with GPRC5D-targeting therapies have been favorable. The sequential use of BCMA and GPRC5D monotherapies will require careful planning to mitigate the potential selection of antigen-negative clones.82 Future strategies involving multiple immunotherapies in rapid succession may aim to eliminate clones before increasing treatment pressures result in immune escape variants.
Conclusion
At present, the use of BCMA as a target in CAR T-cell therapy has achieved unprecedented responses, changing the paradigm in the treatment of R/R MM. Nevertheless, there remain challenges because patients continue to relapse. One of the main mechanisms is antigen downregulation or antigen loss under therapeutic pressure, and using alternative novel targets is a potential strategy to overcome antigen-negative escape.
GPRC5D is the most advanced novel target, with numerous potential treatments undergoing clinical trials. Other targets being explored in clinical trials include SLAMF7, CD138, CD38, CCR10, and MUC-1, whereas most of the other targets described above are in the preclinical stage.
In the dynamic landscape of MM therapeutics, the increasing array of therapeutic agents offers promising avenues for treatment, yet determining the optimal sequencing presents a complex challenge. Strategically designed studies are shedding light on the best treatment selection and sequencing strategies, with the goal of eradicating treatment-resistant tumor clones and maintaining immune health to achieve optimal responses in subsequent therapy lines. Although debates persist regarding the sequence of immunotherapies, current evidence suggests that prioritizing CAR T-cell therapy before TCE may be a rational approach due to favorable response rates, coupled with time off therapy and less predisposition to antigen relapse compared with TCEs for tumor antigen retargeting. Ongoing research underscores the importance of addressing T-cell exhaustion, antigenic loss, and the intricate tumor microenvironment to develop effective salvage strategies.
Future directions point toward innovative strategies involving combination therapies, sequential use of agents targeting diverse TAA, and careful consideration of treatment timing to maximize therapeutic benefits and minimize AEs in the ever-evolving landscape of MM treatment.
Authorship
Contribution: M.T., Y.C., and E.L.S. contributed equally to writing the manuscript.
Conflict-of-interest disclosure: Y.C. reports honoraria from Bristol Myers Squibb, GlaxoSmithKline, and Johnson & Johnson, and consulting fees from Johnson & Johnson and Pfizer. E.L.S. is a paid consultant for Bristol Myers Squibb, Sanofi, Chimeric Therapeutics, Chroma Medicine, ImmuneBridge, Clade Therapeutics, Eureka Therapeutics, Sana Biotech, GC Cell, and ONK Therapeutics; reports licensed patents to Bristol Myers Squibb and Sanofi; research support from Sanofi; in-kind research support from Harbour BioMed; and research funding from the National Cancer Institute, the Parker Institute for Cancer Immunotherapy, the Blavatnik Family Foundation, the Mathers Foundation, Wellcome-Leap, the DeGregorio Family Foundation, the Lavine Family Foundation, the Massachusetts Life Sciences Foundation, and the International Myeloma Society. M.T. declares no competing financial interests.
Correspondence: Eric L. Smith, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; email: ericl_smith@dfci.harvard.edu.
References
Author notes
M.T., Y.C., and E.L.S. contributed equally to this work.