Key Points
Exchange transfusion is used for SCD complications, but there is inadequate evidence of its safety and benefit in pregnancy.
The TAPS2 trial shows the feasibility of conducting a definitive trial of prophylactic exchange transfusion in SCD pregnancy.
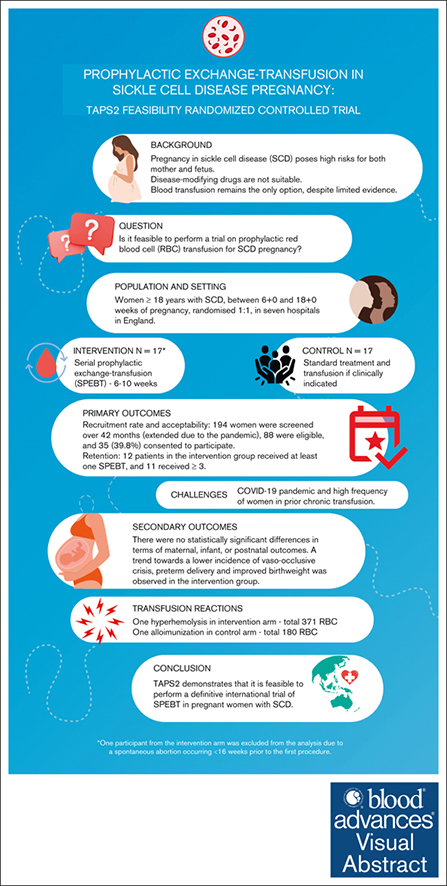
Visual Abstract
Serial prophylactic exchange blood transfusion (SPEBT) is increasingly used in sickle cell disease (SCD) pregnancy, despite a lack of robust evidence. The Transfusion Antenatally in Pregnant Women with Sickle Cell Disease (TAPS2) study assessed the feasibility and acceptability of conducting a definitive randomized controlled trial of SPEBT (intervention) vs standard care (control) in this population. Women aged ≥18 years with SCD, between 6+0 and 18+6 weeks of singleton gestation, were randomized 1:1 every 6 -10 weeks throughout pregnancy in 7 hospitals in England. The main outcomes were recruitment rate (primary outcome), acceptability, and retention. Secondary outcomes were safety and maternal/infant outcomes. In total, 194 women were screened over 42 months (extended because of the pandemic), 88 were eligible, and 35 (39.8%) consented to participate; 18 participants were randomized to intervention, and 17 to control. Follow-up data were collected on all participants. Twelve patients in the intervention group received at least 1 SPEBT, of these, 11 received ≥3. The remaining patient was withdrawn from SPEBT because of transfusion reaction. Sixteen control participants required at least 1 transfusion. There were no statistically significant differences in maternal, infant, and postnatal outcomes. A trend toward a lower incidence of vaso-occlusive crisis, preterm delivery, and improved birthweight was observed in the intervention. The study achieved satisfactory recruitment and retention, confirming its acceptability to participants. TAPS2 demonstrates that it is feasible to perform a definitive international trial of SPEBT in SCD pregnancy. These trials were registered at www.ClinicalTrials.gov as #NCT03975894 and International Standard Randomized Controlled Trial Number (www.isrctn.com; #ISRCTN52684446).
Introduction
Sickle cell disease (SCD) is the most common inherited disease worldwide, characterized by anemia; intermittent painful vaso-occlusive crisis (VOC); and chronic complications including chronic lung disease, sickle renal disease, stroke, and pulmonary hypertension.1
In high-income and some middle-income countries, most children born with SCD will survive to adulthood,2 and have a good expectation of having their own families. In the United Kingdom, ∼110 pregnancies occur annually in women living with SCD.3 Pregnancy in women living with SCD is high risk, with increased risk of perinatal and maternal morbidity and mortality.4-8 Pregnancy also exacerbates SCD-related complications such as anemia, painful crisis, pulmonary complications, and infection.3,5
Current disease–modifying treatments for patients with SCD include hydroxycarbamide, L-glutamine, crizanlizumab, and voxelotor; these are not licensed for use in pregnancy and are therefore not recommended.9 Standard care for pregnant women with SCD in the United Kingdom consists of blood transfusion if clinically indicated.4,10 Transfusion can be given as a simple top-up transfusion, which, while improving oxygen carriage, can potentially cause hyperviscosity and iron overload. The alternative approach is exchange blood transfusion (EBT), which simultaneously removes autologous red blood cells (RBCs) and replaces with healthy RBCs, resulting in a more effective reduction in the percentage of hemoglobin S (HbS%) and consequentially reduces the risk of acute SCD complications.11,12
Outside pregnancy, serial prophylactic EBT (SPEBT) has proven efficacy as a treatment for acute SCD complications, and for the prevention of pain, acute chest syndrome, and strokes in this patient group.12,13 However, there is inadequate evidence for the safety and benefit of SPEBT for SCD during pregnancy.14 One previous randomized control trial (RCT) compared SPEBT vs standard care (N = 72).15 The results identified decreased pain episodes in the intervention group (P < .01) but was underpowered to assess other clinical outcomes. There have been repeated calls for a definitive RCT to establish the effectiveness of SPEBT in pregnancy.14,16
The aim of this study was to assess the feasibility and acceptability of conducting a definitive RCT of SPEBT vs standard care on maternal and fetal outcomes in pregnant women living with SCD. The objectives were to: assess the willingness of pregnant women with SCD to take part; evaluate the acceptability of the intervention and trial conduct; assess adherence and retention; to record safety issues; and to measure clinical outcomes to inform the design of the definitive RCT.
Methods
Trial design
This was a 2-arm randomized controlled trial conducted in 7 hospital trusts in England, including an embedded qualitative study and economic evaluation (these will be published separately). The trial protocol has been published previously.17 Ethical approval was granted on 28 March 2019 from the London-Surrey Borders Research Ethics Committee (reference 18/LO/2070). The trial was registered on National Institutes of Health registry (www.ClinicalTrials.gov; #NCT03975894; registered on 5 June 2019) and International Standard Randomised Controlled Trial Number (www.isrctn.com; registration number ISRCTN52684446; retrospectively registered 2 August 2019).
Participants and recruitment
Eligible participants were pregnant women aged ≥18 years with SCD (sickle genotypes SS, SC, or S+Other) and a singleton pregnancy, recruited between 6+0 to 18+0 weeks’ gestation from either the SCD clinic or antenatal booking clinic at participating hospital trusts. The decision to exclusively include women screened before 18 weeks gestation was predicated on the understanding that anemia, vaso-occlusion, and vasculopathy could impact placental health.7 Thus, there exists a potential for early exchange transfusion to mitigate preeclampsia and fetal growth restriction in women with SCD.18 This is because early aberrant placentation frequently underlies the pathophysiological mechanism for these conditions.19 During the recruitment phase, the study was advertised via the Sickle Cell Society and other patient networks. Participants were excluded if they: were unable or unwilling to give written informed consent; were on long-term transfusion program before pregnancy for the amelioration of SCD; were unable to receive blood transfusion for social, clinical, or religious reasons; had current diagnosis of major medical or psychiatric comorbidity that, in the randomizing clinicians’ opinion, rendered them unable to enter the trial; had had prior hyperhemolysis; and/or had RBC phenotype or antibodies that may prevent likely provision of adequate RBC units to support the SPEBT program.
Potentially eligible participants attending routine antenatal clinics met with the research midwife/nurse at each site and were given the patient information sheet and the opportunity to ask questions in accordance with good clinical practice. Written informed consent was obtained by 1 of the trial staff. Participants were free to withdraw from the study at any time without giving a reason; those who withdrew were asked if they were still willing to provide follow-up data via clinical records.
Intervention and comparator
Participants allocated to the SPEBT arm received their first transfusion shortly after recruitment and ideally within 2 weeks of recruitment. SPEBT was performed every 6 to 10 weeks until the end of pregnancy, aiming to maintain HbS% of <30% or combined HbS and HbC of <30%. The number of units to be transfused in each exchange was calculated using the Optia apheresis machine software, which takes into account the patient's height, weight, preprocedure hematocrit, HbS%, as well as the hematocrit of the RBC units and the target HbS%, but was usually between 6 and 8 units. The RBC units were leukoreduced and phenotypically matched for Rh and K. The procedure was carried out by automated apheresis technology using Spectra Optia (Terumo Blood and Cell Technologies), either on the hematology day unit or the maternity unit in accordance with local provision. Peripheral venous access was used, but when not possible, access was via femoral line. Blood tests (full blood count, HbS%, blood group, and screen) were carried out 1 to 3 days before the procedure and again immediately after procedure to assess treatment impact.
Participants allocated to the control arm received routine National Health Service care based on the National Institute for Health and Care Excellence accredited Royal College of Obstetricians and Gynaecologists guidelines (before 2021) and latterly the British Journal of Haematology guidelines.9,20 This involved multidisciplinary care, and recommended medications including prophylactic penicillin, folic acid, and low-dose aspirin.9,20 Participants in the control arm received transfusion only if clinically indicated. Clinically indicated transfusions may be conducted either as top-up or exchange transfusion in cases of severe anemia, pain unresponsive to opioids, acute chest syndrome, stroke, and other indications deemed necessary by the medical team.
Feasibility outcomes
The primary outcome was recruitment rate, measured as the ratio of women eligible vs women randomized. Secondary feasibility outcomes included: the number of women screened who met the study eligibility criteria; reasons why eligible women declined participation; rate and reasons for attrition; and protocol adherence. Patient experiences and acceptability of the intervention and trial; and the views and experiences of clinical staff involved in the study were explored in the embedded qualitative study. We extracted the details of the safety outcomes from medical records (eg, transfusion reactions and alloimmunization). Although the focus of this study was feasibility, we also collected information on key clinical neonatal and maternal outcomes from medical records and via maternal self-report. Members of the research team in each trust were responsible for collecting and including the data on the secure Internet-based data management system contemporaneously, with the exception of pregnancy outcome follow-up, which was updated postnatally. Additional data were extracted from National Health Service medical records and entered directly onto the system by members of the research team, who were not blinded. Clinical outcomes included: antenatal hospital admissions, painful crisis, other SCD-related complications, number and reason for any transfusion (control arm), neonatal condition at birth (need for resuscitation, and Apgar scores), birthweight, gestation at birth, neonatal intensive care unit (NICU) admission, feeding method at discharge, and postnatal health and complications. Health-related quality-of-life data were collected using both the 3L and 5L EuroQol EQ-5D questionnaires and a specifically devised client service receipt inventory, which recorded expenses to each participant throughout the trial in relation to their care and condition. A priori criteria for progression to a definite trial were defined as adequate recruitment (reach and acceptability), frequency of SPEBT (dose and acceptability), and retention (acceptability).17
Study conduct and assessments
The baseline questionnaire completed at recruitment collected both previous and current medical and obstetric history, and sociodemographic information. Participants were followed up by trial practitioners every 4 to 6 weeks throughout pregnancy to assess and record maternal, sickle and fetal outcomes, service use, and quality-of-life data. Follow-up was timed to coincide with routine follow-up at the specialist sickle-obstetric clinic or high-risk obstetric clinic, when possible. After birth, information on health in late pregnancy, labor and birth, neonatal condition, admission to NICU/special care baby unit, and feeding type were extracted from clinical records. At ∼6 weeks postpartum, postnatal health and complications were recorded by the research team via routine appointment or telephone interview.
Randomization and blinding
Participants were allocated in a ratio of 1:1 to intervention and control arm; randomization was performed using a web-based randomization system (MedSciNet), minimizing on center, SCD genotype, and maternal age. Patients and their clinical teams were not blinded because of the nature of the intervention. Study staff analyzing the data were blinded to treatment allocation.
Sample size
The feasibility study was designed to establish the rates at which women with SCD can be recruited and retained in a future definitive RCT. It was estimated that a sample of 40 women (20 per arm) would allow us to estimate the overall recruitment rate to 10% of the true value, or better using the Clopper-Pearson exact binomial method for a 95% confidence interval (CI).
Statistical methods
The statistical analysis plan has been published previsouly.21 Although underpowered for clinical outcomes, results are presented as an initial assessment of the efficacy and safety of SPEBT. Descriptive statistics including 95% CIs were presented for all baseline data and clinical outcomes, with a focus on estimates of standard deviations and other quantities to inform the sample size calculation for the definitive trial. In addition to the analyses in our published analysis plan,21 we also evaluated a composite maternal end point, defined as ≥1 of the following: admission for SCD, sickle cell crisis (any report), acute chest syndrome, preeclampsia, venous thromboembolism/pulmonary embolism, and/or emergency cesarean delivery. This composite outcome was proposed for a future definitive trial after consultation with our Patient and Public Involvement and Engagement (PPIE) group. Analysis was based on the intention-to-treat principle using all available data. There were minimal missing data, and this was not inputted.
Statistical analysis was conducted using Stata Version 17 (StataCorp, College Station, TX).
Patient and public involvement
A PPIE representative was involved from the design stage onward. A representative of the largest UK charity representing people affected by a sickle cell disorder was involved in discussions and kept informed of study progress. A second PPIE member joined the team once the trial had opened, and contributed to designing recruitment materials and the study website to aid recruitment to the study (https://www.guysandstthomasbrc.nihr.ac.uk/microsites/taps2/). This PPIE member led the PPIE elements of the study, attending monthly core project team meetings, aiding the interpretation of results, and coauthored the present paper. Additionally, this PPIE member has also spoken about her role in the trial at local and national meetings and has published her experience as PPIE group member in this research.22
Results
Participant recruitment and retention
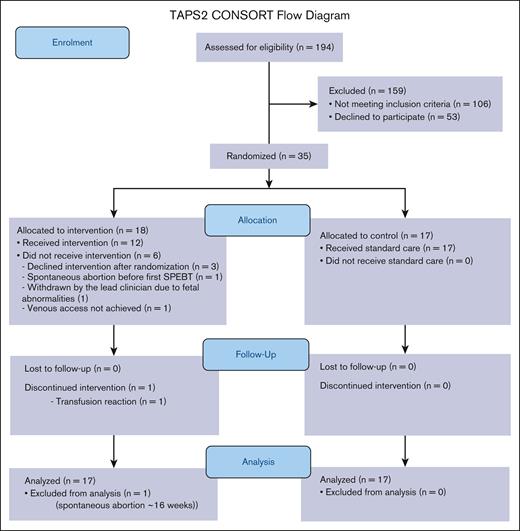
Between April 2019 and October 2022, 194 pregnant women living with SCD were screened for eligibility at participating sites (CONSORT flow diagram presented in Figure 1; site-specific information presented in supplemental Table 1). Of these, 88 (45.4%; 95% CI, 38-53) met the eligibility criteria. The most common reasons for ineligibility were ≥18 weeks at screening (n = 34) or already on an existing SPEBT (n = 30; supplemental Table 2). Of those eligible for the study, 35 were recruited: equivalent to a recruitment rate of 39.8% (95% CI, 29.5-50.8). The mean recruitment rate was 0.19 per center per month (standard deviation [SD], 0.09). Of the 53 eligible women not recruited, 22 (41.5%) cited an unwillingness to be randomized to the intervention arm as their main reason for refusal. Other reasons for refusal included not wanting to take part in research, or practical barriers (eg, time commitment or travel issues; supplemental Table 2).
TAPS2 CONSORT flow diagram. Intervention arm consists of serial prophylactic exchange transfusion; control arm consists of standard care.
TAPS2 CONSORT flow diagram. Intervention arm consists of serial prophylactic exchange transfusion; control arm consists of standard care.
In total, 35 women participated in the trial, 18 of whom were randomized to the intervention arm, and 17 randomized to the control arm. Follow-up data were collected on all 35 (100%) participants. One participant in the intervention group had a spontaneous abortion <16 weeks before first SPEBT and was withdrawn from the trial: clinical outcomes and protocol adherence are not reported for this participant.
Baseline characteristics
Baseline characteristics were broadly similar across both arms (Table 1). The mean age of participants was 31.8 years (SD, 5.61), and approximately half of participants were primiparous. Mean body mass index at recruitment was 26.9 (SD, 5.66), and mean gestational week was 14.2 (SD, 3.09). Most participants had HbSS disease (n = 21, 60%), 12 (34%) had HbSC disease, and the remainder (n = 2, 6%) had Sβ-thalassemia.
Baseline characteristics by trial arm at recruitment
| . | Intervention (n = 18) . | Control (n = 17) . | All participants (N = 35) . |
|---|---|---|---|
| Age, y (SD) | 32.67 (5.09) | 30.94 (6.14) | 31.83 (5.61) |
| Parity | |||
| Primiparous, n (%) | 10 (55.6%) | 7 (41.2%) | 17 (48.6%) |
| Previous miscarriage | 4 (22.2%) | 3 (17.3%) | 7 (20%) |
| Previous stillbirth | 0 | 1 (5.9%) | 1 (2.8%) |
| BMI, kg/m2 (SD) | 26.29 (4.88) | 27.59 (6.53) | 26.90 (5.66) |
| Gestation in weeks at recruitment (SD) | 14.44 (3.09) | 13.87 (3.15) | 14.16 (3.09) |
| Clinical disease | |||
| HbSS, n (%) | 11 (61.1%) | 10 (58.8%) | 21 (60.0%) |
| HbSC, n (%) | 6 (33.3%) | 6 (35.3%) | 12 (34.3%) |
| Sβ-thalassemia, n (%) | 1 (5.6%) | 1 (5.9%) | 2 (5.7%) |
| . | Intervention (n = 18) . | Control (n = 17) . | All participants (N = 35) . |
|---|---|---|---|
| Age, y (SD) | 32.67 (5.09) | 30.94 (6.14) | 31.83 (5.61) |
| Parity | |||
| Primiparous, n (%) | 10 (55.6%) | 7 (41.2%) | 17 (48.6%) |
| Previous miscarriage | 4 (22.2%) | 3 (17.3%) | 7 (20%) |
| Previous stillbirth | 0 | 1 (5.9%) | 1 (2.8%) |
| BMI, kg/m2 (SD) | 26.29 (4.88) | 27.59 (6.53) | 26.90 (5.66) |
| Gestation in weeks at recruitment (SD) | 14.44 (3.09) | 13.87 (3.15) | 14.16 (3.09) |
| Clinical disease | |||
| HbSS, n (%) | 11 (61.1%) | 10 (58.8%) | 21 (60.0%) |
| HbSC, n (%) | 6 (33.3%) | 6 (35.3%) | 12 (34.3%) |
| Sβ-thalassemia, n (%) | 1 (5.6%) | 1 (5.9%) | 2 (5.7%) |
Intervention arm consists of serial prophylactic exchange transfusion; control arm consists of standard care.
BMI, body mass index, HbSC, hemoglobin SC disease; HbSS, sickle cell hemoglobinopathy.
Protocol adherence
Of the 17 intervention participants, 12 received at least 1 SPEBT during pregnancy (70.6%; mean number of SPEBTs, 2.6 [SD, 2.02)]; range, 0-5). The other 5 participants did not receive any SPEBT for the following reasons: 3 withdrew from the SPEBT program after randomization, 1 couldn't achieve venous access, and the fifth participant was withdrawn by the lead clinician. This decision was because of the potential impact on the participant because the pregnancy was found to be affected by fetal abnormality before commencing the intervention. Of the 12 patients who received the first SBEPT (91.6 %), 11 received ≥3 SPEBTs (Table 2); 1 of the participants was withdrawn by the clinician because of a delayed hemolytic transfusion reaction (HTR) (detailed below). Three participants in the intervention arm received a clinically indicated transfusion during pregnancy. Neither was receiving SPEBT.
Protocol adherence and transfusion data
| . | Intervention (n = 17) . | Control (n = 17) . |
|---|---|---|
| At least 1 SPEBT during pregnancy, n (%) | 12∗ (70.6) | - |
| Number of SPEBT during pregnancy, mean (SD); range | 2.6 (2.02); 0-5 | - |
| ≥3 SPEBT during pregnancy†, n (%) | 11‡ (64.7) | - |
| ≥3 SPEBT in participants not withdrawn from the trial for medical reasons, n (%) | 11/14 (78.6) | - |
| Number of patients with at least 1 clinically indicated transfusion during pregnancy, n (%) | 6§ (35.3) | 16 (94.1) |
| Number of clinically indicated transfusion episodes during pregnancy, mean (SD); range | 0.35 (0.49); 0-1 | 2.1|| (1.6); 0-5 |
| ≥2 clinically indicated transfusion episodes during pregnancy, n (%) | 0 (0) | 8 (47.1) |
| Number of RBC units transfused during pregnancy, n | 371 | 180 |
| Number of RBC units transfused during pregnancy, mean (SD); range | 21.8 (15.7); 0-45 | 10.6 (12.7); 0-39 |
| . | Intervention (n = 17) . | Control (n = 17) . |
|---|---|---|
| At least 1 SPEBT during pregnancy, n (%) | 12∗ (70.6) | - |
| Number of SPEBT during pregnancy, mean (SD); range | 2.6 (2.02); 0-5 | - |
| ≥3 SPEBT during pregnancy†, n (%) | 11‡ (64.7) | - |
| ≥3 SPEBT in participants not withdrawn from the trial for medical reasons, n (%) | 11/14 (78.6) | - |
| Number of patients with at least 1 clinically indicated transfusion during pregnancy, n (%) | 6§ (35.3) | 16 (94.1) |
| Number of clinically indicated transfusion episodes during pregnancy, mean (SD); range | 0.35 (0.49); 0-1 | 2.1|| (1.6); 0-5 |
| ≥2 clinically indicated transfusion episodes during pregnancy, n (%) | 0 (0) | 8 (47.1) |
| Number of RBC units transfused during pregnancy, n | 371 | 180 |
| Number of RBC units transfused during pregnancy, mean (SD); range | 21.8 (15.7); 0-45 | 10.6 (12.7); 0-39 |
Intervention arm consists of serial prophylactic exchange transfusion; control arm consists of standard care.
One participant from the intervention arm was excluded from the analysis because of a spontaneous abortion occurring at <16 weeks before the first procedure.
Five women allocated to the intervention arm did not have any SPEBT for the following reasons: complex pregnancy with fetal abnormalities (n = 1), withdrew from SPEBT program after randomization but continued in study (n = 3), venous access not achieved (n = 1).
Three or more SPEBT during pregnancy was identified as an indicator of “dose/acceptability” for the feasibility study.
One woman allocated to the intervention arm had only 1 SPEBT because of delayed hemolytic reaction after first procedure.
Three intervention arm participants who withdrew from SPEBT program received 1 clinically indicated transfusion later in pregnancy.
The indications for transfusion in control arm were recorded in 11 patients and included sickle cell crisis (54.5%), severe anemia (27.3%), previous severe pregnancy complications with intensive care admission and kidney failure (9.1%), and acute chest syndrome (9.1%).
HbS% was evaluated in 42 of 43 SPEBTs performed, and 88% (37/42) achieved a postexchange transfusion HbS% of ≤30% (mean, 21%; SD, 6.3%; range, 13%-35%). The mean preexchange HbS% was 45% (SD, 13.4%; range, 17%-71%).
Clinical outcomes
There were no significant differences between treatment groups in terms of maternal, infant, or postnatal outcomes (Tables 3-5) which may be attributed to the small number of participants in this feasibility study. Most control group participants (n = 16, 94.1%) received at least 1 clinically indicated transfusion during pregnancy, with a mean of 2.1 transfusion episodes (SD, 1.6; range 0-5). Eight (47.1%) control participants had transfusions indicated on ≥2 occasions. (Table 2). Transfusion indications in the control arm were documented for 11 of 16 patients who received transfusions.
Maternal pregnancy and birth outcomes
| . | Intervention (n = 17) . | Control (n = 17) . | Comparison . | |
|---|---|---|---|---|
| RR (95% CI) . | Difference (95% CI) . | |||
| Antenatal admission for any reason, n (%) | 7 (41.2%) | 10 (58.8%) | 0.70 (0.35-1.40) | |
| Inpatient nights for any reason∗, mean (SD) | 4.71 (4.50) | 7.30 (8.93) | −2.59 (−9.64 to 4.47) | |
| Antenatal admission for SCD†, n (%) | 6 (35.3%) | 10 (58.8%) | 0.60 (0.28-1.28) | |
| Inpatient nights for SCD∗, mean (SD) | 3.33 (2.50) | 7.10 (9.05) | −3.77 (−10.35 to 2.81) | |
| Sickle cell crisis during pregnancy∗, n (%) | 8 (47.1%) | 13 (76.5%) | 0.62 (0.35-1.09) | |
| Severe crisis during pregnancy∗,‡, n (%) | 6 (35.3%) | 8 (47.1%) | 0.75 (0.33-1.70) | |
| Acute chest syndrome∗, n (%) | 0 (0.0%) | 1 (5.9%) | - | |
| Preeclampsia∗, n (%) | 0 (0.0%) | 2 (11.8%) | - | |
| Venous/pulmonary embolism in pregnancy∗, n (%) | 1 (5.6%) | 0 (0.0%) | - | |
| Onset of labor | ||||
| Spontaneous, n (%) | 0 (0.0%) | 2 (11.8%) | - | |
| Induced, n (%) | 8 (47.1%) | 10 (58.8%) | - | |
| Cesarean delivery, n (%) | 9 (52.9%) | 5 (29.4%) | - | |
| Emergency cesarean∗, n (%) | 3 (17.6%) | 1 (5.9%) | 3.00 (0.35-26.04) | |
| Elective cesarean, n (%) | 10 (58.8%) | 9 (52.9%) | 1.11 (0.61-2.02) | |
| Postpartum transfusion, n (%) | 2 (11.8%) | 6 (37.5%) | 0.31 (0.07-1.33) | |
| Proposed composite end point for TAPS3§, n (%) | 10 (58.8%) | 15 (88.2%) | 0.67 (0.43-1.03) | |
| . | Intervention (n = 17) . | Control (n = 17) . | Comparison . | |
|---|---|---|---|---|
| RR (95% CI) . | Difference (95% CI) . | |||
| Antenatal admission for any reason, n (%) | 7 (41.2%) | 10 (58.8%) | 0.70 (0.35-1.40) | |
| Inpatient nights for any reason∗, mean (SD) | 4.71 (4.50) | 7.30 (8.93) | −2.59 (−9.64 to 4.47) | |
| Antenatal admission for SCD†, n (%) | 6 (35.3%) | 10 (58.8%) | 0.60 (0.28-1.28) | |
| Inpatient nights for SCD∗, mean (SD) | 3.33 (2.50) | 7.10 (9.05) | −3.77 (−10.35 to 2.81) | |
| Sickle cell crisis during pregnancy∗, n (%) | 8 (47.1%) | 13 (76.5%) | 0.62 (0.35-1.09) | |
| Severe crisis during pregnancy∗,‡, n (%) | 6 (35.3%) | 8 (47.1%) | 0.75 (0.33-1.70) | |
| Acute chest syndrome∗, n (%) | 0 (0.0%) | 1 (5.9%) | - | |
| Preeclampsia∗, n (%) | 0 (0.0%) | 2 (11.8%) | - | |
| Venous/pulmonary embolism in pregnancy∗, n (%) | 1 (5.6%) | 0 (0.0%) | - | |
| Onset of labor | ||||
| Spontaneous, n (%) | 0 (0.0%) | 2 (11.8%) | - | |
| Induced, n (%) | 8 (47.1%) | 10 (58.8%) | - | |
| Cesarean delivery, n (%) | 9 (52.9%) | 5 (29.4%) | - | |
| Emergency cesarean∗, n (%) | 3 (17.6%) | 1 (5.9%) | 3.00 (0.35-26.04) | |
| Elective cesarean, n (%) | 10 (58.8%) | 9 (52.9%) | 1.11 (0.61-2.02) | |
| Postpartum transfusion, n (%) | 2 (11.8%) | 6 (37.5%) | 0.31 (0.07-1.33) | |
| Proposed composite end point for TAPS3§, n (%) | 10 (58.8%) | 15 (88.2%) | 0.67 (0.43-1.03) | |
Intervention arm consists of serial prophylactic exchange transfusion; control arm consists of standard care. One participant from the intervention arm was excluded from the analysis because of a spontaneous abortion occurring <16 weeks before the first procedure.
Outcome contributes to proposed composite end point for TAPS3.
Among those with at least 1 admission.
Defined as sickle cell crisis requiring inpatient admission.
Proposed composite end point for future definitive study, ≥1 of: admission for SCD, sickle cell crisis (any report), acute chest syndrome, preeclampsia, venous/pulmonary embolism, and emergency cesarean delivery.
Infant outcomes
| . | Intervention (n = 17) . | Control (n = 17) . | Comparison . | |
|---|---|---|---|---|
| RR (95% CI) . | Difference (95% CI) . | |||
| Stillbirth | 0 (0.0%) | 0 (0.0%) | - | - |
| Birthweight, g | 3039 (476) | 2833 (446) | 206 (−116 to 528) | |
| Premature delivery, <37 wk n (%) | 1 (5.9%) | 5 (29.4%) | 0.20 (0.03-1.54) | |
| SGA (<10th centile), n (%) | 3 (17.6%) | 3 (17.6%) | 1.00 (0.23-4.27) | |
| Apgar <7 at 5 min∗, n (%) | 1 (5.9%) | 0 (0.0%) | - | |
| NICU admission, n (%) | 2 (11.8%) | 1 (5.9%) | 2.00 (0.20-20.04) | |
| Breastfeeding at discharge, n (%) | 9 (52.9%) | 9 (52.9%) | 1.00 (0.53-1.88) | |
| . | Intervention (n = 17) . | Control (n = 17) . | Comparison . | |
|---|---|---|---|---|
| RR (95% CI) . | Difference (95% CI) . | |||
| Stillbirth | 0 (0.0%) | 0 (0.0%) | - | - |
| Birthweight, g | 3039 (476) | 2833 (446) | 206 (−116 to 528) | |
| Premature delivery, <37 wk n (%) | 1 (5.9%) | 5 (29.4%) | 0.20 (0.03-1.54) | |
| SGA (<10th centile), n (%) | 3 (17.6%) | 3 (17.6%) | 1.00 (0.23-4.27) | |
| Apgar <7 at 5 min∗, n (%) | 1 (5.9%) | 0 (0.0%) | - | |
| NICU admission, n (%) | 2 (11.8%) | 1 (5.9%) | 2.00 (0.20-20.04) | |
| Breastfeeding at discharge, n (%) | 9 (52.9%) | 9 (52.9%) | 1.00 (0.53-1.88) | |
Intervention arm consists of serial prophylactic exchange transfusion; control arm consists of standard care. One participant from the intervention arm was excluded from the analysis because of a spontaneous abortion occurring <16 weeks before the first procedure.
SGA, small for gestational age.
Data available for n = 17 participants in the intervention arm and n = 16 participants in the standard-care arm.
Postpartum outcomes
| . | Intervention (n = 17) . | Control (n = 17) . | Comparison . |
|---|---|---|---|
| RR (95% CI) . | |||
| Pain, n (%) | 4 (23.5%) | 7 (41.2%) | 0.57 (0.20-1.60) |
| Sickle cell crisis, n (%) | 1 (5.9%) | 3 (17.6%) | 0.33 (0.04-2.89) |
| Chest infection, n (%) | 0 (0.0%) | 0 (0.0%) | |
| Venous/pulmonary embolism, n (%) | 0 (0.0%) | 0 (0.0%) | |
| Inpatient admission related to SCD (nights), n (%) | |||
| 0 | 14 (82.4%) | 16 (94.1%) | |
| 1-2 | 2 (11.8%) | 0 (0.0%) | 3.09 (0.29-33.2)∗ |
| ≥3 | 1 (5.9%) | 1 (5.9%) | |
| Inpatient admission not related to SCD (nights), n (%) | |||
| 0 | 17 (100.0%) | 16 (94.1%) | - |
| 1 | 0 (0.0%) | 1 (5.9%) | |
| Number of transfusions in the postnatal period†, n (%) | |||
| 0 | 14 (82.4%) | 14 (87.5%) | |
| 1 | 3 (17.6%) | 2 (12.5%) | 1.61 (0.23-11.09)∗ |
| Day unit attendance in the postnatal period†, n (%) | |||
| 0 | 16 (94.1%) | 16 (94.1%) | |
| 1 | 1 (5.9%) | 1 (5.9%) | 1.00 (0.06-17.4)∗ |
| . | Intervention (n = 17) . | Control (n = 17) . | Comparison . |
|---|---|---|---|
| RR (95% CI) . | |||
| Pain, n (%) | 4 (23.5%) | 7 (41.2%) | 0.57 (0.20-1.60) |
| Sickle cell crisis, n (%) | 1 (5.9%) | 3 (17.6%) | 0.33 (0.04-2.89) |
| Chest infection, n (%) | 0 (0.0%) | 0 (0.0%) | |
| Venous/pulmonary embolism, n (%) | 0 (0.0%) | 0 (0.0%) | |
| Inpatient admission related to SCD (nights), n (%) | |||
| 0 | 14 (82.4%) | 16 (94.1%) | |
| 1-2 | 2 (11.8%) | 0 (0.0%) | 3.09 (0.29-33.2)∗ |
| ≥3 | 1 (5.9%) | 1 (5.9%) | |
| Inpatient admission not related to SCD (nights), n (%) | |||
| 0 | 17 (100.0%) | 16 (94.1%) | - |
| 1 | 0 (0.0%) | 1 (5.9%) | |
| Number of transfusions in the postnatal period†, n (%) | |||
| 0 | 14 (82.4%) | 14 (87.5%) | |
| 1 | 3 (17.6%) | 2 (12.5%) | 1.61 (0.23-11.09)∗ |
| Day unit attendance in the postnatal period†, n (%) | |||
| 0 | 16 (94.1%) | 16 (94.1%) | |
| 1 | 1 (5.9%) | 1 (5.9%) | 1.00 (0.06-17.4)∗ |
Intervention arm consists of SPEBT; control arm consists of standard care. One participant from the intervention arm was excluded from the analysis because of a spontaneous abortion occurring <16 weeks before the first procedure.
Odds ratios derived by ordered logistic regression.
Data available for n = 17 participants in the intervention arm and n = 16 participants in the standard-care arm.
Maternal
We noted a trend toward a lower frequency of VOC in pregnancy in the intervention group compared with the control arm (any crisis [47.1% vs 76.5%; risk ratio (RR), 0.62; 95% CI, 0.35-1.09]; severe crisis requiring hospitalization [35.3% vs 47.1%; RR, 0.75; 95% CI, 0.33-1.70]), and a lower risk of SCD-related antenatal admissions (35.3% vs 58.8%; RR, 0.60; 95% CI, 0.28-1.28; Table 3). There was a trend toward women in the intervention group being at lower risk of needing a transfusion in the immediate postpartum period compared with the control arm (11.8% vs 37.5%; RR 0.31; 95% CI, 0.07-1.33; Table 3). We noted some weak evidence of a lower risk of maternal adverse outcome using our PPIE informed composite end point (58.8% vs 88.2%; RR, 0.67; 95% CI, 0.43-1.03; Table 3). There was 1 case of venous/pulmonary embolism during pregnancy (intervention group), 2 cases of preeclampsia (both in the control arm), and 1 case of acute chest syndrome during pregnancy (control arm). All 34 participants for whom outcomes are reported delivered live-born infants (Table 4).
Infant
The mean birthweight in the intervention group was slightly higher (3039 vs 2833 g; mean difference, 206 g; 95% CI, −116 to 528). There were fewer preterm deliveries in the intervention group (5.9% vs 29.4%; RR, 0.20; 95% CI, 0.03-1.54). A similar proportion of infants (17.6%) across both groups were small for gestational age. One infant had an Apgar score of <7 at 5 minutes (intervention group), and 3 infants required NICU admission (2 in the intervention group, 1 in the control arm; Table 4). There were no cases of stillbirths or neonatal deaths.
Postnatal
At the 6-week postnatal follow-up, there was a trend for fewer women in the intervention group to report pain events and crises (23.5% vs 41.2%; RR for pain, 0.57 [95% CI, 0.20-1.60]; 5.9% vs 17.6%, RR for crisis, 0.33 [95% CI, 0.04-2.89]). There were no cases of venous/pulmonary embolism or chest infection during the postnatal period. Two women (1 intervention and 1 control) reported day unit attendance during the postnatal period; 4 women (3 intervention and1 control) required inpatient admission for SCD-related reasons; and 1 further woman (control arm) required admission for other reasons. Three women in the intervention group and 2 in the standard-care group required at least 1 transfusion in the postnatal period.
Safety outcomes
Two transfusion reactions were recorded (supplemental Table 3). One intervention participant had a delayed HTR. The patient, with no history of prior transfusions, had an anti-Leb antibody documented in November 2020, before allocation. Ten days after the first session of SPEBT, during which 8 Rh, K, and Leb-matched RBCs were transfused, she presented with the HTR and was subsequently admitted to the medical ward. She received IV immunoglobulin (1 g/kg) and methylprednisolone 500 mg for 2 days. Notably, intensive care unit admission was not required during the treatment course. The HTR was attributed to anti-Jkb. One control group participant developed RBC alloimmunization (anti-Lea) after a clinically indicated transfusion in the first trimester. Although this participant subsequently required 3 additional clinically indicated EBTs before delivery, these occurred without further complications. Alloimmunization, with or without HTR, was observed in 0.55 instances per 100 RBC units transfused in the control arm, and 0.27 instances per 100 RBC units transfused in the intervention arm. There were no additional adverse events associated with transfusions in either the intervention or control arms.
Discussion
We report the first RCT of prophylactic automated EBTs in pregnant women living with SCD. Despite considerable COVID-19 pandemic–related recruitment challenges, we achieved a 39% recruitment rate in this shielding population. Protocol adherence was good, with 70.6% of participants in the intervention group receiving at least 1 SPEBT (12/17), and 64.7% receiving ≥3 overall (11/17). Omitting the 3 participants who could not proceed to SPEBT because of medical issues or lack of venous access, resulted in 78.6% compliance of remaining participants (11/14) in the intervention arm, receiving ≥3 SPEBT in pregnancy. Table 2 illustrates compliance and transfusion data.
More than 1 half of women screened for the trial were ineligible. The most common reason for ineligibility was that women were at ≥18 weeks of gestation at assessment, and after qualitative interviews with trial staff it became clear that this was partly a result of COVID-19–related disruption, because most trusts suspended their services temporarily and subsequently decreased the number of patients per clinic to prevent overcrowding and contamination. The second most common reason for ineligibility was that women were on a preexisting SPEBT program. The increasing use of SPEBT, as both a new treatment in pregnancy and continuation of a preexisting treatment regime for SCD, highlights the urgent need for robust evidence for its effectiveness in this population.7,14
Before embarking on this feasibility trial, we established progression criteria against which to assess support for a definitive trial, called TAPS3. We had anticipated challenges to recruiting participants, but the COVID-19 pandemic resulted in additional recruitment barriers. Our study population were “shielding” for much of the recruitment period, so, the possibility of attending a hospital for extra appointments was likely a further disincentive to participation. We also had to deal with complete recruitment site closures for several months, reduced capacity within the hematology and maternity services to perform SPEBT, and research staff deployment for extended periods. Notwithstanding, the recruitment rate was deemed “moderately adequate.” According to the previously published study protocol,17 TAPS3 would have been deemed not feasible if the recruitment rate fell below 30%, or if adherence or retention fell below 50%. In response to the recruitment rate falling into the yellow zone, where changes must be considered according to the protocol, a comprehensive discussion involving the research team, PPI group, and key stakeholders concluded that expanding the TAPS3 trial internationally would expedite patient inclusion within a shorter timeframe. This strategic decision aims to ensure the trial’s timely completion and maximize its potential impact. Because these unique circumstances are unlikely to arise in the future, we do not intend to amend the design of TAPS3. However, trial recruitment is always uncertain, and we will extend the recruitment should it be necessary.
Even with only selective transfusion, 34% to 61% of women with SCD still receive transfusion during pregnancy.10,23,24 In this trial, at least 1 clinically indicated transfusion was administered to most of the participants (94.1%) in the control arm. Despite the clear benefits of transfusion for many SCD complications, there remains a significant risk of transfusion reaction, primarily RBC alloimmunization and HTR.11,14,25-28 In our study, 1 HTR was observed in the intervention group and 1 instance of alloimmunization was reported in the nonintervention group after an emergency transfusion.
Alloimmunization and HTR are associated with blood transfusions in the wider SCD population, with alloimmunization rates as high as 65% with ABO and D matching alone29 and 11% with Rh- and K-matched units,30 so they are not specific to this trial. Although automated exchange demands a larger volume of RBCs, resulting in increased donor exposure, if Rh- and K-matched units are selected, erythrocytapheresis does not seem to elevate the risk of alloimmunization.31-33 Furthermore, despite the lower number of RBC units transfused in the control group, antibody formation frequency was similar to the intervention arm. This may be attributed to the heightened risk of alloimmunization when transfusions are administered in an acute-setting scenario.34 Even patients already immunized, while chronically transfused and in a steady state, have a reduced risk of developing new antibodies compared with patients undergoing occasional transfusions for acute conditions.25,34 In this context, the trial was deemed safe.
For a definitive trial, additional strategies can be used to mitigate alloimmunization risk, such as using extended phenotyped or genotype–matched RBC units. These can be particularly beneficial for patients with a history of few transfusions and/or previous alloimmunization, because they are considered at high risk for antibodies formation.14,35,36 Despite the fact that some authors advocate that, because of the limited availability of phenotypically/genotypically matched RBC, units matched for Fy, Jk, and Ss antigens should be reserved exclusively for transfusing immunized patients in an acute condition,37 this can be a strategy for patients with combined risk factors. This includes those with few transfusions and previous alloimmunization, such as the patient in the intervention arm who presented HTR.
This study aimed to inform a key evidence gap in current guidelines on the management of SCD in pregnancy.20,26 Pregnant women living with SCD experience the dual burden of living with a condition that has been chronically under prioritized,38 while also experiencing inequalities in maternity care because of being predominantly from a black background.39 Given that current disease–modifying treatments for SCD are contraindicated in pregnancy, there is an urgent need to identify strategies that can improve maternal and infant outcomes. Despite recruitment during the pandemic, we were still able to complete the study and demonstrate feasibility. Further strengths of this study were randomized design, the multicenter setting, and the accompanying qualitative substudy, for which analysis is still ongoing. A prepublished data analysis plan was an additional strength.
The lack of blinding, inherent in trials evaluating transfusion, was a limitation of this study. The fact that the study was underpowered to evaluate clinical outcomes could be considered a limitation, however, the primary aim of this study was to assess feasibility. Although this study was not powered to detect differences in clinical outcomes, our results show a signal of efficacy in important maternal clinical outcomes including VOC and frequency of hospital admissions in those receiving intervention. There was a trend toward improved birth weight and reduced frequency of preterm delivery. Although it is crucial not to overinterpret these findings, they align with existing evidence from the previous RCT15 (reduction of VOC) and a meta-analysis.19 The latter suggested that prophylactic transfusion is linked to a decrease in maternal and perinatal mortality, VOC, pulmonary complications, and preterm birth. One important limitation of this trial, which will be addressed before commencing a larger trial, was the challenge of maintaining HbS% of <30%. This HbS% threshold was defined by the research team. However, like many aspects of this trial, it was not solely based on existing literature because of the limited number of studies involving pregnant women. For TAPS3, we aim to outline a more practical approach, targeting HbS of <30% immediately after procedure, and HbS of <50% before exchange. Also, the limitation regarding transfusion indications in the control arm will be addressed in TAPS3 by implementing a customized database with mandatory fields to ensure comprehensive data collection.
Despite the challenges encountered, mainly due to the COVID-19 pandemic and the high frequency of women in previous SPEBT, TAPS2 has shown that it is feasible to perform a RCT of SPEBT in pregnant women living with SCD. Moreover, we advocate for a multinational trial to swiftly enlist a substantial number of participants, enabling us to definitively assess the benefits of SPEBT in mitigating the well-established heightened risks of adverse maternal and infant outcomes in this population.
Acknowledgments
The authors are grateful to the following members, past and present, of the TAPS2 (Transfusion Antenatally in Pregnant Women with Sickle Cell Disease) consortium: Paul Telfer (Barts Health National Health Service (NHS) Trust, London, United Kingdom); Miriam Kabia (Dora Foundation, London, United Kingdom and Patient and Public Involvement and Engagement [PPIE]); Annette Briley (Flinders University, Adelaide, Australia); Mamta Sohal (Imperial College Healthcare NHS Trust, London, United Kingdom); Hayley Martin (King’s College Hospital NHS Foundation Trust, London, United Kingdom); Susan Robinson, and Claire Singh (Kings College London, London, United Kingdom); Pat Doyle (London School of Hygiene & Tropical Medicine, London, United Kingdom); John James (Sickle Cell Society, London, United Kingdom); Asma Khalil, Ingrid Watt-Coote, and Joyce Adu-Amankwah (St George’s University Hospitals NHS Foundation Trust, London, United Kingdom); Kate Ryan (Manchester University NHS Foundation Trust, Manchester, United Kingdom); Natasha N. Archer and Amy Webster (University Hospitals of Leicester NHS Trust, Leicester, United Kingdom); Maria Chorozhoglou and Claire McDermott (University of Southampton, Southampton, United Kingdom); and Emma Drasar and Ryan Mullally (Whittington Health NHS Trust, London, United Kingdom). The authors thank both PPIE group who advised on all aspects of TAPS2 and proposed direction for future related research; and to all of the staff at our sites who worked on, and recruited to, the TAPS2 trial. The authors also thank the members of the TAPS2 trial steering committee: Isabel Reading (chair; University of Southampton), Amma Kyei-Mensah (Whittington Health NHS Trust), Lorna Williamson (Representative of National Blood Transfusion), Jo Howard (Vertex Pharmaceuticals/Guy’s and St Thomas’ NHS Foundation Trust), and Oseme Etomi (Guy’s and St Thomas’ NHS Foundation Trust).
This study presents independent research funded by the National Institute for Health and Care Research (NIHR), Research for Patient Benefit Programme (grant reference number, PB-PG-0317-20024).
The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. The funder and trial sponsor (Guy’s and St. Thomas’ NHS Foundation Trust) had no role in the design of the study, in the writing of the protocol, or the decision to submit for publication.
Authorship
Contribution: E.O.-N. is the chief/principal investigator, led the study design and funding application, and is the guarantor; E.O.-N., L.L.O., and P.T.S. participated in the study design and writing the submission to the funding body; E.O.-N., L.L.O., P.T.S., S.B., V.R., L.M., and H.T. were involved in compiling the study protocol and drafting the application to the ethics committee; M.A., J.S., and J. Johns advised on design, implementation, and conduct of the trial; P.T.S. was the trial statistician; S.B. led the qualitative component; L.L.O. was the qualitative researcher and drafted the initial manuscript; J. Joseph provided Patient and Public Involvement and Engagement input during the study design and trial delivery phases; V.R., L.M., and H.T. had responsibility for the day-to-day coordination of the trial; P.S. conducted the analysis with support from E.O.-N., L.L.O., J.S., and V.R.; D.M.B. analyzed transfusion data and helped draft the final manuscript; and all authors interpreted the study results, and read, reviewed, and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eugene Oteng-Ntim, Department of Women and Children's Health, School of Life Course Sciences, Faculty of Life Sciences and Medicine, King's College London 10th Floor, North Wing St Thomas' Hospital SE1 7EH London, UK; email: Eugene.Oteng-Ntim@gstt.nhs.uk.
References
Author notes
The deidentified data that support the findings of this study are available upon reasonable request from the corresponding author, Eugene Oteng-Ntim (Eugene.Oteng-Ntim@gstt.nhs.uk).
The full-text version of this article contains a data supplement.