Key Points
Dysfunctions in processing speed, and short term and working memory are frequent in adults with SCD and suspected neurological morbidity.
Cognitive dysfunction in adult with SCD are partly associated with brain damage.
Visual Abstract
The prognosis of sickle cell disease (SCD) in adults is determined primarily by damage to targeted organs such as the brain. Cognitive dysfunction in SCD is a common chronic neurological manifestation, but studies remain mostly descriptive in adults. The objective of this study was to better characterize the cognitive profile and the association between cognitive dysfunction and brain lesions. We included adult patients with SCD referred for a neurological assessment. An adapted battery of neuropsychological tests was used to assess cognitive deficits. Brain or arterial abnormalities were assessed using brain magnetic resonance imaging/magnetic resonance angiography and a cervical and transcranial Doppler ultrasound. The cognitive profile of 96 patients was characterized by deficits in processing speed (58%), short-term memory (34%), and working memory (24%). Brain infarcts were found in 56% of patients and intracranial vasculopathy in 49%. Twenty percent of patients had no brain abnormalities. Processing speed dysfunction was associated with territorial infarcts (odds ratio [OR], 3.1; P = .03) and education outside of France (OR, 4.7; P = .02). Short-term memory dysfunction was associated with territorial infarcts (OR, 3.4; P = .01) and a low educational level (OR, 8.2; P = .01). Working memory dysfunction was associated with a low educational level (OR, 4.3; P = .05) and vasculopathy (OR, 3.7; P = .03). Cognitive dysfunction appears to be a hallmark sign of SCD, particularly for adults with sickle cell-related stroke or suspected neurological morbidity. Assessment of such dysfunction could be used in longitudinal follow-up and clinical trials.
Introduction
Sickle cell disease (SCD) is the most common genetic disease in the world and is particularly prevalent in Sub-Saharan Africa, the Mediterranean region, the Middle East, and India,1 and affects >100 000 Americans in the United States.2 Improvements in the management of acute complications of the disease has led to an increase in life expectancy and has unveiled more chronic complications of target organ damage affecting quality of life and autonomy.3 Strokes, both ischemic and hemorrhagic, most frequently related to an intracranial vasculopathy affecting the large vessels of the circle of Willis,4 are a feared complication in patients with SCD. Strokes are common, reported to occur in ∼11% of children with SCD5 although they are less frequent in high-income countries since the advent of transcranial Doppler screening.6 Although less noticeable than strokes, cognitive dysfunction appears to be frequent.7 In children with SCD, studies have shown that the acquisition of cognitive skills is delayed or impaired.8 Working memory, attentional capacities, and executive functioning seem to be particularly affected.9-11 Brain lesions are reported frequently in patients with SCD. White matter lesions, often called silent cerebral infarcts, are the most frequent brain abnormalities, affecting from 22%12 to 39% of children by age 18 years13 and roughly 53% of young adults by age 30 years.14 These lesions are of particular interest because they are also potentially preventable with blood exchange transfusions.15 Although it is still debated,16,17 these brain lesions seem to be associated with cognitive dysfunction in children.8,18 Although recent recommendations have suggested a need for systematic screening for cognitive dysfunction and cognitive evaluation if necessary,19 there are still few data regarding cognitive function in adult patients with SCD. Some studies conducted in both children and adults, mostly using general intelligence evaluation batteries, have suggested that executive functioning could be particularly affected.17,20,21 One issue complicating the neuropsychological evaluation of SCD adult patients is the diversity of patient profiles and their educational or cultural background. In France, most patients with SCD are African migrants or descendants of migrants from Sub-Saharan French-speaking African countries,22 but most neuropsychological tools use verbal material requiring a good level of literacy in French, possibly resulting in some biases. Moreover, educational and cultural background may influence neuropsychological performance.23 Methodological precautions are therefore needed when choosing cognitive tests.
Consequently, the prevalence and phenotype of cognitive dysfunction in adult patients with SCD has been largely unknown to date. We hypothesized that dysfunction in processing speed and executive function would be particularly affected in adults with SCD. The main objective of our study was thus to better characterize the cognitive profile of adult patients with sickle cell–related stroke or suspected neurological morbidity using a rapid battery of tests considered to be less influenced by the patients' cultural and educational background than other batteries. Another objective was to assess the extent to which cognitive dysfunction was associated with lesions on brain magnetic resonance imaging (MRI). Finally, the last objective of the study was to assess the feasibility of a neuropsychological evaluation in everyday practice.
Methods
This article was prepared following the guidelines from Strengthening the Reporting of Observational studies in Epidemiology.24
Population
We included adult patients with SCD at high risk of cerebrovascular complications from the PCDREP (Perfusion Cérébrale DREPanocytose) cohort as well as those consecutively referred to our institution, the “Paris Centre” SCD Referral Center, for a neurological evaluation between January 2020 and September 2021. The inclusion criteria were as follows: age >18 years; a previous history of neurological event such as stroke, transient ischemic attack, chronic headaches, or seizure; and/or a definite or probable intracranial vasculopathy, based on previous cervical and transcranial echo color Doppler ultrasound or magnetic resonance angiography (MRA). Exclusion criteria was an MRI contraindication. These patients were followed-up in the adult referral center of Henri-Mondor Hospital (Créteil, France). The “Paris Centre” SCD Referral Center cohort includes patients from the Necker Hospital and Georges Pompidou European Hospital referred for a neurological assessment as part of their clinical follow-up.
All patients had a multidisciplinary evaluation at GHU Paris Psychiatrie et Neurosciences (Sainte-Anne Hospital, Paris, France), which included a neurological, neuropsychological, and radiological evaluation using cervical and transcranial echo color Doppler ultrasound and multimodal brain MRI.
Clinical data
The following data were systematically collected in a standardized case report form: sex, date of birth, SCD genotype, vascular risk factors (treated hypertension, diabetes mellitus, treated dyslipidemia, and active smoking), history of epilepsy, headache, or stroke (defined as a focal neurological dysfunction lasting >24 hours), baseline treatments (hydroxyurea, exchange and blood transfusions, and antithrombotic therapy), and previously known brain (infarcts or hemorrhage) or arterial abnormalities. We collected information on educational level (EL) as follows: grade 1, when education ended before the ninth grade; grade 2, when ended between ninth and eleventh grade included; and grade 3, when patients continued education after high school. We also recorded the country of education (CE), as follows: France since preschool (CE 1), arrived in France before high school (CE 2), or full education in a country other than France (CE 3). They were then dichotomized into 2 groups: those who had most of their education outside of France (CE 2 and 3) and others (CE1).
Neuropsychological assessment
The assessment included cognitive tests specifically chosen to be appropriate in multicultural populations, and mood scales. The neuropsychological assessment was designed to be completed in ∼30 minutes. Cognitive complaint was assessed as follows: “Do you have cognitive difficulties, meaning difficulties with memory, language, or concentration, for example?” Response was binary (yes, present; or no, absent).
Orientation and memory
Temporospatial orientation was assessed using the corresponding subscale of the Montreal Cognitive Assessment25 global cognitive screening test (score out of 6). The score was interpreted as normal (6), weak (5), or pathological (<5). Episodic memory was assessed using the 9-images test26 (test des neuf images–93 or TNI-93). During the learning phase, 9 images were shown on the same sheet (eg, a duck, a carrot, etc). Patients completed a naming task attributing the semantic category for each image (eg, animal, vegetable, etc). A cued recall was then proposed by giving the semantic category as a cue. After a short interference task (20 seconds), free recall was requested, followed by a cued recall for the forgotten items. The free recall score (out of 9) was specifically collected here as a proxy for episodic memory retrieval in consideration of the predominant executive profile in SCD. Scores were classified as normal or pathological based on the French cutoff scores (<6 for the free recall). Visuospatial, short term, and working memory were assessed respectively using the forward and backward Corsi block-tapping subtests from the Wechsler Adult Intelligence Scale Fourth Edition.27 Raw scores were transformed into standard scores following the Wechsler procedure. Standard scores were considered as normal (>7), weak (6-7), or pathological (<6).28
Executive functioning
The Digit-Symbol Coding subtest (DSST)27 was used to assess processing speed. It consists of 9 digit-symbol pairs followed by a list of digits. Under each digit, patients were asked to write down as many of the corresponding symbols as possible over the course of 120 seconds. Raw scores were transformed into standard scores and classified as normal (>7), weak (6-7), or pathological (<6). Mental flexibility was assessed using the switching fluency test of the district of Seine-Saint-Denis29 (le test des fluences alternées du 93 or TFA-93). After simple fluency tasks, patients were asked to say as many words as possible in 1 minute by alternating between animals and fruit. The switching cost index as a measure of flexibility was calculated by dividing the number of correct responses given in the switching condition by the sum of correct responses given in the 2 semantic fluency tasks. The higher the calculated index, the lower the switching cost. This index was interpreted based on data from 279 native French speaking healthy controls aged 21 to 84 years (mean age: 60.9 ±13.6), 43% (EL1: 39%; EL2: 27%; and EL3: 34%). The switching index was considered as weak if <70%, and pathological if <65%, corresponding respectively to a risk of drawing a wrong inference, <10% (percentile 10) vs <5% (percentile 5).
Mood
The Hospital Anxiety and Depression Scale30 was used to assess the patient’s current emotional state. Scores were classified separately for anxious and depressive symptoms as follows: absence of symptoms (≤7), suspected presence or presence of symptoms (>7).
Radiological workup
The baseline radiological workup included a detailed multimodal brain MRI and a cervical and transcranial Doppler ultrasound to assess any extracranial and/or intracranial arterial vasculopathy. Brain MRI was performed on a 3-T MRI (3T Discovery MR750, GE Healthcare, Milwaukee, WI) and included axial 2-dimensional fluid attenuated inversion recovery, axial diffusion-weighted imaging (b0-b1,000), axial gradient recalled echo T2-weighted imaging, 3-dimensional time of flight (3D-TOF) MRA, and 3D T1-weighted inversion recovery prepared fast spoiled gradient recalled (IR-FSPGR) sequence. The MRI technical parameters met the Standards for Reporting Vascular Changes on Neuroimaging 1.31 MRI parameters were as follows: 2-dimensional fluid attenuated inversion recovery (slice thickness, 3.5 mm; in-plane resolution, 0.9 × 0.9 mm), axial diffusion-weighted imaging (slice thickness, 3.5 mm; in-plane resolution, 1 × 1 mm), T2∗-weighted axial gradient recalled echo (slice thickness, 3.5 mm; in-plane resolution, 0.4 × 0.4 mm), 3D-TOF MRA (slice thickness, 1.2 mm; in-plane resolution, 0.4 × 0.4 mm), 3D T1-weighted inversion recovery prepared fast spoiled gradient recalled sequence (slice thickness, 1.2 mm; in-plane resolution, 0.5 × 0.5 mm).
The following data were evaluated with respect to lateralization: presence of territorial infarcts (dichotomized into subcortical and cortical), the presence of a lacune of presumed vascular origin,32 and the presence of white matter hyperintensities >3 mm and of presumed vascular origin. The intracranial arterial analysis performed on the 3D-TOF MRA was previously reported.32 Briefly, this MR angiography aimed to identify arterial stenosis of the intracranial internal carotid artery, the proximal segment of the middle cerebral artery, and the anterior cerebral artery. Each arterial segment was then classified as (1) normal, (2) stenosis ≥50%, (3) occlusion or subocclusion defined as an interruption of signal on MR angiography sequences. Patients with Moya-Moya syndrome were defined by the presence of steno-occlusive lesions of the intracranial internal carotid artery and/or the proximal portion of the middle cerebral artery or anterior cerebral artery, associated with an abnormal vascular network within the basal ganglia and/or the existence of meningeal anastomoses assessed on the 3D-TOF.
Cervical and transcranial echo color Doppler ultrasound examinations were performed by experts following quality standards33 on a Philips iU22 xMatrix ultrasound system (Eindhoven, The Netherlands). Cervical and transcranial echo color Doppler ultrasound examination protocols have been previously described.34 Examination protocol included visualization and analysis of the brachiocephalic artery trunk, the subclavian arteries at least in their prevertebral segments, all vertebral artery segments from V0 to V3, the primitive carotid arteries, the external carotid arteries, and the internal carotid arteries from the bulb to their entry into the carotid canal. All of these arteries were examined with a 9-3–Mhz linear array probe, and a 5-1–MHz curved probe when necessary (visualization of the subpetrosal segment).
The examination was based on repeated axial and longitudinal sections from the anterior, lateral, and posterolateral views. Each artery was studied in B-mode allowing a morphological analysis of the internal and external sides of the artery wall. Morphological analysis was performed either in color or in energy Doppler mode in order to mold the wall-circulating light interface, taking care to adjust the pulse repetition frequency. The hemodynamic study combined color Doppler to identify areas of acceleration and turbulence in blood flow, and pulsed Doppler to analyze the Doppler spectrum and quantify mean flow velocities (TAMx). All velocity measurements followed the following rules: (i) angle of insonation of <60°; (ii) angle correction by placing the linear marker provided by the scanner software along the long axis of the vessel, under visual guidance on the color image of the artery being insonated; and (iii) maintenance of a similar angle of insonation between 2 segments to be compared. The analyzed segments were: distal intrapetrosal internal carotid artery, carotid siphon, middle cerebral artery (M1), anterior cerebral artery (A1), basilar artery, and the posterior cerebral artery. We considered the intracranial carotid artery, proximal middle cerebral artery, and proximal anterior cerebral artery of the same side, as a single axis. For each axis a TAMx (time averaged maximum velocity) ratio was established using the highest TAMx value recorded on 1 axis, divided by the TAMx value of the ipsilateral extracranial internal carotid artery. Occlusion was defined as no flow or slow flow velocities corresponding to abnormal color flow mode confirming the occlusion. Extracranial vasculopathy was defined as a reduction of diameter associated with focal acceleration of peak systolic velocity leading to poststenotic/prestenotic ratio of >2 at the cervical level. Patients were categorized as having vasculopathy if they had a TAMx ratio of ≥3, an extracranial or intracranial occlusion, or a significant stenosis. Patients were categorized according to the highest abnormality, regardless of the side.
We previously reported the very high sensitivity and specificity cervical and transcranial echo color Doppler ultrasound to identify patients with ≥50% vasculopathy on MRA.34 Moreover, the presence of vasculopathy was confirmed during team meetings on the basis of a consensus reached between a radiologist and a neurosonographer using MRA and cervical and transcranial Doppler ultrasound data. Patients were then classified as exhibiting vasculopathy if they had: an intracranial stenosis ≥50% (or occlusion) with or without Moya-Moya syndrome and/or an extracranial stenosis ≥50% (or occlusion).
Statistical analysis
Quantitative variables were expressed as mean ± standard deviation, and qualitative variables as percentages. The prevalence of cognitive and mood disorders and their 95% confidence intervals (CIs) were calculated. We performed logistic regression to calculate crude odds ratios (ORs) to assess the magnitude of relationships between patient characteristics and the presence of cognitive dysfunction (defined as a pathological standard score on any cognitive test). To clarify the relationships between cognitive dysfunction and brain abnormalities, we decided to separate patients into those with territorial infarcts (cortical and/or subcortical) and those without. In order to identify variables significantly associated with cognitive dysfunction, we included into our multivariable model the presence of territorial infarct, presence of vasculopathy, EL, and CE, according to previous studies16,17,23 and our hypothesis. Statistical analyses were performed on SPSS version 28.0.
Standard protocol approvals, registrations, and patient consents
The PCDREP cohort received ethics committee (Comité de Protection des Personnes Île-de-France Saint-Louis) approval and was conducted in accordance with the Declaration of Helsinki, good clinical practice guidelines, and local laws and regulations. Patients were enrolled after giving their written informed consent. Patients from the “Paris Centre” SCD referral Center were informed of their participation in the study. In accordance with local legislation, because this study only implied retrospective analysis of anonymized data collected as part of routine care and in accordance with local legislation, formal approval by an ethics committee was not required.
Results
Clinical characteristics
During the study period, 121 patients were admitted for a neurological assessment. Twenty-five patients (20%) did not receive a neuropsychological assessment because time constraints during the 1-day hospitalization, psychological distress, uncontrolled pain, severe hearing loss, visual impairment, and/or severe aphasia. Overall, 96 patients were included (56% female, and 95% with a hemoglobin S genotype). The mean age at inclusion was 29.7 (±10.2) years. Forty-one (43%) patients had a history of stroke. Baseline treatments included blood exchange transfusions in 61 patients (63%; with or without hydroxyurea), 37 of whom had an intracranial vasculopathy. With regard to EL, almost half of the patients ended schooling before the twelfth grade, and ∼75% received their education entirely or mostly in France (details in Table 1).
Patient characteristics
| . | n (%) . |
|---|---|
| Age, y (mean ± SD) | 29.7 ± 10.2 |
| Female | 54 (56) |
| Hypertension | 3 (3) |
| Diabetes mellitus | 1 (1) |
| Current smoking | 1 (2) |
| Dyslipidemia | 0 (0) |
| Epileptic seizures | 8 (8) |
| Headaches | 22 (23) |
| Previously suspected TIA | 4 (4) |
| Previously known stroke | 41 (42)∗ |
| Ischemic | 38 (40) |
| Hemorrhagic | 4 (4) |
| Previously suspected or known intracranial vasculopathy | 60 (61) |
| Genotype | |
| SS | 91 (95) |
| Sβ | 2 (2) |
| SC | 3 (3) |
| Treatment | |
| Blood exchange transfusion (±hydroxyurea) | 61 (63) |
| Hydroxyurea only | 25 (26) |
| Cerebral revascularization | 6 (6) |
| None | 10 (10) |
| EL | |
| Grade 1 (education stopped before the ninth grade) | 10 (10) |
| Grade 2 (education stopped before the 12th grade) | 34 (35) |
| Grade 3 (education “after high school”) | 52 (54) |
| CE | |
| France (since preschool, 3) | 66 (69) |
| France (since primary school, 2) | 6 (6) |
| Other (1) | 22 (23) |
| Unknown | 2 (2) |
| Cognitive complaint | |
| Absent | 43 (45) |
| Present | 51 (53) |
| Unknown | 2 (2) |
| . | n (%) . |
|---|---|
| Age, y (mean ± SD) | 29.7 ± 10.2 |
| Female | 54 (56) |
| Hypertension | 3 (3) |
| Diabetes mellitus | 1 (1) |
| Current smoking | 1 (2) |
| Dyslipidemia | 0 (0) |
| Epileptic seizures | 8 (8) |
| Headaches | 22 (23) |
| Previously suspected TIA | 4 (4) |
| Previously known stroke | 41 (42)∗ |
| Ischemic | 38 (40) |
| Hemorrhagic | 4 (4) |
| Previously suspected or known intracranial vasculopathy | 60 (61) |
| Genotype | |
| SS | 91 (95) |
| Sβ | 2 (2) |
| SC | 3 (3) |
| Treatment | |
| Blood exchange transfusion (±hydroxyurea) | 61 (63) |
| Hydroxyurea only | 25 (26) |
| Cerebral revascularization | 6 (6) |
| None | 10 (10) |
| EL | |
| Grade 1 (education stopped before the ninth grade) | 10 (10) |
| Grade 2 (education stopped before the 12th grade) | 34 (35) |
| Grade 3 (education “after high school”) | 52 (54) |
| CE | |
| France (since preschool, 3) | 66 (69) |
| France (since primary school, 2) | 6 (6) |
| Other (1) | 22 (23) |
| Unknown | 2 (2) |
| Cognitive complaint | |
| Absent | 43 (45) |
| Present | 51 (53) |
| Unknown | 2 (2) |
N = 96 patients.
SD, standard deviation; TIA, transient ischemic attack.
One patient had both an ischemic and an hemorrhagic stroke history.
Cognitive and mood disorders
The cognitive profile was characterized by dysfunction in processing speed in 58% (95% CI, 48-68) of patients, dysfunction in visuospatial short-term memory in 34% (95% CI, 24-43), and dysfunction in visuospatial working memory in 24% (95% CI, 15-33). Other cognitive domains (episodic memory and mental flexibility) were relatively intact (Table 2). Depressive symptoms were present in 26% (95% CI, 17-35) of the patients, and anxiety symptoms in 41% (95% CI, 31-51). A cognitive complaint was present for 53% of the patients.
Proportion of dysfunction in each cognitive domain and mood
| . | n (% [95% CI])∗ . |
|---|---|
| Orientation and memory | |
| Temporospatial orientation | 5 (5 [1-9]) |
| Episodic memory | 8 (8 [1-28]) |
| Visuospatial short-term memory | 32/95 (34 [24-44]) |
| Visuospatial working memory | 23/95 (24 [15-33]) |
| Executive functioning | |
| Processing Speed | 54/93 (58 [48-68]) |
| Mental flexibility | 6 (6 [1-11]) |
| Mood | |
| Anxiety | 37/91 (41 [31-51]) |
| Depression | 23/89 (26 [17-35]) |
| . | n (% [95% CI])∗ . |
|---|---|
| Orientation and memory | |
| Temporospatial orientation | 5 (5 [1-9]) |
| Episodic memory | 8 (8 [1-28]) |
| Visuospatial short-term memory | 32/95 (34 [24-44]) |
| Visuospatial working memory | 23/95 (24 [15-33]) |
| Executive functioning | |
| Processing Speed | 54/93 (58 [48-68]) |
| Mental flexibility | 6 (6 [1-11]) |
| Mood | |
| Anxiety | 37/91 (41 [31-51]) |
| Depression | 23/89 (26 [17-35]) |
Percentage of the total population (N = 96), unless indicated otherwise.
Radiological characteristics
Fifty-four (56%; 95% CI, 46-66) patients had infarcts (territorial or lacunar) on brain MRI. Territorial infarcts (subcortical or cortical) were found in 42 (44%; 95% CI, 34-54) patients. More precisely, subcortical infarcts were found in 27 (28%; 95% CI, 19-37) patients, cortical infarcts in 32 (33%; 95% CI, 24-42) patients, and isolated lacunes (without any associated territorial infarct) in 12 (13%; 95% CI, 6-20) patients. In addition, 72 patients (75%; 95% CI, 66-84) had white matter hyperintensities of presumed vascular origin, isolated in 23 (24%; 95% CI, 15-32). Finally, 19 (20%; 95% CI, 11-27) patients had no abnormality on brain MRI.
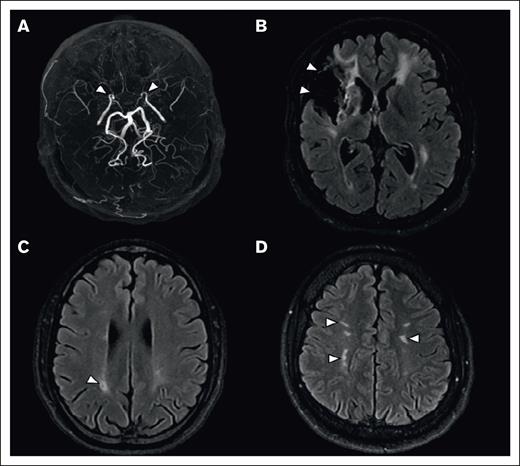
An intracranial vasculopathy was identified in 47 (49%; 95% CI, 39-59) patients, associated with Moya-Moya syndrome in 29 (30%; 95% CI, 21-39) patients. Additionally, 17 (18%; 95% CI, 10-26) patients had an extracranial vasculopathy, which was isolated in 6 patients. Overall, the prevalence of vasculopathy (intra and/or extracranial) was 55% (95% CI, 45-65; n = 53). MRI findings are summarized in Figure 1.
Brain MRI of lesions associated with SCD. (A) Maximal intensity projection reconstruction of a 3D-TOF MRA, showing occlusion of the distal segment of internal carotid arteries (arrowheads). (B-D) Axial 2-dimensional fluid attenuated inversion recovery (2D FLAIR), showing a territorial infarct (B, arrowheads), an isolated lacune (C, arrowhead), and white matter hyperintensities of presumed vascular origin (D, arrowheads).
Brain MRI of lesions associated with SCD. (A) Maximal intensity projection reconstruction of a 3D-TOF MRA, showing occlusion of the distal segment of internal carotid arteries (arrowheads). (B-D) Axial 2-dimensional fluid attenuated inversion recovery (2D FLAIR), showing a territorial infarct (B, arrowheads), an isolated lacune (C, arrowhead), and white matter hyperintensities of presumed vascular origin (D, arrowheads).
Predictors of cognitive dysfunction
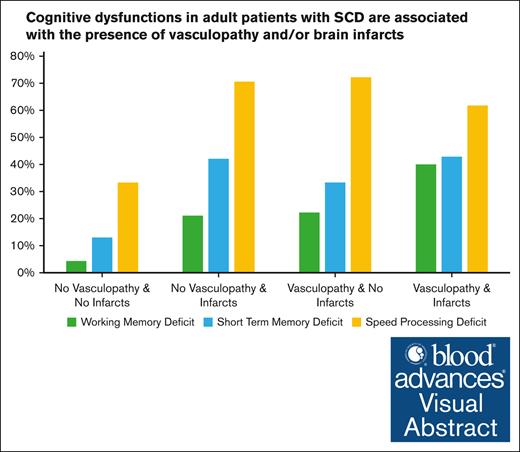
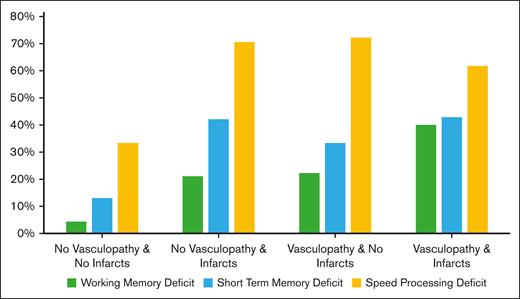
The proportion of cognitive dysfunction according to the presence of vasculopathy and/or cerebral infarcts is presented in Figure 2.
Cognitive dysfunction according to the presence of vasculopathy and/or infarcts.
Cognitive dysfunction according to the presence of vasculopathy and/or infarcts.
The presence of territorial infarcts was associated with the presence of visuospatial short-term memory dysfunction (OR, 3.8; 95% CI 1.6-9.4), visuospatial working memory dysfunction (OR, 3.1; 95% CI, 1.2-8.3), and processing speed dysfunction (OR, 4.2; 95% CI, 1.7-10.4); details in Table 3). Cerebral infarcts were also associated with visuospatial short-term memory and visuospatial working memory dysfunction when lacunes were considered in the group of patients with infarcts compared with those without infarcts (exploratory analysis in supplemental Table 1). Finally, among the patients without any abnormality on brain MRI (n = 19), 9 (47%) showed pathological processing speed, 3 (33%) had pathological scores on short-term memory, and 1 (5%) had a pathological score on visuospatial working memory.
Prevalence of neuropsychological dysfunction according to territorial infarcts and vasculopathy
| . | No territorial infarct . | Territorial infarct . | OR [95% CI] . |
|---|---|---|---|
| n (%) . | n (%) . | ||
| Pathological memory | |||
| Visuospatial short-term memory | 11 (21) | 21 (50) | 3.8 [1.6-9.4] |
| Visuospatial working memory | 8 (15) | 15 (28) | 3.1 [1.2-8.3] |
| Pathological executive functioning | |||
| Processing Speed | 24 (44) | 30 (77) | 4.2 [1.7-10.4] |
| . | No territorial infarct . | Territorial infarct . | OR [95% CI] . |
|---|---|---|---|
| n (%) . | n (%) . | ||
| Pathological memory | |||
| Visuospatial short-term memory | 11 (21) | 21 (50) | 3.8 [1.6-9.4] |
| Visuospatial working memory | 8 (15) | 15 (28) | 3.1 [1.2-8.3] |
| Pathological executive functioning | |||
| Processing Speed | 24 (44) | 30 (77) | 4.2 [1.7-10.4] |
| . | No vasculopathy . | Vasculopathy . | OR [95% CI] . |
|---|---|---|---|
| n (%) . | n (%) . | ||
| Pathological memory | |||
| Visuospatial short-term memory | 11 (27) | 21 (39) | 1.7 [0.7-4.2] |
| Visuospatial working memory | 5 (12) | 18 (50) | 3.6 [1.2-10.7] |
| Pathological executive functioning | |||
| Processing speed | 20 (50) | 34 (64) | 1.8 [0.8-4.1] |
| . | No vasculopathy . | Vasculopathy . | OR [95% CI] . |
|---|---|---|---|
| n (%) . | n (%) . | ||
| Pathological memory | |||
| Visuospatial short-term memory | 11 (27) | 21 (39) | 1.7 [0.7-4.2] |
| Visuospatial working memory | 5 (12) | 18 (50) | 3.6 [1.2-10.7] |
| Pathological executive functioning | |||
| Processing speed | 20 (50) | 34 (64) | 1.8 [0.8-4.1] |
Vasculopathy was associated with visuospatial working memory dysfunction (OR, 3.6; 95% CI, 1.2-10.7). The presence of a low EL was associated with the presence of visuospatial short-term memory dysfunction (OR, 10.2; 95% CI, 2.0-51.4) and visuospatial working memory dysfunction (OR, 6.0; 95% CI, 1.5-23.7). Education in a country outside of France was associated with the presence of processing speed dysfunction (OR, 6.4; 95% CI, 2.0-20.7). The presence of anxiety and/or depression was not associated with any cognitive dysfunction (details in supplemental Table 2), nor was the presence of blood exchange transfusion (details in supplemental Table 3). The presence of a cognitive complaint was associated with processing speed dysfunction (OR, 2.5; 95% CI, 1.1-6.7) but not with visuospatial short-term memory dysfunction (OR, 2.0; 95% CI, 0.8-4.8) or visuospatial working memory dysfunction (OR, 2.3; 95% CI, 0.8-6.2; details in supplemental Table 4). Patients with episodic memory dysfunction were older than those without (P = .002), but age was not associated with other cognitive dysfunction (details in Tables 3 and 4; supplemental Table 5).
Prevalence of neuropsychological dysfunction according to EL and CE
| . | EL 2-3 . | EL 1 . | OR [95% CI] . |
|---|---|---|---|
| n (%) . | n (%) . | ||
| Pathological memory | |||
| Visuospatial short-term memory | 24 (28) | 8 (80) | 10.2 [2.0-51.4] |
| Visuospatial working memory | 17 (20) | 6 (60) | 6.0 [1.5-23.7] |
| Pathological executive functioning | |||
| Processing Speed | 44 (53) | 10 (100) | N.A.∗ |
| . | EL 2-3 . | EL 1 . | OR [95% CI] . |
|---|---|---|---|
| n (%) . | n (%) . | ||
| Pathological memory | |||
| Visuospatial short-term memory | 24 (28) | 8 (80) | 10.2 [2.0-51.4] |
| Visuospatial working memory | 17 (20) | 6 (60) | 6.0 [1.5-23.7] |
| Pathological executive functioning | |||
| Processing Speed | 44 (53) | 10 (100) | N.A.∗ |
| . | CE 2-3 . | CE 1 . | OR [95% CI] . |
|---|---|---|---|
| n (%) . | n (%) . | ||
| Pathological memory | |||
| Visuospatial short-term memory | 20 (30) | 12 (43) | 1.8 [0.7-4.6] |
| Visuospatial working memory | 14 (21) | 9 (32) | 1.7 [0.6-4.6] |
| Pathological executive functioning | |||
| Processing speed | 32 (47) | 22 (78) | 6.4 [2.0-20.7] |
| . | CE 2-3 . | CE 1 . | OR [95% CI] . |
|---|---|---|---|
| n (%) . | n (%) . | ||
| Pathological memory | |||
| Visuospatial short-term memory | 20 (30) | 12 (43) | 1.8 [0.7-4.6] |
| Visuospatial working memory | 14 (21) | 9 (32) | 1.7 [0.6-4.6] |
| Pathological executive functioning | |||
| Processing speed | 32 (47) | 22 (78) | 6.4 [2.0-20.7] |
All patients with a low EL had processing speed disorders, making calculation of the OR impossible.
Multivariable analysis
Visuospatial short-term memory impairment was independently associated with territorial infarcts and EL (OR, 3.4; 95% CI, 1.3-9.2; P = .01; and OR, 8.2; 95% CI, 1.5-45.2; P = .01, respectively). Visuospatial working memory impairment was independently associated with the presence of vasculopathy and a low EL (OR, 3.7; 95% CI, 1.1-11.9; P = .03; and OR, 4.3; 95% CI, 1.0-18.5; P = .05, respectively) but the association with territorial infarct did not reach the threshold for statistical significance (OR, 2.2; 95% CI, 0.8-6.4; P = .14). Processing speed dysfunction was independently associated with the presence of territorial infarcts and education outside of France (CE 2 or CE 3; OR, 3.1; 95% CI, 1.1-8.6; P = .03; and OR, 4.7; 95% CI, 1.6-16.5; P = .02, respectively; details in Table 5).
Multivariable analysis
| . | Memory . | Executive functioning . | |
|---|---|---|---|
| Visuospatial short-term memory . | Visuospatial working memory . | Processing speed . | |
| EL OR [95% CI] | 8.2 [1.5-45.2] (P = .01) | 4.3 [1.0-18.5] (P = .05) | ∗ |
| CE OR [95% CI] | 1.2 [0.4-3.5] (P = .69) | 1.6 [0.5-4.8] (P = .42) | 4.7 [1.6-16.5] (P = .02) |
| Territorial infarcts OR [95% CI] | 3.4 [1.3-9.2] (P = .01) | 2.2 [0.8-6.4] (P = .14) | 3.1 [1.1-8.6] (P = .03) |
| Vasculopathy OR [95% CI] | 1.4 [0.5-3.9] (P = .46) | 3.7 [1.1-11.9] (P = .03) | 1.5 [0.6-3.9] (P = .43) |
| . | Memory . | Executive functioning . | |
|---|---|---|---|
| Visuospatial short-term memory . | Visuospatial working memory . | Processing speed . | |
| EL OR [95% CI] | 8.2 [1.5-45.2] (P = .01) | 4.3 [1.0-18.5] (P = .05) | ∗ |
| CE OR [95% CI] | 1.2 [0.4-3.5] (P = .69) | 1.6 [0.5-4.8] (P = .42) | 4.7 [1.6-16.5] (P = .02) |
| Territorial infarcts OR [95% CI] | 3.4 [1.3-9.2] (P = .01) | 2.2 [0.8-6.4] (P = .14) | 3.1 [1.1-8.6] (P = .03) |
| Vasculopathy OR [95% CI] | 1.4 [0.5-3.9] (P = .46) | 3.7 [1.1-11.9] (P = .03) | 1.5 [0.6-3.9] (P = .43) |
Boldface indicates variables significantly associated (P < 0.05) with cognitive dysfunctions.
Association not measurable.
Discussion
In our study of 96 adult patients with SCD with sickle cell–related stroke or suspected neurological morbidity, we found that cognitive dysfunction was frequent, particularly affecting processing speed and short-term and working memory whereas episodic memory and mental flexibility seemed relatively spared.
The phenotype of cognitive dysfunction found in our study is largely consistent with previous studies conducted in children with SCD which reported dysfunction in selective attention, working memory, processing speed, vocabulary, abstract reasoning, and verbal comprehension.9-11,35,36 However, it should be noted that few patients showed a dysfunction in mental flexibility. This unexpected result may be explained by the test chosen to assess this executive function. The cognitive domains affected in patients included in our study are essential for performing instrumental activities and other complex cognitive functions such as problem-solving and goal-directed thinking.37,38 Although few studies have taken an interest in the impact of cognitive dysfunction in adult patients with SCD, some authors have pointed out that these dysfunctions may negatively affect their health care,39 social functioning,40 and employment.41 In our study, only dysfunction in processing speed was associated with the presence of a cognitive complaint whereas short-term memory dysfunction and working memory dysfunction were not. Although cognitive complaints and cognitive dysfunction assessed by neuropsychological performance may sometimes be discordant,42,43 this result suggests the usefulness of investigating metacognition or symptoms of anosognosia in adult patients with SCD in future studies. We also hypothesize that the severity and relentlessness of SCD complications, especially acute and chronic pain, make cognitive complaints a secondary priority.
Because the presence of a cognitive complaint did not accurately identify patients with cognitive dysfunction in our study and because a systematic cognitive screening in all adult patients with SCD would likely be difficult to implement, the identification of predictive factors could help target a specific high-risk SCD population. One of the main objectives of our study was to assess relationships between cognitive dysfunction and brain imaging abnormalities. As expected, brain territorial infarcts were associated with cognitive dysfunction, particularly visuospatial short-term memory and processing speed. When isolated lacunes were also included in the brain infarct group, that association was still present (data not shown). This association has been questioned in patients with SCD, but no previous study has performed a detailed cognitive, brain imaging, and arterial assessment in the same cohort. Noteworthy, vasculopathy was, in our study, only significantly associated with working memory impairment, independently of MRI brain infarcts.
Beyond brain lesions, other factors could be associated with cognitive dysfunction because half of patients with normal MRI results had at least 1 cognitive dysfunction in our cohort. Although anxiety and/or depressive syndrome are frequently associated with cognitive dysfunction,44 these were not associated with any of the cognitive dysfunction in our study. It is also possible that other factors, not recorded in this study, such as chronic anemia, chronic pain, and sleep disorders, may negatively affect the cognitive functioning of adult patients with SCD.45
Because of the demographic specificities of the SCD population, it is important to also take sociodemographic characteristics into account. One of the strengths of our study is the selection of appropriate tools with regard to patients’ educational background and/or level of French literacy, leading to a lower risk of false positives for cognitive dysfunction. Some tests were suitable for low-educated populations or people with a low level of literacy in French (eg, TNI-93 and TFA-93). However, because these tests were previously validated for the detection of dementia in an older population, they may lead to an underestimate of episodic memory and mental flexibility dysfunction because the sensitivity of norms could be lower for a younger population. To overcome the literacy problem, we also used visual/visuospatial tests (ie, DSST and Corsi), which have the advantage of having short and simple instructions and do not require a high level of language. Further studies are needed to specifically assess visuospatial functioning in adult patients with SCD because it may be altered.46 In children with SCD, socioeconomic level and cultural conditions have been shown to be associated with an increased risk of cognitive dysfunction.47 In our study, sociocultural level negatively influenced cognitive performance in the areas of short-term memory, and work and processing speed, whereas total or partial schooling outside of France negatively influenced processing speed. Thus, despite an upstream selection of our cognitive tests, we clearly show that sociocultural level and CE influence test results. Of note, some of the tests (DSST and Corsi) have not been validated in low-educated populations or people with a low level of literacy. It should also be noted that a lower sociocultural level may be a consequence of cognitive abnormalities beginning in childhood, preventing young patients from continuing their schooling. Future longitudinal studies are needed to clarify these issues. The impact of socioeconomic level and cultural conditions on cognitive dysfunction and the role of access to health care from country to country remain difficult to disentangle.
The assessment of cognitive dysfunction has become increasingly important in the management of SCD because the last few years have been marked by improvements in therapeutic management, leading to a great improvement in life expectancy.48,49 Consequently, clinicians must face new questions and challenges, specifically the frequency and characteristics of cognitive dysfunction. Because of their "silent" manifestations in contrast to those of strokes, these cognitive dysfunctions are probably underestimated in routine clinical care despite their impact on the daily life of these patients. Our study suggests that a rapid and adapted battery of neuropsychological tests can be performed in adult patients with SCD and can be applied routinely according to recent recommendations.19 Further studies are needed to confirm the accuracy and the replicability of this neuropsychological battery.
Our study has some potential limitations. First, we were not able to assess potential biological markers associated with cognitive dysfunction.11 Because more than half of the patients were treated with regular exchange and blood transfusions, the measure of steady-state biological parameters like basal hemoglobin level was not relevant. Second, because of a selection bias, patients included in our study were at high cerebrovascular risk as shown by the prevalence of brain lesions. This selection bias may have resulted in an overestimation of the prevalence of cognitive dysfunction. However, we think that it is very unlikely that the predictive factors associated with cognitive dysfunction would have been different in a population with lower cerebrovascular risk. Lastly, we did not look for the presence of behavioral dysexecutive syndrome, which has been shown to be present in 10% of patients with vascular sequelae despite the absence of cognitive dysexecutive syndrome.50
Conclusion
Cognitive dysfunction is frequent in young adult patients with sickle cell–related stroke or suspected neurological morbidity but is underestimated by the patients themselves. Therefore, screening for cognitive dysfunction should be more routinely done using an adapted set of tests that are more adapted to the sociocultural factors of this population. Further studies are needed to determine the socio-economic consequences of this cognitive dysfunction and to assess the efficacy of therapeutic interventions to preserve cognitive function in patients who are impaired.
Authorship
Contribution: D.M. analyzed and interpreted the data, and drafted and revised the manuscript; H.B. and C.E. contributed to the conception and design of the work, the acquisition and the interpretation of the data, and revised the manuscript; C.P. analyzed the data and revised the manuscript; D.C. contributed to the conception and the design of the work, monitored data collection, contributed to the acquisition, analysis, and interpretation of data, and drafted and revised the manuscript; P.N. contributed to the conception and the design of the work, analysis and interpretation of data, and revised the manuscript; P.B. contributed to the conception and the design of the work, contributed to the acquisition of data, and revised the manuscript; J.-B.A., B.R., L.J., and N.M. contributed to the acquisition of the data and revised the manuscript; and R.K. contributed to the conception and design of the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Calvet, Neurology Department, Université Paris Cité, Institute of Psychiatry and Neuroscience of Paris, INSERM U1266, FHU Neurovasc, 1 rue Cabanis, 75014 Paris, France; email: d.calvet@ghu-paris.fr.
References
Author notes
Data are available on request from the corresponding author, David Calvet (d.calvet@ghu-paris.fr).
The full-text version of this article contains a data supplement.