TO THE EDITOR:
Thrombotic thrombocytopenic purpura (TTP) is a thrombotic microangiopathy (TMA) characterized by severe thrombocytopenia, hemolytic anemia, and end-organ ischemia, which is fatal without prompt recognition and treatment.1 TTP arises from a severe deficiency of ADAMTS13, a metalloprotease responsible for cleaving von Willebrand Factor, allowing the accumulation of ultra-large von Willebrand Factor multimers that precipitate disseminated platelet-rich microthrombi.2 ADAMTS13 deficiency can either be immune-mediated TTP due to autoantibodies or congenital arising from biallelic mutations in the ADAMTS13 gene (cTTP). Pregnancy is a well-known precipitant for acute TTP episodes, particularly cTTP, in some cohorts; 25% to 30% of all obstetrical TTP cases are due to the congenital form.3,4
TTP during pregnancy is fraught with substantial difficulties. It must be rapidly distinguished from other pregnancy-related TMAs, such as preeclampsia or HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome, which may also coexist in cTTP presentation, because maternal and fetal morbidity/mortality are high with delays in therapy.5 Acute cTTP episodes during pregnancy confer a maternal mortality risk of 10%, and the rates of fetal demise are >50% with suboptimal management.6 The mainstay of therapy for acute cTTP is the administration of fresh frozen plasma, either by plasma infusion, typically 10 to 15 mL/kg given once daily until normalization of platelet counts, or plasma exchange. In populations that cannot receive plasma products, such as Jehovah’s Witnesses or those with anaphylaxis to plasma, cTTP management presents a unique challenge. Previously, plasma–derived factor VIII concentrates, which contain trace amounts of ADAMTS13, have been used.7 Recently, the Food and Drug Administration has approved recombinant ADAMTS13 (rADAMTS13) for the treatment of cTTP.8 It’s use has been described in pregnancy-associated cTTP, refractory to initial plasma infusion therapy.9 Here, we describe the first use of rADAMTS13 for acute rescue therapy in a critically ill pregnant patient with cTTP treated without plasma infusion.
A 25-year-old gravida 2, aborta 1, para 0, presented to our clinic to establish prenatal care. She had a significant family history of cTTP in her 2 sisters. Her diagnosis was made at the age of 5 years after presenting with transient episodes of altered mental status and peripheral loss of sensation. She was subsequently found to have ADAMTS13 activity <5% with no inhibitor. Genetic analysis confirmed cTTP, revealing mutations in exon 24 (c.C3178T causing p.R1060W) and exon 26 (c.T3650C causing p.I1217T) in ADAMTS13. She was started on twice-monthly prophylactic fresh frozen plasma transfusion with the resolution of neurological symptoms. After 7 years of chronic transfusion therapy, she developed a severe anaphylactic reaction to plasma. The plasma rechallenge resulted in anaphylaxis; therefore, she was transitioned to weekly infusions of Koate 30 IU/kg, a plasma–derived factor VIII concentrate with residual ADAMTS-13 activity,7 via an indwelling port. She briefly partook in the rADAMTS13 clinical trial (NCT04683003) with an excellent response but withdrew and transitioned back to Koate after a positive pregnancy test and a lack of reported safety data during pregnancy. This pregnancy ultimately resulted in a spontaneous first-trimester miscarriage. She then resumed her weekly Koate infusions, which she continued when she became pregnant once again. She had regular monitoring of her complete blood counts in close coordination with obstetrics, maternal-fetal medicine, and hematology teams. Her fetus experienced normal growth, without concern for anomalies. At ∼31 weeks of gestation, her platelet count decreased to 99 × 109/L (Figure 1, see day -29), and her Koate was increased to twice weekly with the normalization of her platelets.
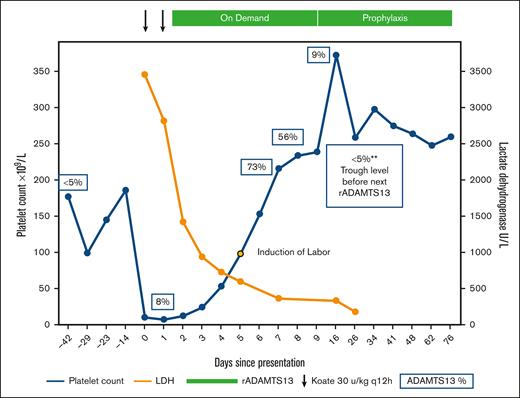
Recombinant ADAMTS13 (rADAMTS13) as rescue therapy for acute cTTP during pregnancy. The patient’s clinical course and treatment are illustrated. Her home dose of Koate was intensified twice daily on days 0 and 1 of the presentation. Given the lack of a response, rADAMTS13 was initiated on day 2. Platelets (blue) and LDH (orange) normalized rapidly. ADAMTS13 activity levels obtained at various time points are shown in blue boxes. Induction of labor commenced on day 5, with successful delivery on day 6. She remains on prophylactic therapy with rADAMTS13. LDH, lactate dehydrogenase.
Recombinant ADAMTS13 (rADAMTS13) as rescue therapy for acute cTTP during pregnancy. The patient’s clinical course and treatment are illustrated. Her home dose of Koate was intensified twice daily on days 0 and 1 of the presentation. Given the lack of a response, rADAMTS13 was initiated on day 2. Platelets (blue) and LDH (orange) normalized rapidly. ADAMTS13 activity levels obtained at various time points are shown in blue boxes. Induction of labor commenced on day 5, with successful delivery on day 6. She remains on prophylactic therapy with rADAMTS13. LDH, lactate dehydrogenase.
At 35 weeks of gestation, she presented to emergency care with generalized abdominal pain, bilateral flank pain, and dark-brown urine. Vital signs for both the mother and fetus were within normal limits for this gestational age. Her initial laboratory values on presentation are summarized in Table 1. Although HELLP and preeclampsia were briefly considered, her normal liver enzymes and blood pressure were most consistent with an acute exacerbation of her cTTP. She was immediately started on Koate 30 IU/kg every 12 hours. A multidisciplinary meeting was held between the maternal-fetal medicine, hematology, transfusion medicine, allergy and immunology, and anesthesiology teams. Immediate cesarean delivery of the fetus was deferred, as this would likely precipitate further propagation of TMA with high bleeding risk while severely thrombocytopenic. Allergy consultation confirmed the anaphylactic nature of the plasma allergy and determined that desensitization would carry an unacceptably high risk of anaphylaxis and injury to both the patient and fetus. Red blood cells were washed to remove any residual plasma and were made available by the transfusion medicine team throughout admission to maintain hemoglobin >7 g/dL. She tolerated red blood cell transfusions without evidence of anaphylaxis. After 36 hours of treatment with intensified Koate administration, the platelet count only marginally improved (Figure 1). In discussion with the patient regarding risks and benefits, along with the relative lack of formal safety data in the pregnant population, the decision was mutually made to use rADAMTS13 given her anaphylaxis to plasma products. On day 2, rADAMTS13 was started at 40 units/kg. The product label recommends dosing 40 units/kg initially, then 20 units/kg, and then 15 units/kg daily until platelet count recovery. However, due to the severity of her presentation, we opted to continue 40 units/kg daily until the platelet count was ≥100 109/L, followed immediately by induction of labor. On day 5, her platelets were adequate, and induction of labor commenced. Immediately before delivery, she began to develop labile blood pressure and evidence of pulmonary edema concerning for preeclampsia with severe features. She underwent uncomplicated vaginal delivery of a 2100 g female neonate. The 1- and 5-minute Apgar scores were 8 and 9, respectively. Placental pathology was notable for features of maternal vascular malperfusion in the setting of preeclampsia and TTP: severe placental growth restriction (<3rd percentile), hypermature chorionic villi with multifocal placental infarction involving ∼10% of the disk volume, and degenerated marginal retroplacental hemorrhage suggestive of remote placental abruption. After delivery, rADAMTS13 dosing was decreased to 20 units/kg and then to 15 units/kg daily. By day 9 (postpartum day 3), she was deemed fit for discharge. Given her prematurity, the neonate was admitted for neonatal intensive care. The neonate’s hospital course was notable for bilirubinemia, briefly requiring phototherapy, which was thought to be due to prematurity and was unrelated to therapy with rADAMTS13. Neonatal platelet counts were normal and she was discharged home on day 22 after meeting the appropriate milestones. The patient was continued on weekly rADAMTS13 at 40 U/kg weekly for 6 weeks postpartum and then transitioned to every other week of prophylaxis. The patient and her baby continued to do well and she remained on prophylactic therapy with rADAMTS13.
Laboratory values on admission
| . | Normal reference range third trimester . | Admission values . |
|---|---|---|
| Chemistry | ||
| Sodium (mmol/L) | 130-148 | 129 |
| Potassium (mmol/L) | 3.3-5.1 | 4.3 |
| Chloride (mEq/L) | 97-109 | 105 |
| CO2 (mmol/L) | 20-24 | 18 |
| Anion gap | 6 | |
| Glucose (mg/dL) | 70-100 | 92 |
| BUN (mg/dL) | 4-18 | 22 |
| Creatinine (mg/dL) | 0.4-0.9 | 1.10 |
| Calcium (mg/dL) | 8.2-9.7 | 8.1 |
| AST (U/L) | 4-32 | 152 |
| ALT (U/L) | 2-25 | 26 |
| Albumin (g/dL) | 2.3-4.2 | 3.2 |
| Total bilirubin (mg/dL) | 0.2-1 | 4.5 |
| LDH (U/L) | 82-524 | 3462 |
| Hematology | ||
| White blood cell count (×109/L) | 5.9-16.9 | 12.04 |
| Hemoglobin (g/dL) | 9.5-15.0 | 9.4 |
| Hematocrit (%) | 28.0-40.0 | 28.3 |
| MCV (fL) | 81-99 | 86.5 |
| Platelet (×109/L) | 186-353 | 10 |
| Neutrophil % | 42.5-73.2 | 80.5 |
| Lymphocytes % | 18.2-47.4 | 8.5 |
| Monocytes % | 4.3-11.0 | 9.6 |
| Eosinophils % | 0.0-3.0 | 0.3 |
| Basophils % | 0.0-0.7 | 0.4 |
| Coagulation studies | ||
| Prothrombin time (s) | 9.6-12.9 | 14.4 |
| International normalized ratio | 0.8-1.09 | 1.1 |
| Partial thromboplastin time (s) | 22.6-35.0 | 31.6 |
| Fibrinogen (mg/dL) | 301-696 | 481 |
| D-dimer (μg/mL) | <0.4 | 11.42 |
| . | Normal reference range third trimester . | Admission values . |
|---|---|---|
| Chemistry | ||
| Sodium (mmol/L) | 130-148 | 129 |
| Potassium (mmol/L) | 3.3-5.1 | 4.3 |
| Chloride (mEq/L) | 97-109 | 105 |
| CO2 (mmol/L) | 20-24 | 18 |
| Anion gap | 6 | |
| Glucose (mg/dL) | 70-100 | 92 |
| BUN (mg/dL) | 4-18 | 22 |
| Creatinine (mg/dL) | 0.4-0.9 | 1.10 |
| Calcium (mg/dL) | 8.2-9.7 | 8.1 |
| AST (U/L) | 4-32 | 152 |
| ALT (U/L) | 2-25 | 26 |
| Albumin (g/dL) | 2.3-4.2 | 3.2 |
| Total bilirubin (mg/dL) | 0.2-1 | 4.5 |
| LDH (U/L) | 82-524 | 3462 |
| Hematology | ||
| White blood cell count (×109/L) | 5.9-16.9 | 12.04 |
| Hemoglobin (g/dL) | 9.5-15.0 | 9.4 |
| Hematocrit (%) | 28.0-40.0 | 28.3 |
| MCV (fL) | 81-99 | 86.5 |
| Platelet (×109/L) | 186-353 | 10 |
| Neutrophil % | 42.5-73.2 | 80.5 |
| Lymphocytes % | 18.2-47.4 | 8.5 |
| Monocytes % | 4.3-11.0 | 9.6 |
| Eosinophils % | 0.0-3.0 | 0.3 |
| Basophils % | 0.0-0.7 | 0.4 |
| Coagulation studies | ||
| Prothrombin time (s) | 9.6-12.9 | 14.4 |
| International normalized ratio | 0.8-1.09 | 1.1 |
| Partial thromboplastin time (s) | 22.6-35.0 | 31.6 |
| Fibrinogen (mg/dL) | 301-696 | 481 |
| D-dimer (μg/mL) | <0.4 | 11.42 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; LDH, lactate dehydrogenase; MCV, mean corpuscular volume. Abnormal values in bold.
Herein we describe, to our knowledge, the first case reported in the literature of the successful administration of rADAMTS13 for acute cTTP in a pregnant patient who had previously had severe anaphylaxis to plasma. Close interdisciplinary collaboration played a crucial role in the successful diagnosis and management of this case. Intensified dosing of rADAMTS13 was chosen in our patient because the severity of her presentation was greater than that typically seen in exacerbations of cTTP, which often presents after mild illness with more modest thrombocytopenia. Although data on pregnancy remains limited, rADAMTS13 rescue therapy proved to be a safe and effective intervention that rapidly improved both clinical and hematologic parameters and resulted in positive maternal and neonatal outcomes. Further prospective studies on rADAMTS13 for cTTP in pregnancy are required.
The report was written with the consent of the patient involved and all data are deidentified.
Contribution: S.D. wrote the manuscript and cared for the patient; S.S. supervised the patient’s management, and reviewed and edited all aspects of the manuscript; and all authors comprehensively reviewed and approved the manuscript.
Conflict-of-disclosure: S.I. reports consultancy for AbbVie, ADC Therapeutics, Sobi, Argenx, and Ipsen. S.S. reports honoraria for scientific advisory board participation for Sanofi-Genzyme. The remaining authors declare no competing financial interests.
Correspondence: Senthil Sukumar, Baylor College of Medicine, 7200 Cambridge St 7th floor, Suite 7B, Houston, TX 77030; email: senthil.sukumar@bcm.edu.
References
Author notes
Individual patient data will not be shared. Queries may be directed to the corresponding author, Senthil Sukumar (senthil.sukumar@bcm.edu).