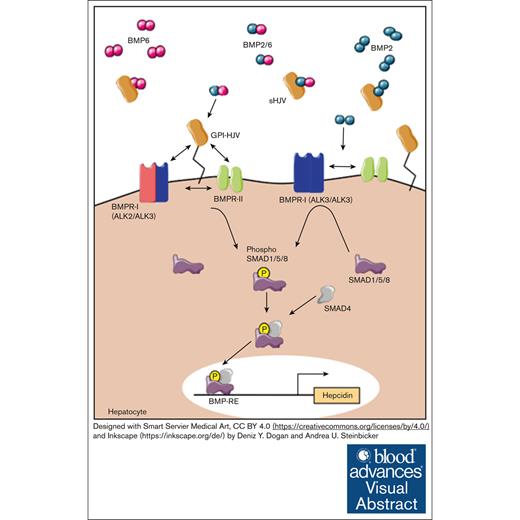
The bone morphogenetic protein type I receptors ALK2 and ALK3 are required for HJV-mediated induction of hepcidin in vivo.
Visual Abstract
Hemojuvelin (HJV) is a glycosylphosphatidylinositol-anchored protein of the repulsive guidance molecule family acting as a bone morphogenetic protein (BMP) coreceptor to induce the hepatic iron regulatory protein hepcidin. Hepcidin causes ubiquitination and degradation of the sole known iron exporter ferroportin, thereby limiting iron availability. The detailed signaling mechanism of HJV in vivo has yet to be investigated. In the current manuscript, we used an established model of adeno-associated virus (AAV)-mediated liver-specific overexpression of HJV in murine models of hepatocyte-specific deficiency of the BMP type I receptors Alk2 or Alk3. In control mice, HJV overexpression increased hepatic Hamp messenger RNA (mRNA) levels, soluble HJV (sHJV), splenic iron content (SIC), as well as phosphorylated small mothers against decapentaplegic protein (pSMAD1/5/8) levels. In contrast, in Alk2fl/fl;Alb-Cre and Alk3fl/fl;Alb-Cre mice, which present with moderate and severe iron overload, respectively, the administration of AAV-HJV induced HJV and sHJV. However, it did not rescue the iron overload phenotypes of those mice. Serum iron levels were induced in Alk2fl/fl;Alb-Cre mice after HJV overexpression. In phosphate-buffered saline–injected Alk3fl/fl;Alb-Cre mice, serum iron levels and the expression of duodenal ferroportin remained high, whereas Hamp mRNA levels were decreased to 1% to 5% of the levels detected in controls. This was reduced even further by AAV-HJV overexpression. SIC remained low in mice with hepatocyte-specific Alk2 or Alk3 deficiency, reflecting disturbed iron homeostasis with high serum iron levels and transferrin saturation and an inability to induce hepcidin by HJV overexpression. The data indicate that ALK2 and ALK3 are both required in vivo for the HJV-mediated induction of hepcidin.
Introduction
Iron homeostasis requires a multitude of regulatory mechanisms including different iron regulatory proteins. The hepatic hormone hepcidin is the key regulatory protein that controls systemic iron homeostasis.1 The main erythroid regulator of hepcidin is erythroferrone (ERFE).2 ERFE controls plasma iron levels and total body iron. It is induced by erythropoietin in the kidney and inhibits hepcidin by binding to bone morphogenetic protein (BMP) ligands, thereby limiting BMP receptor activation.3 Hepcidin induces the ubiquitination and degradation of the sole known iron exporter ferroportin, thereby reducing iron uptake from the diet and iron release from hepatocytes and macrophages.4 Hepcidin is produced in the liver in response to circulating and stored iron and inflammatory mediators such as interleukin-6. At the transcriptional level, the expression of hepcidin is controlled by the BMP-SMAD (small mothers against decapentaplegic protein) signaling pathway.5 In hepatocytes, this pathway is activated by BMP2 and BMP6 ligands that are secreted by liver sinusoidal endothelial cells.6 Upon binding of BMP2 and/or BMP6 to the BMP receptor complex, BMP type II receptors phosphorylate the BMP type I receptors ALK2 and ALK3, which are known to form ALK2/ALK3 heterodimers, ALK3/ALK3 homodimers, and ALK2/ALK2 homodimers.7-9 Both, ALK2 and ALK3 are required for BMP2-, BMP6-, and BMP2/6-mediated hepcidin induction.3,8,10 Hemojuvelin (HJV) is a glycosylphosphatidylinositol (GPI)-anchored protein of the repulsive guidance molecule family that acts as a BMP coreceptor.11 Mutations in the gene encoding Hjv lead to markedly reduced hepcidin levels and thus cause severe iron overload in humans.12 HJV directly binds BMP ligands and activates BMP signaling.11,13 HJV exists in 2 forms: GPI membrane–anchored HJV and soluble plasmatic HJV (sHJV). Already, in 2007, Babitt et al showed that BMP ligands induce hepcidin but that sHJV at increasing concentrations leads to a decrease in hepcidin expression by binding of sHJV to BMP ligands.14
A model by Healey et al, based on in vitro and in silico experiments, suggests that HJV forms a complex with BMP ligands, which is targeted to endosomes. In endosomes, HJV dissociates from BMP ligands and is replaced by BMP type I receptors for downstream signaling.15 The exact mechanism by which HJV enhances BMP signaling is still under investigation. It has been shown that BMP2 has competing binding sites at HJV and the ectodomain of ALK3.15
To further elucidate, which BMP type I receptor(s) are used by HJV to activate BMP signaling in vivo and to elucidate the effects of HJV overexpression on systemic iron homeostasis, we studied the consequences of adeno-associated virus (AAV)-mediated overexpression of HJV as well as the impact of the hepatocyte-specific deficiency of Alk2 or Alk3.8 The data indicate that HJV mediated induction of hepcidin requires both receptors, ALK2 and ALK3, in vivo.
Material and methods
Animals
Animal protocols were approved by the institutional ethical committee of the North Rhine-Westphalian Agency for Nature, Environment, and Consumer Protection (permit number Az. 81.92.04.2019.A207). Hepatocyte-specific Alk2- (Alk2fl/fl;Alb-Cre) or Alk3-deficient (Alk3fl/fl;Alb-Cre) mice and Cre- control littermates on a C57BL/6 background16,17 were fed a standard rodent diet (198 parts per million iron). Eight-week-old male mice were injected intravenously with either 5 × 1011 particles of AAV2/8 expressing Hjv-MycDDK under the control of a liver-specific promoter (AAV2/8-ALB-mHfe2-MycDDK, abbreviated as AAV-HJV; supplemental Figure 1) (Vector BioLabs) or phosphate-buffered saline (PBS) as control.18 PBS was used as control because it has been shown that an AAV2/8 expressing glutaryl-coenzyme A dehydrogenase (a protein unrelated to iron homeostasis) did not cause an induction of inflammatory parameters nor did it affect iron homeostasis.19 Mice were euthanized 2 weeks after virus administration under deep anesthesia, and blood and organs were collected for further analysis.
Hepcidin, hematologic, and iron parameters
Blood was drawn by retro-orbital puncture under deep ketamine/xylazine anesthesia. Serum iron levels and unsaturated binding capacity were determined using the Iron/U.I.B.C Kit (Biolabo) according to the manufacturer’s instructions. Hepcidin levels were determined using the Hepcidin Murine-Compete enzyme-linked immunosorbent assay kit (Intrinsic Lifesciences) according to the manufacturer’s instructions. Nonheme tissue iron content was determined as described by Torrance and Bothwell.20 For histological staining of nonheme iron, paraffin embedded tissue sections (4 μm) were stained with Perls’ Prussian blue stain.
qRT-PCR
Total RNA was extracted from tissue samples using Trizol (Sigma-Aldrich) according to the manufacturer’s instructions. Complementary DNA was synthesized using MMLV-reverse transcriptase (Sigma-Aldrich). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed on the Bio-Rad CFX Connect real-time PCR system using either iTaq Universal SYBR Green supermix (BioRad) or TaqMan universal master mix (Applied Biosystems). TaqMan probes and primer pairs are listed in supplemental Table 1. Target gene expression was normalized to levels of 18S ribosomal RNA and calculated using the relative CT method.21
Immunoblotting and iron staining
Tissue samples were lysed in RIPA buffer supplemented with protease and phosphatase inhibitor cocktails, and protein concentration was determined using the Pierce bicinchoninic acid protein assay kit (Thermo Fisher Scientific). Equal amounts of isolated proteins from tissue samples were separated by electrophoresis using 10% to 16% bis- tris(hydroxymethyl)aminomethane gels. Proteins were transferred on nitrocellulose membranes and incubated with antibodies directed against glyceraldehyde-3-phosphate dehydrogenase (no. G8795, Sigma-Aldrich), HJV (no. AF3634, R&D Systems), ferroportin (no. NBP1-21502, Novus Biologicals), or pSMAD1,5,9 (no. Vl131, Maine Medical Center Research Institute), and Li-Cor secondary antibodies (no. 926-32210 800CW goat anti-mouse immunoglobulin G, no. 926-68073 680RD donkey anti-rabbit immunoglobulin G). Immunoblots were imaged using the LI-COR Odyssey DLx imaging system (LI-COR).
For iron staining of formalin-fixed, paraffin-embedded material, 4-μm-thick slides were stained with 10% potassium hexacyanoferate (Roth). Hematoxylin and eosin–stained slides and FE-stained slides (Perls’ stain) were examined with an optical/light microscope (Scope A1, Zeiss)
Immunofluorescence
Duodenal sections were deparaffinized and blocked (1 hour at room temperature) with PBS containing 5% horse serum and 0.3% Triton X-100, and then incubated overnight (4°C) with a primary antibody directed against ferroportin (no. NBP1-21502, Novus Biologicals). Thereafter, slides were washed with PBS and incubated (2 hours at room temperature) with the corresponding secondary antibody Alexa Fluor 546 (no. A10040, Thermo Fisher Scientific). After repetitive washing with PBS, slides were mounted in glycerin-based Hoechst 33347 containing mounting medium (Sigma-Aldrich). Images were generated using a Zeiss confocal microscope (LSM 780, Zeiss) and were analyzed with Imaris 10.1 (Bitplane) using the surface rendering function to quantify the area of fluorescence signal normalized to area of field view.
Statistical analysis
All data are presented as box plots with individual data points shown and medians. Equality of variances was validated by Levene test and outliers by Grubbs test. The Shapiro-Wilk test was performed to test for normality. Means were compared by Students t test and 1-way analysis of variance or Welch analysis of variance for parametric data, and Mann-Whitney U test and Kruskal Wallis test for nonparametric data sets (Prism 8, GraphPad Software). A P value <.05 was considered statistically significant. Correlations were analyzed using nonparametric Spearman correlation with a 2-tailed P value.
Results
Successful liver-specific overexpression of HJV by AAV-HJV in vivo
Because deficiency of HJV reduces BMP signaling and hepcidin expression and leads to the development of severe iron overload in mice,12 we sought to investigate the role of BMP signaling in HJV induction. Previous studies have shown that the BMP type I receptors ALK2 and ALK3 are crucial for the expression of hepcidin and iron homeostasis.8
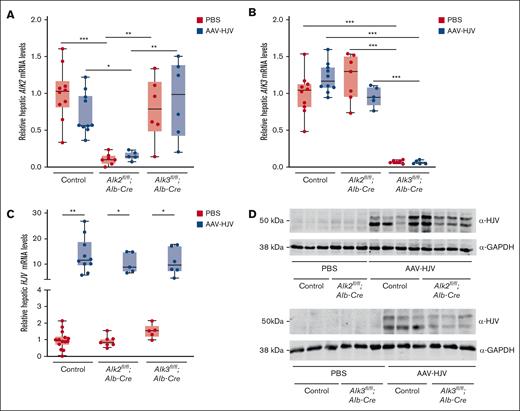
To investigate whether activation of BMP/SMAD signaling and induction of hepcidin expression by HJV are dependent on the BMP type I receptor ALK2 and/or ALK3, mice with hepatocyte-specific deficiency for Alk2 or Alk3 were injected with an AAV2/8 expressing HJV-Myc-DDK under the control of a liver-specific promoter or PBS as a control. Fourteen days after virus administration, blood and organs were harvested and the effect of HJV overexpression on iron parameters and hepcidin expression was investigated. First, the efficiency of Alk2 or Alk3 knockdown and of HJV overexpression were verified. Hepatocyte-specific Alk2-deficient mice had an 84% reduction in Alk2 messenger RNA (mRNA) levels compared with control mice, and hepatocyte-specific Alk3-deficient mice had a 93% reduction in Alk3 mRNA levels compared with control mice (Figure 1A-B). The successful in vivo, liver-specific AAV-HJV–mediated overexpression of HJV was confirmed by qRT-PCR and immunoblotting (Figure 1C-D; also previously reported22). It has been previously published that HJV-Myc-DDK is detected in membrane-enriched fractions of the liver of animals injected with AAV-HJV22 (Figure 1D). In control mice and in mice with hepatocyte-specific Alk2 or Alk3 deficiency, hepatic Hjv mRNA and HJV protein levels (in the form of 2 bands, as described previously22) were increased to a similar extent (Figure 1C; supplemental Figure 2). HJV was not detected in a hepatic sample of a Hjv-knockout mouse (supplemental Figure 2C). Liver-specific targeting of AAV2/823 was confirmed by lack of HJV protein expression in the spleen (supplemental Figure 2D). The data indicate that all mice injected with AAV-HJV were successfully overexpressing HJV in the liver in vivo.
Intravenous AAV2/8-ALB-mHFE2-MycDDK (abbreviated as AAV-HJV) injection induces liver-specific HJV expression. Hepatocyte-specific Alk2-deficient mice (Alk2fl/fl;Alb-Cre), hepatocyte-specific Alk3-deficient mice (Alk3fl/fl;Alb-Cre), and their respective controls (Alk2fl/fl or Alk3fl/fl; ≥5 mice per group) at 8 weeks of age were intravenously injected with 5 × 1011 particles of an AAV2/8 expressing Hjv-MycDDK under the control of a liver-specific promotor or PBS, and euthanized 2 weeks later. (A) Alk2 mRNA levels (control PBS, n = 10; control AAV-HJV, n = 10; Alk2fl/fl;Alb-Cre PBS, n = 7; Alk2fl/fl;Alb-Cre AAV-HJV, n = 5; Alk3fl/fl;Alb-Cre PBS, n = 6; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 6) and (B) Alk3 mRNA levels in the liver were determined by qRT-PCR to verify knockdown efficiency. 18S ribosomal RNA was used as an internal control (control PBS, n = 10; control AAV-HJV, n = 10; Alk2fl/fl;Alb-Cre PBS, n = 7; Alk2fl/fl;Alb-Cre AAV-HJV, n = 5; Alk3fl/fl;Alb-Cre PBS, n = 6; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 6). (C) Relative hepatic Hjv mRNA levels were determined by qRT-PCR. Transcripts were normalized to 18S ribosomal RNA, and the average of control mice treated with PBS was set to 1 (control PBS, n = 14; control AAV-HJV, n = 10; Alk2fl/fl;Alb-Cre PBS, n = 7; Alk2fl/fl;Alb-Cre AAV-HJV, n = 5; Alk3fl/fl;Alb-Cre PBS, n = 5; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 6). (D) HJV protein levels were determined to validate the hepatic overexpression of HJV. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal control. Data are presented as box plots with minimum to maximum whiskers. Significances were presented relative to the indicated control with ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. (Alk2fl/fl PBS, n = 3; Alk2fl/fl;Alb-Cre PBS, n = 3; Alk2fl/fl AAV-HJV, n = 4; Alk2fl/fl;Alb-Cre AAV-HJV, n = 4; Alk3fl/fl PBS, n = 3; Alk3fl/fl;Alb-Cre PBS, n = 3; Alk3fl/fl AAV-HJV, n = 3; Alk3fl/fl;Alb-Cre AAV-HJV, n = 3).
Intravenous AAV2/8-ALB-mHFE2-MycDDK (abbreviated as AAV-HJV) injection induces liver-specific HJV expression. Hepatocyte-specific Alk2-deficient mice (Alk2fl/fl;Alb-Cre), hepatocyte-specific Alk3-deficient mice (Alk3fl/fl;Alb-Cre), and their respective controls (Alk2fl/fl or Alk3fl/fl; ≥5 mice per group) at 8 weeks of age were intravenously injected with 5 × 1011 particles of an AAV2/8 expressing Hjv-MycDDK under the control of a liver-specific promotor or PBS, and euthanized 2 weeks later. (A) Alk2 mRNA levels (control PBS, n = 10; control AAV-HJV, n = 10; Alk2fl/fl;Alb-Cre PBS, n = 7; Alk2fl/fl;Alb-Cre AAV-HJV, n = 5; Alk3fl/fl;Alb-Cre PBS, n = 6; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 6) and (B) Alk3 mRNA levels in the liver were determined by qRT-PCR to verify knockdown efficiency. 18S ribosomal RNA was used as an internal control (control PBS, n = 10; control AAV-HJV, n = 10; Alk2fl/fl;Alb-Cre PBS, n = 7; Alk2fl/fl;Alb-Cre AAV-HJV, n = 5; Alk3fl/fl;Alb-Cre PBS, n = 6; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 6). (C) Relative hepatic Hjv mRNA levels were determined by qRT-PCR. Transcripts were normalized to 18S ribosomal RNA, and the average of control mice treated with PBS was set to 1 (control PBS, n = 14; control AAV-HJV, n = 10; Alk2fl/fl;Alb-Cre PBS, n = 7; Alk2fl/fl;Alb-Cre AAV-HJV, n = 5; Alk3fl/fl;Alb-Cre PBS, n = 5; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 6). (D) HJV protein levels were determined to validate the hepatic overexpression of HJV. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal control. Data are presented as box plots with minimum to maximum whiskers. Significances were presented relative to the indicated control with ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. (Alk2fl/fl PBS, n = 3; Alk2fl/fl;Alb-Cre PBS, n = 3; Alk2fl/fl AAV-HJV, n = 4; Alk2fl/fl;Alb-Cre AAV-HJV, n = 4; Alk3fl/fl PBS, n = 3; Alk3fl/fl;Alb-Cre PBS, n = 3; Alk3fl/fl AAV-HJV, n = 3; Alk3fl/fl;Alb-Cre AAV-HJV, n = 3).
HJV overexpression induced hepcidin levels in control mice
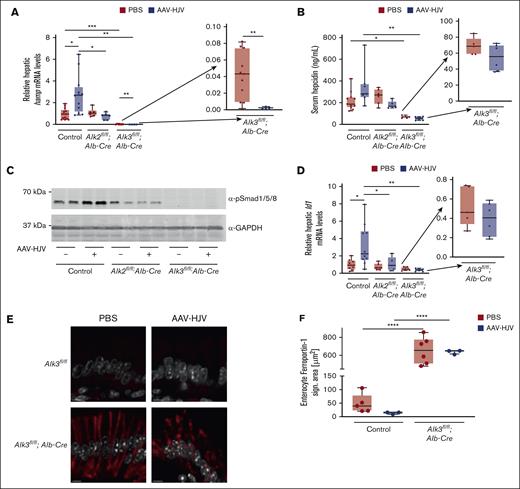
To investigate the effect of HJV overexpression on BMP signaling, hepatic Hamp mRNA and serum hepcidin levels were determined. The overexpression of HJV increased hepatic Hamp mRNA levels in control mice injected with AAV-HJV compared with PBS-injected control mice (Figure 2A). In Alk2fl/fl;Alb-Cre mice the basal expression of Hamp mRNA was not altered, but HJV overexpression resulted in slightly reduced Hamp mRNA expression, albeit not significant. However, in Alk3fl/fl;Alb-Cre mice baseline Hamp mRNA expression was already heavily reduced, which was further suppressed by the administration of AAV-HJV (inlay, Figure 2A).
HJV overexpression in control animals induced BMP signaling and increased hepcidin expression. (A) Relative hepatic hepcidin (Hamp) mRNA levels were determined by qRT-PCR. Transcripts were normalized to 18S ribosomal RNA as internal controls, and the average of respective Alk2fl/fl and Alk3fl/fl control mice was set to 1 (control PBS, n = 13; control AAV-HJV, n = 12; Alk2fl/fl;Alb-Cre PBS, n = 6; Alk2fl/fl;Alb-Cre AAV-HJV, n = 6; Alk3fl/fl;Alb-Cre PBS, n = 11; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 5). (B) Serum hepcidin levels were decreased in hepatocyte-specific Alk3-deficient mice compared with control animals as determined by enzyme-linked immunosorbent assay (control PBS, n = 11; control AAV-HJV, n = 7; Alk2fl/fl;Alb-Cre PBS, n = 6; Alk2fl/fl;Alb-Cre AAV-HJV, n = 5; Alk3fl/fl;Alb-Cre PBS, n = 5; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 6). (C) HJV overexpression in control animals increased the level of pSMAD1/5/8 determined by immunoblotting. GAPDH was used as a loading control. (D) Hepatic Id1 mRNA levels were determined by qRT-PCR. Transcripts were normalized to 18S ribosomal RNA, and the average of control mice treated with PBS was set to 1 (control PBS, n = 8; control AAV-HJV, n =10; Alk2fl/fl;Alb-Cre PBS, n = 6; Alk2fl/fl;Alb-Cre AAV-HJV, n = 5; Alk3fl/fl;Alb-Cre PBS, n = 5; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 6). (E) Ferroportin staining (red) and DAPI (4′,6-diamidino-2-phenylindole) staining (white) of representative duodenal sections of control mice and Alk3fl/fl;Alb-Cre mice injected with PBS or AAV-HJV are shown. (F) In Alk3fl/fl;Alb-Cre mice the ferroportin signal is increased compared with control littermates. AAV-HJV injection in control mice decreased the ferroportin signal, albeit not significant. In Alk3fl/fl;Alb-Cre mice there is no difference in ferroportin signal detectable between AAV-HJV–injected mice and PBS-injected mice (control PBS, n = 5; control AAV-HJV, n = 3; Alk3fl/fl;Alb-Cre PBS, n = 6; and Alk2fl/fl;Alb-Cre AAV-HJV, n = 3). Data are presented as box plots with minimum to maximum whiskers and medians. Significances were presented relative to the indicated control with ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001.
HJV overexpression in control animals induced BMP signaling and increased hepcidin expression. (A) Relative hepatic hepcidin (Hamp) mRNA levels were determined by qRT-PCR. Transcripts were normalized to 18S ribosomal RNA as internal controls, and the average of respective Alk2fl/fl and Alk3fl/fl control mice was set to 1 (control PBS, n = 13; control AAV-HJV, n = 12; Alk2fl/fl;Alb-Cre PBS, n = 6; Alk2fl/fl;Alb-Cre AAV-HJV, n = 6; Alk3fl/fl;Alb-Cre PBS, n = 11; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 5). (B) Serum hepcidin levels were decreased in hepatocyte-specific Alk3-deficient mice compared with control animals as determined by enzyme-linked immunosorbent assay (control PBS, n = 11; control AAV-HJV, n = 7; Alk2fl/fl;Alb-Cre PBS, n = 6; Alk2fl/fl;Alb-Cre AAV-HJV, n = 5; Alk3fl/fl;Alb-Cre PBS, n = 5; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 6). (C) HJV overexpression in control animals increased the level of pSMAD1/5/8 determined by immunoblotting. GAPDH was used as a loading control. (D) Hepatic Id1 mRNA levels were determined by qRT-PCR. Transcripts were normalized to 18S ribosomal RNA, and the average of control mice treated with PBS was set to 1 (control PBS, n = 8; control AAV-HJV, n =10; Alk2fl/fl;Alb-Cre PBS, n = 6; Alk2fl/fl;Alb-Cre AAV-HJV, n = 5; Alk3fl/fl;Alb-Cre PBS, n = 5; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 6). (E) Ferroportin staining (red) and DAPI (4′,6-diamidino-2-phenylindole) staining (white) of representative duodenal sections of control mice and Alk3fl/fl;Alb-Cre mice injected with PBS or AAV-HJV are shown. (F) In Alk3fl/fl;Alb-Cre mice the ferroportin signal is increased compared with control littermates. AAV-HJV injection in control mice decreased the ferroportin signal, albeit not significant. In Alk3fl/fl;Alb-Cre mice there is no difference in ferroportin signal detectable between AAV-HJV–injected mice and PBS-injected mice (control PBS, n = 5; control AAV-HJV, n = 3; Alk3fl/fl;Alb-Cre PBS, n = 6; and Alk2fl/fl;Alb-Cre AAV-HJV, n = 3). Data are presented as box plots with minimum to maximum whiskers and medians. Significances were presented relative to the indicated control with ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001.
There is a positive correlation between hepatic Hamp mRNA levels and Hjv mRNA levels in control mice (R = 0.66; supplemental Figure 3A), whereas hepatic Hamp mRNA levels and Hjv mRNA levels negatively correlated in Alk2fl/fl;Alb-Cre and Alk3fl/fl;Alb-Cre mice (R = −0.44; supplemental Figure 3B). The data indicated a dependency of HJV and hepcidin that is absent in mice with a deficiency of either of the 2 BMP type I receptors Alk2 or Alk3. Serum hepcidin levels were also measured and showed a similar trend as that of the Hamp mRNA levels, albeit differences were not statistically significant (Figure 2B).
In order to show that HJV overexpression induces hepcidin levels by activation of the BMP signaling pathway, the phosphorylation of SMAD1/5/8 proteins and the expression levels of the BMP target gene Id1 were investigated. pSMAD1/5/8 protein levels were mildly increased in AAV-HJV control mice when compared with PBS-injected mice, whereas there was little signaling activity in Alk2fl/fl;Alb-Cre mice and no activity in Alk3fl/fl;Alb-Cre mice (Figure 2C; supplemental Figure 3C), which confirmed our previous finding that Alk3fl/fl;Alb-Cre mice are characterized by very low pSMAD1/5/8 levels.8 The expression levels of Id1 were increased in control mice injected with AAV-HJV, whereas the administration of AAV-HJV had no effect on Id1 mRNA levels in Alk2fl/fl;Alb-Cre and Alk3fl/fl;Alb-Cre mice (Figure 2D). Because hepcidin directly regulates the surface expression of ferroportin, we assessed its level in intestine using immunofluorescence and confocal microscopy (Figure 2E-F). In untreated control mice ferroportin was barely detectable (Figure 2E, top panel); despite this, ferroportin level tended to decrease after the application of AAV-HJV (Figure 2F). In mice lacking ALK3 in hepatocytes (Alk3fl/fl;Alb-Cre mice) ferroportin levels were markedly increased because of hepcidin deficiency (Figure 2E, bottom). Hamp mRNA was even further decreased in the latter mice after HJV injection, and ferroportin protein levels remained high (Figure 2F). The data indicate that HJV overexpression induces hepcidin in control mice but not in Alk2fl/fl;Alb-Cre and Alk3fl/fl;Alb-Cre mice by activation of the BMP-SMAD signaling pathway. Interestingly, the loss of BMP signal transduction in Alk2fl/fl;Alb-Cre and Alk3fl/fl;Alb-Cre mice could not be rescued by HJV overexpression, but rather this abolished the positive correlation of HJV to hepcidin that was detected in control mice injected with AAV-HJV.
Effect of HJV overexpression on iron homeostasis
Hepcidin is the major regulator of systemic iron homeostasis and overexpression of HJV induced hepcidin levels in controls but not in Alk2fl/fl;Alb-Cre and Alk3fl/fl;Alb-Cre mice. Because hepatocyte-specific Alk2 and Alk3 deficiency causes moderate and severe iron overload, respectively,8 we investigated the effect of HJV overexpression on systemic iron homeostasis, serum iron parameters, and tissue iron contents in Alk2fl/fl;Alb-Cre and Alk3fl/fl;Alb-Cre mice.
Serum iron levels and transferrin saturation were unchanged, with a slight trend to a reduction in control animals upon overexpression of HJV- compared with PBS-injected animals (Figure 3A-B). In Alk2fl/fl;Alb-Cre mice, the injection of AAV-HJV resulted in the opposite effect: an increase in serum iron levels and transferrin saturation compared with PBS-injected littermates. This effect is in line with a trend of decreased hepcidin levels and the lack of increased BMP signaling transduction in these mice. The increase in serum iron, however, was stronger than the observed decrease in hepcidin. In Alk3fl/fl;Alb-Cre mice with already severe iron overload and increased iron parameters, AAV-HJV did not further change iron parameters compared with PBS-injected littermates.
HJV overexpression affects iron homeostasis in mice. (A) Serum iron levels (control PBS, n = 8; control AAV-HJV, n = 5; Alk2fl/fl;Alb-Cre PBS, n = 6; Alk2fl/fl;Alb-Cre AAV-HJV, n = 5; Alk3fl/fl;Alb-Cre PBS, n = 5; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 6) and (B) transferrin saturation were determined to analyze systemic iron homeostasis (control PBS, n = 9; control AAV-HJV, n = 5; Alk2fl/fl;Alb-Cre PBS, n = 6; Alk2fl/fl;Alb-Cre AAV-HJV, n = 5; Alk3fl/fl;Alb-Cre PBS, n = 5; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 6). (C) Liver iron content was measured to determine tissue iron retention (control PBS, n = 11; control AAV-HJV, n = 9; Alk2fl/fl;Alb-Cre PBS, n = 7; Alk2fl/fl;Alb-Cre AAV-HJV, n = 5; Alk3fl/fl;Alb-Cre PBS, n = 6; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 6). (D) Hepatic Bmp6 mRNA levels were determined by qRT-PCR. 18S ribosomal RNA was used as an internal control and the average of control mice treated with PBS was set to 1 (control PBS, n = 10; control AAV-HJV, n = 10; Alk2fl/fl;Alb-Cre PBS, n = 7; Alk2fl/fl;Alb-Cre AAV-HJV, n = 5; Alk3fl/fl;Alb-Cre PBS, n = 6; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 6). Data are presented as box plots with minimum to maximum whiskers and medians. Significances were presented relative to the indicated control with ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
HJV overexpression affects iron homeostasis in mice. (A) Serum iron levels (control PBS, n = 8; control AAV-HJV, n = 5; Alk2fl/fl;Alb-Cre PBS, n = 6; Alk2fl/fl;Alb-Cre AAV-HJV, n = 5; Alk3fl/fl;Alb-Cre PBS, n = 5; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 6) and (B) transferrin saturation were determined to analyze systemic iron homeostasis (control PBS, n = 9; control AAV-HJV, n = 5; Alk2fl/fl;Alb-Cre PBS, n = 6; Alk2fl/fl;Alb-Cre AAV-HJV, n = 5; Alk3fl/fl;Alb-Cre PBS, n = 5; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 6). (C) Liver iron content was measured to determine tissue iron retention (control PBS, n = 11; control AAV-HJV, n = 9; Alk2fl/fl;Alb-Cre PBS, n = 7; Alk2fl/fl;Alb-Cre AAV-HJV, n = 5; Alk3fl/fl;Alb-Cre PBS, n = 6; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 6). (D) Hepatic Bmp6 mRNA levels were determined by qRT-PCR. 18S ribosomal RNA was used as an internal control and the average of control mice treated with PBS was set to 1 (control PBS, n = 10; control AAV-HJV, n = 10; Alk2fl/fl;Alb-Cre PBS, n = 7; Alk2fl/fl;Alb-Cre AAV-HJV, n = 5; Alk3fl/fl;Alb-Cre PBS, n = 6; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 6). Data are presented as box plots with minimum to maximum whiskers and medians. Significances were presented relative to the indicated control with ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
As previously published,8 liver iron content was increased in hepatocyte-specific Alk3-deficient mice and was modestly greater in Alk2fl/fl;Alb-Cre mice than in control animals, albeit not significant (P = .06) in contrast to our previous publication (characterization of 12-week-old female mice with a higher number of mice per group). The overexpression of HJV had no effect on liver iron content in all groups of mice (Figure 3C). The expression of the BMP ligand BMP6 is induced by the transcription factor NRF2 in response to iron loading in the liver.24 Because prolonged HJV overexpression over 2 weeks had no effect on liver iron content, BMP6 mRNA levels were unchanged in AAV-HJV–injected controls, as expected. In AAV-HJV–injected Alk3fl/fl;Alb-Cre mice compared with AAV-HJV–injected controls, BMP6 levels were higher (Figure 3D). This effect is provoked by the Alk3 deficiency with severe iron overload, because Bmp6 mRNA levels are induced in response to high hepatic iron loading.8
The amount of erythroid progenitor cells was determined by CD44/TER119 staining via flow cytometry analysis. A reduction in erythropoiesis as potential cause for the increased serum iron concentration was excluded, because erythropoiesis was similar in all groups of mice with and without AAV-HJV overexpression (data not shown). Therefore, the increase in serum iron in hepatocyte-specific Alk2-deficient mice is most likely caused by the hepcidin induction.
HJV overexpression increased SIC in control mice
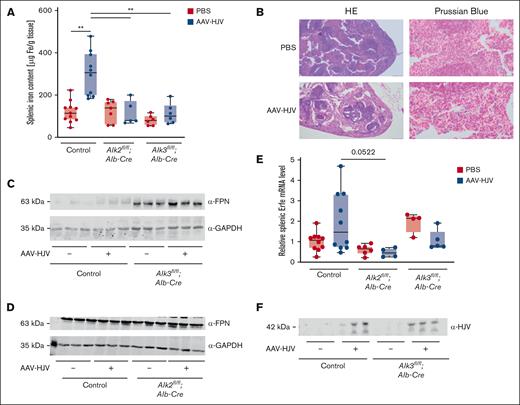
Because serum iron content seemed to be more affected by HJV overexpression than liver iron content, we also investigated splenic iron content (SIC). In control mice, HJV overexpression led to an increase in SIC compared with PBS-injected controls, but not in Alk2fl/fl;Alb-Cre mice nor Alk3fl/fl;Alb-Cre mice injected with AAV-HJV (Figure 4A). Also, Prussian blue staining revealed iron retention in the spleen in HJV-overexpressing control mice (Figure 4B).
HJV overexpression causes splenic iron retention in control animals. (A) SIC was increased in control animals after HJV overexpression (control PBS, n = 11; control AAV-HJV, n = 10; Alk2fl/fl;Alb-Cre PBS, n = 7; Alk2fl/fl;Alb-Cre AAV-HJV, n = 5; Alk3fl/fl;Alb-Cre PBS, n = 6; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 6). (B) Iron retention in the spleen was confirmed by Prussian blue staining. (C-D) Splenic ferroportin protein levels were determined by immunoblotting. GAPDH was used as internal control. (E) Splenic Erfe mRNA levels were determined by qRT-PCR. 18S ribosomal RNA was used as an internal control (control PBS, n = 10; control AAV-HJV, n = 10; Alk2fl/fl;Alb-Cre PBS, n = 6; Alk2fl/fl;Alb-Cre AAV-HJV, n = 4; Alk3fl/fl;Alb-Cre PBS, n = 4; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 5). (F) Serum sHJV protein levels were determined by immunoblotting; n = 3 per group. Significances were presented relative to the indicated control with ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
HJV overexpression causes splenic iron retention in control animals. (A) SIC was increased in control animals after HJV overexpression (control PBS, n = 11; control AAV-HJV, n = 10; Alk2fl/fl;Alb-Cre PBS, n = 7; Alk2fl/fl;Alb-Cre AAV-HJV, n = 5; Alk3fl/fl;Alb-Cre PBS, n = 6; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 6). (B) Iron retention in the spleen was confirmed by Prussian blue staining. (C-D) Splenic ferroportin protein levels were determined by immunoblotting. GAPDH was used as internal control. (E) Splenic Erfe mRNA levels were determined by qRT-PCR. 18S ribosomal RNA was used as an internal control (control PBS, n = 10; control AAV-HJV, n = 10; Alk2fl/fl;Alb-Cre PBS, n = 6; Alk2fl/fl;Alb-Cre AAV-HJV, n = 4; Alk3fl/fl;Alb-Cre PBS, n = 4; and Alk3fl/fl;Alb-Cre AAV-HJV, n = 5). (F) Serum sHJV protein levels were determined by immunoblotting; n = 3 per group. Significances were presented relative to the indicated control with ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Retention of iron in the spleen is known as an effect to prevent iron release in settings of hepcidin induction, here, mediated via AAV-HJV. In Alk3fl/fl;Alb-Cre mice but not in Alk2fl/fl;Alb-Cre mice, the protein expression of hepatic ferritin heavy chain, the iron storage protein, was further decreased after HJV overexpression (supplemental Figure 4A-D), which is in line with reduced hepatic hepcidin levels (Figure 2A). These data indicate that HJV overexpression causes hepcidin-mediated iron uptake and retention in the spleen of control mice but not in hepatocyte-specific Alk2- or Alk3-deficient mice. In order to determine whether splenic iron retention in AAV-HJV–injected control mice was caused by degradation of ferroportin, splenic ferroportin protein levels were analyzed. In Alk3fl/fl;Alb-Cre mice, ferroportin protein expression was increased, which is in line with the reported severe iron overload of the phenotype and hepcidin deficiency, and the injection of AAV-HJV had no impact on ferroportin protein levels (Figure 4C-D). In PBS-injected control mice, splenic ferroportin protein levels were low, and the overexpression of HJV slightly increased ferroportin protein levels. A potential explanation is the previously reported occlusion of ferroportin by hepcidin in AAV-HJV injected control mice.25,26
sHJV is increased by AAV-HJV overexpression
The effect of HJV-mediated hepcidin induction and splenic iron retention in control mice was absent in hepatocyte-specific Alk2- and Alk3-deficient mice. Interestingly, the overexpression of HJV suppressed Hamp mRNA levels in hepatocyte-specific Alk3-deficient mice and to a lesser extent in hepatocyte-specific Alk2-deficient mice but led to a serum iron and transferrin saturation increase in Alk2fl/fl;Alb-Cre mice. Both findings indicate a reduced activation of the BMP-SMAD signaling pathway. To validate whether ERFE, a known hepcidin antagonist, is responsible for the observed decrease in hepcidin expression in hepatocyte-specific Alk3 mice, splenic Erfe mRNA levels were measured. In controls, the average of Erfe mRNA levels did not change upon HJV overexpression when compared to controls. In mice with hepatocyte-specific Alk2 deficiency, splenic Erfe mRNA levels trended to be lower than in HJV-overexpressed controls (Figure 4E, P = 0.0522). sHJV is known to circulate in the plasma and act as a decoy receptor. It binds BMP ligands and thereby inhibits the induction of hepcidin and even leads to a decrease.14 To consider this second possibility that plasmatic sHJV is responsible for the observed effects, sHJV was determined in serum samples of AAV-HJV–injected animals. In comparison with PBS-injected littermates, a 42-kDa sHJV form was detected in the sera of all AAV-HJV–injected mice (Figure 4F).
Discussion
HJV is a GPI-anchored protein of the repulsive guidance molecule family that acts as a BMP coreceptor. This work further investigated the role of HJV in iron homeostasis. We show that HJV signals via both BMP type I receptors ALK2 and ALK3 in vivo using an established model of AAV-mediated overexpression of HJV in hepatocyte-specific Alk2- or Alk3-deficient murine models. HJV overexpression in control mice causes an induction of HJV, sHJV, Hamp, and Id1 mRNA levels, potentially by inducing pSMAD1/5/8 levels, along with decreased serum iron and transferrin saturation. Although the liver iron content remains unchanged, SIC increases. The requirement of ALK2 is shown, because there is no induction of BMP signaling in hepatocyte-specific Alk2-deficient mice after HJV overexpression. Interestingly, the contrary occurs: serum iron and transferrin saturation are highly induced, whereas Hamp mRNA levels show a decreased trend. These data indicate a further reduction in BMP-SMAD signaling. In mice with hepatocyte-specific Alk2 deficiency, ALK3 is still present, but unable to mediate hepcidin induction via HJV. The necessity for ALK3 in HJV-mediated hepcidin induction is shown, because hepatocyte-specific Alk3-deficient mice have severely decreased BMP signaling that cannot be activated by HJV.
Our data are in line with results that have been published by Xia et al, who reported that HJV can use all three BMP type I receptors (ALK2, ALK3, and ALK6) in vitro to induce hepcidin expression.13 To the best of our knowledge, in vivo studies have not yet been performed. Because only ALK2 and ALK3 are detected in the human liver13 and are required for hepcidin induction and systemic iron homeostasis,8 the role of these two receptors in HJV-mediated BMP signaling have been demonstrated in vivo. Previous studies suggest the formation of functionally active ALK3/ALK3 homodimers, ALK2/ALK3 heterodimers,9 and ALK2/ALK2 homodimers.7 In this manuscript it was demonstrated that both ALK2 and ALK3 are required for intact HJV signaling in vivo, suggesting that HJV may signal predominantly via the heterodimeric BMP type I receptor complex of ALK2/ALK3 and not via the homodimeric equivalent. Of note, in mice with hepatocyte-specific Alk2 deficiency, signaling via ALK3/ALK3 homodimers still persists and hepcidin levels are maintained within normal, to mildly depleted, levels. Induction of the BMP pathway by iron or BMP ligands is, however, not feasible.8 In contrast, in mice with a hepatocyte-specific Alk3 deficiency, neither the ALK3/ALK3 homodimers nor the ALK2/ALK3 heterodimers exist and BMP signaling is almost completely abrogated under basal, as well as stimulatory, conditions.8,27 These data raise the question whether the iron overload phenotype of Alk3-deficient mice and Hjv-deficient mice is of similar severity. To date, no experiments have been performed to directly compare the iron overload phenotypes of Alk3fl/fl;Alb-Cre mice with the iron overload phenotype of Hjv knockout mice. In terms of the importance of ALK2 and ALK3 in iron homeostasis, it is well known that ALK3 plays a more important role in vivo than ALK2.
It is therefore reasonable, and prior data indicate, that ALK3 homodimers play a more important role in hepcidin induction than ALK2 homodimers.9 However, the hepatocyte-specific deletion of both Alk2 and Alk3 lead to a more severe iron overload phenotype than the hepatocyte-specific deletion of Alk3 alone, suggesting that ALK2/ALK3 heterodimers are essential for HJV-mediated hepcidin signaling in vivo.9
Here, the overexpression of HJV in Alk2fl/fl;Alb-Cre mice and in Alk3fl/fl;Alb-Cre mice did not rescue the loss in BMP-SMAD signaling but led to an increase in circulating serum iron levels and transferrin saturation. The observed decrease in Hamp mRNA levels in hepatocyte-specific Alk3-deficient mice and to a lesser extent in hepatocyte-specific Alk2-deficient mice may be caused by unknown mechanisms induced by sHJV in vivo. Cleavage of HJV either at its furine cleavage site, the TMPRSS6 site, or at the autocleavage site, results in the smaller non–membrane bound form sHJV.12,28 sHJV inhibits BMP signaling and reduces hepcidin expression in vitro by binding and sequestering BMP ligands, predominately BMP6. As a consequence, BMP6 cannot interact with its receptors, and fails to induce hepcidin expression.29 sHJV can also bind BMP ligands in mice with hepatocyte-specific Alk2 or Alk3 deficiency. Therefore, binding of sHJV may result in reduced hepcidin expression and increased serum iron levels as we observed in mice with hepatocyte-specific Alk2 or Alk3 deficiency. In control animals, the overexpression of HJV also causes an increase in sHJV, but the BMP receptors required are functional and therefore BMP-SMAD signaling might be dominant over sHJV, and BMP ligand binding induces hepcidin expression.
One possible limitation of our study is that the overexpression of HJV may result in supraphysiological amounts of HJV and sHJV. All changes observed in iron homeostasis and BMP-SMAD signaling might not occur under physiological conditions. Increased HJV and sHJV levels may have an artificial effect on hepcidin synthesis and may cause a higher activation of BMP-SMAD signaling and hepcidin synthesis than in nonstimulated scenarios. However, to investigate the interaction of HJV in vivo, the method of AAV-HJV overexpression allows an in vivo setting, which is preferable to cellular models.
The observed effect of HJV overexpression on serum iron levels in hepatocyte-specific Alk2-deficient mice was impressive compared with the moderate hepcidin decrease. In comparison with hepatocyte-specific Alk3-deficient mice the induction was also more dominant. This may reflect the high baseline iron levels of hepatocyte-specific Alk3-deficient mice because of very low hepcidin levels and the severe iron overload phenotype of these mice.8 Therefore, the overexpression of HJV and the increase in sHJV does not lead to a change in serum iron levels in hepatocyte-specific Alk3-deficient mice, because serum iron and transferrin saturation are already saturated. The strong serum iron induction in mice with hepatocyte-specific Alk2 deficiency injected with AAV-HJV raises the question of where the iron comes from. There are several hypotheses: first, HJV causes hepcidin-mediated inactivation of ferroportin and thereby decreases serum iron levels. In mice with Alk2 deficiency, hepcidin induction does not occur because of deficiency of the BMP type I receptor ALK2. Therefore, ferroportin remains expressed and leads to an induction of serum iron. A second possibility might be that sHJV directly activates ferroportin in the setting of low hepcidin levels to increase iron absorption from the intestine, thereby increasing iron parameters in hepatocyte-specific Alk2-deficient mice. And third, another independent different mechanism could interfere and lead to a release of iron from iron storage cells. The hepcidin antagonist ERFE might be a potential candidate. The overexpression of HJV led to an induction of hepcidin. In splenic Erfe mRNA levels, there was a high variability observed in control mice in terms of induction and suppression of single values, which was missing in mice with hepatocyte-specific Alk2 or Alk3 deficiency.
In this study, the overexpression of HJV in control animals caused an induction in hepcidin expression and consequently splenic iron retention. These results are in contrast to those of Zhang et al, who reported that the AAV-mediated overexpression of HJV had no effect on iron status and hepcidin expression in wild-type mice.30 In this study, mice on a C57BL/6 background were used, whereas Zhang et al characterized the effect of HJV overexpression in mice on a 129/SvEvTac background. Mice with a different genetic background show differences in iron homeostasis31, and it is suggested that the contradictory results are a result of the different genetic mouse backgrounds used in both studies.
In conclusion, the data indicate that both ALK2 and ALK3 are functionally important for HJV-mediated hepcidin induction. sHJV induced by AAV-HJV can bind and thereby decrease BMP-SMAD signaling in vivo. HJV overexpression did not induce hepcidin expression in neither hepatocyte-specific Alk2- nor hepatocyte-specific Alk3-deficient mice, as it does in controls. Therefore, the use of the ALK2/3 heterodimer is most probably required for signaling and should be investigated in future studies.
Acknowledgments
The authors thank Verena Gröning for helping with the AAV2/8-ALB-mHFE2-Myc-DDK injection.
This work was supported by a grant from the German Research Foundation within the research consortium FerrOs FOR5146 (STE 1895/9-1; to A.U.S).
D.Y.D, I.H., L.S., P.K., and U.K. are part of the FerrOs consortium.
Authorship
Contribution: D.Y.D. performed experiments, interpreted data, and wrote the manuscript; E.I.U., I.H., P.K., D.O., F.M.F., L.H.N., F.R., E.G., A.V.K., R.P., and I.F. performed experiments; U.K. and K.Z. interpreted data and wrote the manuscript; L.S. oversaw the study, performed experiments, interpreted data, and wrote the manuscript; and A.U.S. planned the project, conceived and oversaw the study, performed experiments, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: K.Z. declares that the Department of Anaesthesiology, Intensive Care Medicine & Pain Therapy of the University Hospital Frankfurt, Goethe University received support from B. Braun Melsungen, CSL Behring, Fresenius Kabi, and Vifor Pharma for the implementation of Frankfurt‘s Patient Blood Management program. K.Z. has received honoraria for participation in advisory board meetings for Haemonetics and Vifor; received speaker fees from CSL Behring, Masimo, Pharmacosmos, Boston Scientific, Salus, iSEP, and Edwards and GE Healthcare; and serves as the principal investigator of the EU-Horizon 2020 project ENVISION (Intelligent plug-and-play digital tool for real-time surveillance of patients with COVID-19 and smart decision-making in intensive care units) and the Horizon Europe 2021 project COVend (Biomarker and AI-supported FX06 therapy to prevent progression from mild and moderate to severe stages of COVID-19). The remaining authors declare no competing financial interests.
Correspondence: Andrea U. Steinbicker, Department of Anesthesiology, Intensive Care and Pain Medicine, University Hospital Frankfurt, Goethe University Frankfurt, Theodor-Stern-Kai 7, Building 13, 60590 Frankfurt Am Main, Germany; email: steinbicker@em.uni-frankfurt.de.
References
Author notes
L.S. and A.U.S. contributed equally to this study.
Protocols and data sets are available on request from the corresponding author, Andrea U. Steinbicker (steinbicker@em.uni-frankfurt.de).
The full-text version of this article contains a data supplement.