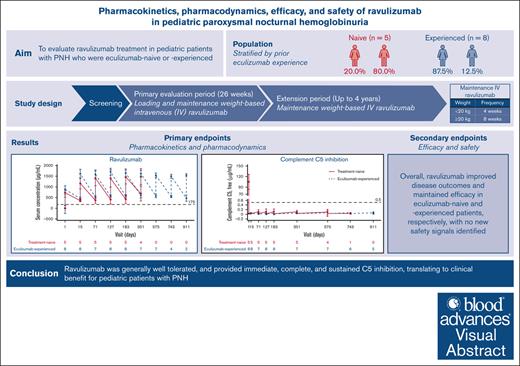
Key Points
PNH is a rare blood disease that can affect children, with symptoms that may result in fatal complications if left untreated.
In children with PNH ravulizumab controlled terminal complement and relieved symptoms with mostly mild treatment-related side effects.
Visual Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare hematologic disease of uncontrolled terminal complement activation leading to intravascular hemolysis, thrombotic events and increased morbidity and mortality. This phase 3, open-label, single-arm, multicenter study evaluated ravulizumab treatment in eculizumab-naive or -experienced pediatric patients (aged <18 years) with PNH over a 26-week primary evaluation period (PEP) and 4-year extension period (EP). Patients included in the study received weight–based intravenous ravulizumab dosing. Primary end points were pharmacokinetic and pharmacodynamic parameters to confirm complement component 5 (C5) inhibition by ravulizumab; secondary end points assessed the efficacy (including percentage change in lactate dehydrogenase levels over time) and safety of ravulizumab. Thirteen patients, 5 (38.5%) eculizumab-naive and 8 (61.5%) eculizumab-experienced, were enrolled. Ravulizumab Ctrough levels were above the pharmacokinetic threshold of 175 μg/mL in the PEP and EP except in 1 patient. At the end of the study, pre- and post-infusion mean ± standard deviation serum ravulizumab concentrations were 610.50 ± 201.53 μg/mL and 518.29 ± 109.67 μg/mL for eculizumab-naive and eculizumab-experienced patients, respectively. After the first ravulizumab infusion, serum-free C5 concentrations were <0.5 μg/mL in both cohorts until the end of the study (0.061 ± 0.021 μg/mL and 0.061 ± 0.018 μg/mL for eculizumab-naive and eculizumab-experienced patients, respectively). Compared with baseline, ravulizumab improved and maintained efficacy outcomes in both groups. Ravulizumab had an acceptable safety profile with no new safety signals identified, and provided immediate, complete, and sustained terminal complement inhibition, translating to clinical benefit for pediatric patients with PNH. This trial was registered at www.ClinicalTrials.gov as #NCT03406507.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, chronic hematologic disorder associated with uncontrolled terminal complement activation on the surface of blood cells resulting in intravascular hemolysis (IVH), a risk of thromboembolic events, and organ damage (eg, kidney impairment and pulmonary hypertension). This directly translates into increased morbidity and mortality if PNH is untreated.1 Patients with PNH usually report symptoms of hemolytic anemia, fatigue, and shortness of breath, which can negatively affect patients’ quality of life.2,3
Onset of hemolytic PNH (including the classical form of PNH or PNH with aplastic anemia) during childhood is less common than during adulthood.4 The onset of pediatric hemolytic PNH has been reported to be 14%, with 86% of hemolytic PNH onset taking place during adulthood.4 According to a study of 18 patients, the proportions of pediatric patients with classical PNH and PNH with associated bone marrow disorder are 28% and 72%, respectively.5 In children, PNH is associated with a higher incidence of acquired bone marrow failure, less severe hemolysis, and possibly a lower incidence of hemoglobinuria compared with adults with the disease.6,7 Both pediatric and adult populations with PNH have considerable morbidity resulting from hemoglobinuria, infection, and thrombosis.8 Although the incidence of thrombosis may be lower in children with PNH compared with adults, it is an important complication of the disease that can result in mortality.7,9 As in adults, early diagnosis and treatment of PNH are key to improving outcomes in pediatric patients.10,11
PNH is caused by the clonal expansion of a hematopoietic progenitor cell lacking glycosylphosphatidylinositol anchored proteins CD55 and CD59 as a result of a somatic PIGA gene mutation, leading to uncontrolled complement-mediated IVH.1,3,12 The only known curative treatment for PNH is hematopoietic stem cell transplantation. Owing to the complications associated with this procedure, hematopoietic stem cell transplantation may not be suitable for all patients.10,13 The terminal complement component 5 (C5) inhibitors eculizumab and ravulizumab are the current standards of care for treating adult patients with PNH since their approval by the US Food and Drug Administration and the European Medicines Agency in 2007 and 2018, respectively.14-17 The main objective of effective treatment for PNH with targeted therapy is to inhibit terminal complement activity to block IVH and prevent thrombosis. It is therefore important to assess the pharmacokinetic (PK) and pharmacodynamic (PD) activity of these treatments on complement C5 and to ensure immediate and complete terminal complement blockade in pediatric patients with PNH. The PK, PD, efficacy, and safety profiles of ravulizumab have been demonstrated in adult patients with PNH. In 2 phase 3 studies of adult patients with PNH, ravulizumab demonstrated noninferiority to eculizumab in complement C5 inhibitor-naive and -experienced patients (301 [NCT02946463] and 302 [NCT03056040] studies, respectively).18-20 Ravulizumab has been shown to result in immediate, complete, and sustained C5 inhibition and control of IVH, and has an approximately 4-times longer terminal half-life than eculizumab. There are reports of a lower rate of intravascular breakthrough hemolysis (BTH) with ravulizumab compared with eculizumab, owing to its improved PK and PD profile.21 Moreover, ravulizumab offers weight-based dosing and a less burdensome dosing regimen than eculizumab; intravenous (IV) infusions are required every 8 weeks (Q8W) with ravulizumab (for adult and pediatric patients weighing ≥20 kg, or every 4 weeks [Q4W] for patients <20 kg)15 as opposed to every 2 weeks (Q2W) with eculizumab.14,22 In pediatric patients with PNH, the PK, PD, efficacy, and safety profiles for ravulizumab may be different to that seen in adults, and therefore it is important to confirm the response to ravulizumab in this population. Additionally, owing to the rarity of PNH in the pediatric population, the PK/PD profile was chosen as the primary end point of the present study.
Here we report the results from the primary evaluation period (PEP) and extension period (EP) from a phase 3 study evaluating the long-term PKs, PDs, efficacy, and safety of ravulizumab treatment in pediatric patients with PNH, who were either eculizumab treatment-naive or treatment-experienced.
Methods
Study design
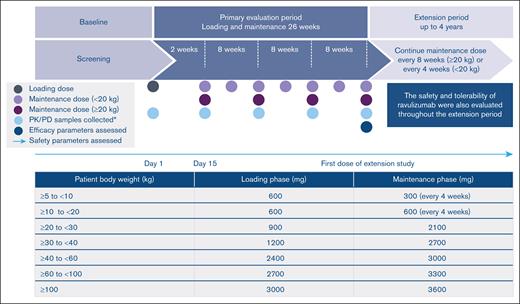
This was a phase 3, open-label, single-arm, multicenter study in pediatric patients with PNH (NCT03406507), who were screened and enrolled across 6 countries (France, the Netherlands, Norway, Russia, the United Kingdom, and the United States). The study included a 4-week screening period, a 26-week PEP, and an EP of up to 4 years (Figure 1).
Study design and weight-based dosing regimen.∗Baseline was defined as last assessment before first ravulizumab dose.
Study design and weight-based dosing regimen.∗Baseline was defined as last assessment before first ravulizumab dose.
This trial was conducted in compliance with the ethical principles of Good Clinical Practice, following the principles of the Declaration of Helsinki and International Conference on Harmonisation. Before protocol procedures were carried out, each patient or their legal representative provided informed consent or assent if applicable. An independent Data Monitoring Committee monitored the study data for patient safety on a regular basis to make recommendations on continuation of study drug administration or termination of the study. All authors had access to primary clinical trial data.
Patients
Male and female patients aged <18 years with a documented diagnosis of PNH, confirmed by high-sensitivity flow cytometry evaluation23 of red and white blood cells with granulocyte or monocyte clone size of ≥5%, were eligible for participation if they met the inclusion criteria. These included a body weight of ≥5 kg at the time of consent; for eculizumab-naive patients, they must have experienced ≥1 of the following PNH symptoms in the 3 months before screening: fatigue, hemoglobinuria, abdominal pain, shortness of breath, anemia, history of a major adverse vascular event (MAVE) (including thrombosis), dysphagia, erectile dysfunction, or had undergone packed red blood cell (pRBC) transfusion owing to PNH; and had lactate dehydrogenase (LDH) values at screening of ≥1.5× upper limit of normal (ULN) for eculizumab-naive patients or ≤1.5× ULN for eculizumab-experienced patients. The LDH normal range in pediatric patients varies depending on age and sex, and increased LDH levels were assessed in all cases according to normalized values. Patients must have been vaccinated against Neisseria meningitidis in the 3 years before, or at time of, initiating study drug, Hemophilus influenzae type b, and Streptococcus pneumoniae, according to national and local vaccination schedule guidelines. Eculizumab-experienced patients were included if they had been treated with eculizumab for ≥6 months before the first day of ravulizumab dosing.
Exclusion criteria for the study included a platelet count of <30 × 109/L at screening, an absolute neutrophil count of <0.5 × 109/L at screening, a history of bone marrow transplantation, and a history of N meningitidis infection or unexplained, recurrent infection. Complete inclusion and exclusion criteria are provided in supplemental Table 1.
History of aplastic anemia was recorded and determined by each principal investigator at their discretion.
Treatment
Ravulizumab dosing was based on patients’ body weight recorded on the day of dosing or the most recently recorded weight; patients received a loading dose of ravulizumab IV infusion on day 1, followed by maintenance dosing of ravulizumab on day 15 and subsequently Q8W for patients weighing ≥20 kg or Q4W for patients <20 kg enrolled in the study (Figure 1). For patients entering the study on eculizumab therapy, day 1 of ravulizumab treatment occurred 2 weeks from the patient’s last dose of eculizumab. Changes in dose regimen (dose level or frequency) were based on the patient’s body weight. If a patient’s weight changed from <20 kg to ≥20 kg on a Q4W visit, the patient received the Q4W dose that day; at the patient’s next Q8W visit, the new Q8W dose was given. To minimize the risk of severe acute respiratory syndrome coronavirus 2 infection during the COVID-19 pandemic, patients who were not able to reach the study sites could receive ravulizumab administration remotely at a medical facility located near or at their home. Routine prophylactic antibiotic treatment was at the discretion of the treating physician.
End points
Primary, secondary, and safety end points were evaluated from baseline through to the end of the EP.
Primary end points
PK parameters consisted of the maximum serum concentration (measured at the end of infusion; Cmax), trough serum concentration (measured at the end of the dosing interval; Ctrough), and accumulation ratio (calculated as Cmax from the last maintenance dose divided by Cmax from the first maintenance dose). PD parameters consisted of the change in free complement C5 concentrations and in chicken red blood cell hemolytic activity (Alexion, AstraZeneca Rare Disease, data on file).
Secondary end points
Secondary end points included the percentage change in LDH from baseline, transfusion avoidance defined as the proportion of patients who remained transfusion-free and did not require a transfusion throughout the study, and the change in fatigue from baseline, as measured by the pediatric Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) questionnaire. Other secondary outcomes included: the proportion of patients with stabilized hemoglobin (Hb), defined as avoidance of ≥20 g/L decrease in Hb from baseline in the absence of transfusion; the percentage change in free Hb from baseline; and the proportion of patients with BTH, defined as ≥1 new or worsening symptom of IVH (fatigue, hemoglobinuria, abdominal pain, shortness of breath, anemia, MAVE [including thrombosis], dysphagia, or erectile dysfunction), in the presence of LDH ≥2 × ULN after prior LDH reduction to <1.5 × ULN on therapy (eculizumab-naive patients) or after stabilized LDH levels (eculizumab-experienced).
Safety end points
Safety analyses were performed on all patients who received ≥1 dose of ravulizumab. Safety end points included adverse events (AEs), serious AEs (SAEs), AEs or SAEs leading to discontinuation, meningococcal infections, MAVEs, and deaths.
Statistical analysis
Sample size determination
Owing to the rarity of PNH in pediatric patients, this study was not statistically powered for hypothesis testing; a sample size of 10 was expected to be sufficient to describe the PKs, PDs, efficacy, and safety in this population.
Primary analyses
PK and PD analyses were conducted for all patients who received ≥1 dose of ravulizumab and who had evaluable PK (PK set) or PD (PD set) data. Individual serum concentration data were used to derive ravulizumab PK parameters. Descriptive statistics were presented for all PD end points at each sampling time.
Secondary analyses
Analyses of secondary efficacy end points were performed on the full analysis set, which included all patients who received ≥1 ravulizumab dose and had ≥1 efficacy assessment after the first IV infusion.
Institutional review board/institutional (or independent) ethics committee approval was obtained at all participating sites to conduct the study.
Results
Patient demographics and clinical characteristics
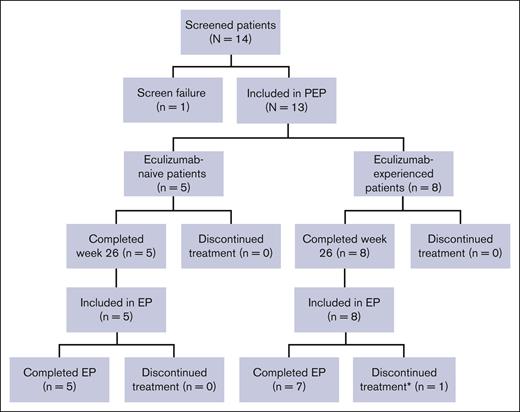
Of 14 screened patients, 13 (93%) were enrolled into the study (eculizumab-naive, n = 5; eculizumab-experienced, n = 8). One patient (7%) did not meet the LDH criteria and was therefore excluded from the study. All 13 enrolled patients completed the PEP and entered the EP; 12 patients (92%) completed the EP and 1 eculizumab-experienced patient (8%) discontinued treatment on day 341 of the EP owing to their decision to undergo bone marrow transplantation (Figure 2). Demographics, presenting symptoms, and clinical characteristics (including PNH clone size) of the patients are summarized in Table 1. Overall, mean ± standard deviation (SD, range) treatment duration was 868.0 ± 98.9 (692-916) days in the eculizumab-naive group and 1022.6 ± 384.6 (341-1596) days in the eculizumab-experienced group. Mean age at first infusion was 14.4 ± 2.7 (9-17) years, 8 patients (61.5%) were female, and 8 (61.5%) were Caucasian. The mean age at PNH diagnosis was slightly higher for the eculizumab-naive patients compared with the eculizumab-experienced patients (13.8 ± 2.4 [11-17] years vs 12.3 ± 3.1 [7-16] years, respectively). Baseline LDH levels were higher for eculizumab-naive patients compared with eculizumab-experienced patients (957.0 ± 757.2 [444.0-2269.7] U/L vs 262.8 ± 106.0 [140.5-487.0] U/L), as were the mean total units of pRBC/whole blood transfusion in the 12 months before the first dose of ravulizumab (7.0 ± 5.7 vs 2.0). At any time before enrollment, 3 treatment-naive patients (60%) and 1 eculizumab-experienced patient (12.5%) had aplastic anemia.
Patient disposition. ∗Discontinued treatment to undergo bone marrow transplantation.
Patient disposition. ∗Discontinued treatment to undergo bone marrow transplantation.
Patient disposition
| Variable . | Eculizumab-naive (n = 5) . | Eculizumab-experienced (n = 8) . | Total (N = 13) . |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 4 (80.0) | 1 (12.5) | 5 (38.5) |
| Female | 1 (20.0) | 7 (87.5) | 8 (61.5) |
| Ravulizumab treatment duration, d, mean ± SD (range) | 883.4 ± 102.7 (700-937) | 1040.4 ± 381.8 (369-1610) | 980.0 ± 308.0 (369-1610) |
| Age at first ravulizumab infusion, y, mean ± SD (range) | 14.4 ± 2.2 (11.0-17.0) | 14.4 ± 3.1 (9.0-17.0) | 14.4 ± 2.7 (9.0-17.0) |
| Age category, y, n (%) | |||
| 0-12 | 1 (20.0) | 1 (12.5) | 2 (15.4) |
| >12 | 4 (80.0) | 7 (87.5) | 11 (84.6) |
| Race, n (%) | |||
| Caucasian | 5 (100) | 3 (37.5) | 8 (61.5) |
| African American | 0 (0) | 2 (25.0) | 2 (15.4) |
| Not reported | 0 (0) | 2 (25.0) | 2 (15.4) |
| Other | 0 (0) | 1 (12.5) | 1 (7.7) |
| Body weight, kg, mean ± SD (range) | 56.3 ± 11.6 (39.5-72.0) | 56.3 ± 12.2 (36.7-69.0) | 56.3 ± 11.5 (36.7-72.0) |
| Weight category, kg, n (%) | |||
| ≥30 to <40 | 1 (20.0) | 1 (12.5) | 2 (15.4) |
| ≥40 to <60 | 3 (60.0) | 4 (50.0) | 7 (53.8) |
| ≥60 to <100 | 1 (20.0) | 3 (37.5) | 4 (30.8) |
| Height, cm, mean ± SD (range) | 163.4 ± 11.8 (143.0-171.0) | 161.0 ± 9.4 (146.0-176.2) | 161.9 ± 9.9 (143.0-176.2) |
| Presenting symptoms, n (%) | |||
| Any PNH symptoms before informed consent | 5 (100) | 7 (87.5) | 12 (92.3) |
| Fatigue/asthenia | 5 (100) | 7 (87.5) | 12 (92.3) |
| Abdominal pain | 3 (60.0) | 5 (62.5) | 8 (61.5) |
| Red/dark urine | 4 (80.0) | 4 (50.0) | 8 (61.5) |
| Jaundice | 4 (80.0) | 3 (37.5) | 7 (53.8) |
| CNS-related symptoms∗ | 2 (40.0) | 4 (50.0) | 6 (46.2) |
| Back or flank pain | 0 (0) | 3 (37.5) | 3 (23.1) |
| Chest pain | 0 (0) | 2 (25.0) | 2 (15.4) |
| Dysphagia | 0 (0) | 1 (12.5) | 1 (7.7) |
| Erectile dysfunction | 0 (0) | 1 (12.5) | 1 (7.7) |
| Leg pain | 0 (0) | 1 (12.5) | 1 (7.7) |
| Dyspnea | 0 (0) | 1 (12.5) | 1 (7.7) |
| Other | 0 (0) | 1 (12.5) | 1 (7.7) |
| Age at PNH diagnosis, y, mean ± SD (range) | 13.8 ± 2.4 (11.0-17.0) | 12.3 ± 3.1 (7.0-16.0) | |
| Time from PNH diagnosis to informed consent, months, mean ± SD (range) | 8.9 ± 1.4 (0-39.6) | 24.1 ± 1.0 (13.2-45.6) | |
| History of pRBC/whole blood transfusion† , n (%) | 2 (40.0) | 2 (25.0) | |
| Units of pRBC/whole blood transfusion, total, mean ± SD (range) | 14.0 7.0 ± 5.7 (3.0-11.0) | 2.0 2.0 (2.0-2.0) | |
| History of aplastic anemia, n (%) | 3 (60.0) | 1 (12.5) | |
| LDH at baseline (U/L), mean ± SD (range)‡ | 957.0 ± 757.2 (444.0-2269.7) | 262.8 ± 106.0 (140.5-487.0) | |
| PNH clone size, mean ± SD (range) | |||
| RBC type II | 18.7 ± 19.5 (0.7-41.4)§ | 10.6 ± 16.4 (0.6-42.6)‖ | |
| RBC type III | 19.2 ± 12.9 (6.2-39.9) | 54.5 ± 22.2 (20.6-80.8) | |
| Total RBC | 38.8 ± 31.5 (6.9-68.1)§ | 65.7 ± 22.7 (21.2-85.4)‖ | |
| Granulocytes | 68.1 ± 26.4 (36.8-99.0) | 82.9 ± 26.0 (20.3-97.6) | |
| Monocytes | 75.2 ± 24.0 (34.9-98.9) | 91.9 ± 5.5 (81.3-97.7) |
| Variable . | Eculizumab-naive (n = 5) . | Eculizumab-experienced (n = 8) . | Total (N = 13) . |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 4 (80.0) | 1 (12.5) | 5 (38.5) |
| Female | 1 (20.0) | 7 (87.5) | 8 (61.5) |
| Ravulizumab treatment duration, d, mean ± SD (range) | 883.4 ± 102.7 (700-937) | 1040.4 ± 381.8 (369-1610) | 980.0 ± 308.0 (369-1610) |
| Age at first ravulizumab infusion, y, mean ± SD (range) | 14.4 ± 2.2 (11.0-17.0) | 14.4 ± 3.1 (9.0-17.0) | 14.4 ± 2.7 (9.0-17.0) |
| Age category, y, n (%) | |||
| 0-12 | 1 (20.0) | 1 (12.5) | 2 (15.4) |
| >12 | 4 (80.0) | 7 (87.5) | 11 (84.6) |
| Race, n (%) | |||
| Caucasian | 5 (100) | 3 (37.5) | 8 (61.5) |
| African American | 0 (0) | 2 (25.0) | 2 (15.4) |
| Not reported | 0 (0) | 2 (25.0) | 2 (15.4) |
| Other | 0 (0) | 1 (12.5) | 1 (7.7) |
| Body weight, kg, mean ± SD (range) | 56.3 ± 11.6 (39.5-72.0) | 56.3 ± 12.2 (36.7-69.0) | 56.3 ± 11.5 (36.7-72.0) |
| Weight category, kg, n (%) | |||
| ≥30 to <40 | 1 (20.0) | 1 (12.5) | 2 (15.4) |
| ≥40 to <60 | 3 (60.0) | 4 (50.0) | 7 (53.8) |
| ≥60 to <100 | 1 (20.0) | 3 (37.5) | 4 (30.8) |
| Height, cm, mean ± SD (range) | 163.4 ± 11.8 (143.0-171.0) | 161.0 ± 9.4 (146.0-176.2) | 161.9 ± 9.9 (143.0-176.2) |
| Presenting symptoms, n (%) | |||
| Any PNH symptoms before informed consent | 5 (100) | 7 (87.5) | 12 (92.3) |
| Fatigue/asthenia | 5 (100) | 7 (87.5) | 12 (92.3) |
| Abdominal pain | 3 (60.0) | 5 (62.5) | 8 (61.5) |
| Red/dark urine | 4 (80.0) | 4 (50.0) | 8 (61.5) |
| Jaundice | 4 (80.0) | 3 (37.5) | 7 (53.8) |
| CNS-related symptoms∗ | 2 (40.0) | 4 (50.0) | 6 (46.2) |
| Back or flank pain | 0 (0) | 3 (37.5) | 3 (23.1) |
| Chest pain | 0 (0) | 2 (25.0) | 2 (15.4) |
| Dysphagia | 0 (0) | 1 (12.5) | 1 (7.7) |
| Erectile dysfunction | 0 (0) | 1 (12.5) | 1 (7.7) |
| Leg pain | 0 (0) | 1 (12.5) | 1 (7.7) |
| Dyspnea | 0 (0) | 1 (12.5) | 1 (7.7) |
| Other | 0 (0) | 1 (12.5) | 1 (7.7) |
| Age at PNH diagnosis, y, mean ± SD (range) | 13.8 ± 2.4 (11.0-17.0) | 12.3 ± 3.1 (7.0-16.0) | |
| Time from PNH diagnosis to informed consent, months, mean ± SD (range) | 8.9 ± 1.4 (0-39.6) | 24.1 ± 1.0 (13.2-45.6) | |
| History of pRBC/whole blood transfusion† , n (%) | 2 (40.0) | 2 (25.0) | |
| Units of pRBC/whole blood transfusion, total, mean ± SD (range) | 14.0 7.0 ± 5.7 (3.0-11.0) | 2.0 2.0 (2.0-2.0) | |
| History of aplastic anemia, n (%) | 3 (60.0) | 1 (12.5) | |
| LDH at baseline (U/L), mean ± SD (range)‡ | 957.0 ± 757.2 (444.0-2269.7) | 262.8 ± 106.0 (140.5-487.0) | |
| PNH clone size, mean ± SD (range) | |||
| RBC type II | 18.7 ± 19.5 (0.7-41.4)§ | 10.6 ± 16.4 (0.6-42.6)‖ | |
| RBC type III | 19.2 ± 12.9 (6.2-39.9) | 54.5 ± 22.2 (20.6-80.8) | |
| Total RBC | 38.8 ± 31.5 (6.9-68.1)§ | 65.7 ± 22.7 (21.2-85.4)‖ | |
| Granulocytes | 68.1 ± 26.4 (36.8-99.0) | 82.9 ± 26.0 (20.3-97.6) | |
| Monocytes | 75.2 ± 24.0 (34.9-98.9) | 91.9 ± 5.5 (81.3-97.7) |
CNS, central nervous system; LDH, lactate dehydrogenase; PNH, paroxysmal nocturnal hemoglobinuria; pRBC, packed red blood cell; RBC, red blood cell; SD, standard deviation; y, years.
For example headache, dizziness, or difficulty concentrating.
In the 12 months before first dose of ravulizumab.
There are multiple LDH normal ranges depending on pediatric age and sex (100-220, 100-242, 100-275, 120-290 and 140-280).
n = 4.
n = 6.
Primary end points
PKs
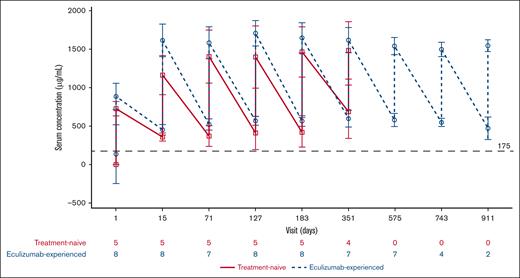
Ravulizumab Ctrough levels were >175 μg/mL (PK threshold) in the PEP and EP, with the exception of 1 patient from the eculizumab-naive cohort on visit days 127 and 183 before ravulizumab dosing (Figure 3). At the end of the study (including both before ravulizumab dosing and after infusion), serum ravulizumab concentrations (mean ± SD [range]) were 610.50 ± 201.53 (468-753) μg/mL and 518.29 ± 109.67 (408-744) μg/mL for eculizumab-naive and eculizumab-experienced patients, respectively. One patient in the eculizumab-naive cohort had received pRBC transfusions on days 44, 46, and 58 as a result of septic shock and multiple organ dysfunction syndrome. Owing to this, the patient who received pRBC transfusions was excluded from the PK analysis.
Mean (SD) change in serum ravulizumab concentration over time. The graph presents values before ravulizumab (lower values) and after ravulizumab (higher values) dosing at every visit for each patient subgroup. Dashed horizontal line indicates 175 μg/mL, the threshold for complete C5 inhibition. EOS, end of study.
Mean (SD) change in serum ravulizumab concentration over time. The graph presents values before ravulizumab (lower values) and after ravulizumab (higher values) dosing at every visit for each patient subgroup. Dashed horizontal line indicates 175 μg/mL, the threshold for complete C5 inhibition. EOS, end of study.
PDs
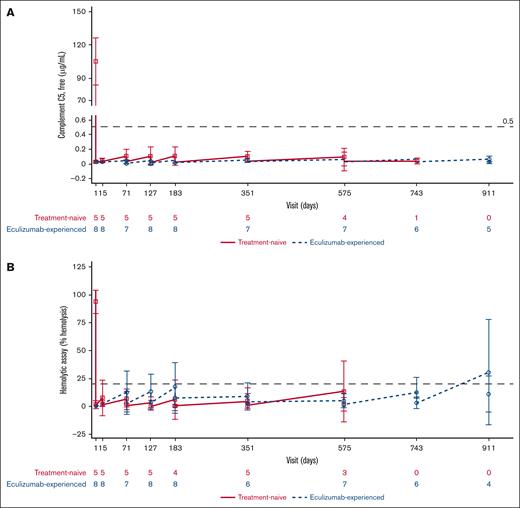
As would be anticipated, baseline serum-free complement C5 levels were <0.5 μg/mL in the eculizumab-experienced cohort. After the first ravulizumab dose infusion, serum-free C5 values were <0.5 μg/mL in both cohorts throughout the PEP and EP, indicating that ravulizumab provided immediate, complete, and sustained terminal complement C5 inhibition (Figure 4A). At the end of the study, serum-free C5 concentrations (mean ± SD [range]) were 0.061 ± 0.021 (0.05-0.08) μg/mL and 0.061 ± 0.018 (0.03-0.08) μg/mL for eculizumab-naive and eculizumab-experienced patients, respectively.
PD parameters. (A) Mean (95% CI) serum-free complement C5 concentration over time. (B) Mean (95% CI) cRBC hemolysis over time. The graph presents values before ravulizumab (lower values) and after ravulizumab (higher values) dosing at every visit for each patient subgroup. Dashed horizontal lines indicate 0.5 μg/mL (A) and 20% hemolysis (B), the thresholds for complete C5 inhibition. BL, baseline; cRBC, chicken red blood cells.
PD parameters. (A) Mean (95% CI) serum-free complement C5 concentration over time. (B) Mean (95% CI) cRBC hemolysis over time. The graph presents values before ravulizumab (lower values) and after ravulizumab (higher values) dosing at every visit for each patient subgroup. Dashed horizontal lines indicate 0.5 μg/mL (A) and 20% hemolysis (B), the thresholds for complete C5 inhibition. BL, baseline; cRBC, chicken red blood cells.
Overall, mean hemolytic activity was held below 20% after the first ravulizumab infusion, indicating complete inhibition of C5 for both patient cohorts, except on days 911 and 1079 (data not shown). On these respective days, percentage mean hemolysis was 30.8% and 32.2% before ravulizumab dosing. This was based on data from 4 (50%) and 3 (37.5%) eculizumab-experienced patients (Figure 4B). At the end of the study, percentage hemolysis (mean ± SD [range]) was 21.9 ± 5.6% (17.9-25.8) and 31.9 ± 33.6% (0.0-79.7) for eculizumab-naive and eculizumab-experienced patients, respectively.
Secondary end points
Lactate dehydrogenase
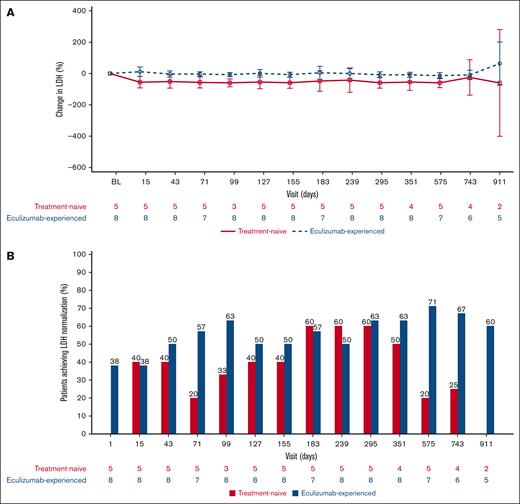
After the first ravulizumab IV infusion, mean LDH values decreased in the eculizumab-naive cohort, an effect that was sustained throughout the PEP and EP. The percentage changes in LDH from baseline (mean [range]) on days 15, 183, and 911 (last available value) were −55.52% (−83.3 to −7.9), −47.91% (−91.4 to 43.5), and −60.15% (−87.0 to −33.3), respectively. LDH levels were maintained throughout the PEP and EP in the eculizumab-experienced cohort. In this cohort, the percentage changes in LDH from baseline (mean [range]) on days 15, 183, and 911 were 11.50% (−48.0 to 76.0), 4.65% (−41.3 to 100.7), and 63.66% (−10.9 to 254.9), respectively (Figure 5A).
Changes in LDH from baseline over time. (A) Mean (95% CI) percentage change from baseline in LDH over time. The graph presents values before ravulizumab (lower values) and after ravulizumab (higher values) dosing at every visit for each patient subgroup. (B) Proportion of patients achieving LDH normalization by visit. BL, baseline.
Changes in LDH from baseline over time. (A) Mean (95% CI) percentage change from baseline in LDH over time. The graph presents values before ravulizumab (lower values) and after ravulizumab (higher values) dosing at every visit for each patient subgroup. (B) Proportion of patients achieving LDH normalization by visit. BL, baseline.
The proportion of patients in the eculizumab-naive cohort who had LDH levels at or below the ULN increased from baseline at all visits except day 911, when neither of the 2 patients who had evaluable data achieved normalization (Figure 5B). This was also the case for the eculizumab-experienced cohort, except on day 15 for which there was no difference from baseline.
Transfusion avoidance
In the PEP, 3 patients (60%) and 8 patients (100%) avoided pRBC or whole blood transfusions from the eculizumab-naive and eculizumab-experienced cohorts, respectively. The mean (range) number of transfusions that took place during this period in the eculizumab-naive cohort was 2.0 (1.0-3.0) and the total units of pRBC or whole blood transfusion was 2.5 (2.0-3.0). Throughout the EP, 4 eculizumab-naive patients (80%) and 7 eculizumab-experienced patients (87%) avoided transfusions. One eculizumab-experienced patient (13%) met the study transfusion guideline but did not receive a transfusion. The number of transfusions throughout the EP was 8.0 (8.0-8.0) and 4.0 (4.0-4.0) for the eculizumab-naive and eculizumab-experienced cohorts, respectively, with 15.0 (15.0-15.0) and 7.0 (7.0-7.0) total units transfused in each cohort. Notably, of the 3 (23.1%) patients who did not avoid transfusions throughout the study, 2 (66.7%) had a history of aplastic anemia.
Stabilized Hb
During the PEP, Hb stabilized in 3 eculizumab-naive patients (60% [95% confidence interval (CI), 14.7-94.7]) and 6 eculizumab-experienced patients (75% [95% CI, 34.9-96.8]); meanwhile in the EP, Hb stabilization was observed in 4 eculizumab-naive patients (80.0% [95% CI, 28.4-99.5]) and 4 eculizumab-experienced patients (50.0% [95% CI, 15.7-84.3]).
Free Hb
In the eculizumab-naive cohort, percentage change in free Hb decreased from baseline at most time points. On days 15, 183, and 911 (last available data), mean (range) free Hb was −20.4 (−80.7 to 43.4) mg/dL, 87.3 (−35.9 to 492.1) mg/dL, and −62.9 (−87.6 to −38.2) mg/dL, respectively. In the eculizumab-experienced cohort on days 15, 183, and 911, free Hb was 423.7 (−63.9 to 3361.5) mg/dL, −15.3 (−89.6 to 138.1) mg/dL, and 32.0 (−23.8 to 150.0) mg/dL.
Intravascular BTH
No patients experienced intravascular BTH during the PEP. Two eculizumab-experienced patients (25% [95% CI, 3.2-65.1]) experienced intravascular BTH during the EP. One BTH event took place between months 6 and 18 of the study, the other between months 18 and 30. None of these were associated with suboptimal C5 inhibition; serum-free C5 levels were not ≥0.5 μg/mL in any patient at any visit throughout the study. Also, the events were not clearly a result of complement-amplifying conditions. Note that the eculizumab-experienced patient who withdrew from the EP to undergo bone marrow transplantation was included in the group with BTH. This was because of classification by the investigator as per protocol–defined criteria. However, this patient did not experience BTH, as their LDH levels were within the normal range throughout the study.
FACIT-F
Overall, treatment with ravulizumab resulted in small improvements in fatigue in eculizumab-naive patients, as shown by an increase in FACIT-F score from baseline (indicating improved quality of life). On days 15, 183, and 911 (last available data), the mean (range) change from baseline was 2.2 (−2.0 to 7.0), 3.4 (−5.0 to 11.0), and 3.8 (−4.0 to 9.0), respectively. Ravulizumab maintained FACIT-F scores in eculizumab-experienced patients. On days 15, 183, and 911, the change from baseline was 0.0 (−9.0 to 6.0), 1.3 (−4.0 to 12.0), and −1.6 (−17.0 to 10.0), respectively.
Safety
The safety profile of ravulizumab in pediatric patients with PNH was consistent with previous studies of ravulizumab; in total, 4 patients (30.8%) experienced SAEs. One eculizumab-naive patient (7.7%) experienced peripherally inserted central catheter line-related sepsis related to staphylococcal infection on day 43. This was followed by a MAVE on day 44, characterized as device-related thrombosis resulting from a central line catheter. On this same day, the patient experienced multiple organ dysfunction syndrome and septic shock. There were no reported deaths, meningococcal infections, AEs, or SAEs leading to drug withdrawal. However, all patients experienced treatment-emergent AEs (TEAEs), with a total of 120 events (Table 2). The most common TEAEs were upper abdominal pain, nausea, COVID-19, nasopharyngitis, and headache (3 patients [23.1%] each). Most TEAEs were mild in nature; 10 patients (76.9%) experienced grade 1 events, 9 (69.2%) grade 2, 3 (32.1%) grade 3, and 1 (7.7%) grade 4. No patients experienced grade 5 TEAEs.
Most common TEAEs throughout the study
| Most common TEAEs by organ class (≥2 patients) . | Eculizumab-naive (n = 5) . | Eculizumab-experienced (n = 8) . | Total (N = 13) . | |||
|---|---|---|---|---|---|---|
| n (%) . | E . | n (%) . | E . | n (%) . | E . | |
| Total patient-years of exposure to ravulizumab, y | 11.9 | 22.4 | 34.3 | |||
| All TEAEs | 5 (100) | 28 | 8 (100) | 92 | 13 (100) | 120 |
| Blood and lymphatic system | ||||||
| Anemia | 0 (0) | 0 | 2 (25.0) | 5 | 2 (15.4) | 5 |
| Gastrointestinal | ||||||
| Abdominal pain | 0 (0) | 0 | 3 (37.5) | 3 | 3 (23.1) | 3 |
| Upper abdominal pain | 0 (0) | 0 | 3 (37.5) | 3 | 3 (23.1) | 3 |
| Nausea | 0 (0) | 0 | 3 (37.5) | 3 | 3 (23.1) | 3 |
| Constipation | 0 (0) | 0 | 2 (25.0) | 2 | 2 (15.4) | 2 |
| Diarrhea | 0 (0) | 0 | 2 (25.0) | 2 | 2 (15.4) | 2 |
| General | ||||||
| Fatigue | 0 (0) | 0 | 2 (25.0) | 2 | 2 (15.4) | 2 |
| Pyrexia | 1 (20.0) | 1 | 1 (12.5) | 1 | 2 (15.4) | 2 |
| Infections | ||||||
| COVID-19 | 2 (40.0) | 2 | 1 (12.5) | 1 | 3 (23.1) | 3 |
| Nasopharyngitis | 1 (20.0) | 1 | 2 (25.0) | 2 | 3 (23.1) | 3 |
| Upper respiratory tract infection | 0 (0) | 0 | 2 (25.0) | 3 | 2 (15.4) | 3 |
| Urinary tract infection | 0 (0) | 0 | 2 (25.0) | 2 | 2 (15.4) | 2 |
| Viral upper respiratory tract infection | 0 (0) | 0 | 2 (25.0) | 2 | 2 (15.4) | 2 |
| Musculoskeletal and connective tissue | ||||||
| Pain in extremity | 0 (0) | 0 | 2 (25.0) | 2 | 2 (15.4) | 2 |
| Nervous system | ||||||
| Headache | 1 (20.0) | 1 | 2 (25.0) | 3 | 3 (23.1) | 4 |
| Respiratory, thoracic, and mediastinal | ||||||
| Oropharyngeal pain | 0 (0) | 0 | 2 (25.0) | 2 | 2 (15.4) | 2 |
| Most common TEAEs by organ class (≥2 patients) . | Eculizumab-naive (n = 5) . | Eculizumab-experienced (n = 8) . | Total (N = 13) . | |||
|---|---|---|---|---|---|---|
| n (%) . | E . | n (%) . | E . | n (%) . | E . | |
| Total patient-years of exposure to ravulizumab, y | 11.9 | 22.4 | 34.3 | |||
| All TEAEs | 5 (100) | 28 | 8 (100) | 92 | 13 (100) | 120 |
| Blood and lymphatic system | ||||||
| Anemia | 0 (0) | 0 | 2 (25.0) | 5 | 2 (15.4) | 5 |
| Gastrointestinal | ||||||
| Abdominal pain | 0 (0) | 0 | 3 (37.5) | 3 | 3 (23.1) | 3 |
| Upper abdominal pain | 0 (0) | 0 | 3 (37.5) | 3 | 3 (23.1) | 3 |
| Nausea | 0 (0) | 0 | 3 (37.5) | 3 | 3 (23.1) | 3 |
| Constipation | 0 (0) | 0 | 2 (25.0) | 2 | 2 (15.4) | 2 |
| Diarrhea | 0 (0) | 0 | 2 (25.0) | 2 | 2 (15.4) | 2 |
| General | ||||||
| Fatigue | 0 (0) | 0 | 2 (25.0) | 2 | 2 (15.4) | 2 |
| Pyrexia | 1 (20.0) | 1 | 1 (12.5) | 1 | 2 (15.4) | 2 |
| Infections | ||||||
| COVID-19 | 2 (40.0) | 2 | 1 (12.5) | 1 | 3 (23.1) | 3 |
| Nasopharyngitis | 1 (20.0) | 1 | 2 (25.0) | 2 | 3 (23.1) | 3 |
| Upper respiratory tract infection | 0 (0) | 0 | 2 (25.0) | 3 | 2 (15.4) | 3 |
| Urinary tract infection | 0 (0) | 0 | 2 (25.0) | 2 | 2 (15.4) | 2 |
| Viral upper respiratory tract infection | 0 (0) | 0 | 2 (25.0) | 2 | 2 (15.4) | 2 |
| Musculoskeletal and connective tissue | ||||||
| Pain in extremity | 0 (0) | 0 | 2 (25.0) | 2 | 2 (15.4) | 2 |
| Nervous system | ||||||
| Headache | 1 (20.0) | 1 | 2 (25.0) | 3 | 3 (23.1) | 4 |
| Respiratory, thoracic, and mediastinal | ||||||
| Oropharyngeal pain | 0 (0) | 0 | 2 (25.0) | 2 | 2 (15.4) | 2 |
E, events; TEAEs, treatment-emergent adverse events.
Discussion
To our knowledge, this is the largest clinical trial to date in pediatric patients with PNH treated with complement C5 inhibitors. PK and PD data from the PEP and the 4-year EP, with mean treatment durations of 868.0 and 1022.6 days in the treatment-naive and treatment-experienced groups, respectively, showed that ravulizumab provided immediate, complete, and sustained terminal complement inhibition, irrespective of prior treatment with eculizumab. Similar to the clinical trials of ravulizumab in adults with PNH (301 and 302 studies), there were differences in the clinical characteristics between the 2 patient cohorts at baseline,19,20 because eculizumab-experienced patients had previously benefited from C5 inhibitor therapy.
Elevated IVH, measured by LDH levels ≥1.5 × ULN, is an indicator of disease severity in patients with PNH.18 In eculizumab-naive patients, ravulizumab treatment resulted in decreased LDH levels from baseline. However, in eculizumab-experienced patients, LDH levels were already generally maintained within the normal range that was achieved during prior treatment with eculizumab. In addition, the proportion of patients who had normalized LDH levels increased from baseline except in 1 visit for each group (eculizumab-naive, day 911; eculizumab-experienced, day 15). Eculizumab-naive patients also experienced small improvements in fatigue from baseline, whereas levels were generally maintained from baseline in the eculizumab-experienced cohort. These results are consistent with those from the 301 and 302 studies, in which eculizumab-naive adult patients with PNH experienced improvements in LDH levels and fatigue, whereas in eculizumab-experienced patients, ravulizumab maintained the improvement already achieved with eculizumab treatment.19,20
The majority of patients avoided transfusions throughout both the PEP and EP, and 2 eculizumab-experienced patients experienced BTH during the EP. These events were unrelated to the PD profile of ravulizumab, since complement C5 was adequately inhibited, evidenced by C5 levels of <0.5 μg/mL throughout the study The proportion of patients who avoided transfusions throughout the study was similar to that previously reported in adults with this disease.18 Patients from both cohorts experienced suppressed hemolytic activity and stabilized Hb.
Regarding the safety of ravulizumab, there were no new safety signals raised in this study and no AEs or SAEs resulted in participant discontinuation. The majority of TEAEs were mild in nature, the most common included abdominal pain and headache. The AEs observed throughout this study were comparable to those previously reported in adults with PNH,18 and pediatric patients with atypical hemolytic uremic syndrome.24 In pediatric patients with atypical hemolytic uremic syndrome, switching from eculizumab to ravulizumab was also found to be an acceptable approach for the treatment of this complement-mediated rare disease. After transitioning from eculizumab to ravulizumab treatment, efficacy end points and C5 inhibition remained stable in these patients.24
Although, to our knowledge, this was the largest study of pediatric patients with PNH treated with a complement C5 inhibitor, the sample size was still relatively small owing to the rarity of PNH in this population. The single-arm nature of the study was also a limitation, as it did not include comparisons with eculizumab or no treatment. Another limitation was the lack of data on extravascular hemolysis and complement component 3 fragment deposition.25 Furthermore, 2 of 3 patients who required pRBC transfusions had a history of aplastic anemia, which prevented the correlation between ravulizumab treatment and transfusion avoidance from being accurately established. Finally, whereas ravulizumab provides sustained efficacy in patients, it does not resolve the underlying cause of PNH, the glycosylphosphatidylinositol anchored protein-deficient cells. A potential limitation owing to the lack of current long-term follow-up of pediatric patients is that, it is not yet understood whether bone marrow transplantation may be required at a later stage.
Since their approvals for the treatment of PNH, both eculizumab and ravulizumab have been effective treatment options for patients with the disease owing to their similar efficacy and safety profiles, although ravulizumab has shown to have an improved PK and PD profile.14,15,22 In adult patients with PNH, the improved profile of ravulizumab has been shown to reduce intravascular BTH associated with suboptimal C5 inhibition compared with eculizumab.21 Another benefit arising from ravulizumab is its dosing regimen, which includes the weight-based dosing and reduced dosing frequency compared with eculizumab. These differences have been found to have a substantially positive impact on the quality of life of patients.26
The PK, PD, efficacy, and safety findings of this long-term study support the conclusion that pediatric patients with PNH may initiate ravulizumab and experience improvements in disease outcomes or switch from eculizumab to ravulizumab without loss of efficacy or change in safety. Importantly, this would allow physicians managing pediatric patients with PNH to consider ravulizumab as a treatment option with less impact on the daily lives of patients and their accompanying caregivers compared with eculizumab.
Acknowledgments
The authors thank the patient advocate for reviewing the plain language summary. Medical writing support was provided by Rebecca Spencer Martín and Rebecca Hornby of Oxford PharmaGenesis, Oxford, United Kingdom, with funding from Alexion, AstraZeneca Rare Disease.
Authorship
Contributions: S.C., M.O., J.Y., and A.G.K. contributed to research design; S.C., A.K., J.B., R.P., M.R., M.O., A.B., and A.G.K. performed research. J.Y. contributed vital new reagents or analytical tools; S.C., A.K., A.M., M.B., J.B., R.P., M.R., E.H., J.Y., A.B., and A.G.K. collected data; S.C., A.K., A.M., M.B., J.B., M.O., E.H., J.Y., A.B., and A.G.K. analyzed and interpreted data; J.Y. performed statistical analysis; and all authors contributed to manuscript development and/or revisions.
Conflict-of-interest disclosure: S.C. received consulting fees from Agios, Alexion, AstraZeneca Rare Disease, Amgen; received research funding from Alexion, AstraZeneca Rare Disease, Novartis, Global Blood Therapeutics/Pfizer; has a membership on an entity’s board of directors or advisory committees for Agios, Novartis, Roche/Genentech; and has received other fees for Alexion, AstraZeneca Rare Disease. A.K. has received honoraria, consulting fees, and research support (to Pavlov University) from Alexion, AstraZeneca Rare Disease. J.B. has received consulting fees from Novartis. M.O. is an employee and equity holder of Alexion, AstraZeneca Rare Disease. E.H. and J.Y. are employees of Alexion, AstraZeneca Rare Disease. A.B. received honoraria from AstraZeneca, Clinigen, Jazz, Novartis, and Servier, all unrelated to the subject of this publication. A.G.K. has received honoraria from Alexion, AstraZeneca Rare Disease, Amgen, Celgene/Bristol Myers Squibb, Novartis, and Ra Pharma; is on the board of directors or is an advisory board member for Alexion, AstraZeneca Rare Disease, Amgen, Celgene/Bristol Myers Squibb, Novartis, Pfizer, Roche, and Ra Pharma; and has received consulting fees from Achillion, Akari Therapeutics, Alexion, AstraZeneca Rare Disease, Biocryst, Celgene/Bristol Myers Squibb, Janssen Pharmaceuticals, Novartis, Novo Nordisk, Pfizer, Roche, and Samsung. The remaining authors declare no competing financial interests.
Correspondence: Satheesh Chonat, Department of Pediatrics, Emory University School of Medicine and Aflac Cancer and Blood Disorders Center, Children’s Healthcare of Atlanta, 2015 Uppergate Dr, Atlanta, GA 30322; email: satheesh.chonat@emory.edu.
References
Author notes
Requests for disclosure of clinical study participant-level data will be considered, provided that participant privacy is assured through methods such as data deidentification, pseudonymization, or anonymization (as required by applicable law), and on condition that such disclosure is included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to the study.
The full-text version of this article contains a data supplement.