Empagliflozin decreases the susceptibility to infection in glycogen storage disease type 1b.
Empagliflozin significantly improves neutrophil population and functions in GSD1b, surpassing G-CSF limited efficacy.
Visual Abstract
Neutropenia and neutrophil dysfunction in glycogen storage disease type 1b (GSD1b) are caused by the accumulation of 1,5-anhydroglucitol-6-phosphate in granulocytes. The antidiabetic drug empagliflozin reduces the concentration of 1,5-anhydroglucitol (1,5-AG), thus restoring neutrophil counts and functions, leading to promising results in previous case reports. Here, we present a comprehensive analysis of neutrophil function in 7 patients with GSD1b and 11 healthy donors, aiming to evaluate the immediate (after 3 months) and long-term (after 12 months) efficacy of empagliflozin compared with the reference treatment with granulocyte-colony stimulating factor (G-CSF). We found that most patients receiving G-CSF remained neutropenic with dysfunctional granulocytes, whereas treatment with empagliflozin increased neutrophil counts and improved functionality by inhibiting apoptosis, restoring phagocytosis and the chemotactic response, normalizing the oxidative burst, and stabilizing cellular and plasma levels of defensins and lactotransferrin. These improvements correlated with the decrease in serum 1,5-AG levels. However, neither G-CSF nor empagliflozin overcame deficiencies in the production of cathelicidin/LL-37 and neutrophil extracellular traps. Given the general improvement promoted by empagliflozin treatment, patients were less susceptible to severe infections. G-CSF injections were therefore discontinued in 6 patients (and the dose was reduced in the seventh) without adverse effects. Our systematic analysis, the most extensive reported thus far, has demonstrated the superior efficacy of empagliflozin compared with G-CSF, restoring the neutrophil population and normal immune functions. This trial was registered as EudraCT 2021-000580-78.
Introduction
Glycogen storage disease type 1b (GSD1b, OMIM: no. 232220) is a rare metabolic defect with a prevalence of 1 in 500 000 people, predominantly affecting carbohydrate metabolism.1 It is caused by biallelic mutations in the SLC37A4 gene, resulting in a deficient glucose-6-phosphate transporter (translocase G6P) in the endoplasmic reticulum (ER) membrane.2 The final step in gluconeogenesis is G6P hydrolysis to glucose, which only takes place in the ER.3 Translocase G6P deficiency therefore leads to episodes of hypoglycemia and elevated levels of G6P in the cytoplasm.4 The accumulation of G6P triggers abnormal metabolic processes that typify GSD1b, including hyperlactatemia, hypertriglyceridemia, hyperuricemia, and hypercholesterolemia.4,5 Furthermore, the storage of excess glycogen and fats causes the enlargement and dysfunction of the liver and kidneys.
The primary treatment for severe hypoglycemia accompanied by metabolic disruption is the frequent consumption of protein-rich meals with minimal levels of simple sugars, including feeding infants at 2-hour intervals day and night.4 However, GSD1b also causes neutrophil deficiency and dysfunction, which has a severe impact on quality of life from the neonatal period onward by making patients susceptible to frequent, life-threatening infection.4,6 Later in life, neutropenia causes other comorbidities such as inflammatory bowel disease (IBD) and recurrent aphthous stomatitis. Until recently, granulocyte-colony stimulating factor (G-CSF) was the only approved treatment for congenital neutropenia.4,7 However, although G-CSF can restore the neutrophil count, its ability to address neutrophil dysfunction is more limited and treatment may increase long-term susceptibility to acute myeloid leukemia, myelodysplasia, and other forms of cancer.8-11
The accumulation of 1,5 anhydroglucitol-6-phosphate (1,5-AG6P) in granulocytes was recently identified as the cause of neutropenia in GSD1b.2 This compound is formed when 1,5-anhydroglucitol (1,5-AG), a glucose analog found in the diet, is phosphorylated by hexokinases. Normally, 1,5-AG6P is transported from the cytosol of neutrophils to the ER, where it is dephosphorylated by glucose-6-phosphatase and then eliminated. But a deficiency for translocase G6P causes 1,5-AG6P to accumulate in the cytosol, in which it inhibits glucose phosphorylation by hexokinases, thereby preventing glycolysis, the primary energy source for mature neutrophils in the absence of mitochondria.3,12 These deficiencies impair the bactericidal activity of neutrophils, and cause them to undergo premature apoptosis.13-15
The discovery of the underlying cause of this congenital form of neutropenia has facilitated the pursuit of more targeted treatment options. Inhibitors of renal sodium-glucose cotransporter type 2 (SGLT2), such as empagliflozin, appear to be the most promising options.3,8 Empagliflozin is used to prevent the reabsorption of glucose and its derivatives in the proximal tubule of the nephron in patients with diabetes.16,17 In GSD1b, empagliflozin also inhibits the feedback of 1,5-AG and reduces 1,5-AG6P levels in the blood and granulocytes.8 Empagliflozin has been shown to increase the population of viable and functional neutrophils in a limited number of patients.3,8 Furthermore, retrospective analysis have shown that empagliflozin have positive effects on neutrophil dysfunction–related symptoms including IBD, oral and urogenital mucosal lesions, infections, or skin abscesses.18-20 Here, we present the first prospective clinical trial evaluating the effectiveness of SGLT2 inhibition in GSD1b with the most extensive and comprehensive analysis of neutrophil dysfunction in patients thus far, in order to investigate both the immediate and long-term efficacy of empagliflozin. We evaluated the effect of empagliflozin on neutrophil apoptosis, phagocytosis, chemotaxis, the oxidative burst, cellular and plasma levels of antimicrobial peptides (AMPs) and LL-37, and the formation of neutrophil extracellular traps (NETs) compared with the control treatment with G-CSF.
Materials and methods
EMPAtia clinical trial
The EMPAtia clinical trial “Evaluation of the effectiveness and safety of empagliflozin in treating neutropenia in patients with GSD1b” (https://www.clinicaltrialsregister.eu/ctr-search/trial/2021-000580-78/PL) has been running in Poland since March 2022. This trial, funded by the Medical Research Agency, was designed by researchers from the Children’s Memorial Health Institute in Warsaw and has enrolled 92% of identified pediatric patients with GSD1b in Poland (12 patients in total). Informed written consent to participate in the EMPAtia clinical trial was obtained from the patients and/or their parents. In collaboration with the FixNet project (Fix Neutropenia: focusing on neutrophil proteases defect, which serve as novel diagnostic and therapeutic options), 7 patients (58%) qualified for the additional assessment of neutrophil functions according to the EMPAtia/FixNet substudy protocol (supplemental Figure 1). Given the use of empagliflozin for an average of 1 year, 4 patients were not included because neutrophils could not be collected at baseline before the initiation of treatment, and 1 additional patient was excluded because the parents did not provide consent. The EMPAtia substudy was reviewed and approved by the bioethics commission at the Children's Memorial Health Institute in Warsaw (16/KBE/2021). It was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. No personal or identifiable data relating to the substudy are included in this article. Healthy controls were originally enrolled in the FixNet project from the Foundation for Polish Science with informed written consent (approved by the Human Research Ethics Committee, Medical University of Łódź [RNN/211/22/KE and RNN/353/19/KE]).
Before the trial started, all 7 patients were treated daily with G-CSF. This was initially maintained when daily treatment with empagliflozin commenced (5 mg at weights below 20 kg, 10 mg at weights of 20-39 kg, and 20 mg at weights of >39 kg). The administration of G-CSF was stopped when the neutrophil count exceeded 1 × 103 cells per L at follow-up visits scheduled in the protocol (every 3 months on average). However, G-CSF treatment was reinstated if neutrophil numbers dipped below this threshold. Inclusion and exclusion criteria, as well as the protocol used to address the potential side effects of empagliflozin, are provided in the supplemental Materials and methods. Neutrophils were isolated from the peripheral blood of patients and healthy donors, as previously described.21 The complete protocol is provided in the supplemental Materials. Blood was collected before the trial as well as 3 and 12 months after the initiation of empagliflozin treatment. Complete blood counts were determined at the same times.
Apoptosis assay
Freshly isolated neutrophils were labeled with annexin V (BD Pharmingen) and apoptotic cells were detected by flow cytometry using a BD FACSLyric system.
Migration and chemotactic response assay
Neutrophil migration and chemotaxis in response to 10% human serum (Sigma-Aldrich) were determined using a Boyden chamber model and the fluorogenic CytoSelect 96-well cell migration assay with 3-μm ports (Cell Biolabs) according to the manufacturer’s instructions.
Induction and quantification of NETs
Freshly isolated neutrophils were stimulated with 25 nM phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) and extracellular DNA was recovered after 3 hours and digested with micrococcal nuclease (Thermo Fisher Scientific). The quantity of DNA was determined using SYTOX Green Nucleic Acid Stain (Thermo Fisher Scientific) at an excitation wavelength of 485 nm and an emission wavelength of 535 nm. NETs were also identified by confocal microscopy, as previously described.21 Cells were stained with a rabbit anti-human neutrophil elastase antibody (no. 01-14-051200, Athens Research and Technology) diluted at 1:500 in phosphate-buffered saline containing 3% bovine serum albumin and 0.1% saponin for 1 hour, followed by APC-conjugated goat anti-rabbit immunoglobulin G F(ab′)2 (no. 111-136-144, Jackson ImmunoResearch Laboratories) diluted at 1:1000 for 45 minutes. Nuclei were counterstained with 1 μg/mL Hoechst 33342 (Invitrogen).
Reduced NADP oxidase assay
The ability of neutrophils to generate reactive oxygen species via reduced NADP oxidase was determined using a dihydrorhodamine 123 (DHR) assay followed by flow cytometry, as previously described.22
Plasma concentration of 1,5-AG
Serum (50 μL) was spiked with 10 μL of the internal standard (100 μg/mL 1,5-AG-13C6) and 150 μL of acetonitrile to precipitate proteins. Samples were vortexed for 2 minutes and centrifuged before plasma 1,5-AG concentration was measured by liquid chromatography–tandem mass spectrometry (LC-MS/MS) using a Waters ACQUITY UPLC glycan BEH amide column (2.1 × 150 mm, 1.7 μm particle size) with isocratic elution and a total runtime of 10 minutes. The mobile phase consisted of solvent A (water with 0.1% (volume-per-volume ammonium hydroxide) and solvent B (acetonitrile with 0.1% [volume-per-volume] ammonium hydroxide). Eluted fractions were injected into the mass spectrometer followed by negative electrospray ionization and analysis by multiple-reaction monitoring with the quantification range of 1 to 100 μg/mL.
ELISA
The following commercially available enzyme-linked immunosorbent assay (ELISA) assay kits were used to quantify various AMPs in cell lysates and plasma samples according to the manufacturers’ instructions: Human G-CSF Instant ELISA kit (Invitrogen, BMS2001NST), EnzCheck Myeloperoxidase (MPO) Activity Assay kit (Invitrogen, E33856), Human LTF/LF (lactoferrin) ELISA kit (Elabscience, E-EL-H5200), Human CAMP/cathelicidin antimicrobial ELISA kit (EIAab, E1046h), and Human HNP1-3 (neutrophil peptide 1-3) ELISA kit (Elabscience, E-EL-H2299).
Statistical analysis
Data were analyzed and visualized using Graphpad Prism (Graphpad Software). Statistical differences between patients and controls were determined by ordinary 1-way analysis of variance for unpaired data. Statistical differences between data sets collected before and during empagliflozin treatment (0, 3, and 12 months) were determined by mixed-effect analysis. Two-tailed nonparametric Spearman correlation assay was used to determine potential correlations with plasma 1,5-AG concentration or plasma G-CSF concentration. Statistical significance was defined as P < .05.
The EMPAtia study was approved by bioethics commission at the Children's Memorial Health Institute in Warsaw no 16/KBE/2021 and FixNet project was approved by the Human Research Ethics Committee, Medical University of Łódź (RNN/211/22/KE and RNN/353/19/KE).
Results
Clinical course during empagliflozin treatment
Overall, 7 pediatric patients (3 girls and 4 boys; median age 11 years; age range, 3-19 years) with various biallelic mutations in the SLC37A4 gene were enrolled for neutrophil functional tests (Tables 1 and 2; Figure 1). Before empagliflozin treatment commenced, all 7 patients were treated with G-CSF (median dose, 2 μg/kg per day; dose range, 1-6.5 μg/kg per day). The median dose of empagliflozin as the trial began was 0.42 mg/kg per day (range, 0.3-0.47 mg/kg per day).
Pediatric patients with GSD1b recruited for the EMPAtia/Fixnet observational substudy
| . | Sex . | Age (y) . | SLC374A mutation NM_001164278.1 . | Protein mutation NP_001157750 . | G-CSF (μg/kg per day) . | Empagliflozin (mg/kg per day) . | Antibiotics requiring infections . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 . | 3 mo . | 12 mo . | 0 . | 3 mo . | 12 mo . | 1 y before . | 1 y after . | |||||
| P1 | M | 17 | c.[1042_1043del];[1042_1043del] | p.[(Leu370Valfs)];[(p.Leu370Valfs)] | 2 | 1 | 0 | 0.45 | 0.45 | 0.44 | 3 | 1 |
| P2 | M | 3 | c.[1042_1043del];[1042_1043del] | p.[(Leu370Valfs)];[(p.Leu370Valfs)] | 5.4 | 2.5 | 2.5 | 0.3 | 0.3 | 0.27 | 6 | 4 |
| P3 | M | 11 | c.[898C>T];[1042_1043del] | p.[(Arg300Cys)];[(p.Leu370Valfs)] | 2 | 0 | 0 | 0.47 | 0.47 | 0.46 | 7 | 6 |
| P4 | F | 7 | c.[1042_1043del];[1042_1043del] | p.[(Leu370Valfs)];[(p.Leu370Valfs)] | 1 | 0 | 0 | 0.44 | 0.44 | 0.42 | 0 | 1 |
| P5 | M | 14 | c.[1015G>T];[1042_1043del] | p.[(Gly361Cys)];[(p.Leu370Valfs)] | 6 | 0 | 0 | 0.43 | 0.42 | 0.4 | 1 | 1 |
| P6 | F | 19 | c.[1042_1043del];[1042_1043del] | p.[(Leu370Valfs)];[(p.Leu370Valfs)] | 4 | 0 | 0 | 0.3 | 0.3 | 0.27 | 4 | 0 |
| P7 | F | 8 | c.[1015G>T];[1042_1043del] | p.[(Gly361Cys)];[(p.Leu370Valfs)] | 2 | 1 | 0 | 0.3 | 0.3 | 0.27 | 2 | 0 |
| . | Sex . | Age (y) . | SLC374A mutation NM_001164278.1 . | Protein mutation NP_001157750 . | G-CSF (μg/kg per day) . | Empagliflozin (mg/kg per day) . | Antibiotics requiring infections . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 . | 3 mo . | 12 mo . | 0 . | 3 mo . | 12 mo . | 1 y before . | 1 y after . | |||||
| P1 | M | 17 | c.[1042_1043del];[1042_1043del] | p.[(Leu370Valfs)];[(p.Leu370Valfs)] | 2 | 1 | 0 | 0.45 | 0.45 | 0.44 | 3 | 1 |
| P2 | M | 3 | c.[1042_1043del];[1042_1043del] | p.[(Leu370Valfs)];[(p.Leu370Valfs)] | 5.4 | 2.5 | 2.5 | 0.3 | 0.3 | 0.27 | 6 | 4 |
| P3 | M | 11 | c.[898C>T];[1042_1043del] | p.[(Arg300Cys)];[(p.Leu370Valfs)] | 2 | 0 | 0 | 0.47 | 0.47 | 0.46 | 7 | 6 |
| P4 | F | 7 | c.[1042_1043del];[1042_1043del] | p.[(Leu370Valfs)];[(p.Leu370Valfs)] | 1 | 0 | 0 | 0.44 | 0.44 | 0.42 | 0 | 1 |
| P5 | M | 14 | c.[1015G>T];[1042_1043del] | p.[(Gly361Cys)];[(p.Leu370Valfs)] | 6 | 0 | 0 | 0.43 | 0.42 | 0.4 | 1 | 1 |
| P6 | F | 19 | c.[1042_1043del];[1042_1043del] | p.[(Leu370Valfs)];[(p.Leu370Valfs)] | 4 | 0 | 0 | 0.3 | 0.3 | 0.27 | 4 | 0 |
| P7 | F | 8 | c.[1015G>T];[1042_1043del] | p.[(Gly361Cys)];[(p.Leu370Valfs)] | 2 | 1 | 0 | 0.3 | 0.3 | 0.27 | 2 | 0 |
F, female; M, male.
Effect of G-CSF and empagliflozin on neutrophils
| . | Control donors . | Patients . | Patients vs control donors (P values) . | Empagliflozin vs G-CSF (P values) . | Correlation with plasma 1,5-AG concentration (P values) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Under G-CSF (0) . | 3 mo . | 12 mo . | G-CSF vs control . | 3 mo vs control . | 12 mo vs control . | 3 mo vs G-CSF . | 12 mo vs G-CSF . | |||
| Number of samples | 11 | 7 | 7 | 6 | ||||||
| Sex | 6M/5F | 4M/3F | 4M/3F | 3M/3F | ||||||
| Age (y) | 14 (9-17) | 11.5 (7.5-15.5) | ||||||||
| Severe infections per y | n.d. | 3 (1.5-5) | 1 (0.5-2.5) | n.d. | .0582 | |||||
| Plasma 1,5-AG concentration (μg/mL) | n.d. | 47.2 (43.35-52.95) | 6.2 (2.85-11.2) | 5.3 (4.6-9.45) | n.d. | n.d. | n.d. | <.0001 | <.0001 | |
| Plasma G-CSF concentration (ng/mL) | 17.9 (15.8-20.8) | 135.0 (46.9-147.9) | 43.1 (23.8-72.3) | 21.4 (18.5-29.9) | <.0001 | .2546 | .9669 | .2467 | .09 | |
| Leukocytes count (×1000 cells per μL) | 6.21 (5.83-7.46) | 2.55 (2.24-4.55) | 4.4 (3.76-6.92) | 3.98 (3.62-4.34) | .0021 | .38 | .0875 | .0352 | .3783 | .0473 |
| Neutrophils count (×1000 cells per μL) | 3.10 (2.69-3,61) | 0.86 (0.58-1.18) | 1.95 (1.44-2.78) | 2.24 (2.13-2.50) | .001 | .2347 | .3247 | .0699 | .0945 | .018 |
| Lymphocytes count (×1000 cells per μL) | 2.24 (1.8-3.10) | 1.59 (1.18-2.52) | 1.83 (1.56-3.15) | 1.26 (1.12-1.83) | .2934 | .8721 | .2044 | .1034 | .7548 | .3620 |
| Monocytes count (×1000 cells per μL) | 0.56 (0.51-0.64) | 0.26 (0.21-0.44) | 0.30 (0.25-0.49) | 0.32 (0.30-0.39) | .0875 | .4193 | .6111 | .1354 | .4787 | .1945 |
| Healthy annexin-V negative (neutrophils %) | 77.0 (73.2-78.2) | 34.8 (30.9-76.3) | 61.7 (44.9-71.3) | 67.8 (57.1.1-74.6) | .0183 | .0605 | .5127 | .9597 | .5335 | .1979 |
| Apoptotic annexin-V positive (neutrophils %) | 23.0 (19.7-27.5) | 65.1 (23.5-68.5) | 38.3 (28.7-55.1) | 32.2 (25.2-42.8) | .0181 | .0567 | .4961 | .9643 | .5381 | .1915 |
| Migration (cells) | 1010 (936-1201) | 192 (124-420) | 350 (196-450) | 616 (558-653) | <.0001 | <.0001 | .0132 | .9083 | .1676 | .4290 |
| Chemotaxis response to 10 % HS (cells) | 3735 (3071-4828) | 1546 (1343-2298) | 3110 (2837-4462) | 3513 (2924-4065) | .0345 | .9977 | .9971 | .0844 | .0293 | .0457 |
| NETosis (PMA induction fold) | 4.33 (2.84-4.77) | 1.03 (0.98-1.07) | 1.16 (1.05-1.27) | 1.58 (1.41-1.80) | <.0001 | <.0001 | <.0001 | .1826 | .02 | .0007 |
| Phagocytosis of S aureus bioparticles (FU) | 3352 (2471-4440) | 1365 (774-1424) | 1809 (1342-2046) | 2470 (1904-2920) | <.0001 | .0143 | .1231 | .1656 | .028 | .0328 |
| Oxidative burst, index DHR (%) | n.d. | 0.89 (0.845-0.955) | n.d. | 0.96 (0.93-1.015) | n.d. | n.d. | n.d. | n.d. | .1292 | .1905 |
| Oxidative burst, index DHR (FMI) | n.d. | 0.97 (0.955-0.985) | n.d. | 0.94 (0.925-1.01) | n.d. | n.d. | n.d. | n.d. | .9444 | .5977 |
| cell. MPO concentration (ng/μg) | 55.3 (40.5-63.0) | 47.3 (37.2-53.0) | 43.9 (37.4-62.0) | 58.7 (46.9-61.6) | .9485 | .8253 | .9760 | .9833 | .8152 | .2521 |
| cell. LTF concentration (ng/μg) | 11.84 (10.96-13.51) | 9.52 (8.24-10.82) | 8.59 (6.06-8.92) | 8.96 (7.72-11.72) | .1982 | .0073 | .2876 | .3453 | .9954 | .7215 |
| cell. defensins concentration (ng/μg) | 44.2 (35.0-46.2) | 56.9 (42.2-72.4) | 17.15 (15.0-44.9) | 22.36 (20.72-38.69) | .2893 | .3701 | .2920 | .0451 | .0419 | .0316 |
| [plasma LTF] (ng/mL) | 0.32 (0.27-0.36) | 0.74 (0.61-0.83) | 0.19 (0.15-0.21) | 0.18 (0.10-0.34) | <.0001 | .1757 | .4234 | .0061 | .0045 | .0005 |
| Plasma defensins concentration (ng/mL) | 0.53 (0.33-0.82) | 3.06 (2.24-3.21) | 2.25 (1.46-2.31) | 1.17 (1.01-1.60) | <.0001 | .0003 | .0759 | .0098 | .0121 | .2356 |
| Plasma CAMP/LL37 concentration (ng/mL) | 7.16 (5.38-8.03) | 1.36 (0.48-3.83) | 1.04 (0.165-2.12) | 0.29 (0-1.36) | .0005 | <.0001 | <.0001 | .5726 | .4034 | .8343 |
| . | Control donors . | Patients . | Patients vs control donors (P values) . | Empagliflozin vs G-CSF (P values) . | Correlation with plasma 1,5-AG concentration (P values) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Under G-CSF (0) . | 3 mo . | 12 mo . | G-CSF vs control . | 3 mo vs control . | 12 mo vs control . | 3 mo vs G-CSF . | 12 mo vs G-CSF . | |||
| Number of samples | 11 | 7 | 7 | 6 | ||||||
| Sex | 6M/5F | 4M/3F | 4M/3F | 3M/3F | ||||||
| Age (y) | 14 (9-17) | 11.5 (7.5-15.5) | ||||||||
| Severe infections per y | n.d. | 3 (1.5-5) | 1 (0.5-2.5) | n.d. | .0582 | |||||
| Plasma 1,5-AG concentration (μg/mL) | n.d. | 47.2 (43.35-52.95) | 6.2 (2.85-11.2) | 5.3 (4.6-9.45) | n.d. | n.d. | n.d. | <.0001 | <.0001 | |
| Plasma G-CSF concentration (ng/mL) | 17.9 (15.8-20.8) | 135.0 (46.9-147.9) | 43.1 (23.8-72.3) | 21.4 (18.5-29.9) | <.0001 | .2546 | .9669 | .2467 | .09 | |
| Leukocytes count (×1000 cells per μL) | 6.21 (5.83-7.46) | 2.55 (2.24-4.55) | 4.4 (3.76-6.92) | 3.98 (3.62-4.34) | .0021 | .38 | .0875 | .0352 | .3783 | .0473 |
| Neutrophils count (×1000 cells per μL) | 3.10 (2.69-3,61) | 0.86 (0.58-1.18) | 1.95 (1.44-2.78) | 2.24 (2.13-2.50) | .001 | .2347 | .3247 | .0699 | .0945 | .018 |
| Lymphocytes count (×1000 cells per μL) | 2.24 (1.8-3.10) | 1.59 (1.18-2.52) | 1.83 (1.56-3.15) | 1.26 (1.12-1.83) | .2934 | .8721 | .2044 | .1034 | .7548 | .3620 |
| Monocytes count (×1000 cells per μL) | 0.56 (0.51-0.64) | 0.26 (0.21-0.44) | 0.30 (0.25-0.49) | 0.32 (0.30-0.39) | .0875 | .4193 | .6111 | .1354 | .4787 | .1945 |
| Healthy annexin-V negative (neutrophils %) | 77.0 (73.2-78.2) | 34.8 (30.9-76.3) | 61.7 (44.9-71.3) | 67.8 (57.1.1-74.6) | .0183 | .0605 | .5127 | .9597 | .5335 | .1979 |
| Apoptotic annexin-V positive (neutrophils %) | 23.0 (19.7-27.5) | 65.1 (23.5-68.5) | 38.3 (28.7-55.1) | 32.2 (25.2-42.8) | .0181 | .0567 | .4961 | .9643 | .5381 | .1915 |
| Migration (cells) | 1010 (936-1201) | 192 (124-420) | 350 (196-450) | 616 (558-653) | <.0001 | <.0001 | .0132 | .9083 | .1676 | .4290 |
| Chemotaxis response to 10 % HS (cells) | 3735 (3071-4828) | 1546 (1343-2298) | 3110 (2837-4462) | 3513 (2924-4065) | .0345 | .9977 | .9971 | .0844 | .0293 | .0457 |
| NETosis (PMA induction fold) | 4.33 (2.84-4.77) | 1.03 (0.98-1.07) | 1.16 (1.05-1.27) | 1.58 (1.41-1.80) | <.0001 | <.0001 | <.0001 | .1826 | .02 | .0007 |
| Phagocytosis of S aureus bioparticles (FU) | 3352 (2471-4440) | 1365 (774-1424) | 1809 (1342-2046) | 2470 (1904-2920) | <.0001 | .0143 | .1231 | .1656 | .028 | .0328 |
| Oxidative burst, index DHR (%) | n.d. | 0.89 (0.845-0.955) | n.d. | 0.96 (0.93-1.015) | n.d. | n.d. | n.d. | n.d. | .1292 | .1905 |
| Oxidative burst, index DHR (FMI) | n.d. | 0.97 (0.955-0.985) | n.d. | 0.94 (0.925-1.01) | n.d. | n.d. | n.d. | n.d. | .9444 | .5977 |
| cell. MPO concentration (ng/μg) | 55.3 (40.5-63.0) | 47.3 (37.2-53.0) | 43.9 (37.4-62.0) | 58.7 (46.9-61.6) | .9485 | .8253 | .9760 | .9833 | .8152 | .2521 |
| cell. LTF concentration (ng/μg) | 11.84 (10.96-13.51) | 9.52 (8.24-10.82) | 8.59 (6.06-8.92) | 8.96 (7.72-11.72) | .1982 | .0073 | .2876 | .3453 | .9954 | .7215 |
| cell. defensins concentration (ng/μg) | 44.2 (35.0-46.2) | 56.9 (42.2-72.4) | 17.15 (15.0-44.9) | 22.36 (20.72-38.69) | .2893 | .3701 | .2920 | .0451 | .0419 | .0316 |
| [plasma LTF] (ng/mL) | 0.32 (0.27-0.36) | 0.74 (0.61-0.83) | 0.19 (0.15-0.21) | 0.18 (0.10-0.34) | <.0001 | .1757 | .4234 | .0061 | .0045 | .0005 |
| Plasma defensins concentration (ng/mL) | 0.53 (0.33-0.82) | 3.06 (2.24-3.21) | 2.25 (1.46-2.31) | 1.17 (1.01-1.60) | <.0001 | .0003 | .0759 | .0098 | .0121 | .2356 |
| Plasma CAMP/LL37 concentration (ng/mL) | 7.16 (5.38-8.03) | 1.36 (0.48-3.83) | 1.04 (0.165-2.12) | 0.29 (0-1.36) | .0005 | <.0001 | <.0001 | .5726 | .4034 | .8343 |
Results are presented as median values with first and third quartile (Q1 and Q3). Statistically significant differences between patients and healthy controls were determined by ordinary 1-way analysis of variance for unpaired data, whereas mixed-effect analysis for paired data was used to compare values at 3 and 12 months after the start of empagliflozin treatment with values obtained before the start of treatment. The potential correlation between each parameter and plasma 1,5-AG concentration was analyzed by 2-tailed Spearman correlation assay.
Cel., intra-cellular; F, female; FI, fluoresence intensity; FMI, fluroresence mean intensity; FU, fluoresence unit; HS, human serum; M, male.
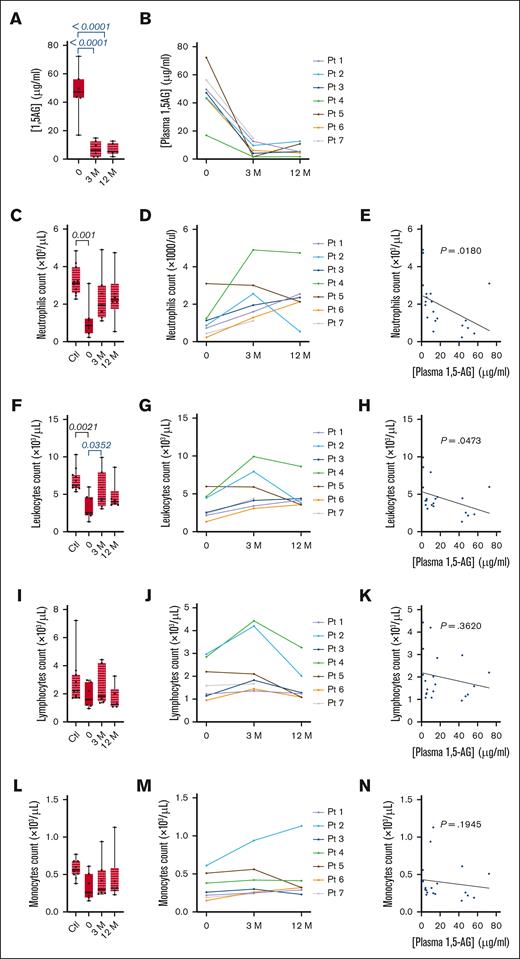
Empagliflozin lowers plasma 1,5-AG concentration and increases the neutrophil count. (A-B) The plasma concentration of 1,5-AG was determined before and 3 and 12 months after the start of empagliflozin treatment in 7 patients with GSD1b and 11 healthy controls: (A) median ± quartiles for the patients and controls, and (B) individual patient profiles. (C-D) Neutrophil counts: (C) median ± quartiles for the patients and controls, and (D) individual profiles. (E) Spearman correlation analysis between plasma 1,5-AG concentration and neutrophil count. (F) median ± quartiles of leukocyte count for the patients and controls, and (G) individual patient profiles. (H) Spearman correlation analysis between plasma 1,5-AG concentration and leukocyte count. (I) median ± quartiles of lymphocyte count for the patients and controls, and (J) individual profiles. (K) Spearman correlation analysis between plasma 1,5-AG concentration and lymphocyte count. (L) median ± quartiles of monocytes count for the patients and controls, and (M) individual profiles. (N) Spearman correlation analysis between plasma 1,5-AG concentration and monocyte count. Statistically significant differences between patients and healthy controls (black) were determined by ordinary 1-way analysis of variance (ANOVA) for unpaired data, whereas mixed-effect analysis for paired data was used to compare values 3 and 12 months after the start of empagliflozin treatment with values obtained before the start of treatment (blue). For clarity, only P values <.5 are shown.
Empagliflozin lowers plasma 1,5-AG concentration and increases the neutrophil count. (A-B) The plasma concentration of 1,5-AG was determined before and 3 and 12 months after the start of empagliflozin treatment in 7 patients with GSD1b and 11 healthy controls: (A) median ± quartiles for the patients and controls, and (B) individual patient profiles. (C-D) Neutrophil counts: (C) median ± quartiles for the patients and controls, and (D) individual profiles. (E) Spearman correlation analysis between plasma 1,5-AG concentration and neutrophil count. (F) median ± quartiles of leukocyte count for the patients and controls, and (G) individual patient profiles. (H) Spearman correlation analysis between plasma 1,5-AG concentration and leukocyte count. (I) median ± quartiles of lymphocyte count for the patients and controls, and (J) individual profiles. (K) Spearman correlation analysis between plasma 1,5-AG concentration and lymphocyte count. (L) median ± quartiles of monocytes count for the patients and controls, and (M) individual profiles. (N) Spearman correlation analysis between plasma 1,5-AG concentration and monocyte count. Statistically significant differences between patients and healthy controls (black) were determined by ordinary 1-way analysis of variance (ANOVA) for unpaired data, whereas mixed-effect analysis for paired data was used to compare values 3 and 12 months after the start of empagliflozin treatment with values obtained before the start of treatment (blue). For clarity, only P values <.5 are shown.
During G-CSF treatment, in the year before the start of the trial, the median number of infections requiring antibiotics was 3 (first quartile [Q1] = 1.5; third quartile [Q3] = 5; Tables 1 and 2). The year after the start of empagliflozin treatment, the median number of infections decreased to 1 (Q1 = 0.5; Q3 = 2.5) and only patient 4 experienced an increase in the number of infections, from 0 to 1 the following year (Table 1). One patient was diagnosed with IBD before the study. During the first year of empagliflozin treatment, IBD symptoms did not become any more severe in this patient and were not observed in the other 6 patients. There was also no significant increase in the frequency of hypoglycemic episodes or side effects during the first year of empagliflozin treatment in any of the patients.
Measurement of plasma 1,5-AG concentration
Before empagliflozin treatment, the median concentration of 1,5-AG in the plasma of 7 patients was 47.2 μg/mL (Q1 = 43.35 μg/mL; Q3 = 52.95 μg/mL; Table 2; Figure 1A). This decreased significantly (P < .0001) to 6.2 μg/mL (Q1 = 2.85 μg/mL; Q3 = 11.2 μg/mL) after 3 months, and to 5.3 μg/mL (Q1 = 4.6 μg/mL; Q3 = 9.45 μg/mL) after 12 months (Table 2; Figure 1A). Substantially lower 1,5-AG levels were observed in all 7 patients (Figure 1B).
Leukocyte and neutrophil counts
Under G-CSF treatment, the median neutrophil count in the 7 patients was 0.86 × 103 cells per μL (Table 2; Figure 1C). This was significantly lower than the median count of 3.1 × 103 cells per μL in the 11 control donors (P = .001). During empagliflozin treatment, the median neutrophil count of 7 patients increased to 1.95 × 103 cells per μL after 3 months, and 2.24 × 103 cells per μL after 12 months (Table 2; Figure 1C). Only patient 2 showed a significant decline in the neutrophil count between 3 and 12 months of empagliflozin treatment (Figure 1D). G-CSF was therefore discontinued in 6 of 7 patients, and the dosage was reduced in patient 2 (Table 1). Spearman analysis revealed a correlation between the neutrophil count and plasma 1,5-AG concentration in the patients (P = .018; Figure 1E). G-CSF was therefore discontinued in 5 of 7 patients during the first 3 months, and only 1 patient still required G-CSF after 1 year, at a reduced dosage (Table 1). This increase in neutrophil count also affected the leukocyte count, which similarly increased after empagliflozin treatment (Table 2; Figure 1F-H). However, there was no significant effect on the lymphocyte or monocyte populations (Table 2; Figure 1I-N).
Neutrophil viability
The proportion of viable neutrophils (negative for annexin V) varied widely among the 7 patients during G-CSF treatment, with a median of 34.8% (Q1 = 30.9%; Q3 = 76.3%; Table 2; Figure 2A). After 3 and 12 months on empagliflozin, the proportion increased to 61.7% (Q1 = 44.9%; Q3 = 71.3%) and 67.8% (Q1 = 57.1%; Q3 = 74.6%), respectively (Table 2; Figure 2A). However, the effect varied greatly, improving cell viability in patients with the lowest initial viability under G-CSF treatment but achieving limited or no improvement in patients with a higher initial proportion of viable neutrophils (Figure 2B). Accordingly, there was no clear correlation between cell viability and plasma 1,5-AG concentration (P = .1979; Figure 2C). Given the inverse relationship between the proportions of viable and apoptotic cells, empagliflozin reduced the frequency of apoptosis in neutrophils when it was high under G-CSF treatment (Table 2; Figure 2D–F).
Effect of empagliflozin on neutrophil viability and the chemotactic response. (A-F) Cell viability and the frequency of apoptosis in freshly isolated neutrophils were determined in 7 patients with GSD1b and 11 healthy controls at 3 time points by labeling with annexin V. (A) Median ± quartiles of neutrophil viability for the patients and controls, and (B) individual profiles. (D) Median ± quartiles of apoptotic frequency for the patients and controls, and (E) individual profiles. Spearman correlation analysis between plasma 1,5-AG concentration and neutrophil viability (C) and apoptosis (F). (G-L) The mobility and chemotactic response of neutrophils determined in a Boyden’s chamber model with or without human serum. (G) Median ± quartiles of the number of migrating neutrophils in the absence of human serum for the patients and controls, and (H) individual profiles. (J) Median ± quartiles of the number of migrating neutrophils in in response to human serum for the patients and controls, and (K) the individual profiles. Spearman correlation analysis between plasma 1,5-AG concentration and (I) neutrophil mobility and (L) chemotactic response. Statistically significant differences between patients and healthy controls (black) were determined by ordinary 1-way ANOVA for unpaired data, whereas mixed-effect analysis for paired data was used to compare values 3 and 12 months after the start of empagliflozin treatment to values obtained before the start of treatment (blue). For clarity, only P values <.5 are shown.
Effect of empagliflozin on neutrophil viability and the chemotactic response. (A-F) Cell viability and the frequency of apoptosis in freshly isolated neutrophils were determined in 7 patients with GSD1b and 11 healthy controls at 3 time points by labeling with annexin V. (A) Median ± quartiles of neutrophil viability for the patients and controls, and (B) individual profiles. (D) Median ± quartiles of apoptotic frequency for the patients and controls, and (E) individual profiles. Spearman correlation analysis between plasma 1,5-AG concentration and neutrophil viability (C) and apoptosis (F). (G-L) The mobility and chemotactic response of neutrophils determined in a Boyden’s chamber model with or without human serum. (G) Median ± quartiles of the number of migrating neutrophils in the absence of human serum for the patients and controls, and (H) individual profiles. (J) Median ± quartiles of the number of migrating neutrophils in in response to human serum for the patients and controls, and (K) the individual profiles. Spearman correlation analysis between plasma 1,5-AG concentration and (I) neutrophil mobility and (L) chemotactic response. Statistically significant differences between patients and healthy controls (black) were determined by ordinary 1-way ANOVA for unpaired data, whereas mixed-effect analysis for paired data was used to compare values 3 and 12 months after the start of empagliflozin treatment to values obtained before the start of treatment (blue). For clarity, only P values <.5 are shown.
Neutrophil mobility and chemotactic response
The mobility and chemotactic response of freshly isolated neutrophils were examined using a Boyden chamber model. In the absence of a chemoattractant, the mobility of neutrophils from patients receiving G-CSG was deficient compared with neutrophils from healthy donors (median of 192 vs 1010 migrating cells, P < .001; Table 2; Figure 2G). After 12 months of empagliflozin treatment, median neutrophil mobility had increased in the 7 patients but still lagged behind the healthy donors. Furthermore, neutrophil mobility decreased in some patients treated with empagliflozin and we observed no clear correlation between neutrophil mobility and plasma 1,5-AG concentration (Figure 2H-I). The chemotactic response of neutrophils to human serum was deficient in patients receiving G-CSG compared with neutrophils from healthy donors (1421 vs 2487 migrating cells, P = .345; Table 2; Figure 2J). After empagliflozin treatment, the median neutrophil chemotactic response in the 7 patients was restored to the level seen in healthy donors (Figure 2J). Chemotaxis increased in all patients, and was inversely correlated with plasma 1,5-AG concentration (P = .0457; Figure 2K-L).
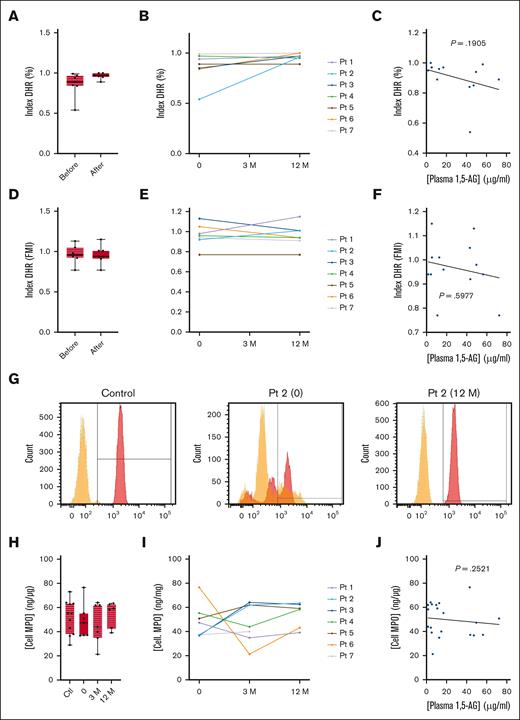
Oxidative burst
We also analyzed the PMA-induced oxidative burst in neutrophils using a DHR test. Only patient 2 showed a defective oxidative system, with a low proportion of positive neutrophils under G-CSF treatment (Figure 3A-B). This deficiency was corrected by empagliflozin, and no differences were observed in the other patients when G-CSF treatment was discontinued (Figure 3B). Therefore, there was no significant correlation between the DHR index and plasma 1,5-AG concentration (P = .1905; Figure 3C). There was also no significant difference in signal intensity between G-CSF and empagliflozin treatment (Figure 3D-F). The median intracellular concentration of MPO in neutrophils from patients treated with G-CSF and empagliflozin treatment was similar to that of healthy donors (Table 2; Figure 3H). Individual variations were observed, but there was no overall increase in MPO levels in patients treated with empagliflozin (Figure 3I). Similarly, there was no correlation between intracellular MPO concentration and plasma 1,5-AG concentration (Figure 3J).
Effect of empagliflozin on the oxidative burst response and intracellular MPO concentration in neutrophils. (A-F) The neutrophil oxidative burst response to PMA stimulation in the 7 patients with GSD1b and 11 healthy controls determined using a DHR assay. (A) Median ± quartiles of the percentage of responsive cells (DHR index) for the patients and controls, and (B) the individual profiles. (D) Median ± quartiles of fluorescence medium intensity (DHR index FMI) for the same neutrophils, and (E) the individual profiles. Spearman correlation analysis between plasma 1,5-AG concentration and (C) DHR index and (F) DHR index FMI. (G) Representative flow cytometry analysis for a control donor and patient 2. (H-I) Intracellular MPO concentrations determined by ELISA: median ± quartiles of (H) MPO concentration for the patients and controls, and (I) the individual profiles. (J) Spearman correlation analysis between plasma 1,5-AG concentration and intracellular MPO concentration. Statistically significant differences between patients and healthy controls (black) were determined by ordinary 1-way ANOVA for unpaired data, whereas mixed-effect analysis for paired data was used to compare values at 3 and 12 months after the start of empagliflozin treatment with values obtained before the start of treatment (blue). For clarity, only P values <.5 are shown.
Effect of empagliflozin on the oxidative burst response and intracellular MPO concentration in neutrophils. (A-F) The neutrophil oxidative burst response to PMA stimulation in the 7 patients with GSD1b and 11 healthy controls determined using a DHR assay. (A) Median ± quartiles of the percentage of responsive cells (DHR index) for the patients and controls, and (B) the individual profiles. (D) Median ± quartiles of fluorescence medium intensity (DHR index FMI) for the same neutrophils, and (E) the individual profiles. Spearman correlation analysis between plasma 1,5-AG concentration and (C) DHR index and (F) DHR index FMI. (G) Representative flow cytometry analysis for a control donor and patient 2. (H-I) Intracellular MPO concentrations determined by ELISA: median ± quartiles of (H) MPO concentration for the patients and controls, and (I) the individual profiles. (J) Spearman correlation analysis between plasma 1,5-AG concentration and intracellular MPO concentration. Statistically significant differences between patients and healthy controls (black) were determined by ordinary 1-way ANOVA for unpaired data, whereas mixed-effect analysis for paired data was used to compare values at 3 and 12 months after the start of empagliflozin treatment with values obtained before the start of treatment (blue). For clarity, only P values <.5 are shown.
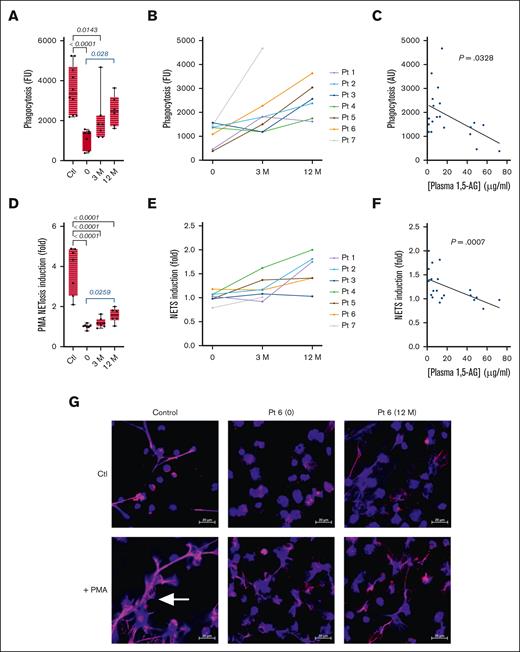
Phagocytosis
Neutrophil-mediated phagocytosis was assessed using fluorescent and pH-sensitive particles of Staphylococcus aureus. The median phagocytic activity of neutrophils from patients treated with G-CSF was significantly lower than that of healthy donors (1546 vs 3735 fluorescence units; P < .0001; Table 2; Figure 4A). However, after 12 months of empagliflozin treatment, the median phagocytic activity increased significantly (P = .028). All individual patients showed an increase in phagocytosis (Figure 4B) and there was a negative correlation between phagocytic activity and plasma 1,5-AG concentration (P = .0328).
Effect of empagliflozin on phagocytosis and NETosis. (A-C) The phagocytic activity of freshly isolated neutrophils was determined in the 7 patients with GSD1b and 11 healthy donors at 3 time points using pH-sensitive particles of S aureus: (A) median ± quartiles of phagocytosis (fluorescence units) for the patients and controls, and (B) the individual profiles. (C) Spearman correlation analysis between plasma 1,5-AG concentration and neutrophil phagocytosis. (D-F) NET production determined by the quantification of extracellular DNA before and after PMA stimulation: (D) median ± quartiles of NETosis induction for the patients and controls, and (E) the individual profiles. (F) Spearman correlation analysis between plasma 1,5-AG concentration and neutrophil NETosis. (G) Representative confocal images of NETosis based on the labeling of DNA (blue) and neutrophil elastase (pink). The scale bar represents 20 μM, and white arrows indicate NETs structures. Statistically significant differences between patients and healthy controls (black) were determined by ordinary 1-way ANOVA for unpaired data, whereas mixed-effect analysis for paired data was used to compare values at 3 and 12 months after the start of empagliflozin treatment with values obtained before the start of treatment (blue). For clarity, only P values <.5 are shown.
Effect of empagliflozin on phagocytosis and NETosis. (A-C) The phagocytic activity of freshly isolated neutrophils was determined in the 7 patients with GSD1b and 11 healthy donors at 3 time points using pH-sensitive particles of S aureus: (A) median ± quartiles of phagocytosis (fluorescence units) for the patients and controls, and (B) the individual profiles. (C) Spearman correlation analysis between plasma 1,5-AG concentration and neutrophil phagocytosis. (D-F) NET production determined by the quantification of extracellular DNA before and after PMA stimulation: (D) median ± quartiles of NETosis induction for the patients and controls, and (E) the individual profiles. (F) Spearman correlation analysis between plasma 1,5-AG concentration and neutrophil NETosis. (G) Representative confocal images of NETosis based on the labeling of DNA (blue) and neutrophil elastase (pink). The scale bar represents 20 μM, and white arrows indicate NETs structures. Statistically significant differences between patients and healthy controls (black) were determined by ordinary 1-way ANOVA for unpaired data, whereas mixed-effect analysis for paired data was used to compare values at 3 and 12 months after the start of empagliflozin treatment with values obtained before the start of treatment (blue). For clarity, only P values <.5 are shown.
NETosis
Patients treated with G-CSF were deficient for NETosis after stimulation with PMA compared with healthy donors (P < .0001; Table 2; Figure 4D). Treatment with empagliflozin caused a significant increase in NETosis (P < .05), however not to the level observed in healthy donor neutrophils (Figure 4D). This slight increase was apparent in all 7 patients (Figure 4E) and we observed a negative correlation between the induction of NETosis and plasma 1,5-AG concentration (P = .0007; Figure 4F). Furthermore, confocal microscopy demonstrated that neutrophils from patients, with G-CSF or empagliflozin, failed to produce visible NETs structures after PMA stimulation, in contrast to neutrophils from the control donor (Figure 4G).
Production of AMPs
Cellular and secreted AMPs such as lactoferrin (LTF), defensins, and LL-37 play a significant role in the antibacterial response of neutrophils. Median intracellular LTF concentrations were similar in patients treated with G-CSF or empagliflozin and in healthy donors and there was no consistent LTF profile at the individual level once empagliflozin treatment had commenced (Table 2; Figure 5A-C). However, the median concentrations of intracellular defensins, plasma LTF, and plasma defensins were significantly higher in patients treated with G-CSF than healthy controls (Table 2; Figure 5E,I,M). Empagliflozin treatment resulted in a significant decrease in the concentration of these AMPs in all patients (Figure 5E,J,N). It is unclear whether the concentrations of these AMPs were higher in patients than healthy controls because of the disease or the high concentration of plasma G-CSF (supplemental Figure 2). The median levels of intracellular defensins, plasma LTF, and plasma defensins were negatively correlated with plasma 1.5-AG concentration and positively correlated with plasma G-CSF concentration in all 7 patients (Table 2; Figure 5). The median plasma CAMP/LL-37 concentration in all 7 patients was significantly lower (P < .0001) during treatment with G-CSF or empagliflozin than in healthy donors (Table 2; Figure 5Q). At the individual level, plasma CAMP/LL-37 concentration decreased in all patients after the start of empagliflozin treatment, but there was no correlation between plasma CAMP/LL-37 concentration and plasma 1,5-AG concentration, or between plasma CAMP/LL-37 concentration and plasma G-CSF concentration (Figure 5R-T).
Effect of empagliflozin on intracellular and plasma AMPs. (A) Median ± quartiles of intracellular LTF concentration for the 7 patients with GSD1b and 11 healthy controls, and (B) the individual profiles. (E) Median ± quartiles of intracellular defensins concentration for the patients and controls, and (F) the individual profiles. (I) Median ± quartiles of plasma LTF concentration for the patients and controls, and (J) the individual profiles. (M) Median ± quartiles of [plasma defensins] for the patients and controls, and (N) the individual profiles. (Q) Median ± quartiles of plasma LL-37 concentration for the patients and controls, and (R) the individual profiles. Spearman correlation analysis between plasma 1,5-AG concentration or plasma G-CSF concentration and intracellular LTF concentration (C-D), intracellular defensins concentration (G-H), plasma LTF concentration (K-L), plasma defensins concentration (O-P) and plasma LL-37 concentration (S-T). Statistically significant differences between patients and healthy controls (black) were determined by ordinary 1-way ANOVA for unpaired data, whereas mixed-effect analysis for paired data was used to compare values at 3 and 12 months after the start of empagliflozin treatment with values obtained before the start of treatment (blue). For clarity, only P values <.5 are shown.
Effect of empagliflozin on intracellular and plasma AMPs. (A) Median ± quartiles of intracellular LTF concentration for the 7 patients with GSD1b and 11 healthy controls, and (B) the individual profiles. (E) Median ± quartiles of intracellular defensins concentration for the patients and controls, and (F) the individual profiles. (I) Median ± quartiles of plasma LTF concentration for the patients and controls, and (J) the individual profiles. (M) Median ± quartiles of [plasma defensins] for the patients and controls, and (N) the individual profiles. (Q) Median ± quartiles of plasma LL-37 concentration for the patients and controls, and (R) the individual profiles. Spearman correlation analysis between plasma 1,5-AG concentration or plasma G-CSF concentration and intracellular LTF concentration (C-D), intracellular defensins concentration (G-H), plasma LTF concentration (K-L), plasma defensins concentration (O-P) and plasma LL-37 concentration (S-T). Statistically significant differences between patients and healthy controls (black) were determined by ordinary 1-way ANOVA for unpaired data, whereas mixed-effect analysis for paired data was used to compare values at 3 and 12 months after the start of empagliflozin treatment with values obtained before the start of treatment (blue). For clarity, only P values <.5 are shown.
Discussion
The accumulation of 1,5-AG6P within the neutrophil cytosol has recently been identified as the cause of neutropenia in GSD1b. Lowering the plasma concentration of 1,5-AG using antidiabetic SGLT2 inhibitors such as empagliflozin has shown promising results.2,3 Several case reports have indicated that empagliflozin normalizes the neutrophil count and partially restores neutrophil function in some patients.8,23-28 In this setting, the EMPAtia clinical trial was initiated to evaluate the efficacy and safety of empagliflozin for the treatment of neutropenia in patients with GSD1b, on a larger scale. Twelve children, representing 92% of identified pediatric patients with GSD1b in Poland, have been enrolled thus far. Seven were qualified for the additional assessment of neutrophil function under G-CSF treatment as well as 3 and 12 months after the initiation of empagliflozin therapy. We observed no side effects such as changes in the frequency of hypoglycemic episodes. Moreover, 1 patient already diagnosed with IBD did not experience any deterioration of the condition.
The ability of G-CSF to restore the immune functions of neutrophils in patients with GSD1b has not been addressed adequately because there is only a small pool of patients and many of them do not produce enough neutrophils for testing.9 During G-CSF treatment, before the administration of empagliflozin, 6 of 7 patients remained neutropenic with a neutrophil count of <1 × 103 cells per μL (Table 2; Figure 2). The neutrophils from these patients showed various deficiencies despite the G-CSF treatment, including an inconsistent rate of apoptosis, lower mobility, a weaker chemotactic response, ineffective phagocytic activity, and impaired production of NETs after stimulation. As a result, these patients experienced frequent serious infections during the year preceding the start of the trial (median of 3 infections per year). However, with the exception of 1 patient, there was no serious deficiency in the oxidative burst, which suggests that G-CSF can, at least, restore this function. This last observation agrees with a previous report showing that most patients with GSD1b treated with G-CSF have a normal oxidative system8
The dose of empagliflozin for pediatric patients with GSD1b was based on the recommendations for adults with diabetes. We used a dose of 0.43 mg/kg per day, which is lower than that used in previous studies (0.7 mg/mL).8 Even so, this lower dose was sufficient to reduce the median plasma 1,5-AG concentration in the patients from 47.2 to 6 μg/mL (Figure 1). We also observed higher neutrophil counts in 6 of 7 patients, with median values of 1.95 × 103 cells per μL after 3 months and 2.24 × 103 cells per μL after 12 months of empagliflozin treatment. The neutrophil count declined in 1 patient between months 3 and 12 despite an initial rise at the beginning of the trial. G-CSF treatment was therefore discontinued in 6 patients with higher neutrophil counts and reduced in the other patient. Empagliflozin did not significantly affect the monocyte or lymphocyte populations in any of the patients.
Empagliflozin reduced the inconsistent viability of neutrophils in the 7 patients, indicating its ability to inhibit apoptosis compared to the limited efficacy of G-CSF. However, it did not fully restore the viability observed in healthy donors. Empagliflozin had only a mild impact on neutrophil mobility, but it increased the chemotactic response to human serum in all patients. This is likely to facilitate the recruitment of neutrophils to infection sites. Similarly, empagliflozin partially restored the phagocytic activity of neutrophils, which is necessary to eliminate pathogens. Despite an overall increase in NETosis after the administration of empagliflozin, the response still lagged behind that observed in healthy donors. Furthermore, we observed inverse correlations between plasma 1,5-AG concentration and the neutrophil count, chemotactic response, phagocytosis, and NETosis. This highlights the central role of 1,5-AG and its derivative 1,5-AG6P in patients with GSD1b and confirms the mode of action of empagliflozin. Neutrophils from all patients treated with empagliflozin retained their normal oxidative burst after stimulation, as observed in previous studies. The lower number of infections probably reflects the enhancement of neutrophil antimicrobial defense mechanisms, improving the quality of life for patients by reducing the number and duration of hospital stays, and allowing the treatment of infections in an outpatient setting.
To the best of our knowledge, the production of AMPs by neutrophils in patients with GSD1b has not been studied before. During treatment with G-CSF, the concentration of intracellular and plasma defensins and plasma LTF increased in all 7 patients, which may reflect the dysfunctional neutrophils or the administration of G-CSF. Empagliflozin treatment reduced the levels of these AMPs, but the absence of untreated patients in the trial made it impossible to determine whether these AMP concentrations correlated with plasma 1,5-AG concentration or plasma G-CSF concentration. Interestingly, neither G-CSF nor empagliflozin were able to restore the production of CAMP/LL-37 and effective NETosis, despite the inverse correlation between NETosis and plasma 1,5AG concentration. Accordingly, additional pathways that do not involve 1,5-AG or 1,5-AG6P must also be deficient in patients with GSD1b.
This study included the most extensive and comprehensive examination of neutrophil functions in patients with GSD1b reported thus far and demonstrated the inability of G-CSF to reverse neutropenia and restore normal neutrophil antimicrobial functions. In contrast, our analysis of the immediate and long-term effects of empagliflozin confirms the efficacy and safety of this antidiabetic drug for the treatment of GSD1b.
Acknowledgments
The Foundation for Polish Science TEAM NET Programme, POIR.04.04.00–00–1603/18; project title: “Fix Neutropenia (FIXNET): focusing on neutrophil proteases defects which serve as novel diagnostic and therapeutic options” and the EMPAtia project financed by the Medical Research Agency grant no. 2020/ABM/01/00047-00. The research by F.V., J.P., B.C.-S., D.B., E.D., J.F., and S.M. has also been supported by a grant from the Priority Research Area BioS under the Strategic Programme Excellence Initiative at the Jagiellonian University.
Authorship
Contribution: D.R., F.V., W.M., J.P., and M.K. conceptualized and designed the study; M.K, M.G., D.W.-K., J.K., and D.R. provided direct patient care and collect patient data; W.M. provided access to control donors; M.K., S.M., J.F., E.D., D.B., B.C.-S., B.P., E.S., J.M., E.E.v.E., and M.H. carried out experiments, data analysis, and figures preparation; F.V., and M.K wrote the manuscript with critical evaluations and revisions from D.R., W.M., J.P., and J.K.; and all authors reviewed and approved the article and participated in the discussion and interpretation of data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Florian Veillard, Department of Microbiology, Faculty of Biochemistry, Biophysics and Biotechnologies, Jagiellonian University, ul Gronostajowa 7, N/A 30-87 Krakow, Poland; email: florian.veillard@uj.edu.pl.
References
Author notes
M.K. and S.M. are joint first authors.
The data that support the findings of this study are available upon reasonable request from the corresponding author, Florian Veillard (florian.veillard@uj.edu.pl). The data are not publicly available because of privacy or ethical restrictions.
The full-text version of this article contains a data supplement.



![Effect of empagliflozin on intracellular and plasma AMPs. (A) Median ± quartiles of intracellular LTF concentration for the 7 patients with GSD1b and 11 healthy controls, and (B) the individual profiles. (E) Median ± quartiles of intracellular defensins concentration for the patients and controls, and (F) the individual profiles. (I) Median ± quartiles of plasma LTF concentration for the patients and controls, and (J) the individual profiles. (M) Median ± quartiles of [plasma defensins] for the patients and controls, and (N) the individual profiles. (Q) Median ± quartiles of plasma LL-37 concentration for the patients and controls, and (R) the individual profiles. Spearman correlation analysis between plasma 1,5-AG concentration or plasma G-CSF concentration and intracellular LTF concentration (C-D), intracellular defensins concentration (G-H), plasma LTF concentration (K-L), plasma defensins concentration (O-P) and plasma LL-37 concentration (S-T). Statistically significant differences between patients and healthy controls (black) were determined by ordinary 1-way ANOVA for unpaired data, whereas mixed-effect analysis for paired data was used to compare values at 3 and 12 months after the start of empagliflozin treatment with values obtained before the start of treatment (blue). For clarity, only P values <.5 are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/11/10.1182_bloodadvances.2023012403/2/m_blooda_adv-2023-012403-gr5.jpeg?Expires=1765908208&Signature=T3IZWtmwAREsppIWzIsVQZlLJkjdAp2MDI5CNYlB7~Q4kMEJrCa3GeWKiaScFi30nby4Mp-tPLW9e5QjCfu1YutZbamq6XgVZrtDpjsE9~mHEXqoFFozyI3fV2ZVrqGTP0ATtpXWUAQLC-ptrsJl0cFhwkm8MAzl7Ieqvzk575ygWcQQgLPfmMaVTZiH6w1SQ2tZp68rRlFIQHCVgLpC~4iLIdjLot0Qybv05WH8IInBCJf2vyP1Vjia~V7pP6fPBuAr7rsTumYrx6y-7PlUDw96vWo-bRm1ItTJXqBP-kMnyC7wJRjq49rnR44pUgSuk4zuvhhW8BZ7KRX8k~2G9g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)