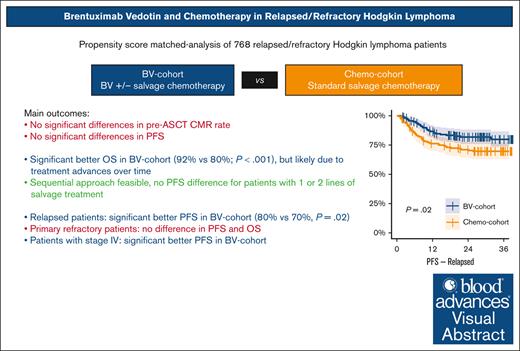
BV with or without chemotherapy does not increase CMR rates or PFS in R/R cHL but seems to increase PFS in patients with relapsed or stage IV disease.
Sequential treatment with BV and chemotherapy is feasible and could spare salvage chemotherapy in a subset of fast responding patients.
Visual Abstract
Several single-arm studies have explored the inclusion of brentuximab vedotin (BV) in salvage chemotherapy followed by autologous stem cell transplantation (ASCT) for relapsed/refractory (R/R) classical Hodgkin lymphoma (cHL). However, no head-to-head comparisons with standard salvage chemotherapy have been performed. This study presents a propensity score–matched analysis encompassing individual patient data from 10 clinical trials to evaluate the impact of BV in transplant-eligible patients with R/R cHL. We included 768 patients, of whom 386 were treated with BV with or without chemotherapy (BV cohort), whereas 382 received chemotherapy alone (chemotherapy cohort). Propensity score matching resulted in balanced cohorts of 240 patients each. No significant differences were observed in pre-ASCT complete metabolic response (CMR) rates (P = .69) or progression free survival (PFS; P = .14) between the BV and chemotherapy cohorts. However, in the BV vs chemotherapy cohort, patients with relapsed disease had a significantly better 3-year PFS of 80% vs 70%, respectively (P = .02), whereas there was no difference for patients with primary refractory disease (56% vs 62%, respectively; P = .67). Patients with stage IV disease achieved a significantly better 3-year PFS in the BV cohort (P = .015). Post-ASCT PFS was comparable for patients achieving a CMR after BV monotherapy and those receiving BV followed by sequential chemotherapy (P = .24). Although 3-year overall survival was higher in the BV cohort (92% vs 80%, respectively; P < .001), this is likely attributed to the use of other novel therapies in later lines for patients experiencing progression, given that studies in the BV cohort were conducted more recently. In conclusion, BV with or without salvage chemotherapy appears to enhance PFS in patients with relapsed disease but not in those with primary refractory cHL.
Introduction
For the past 30 years, standard treatment of patients with classical Hodgkin lymphoma (cHL) who are primary refractory or relapse (R/R) after first-line (primary) treatment, has been to test for chemosensitivity with salvage chemotherapy and, upon response, to treat with myeloablative high-dose chemotherapy followed by autologous stem cell transplantation (ASCT).1-3 With this strategy ∼70% to 80% of patients respond to salvage chemotherapy of whom ∼60% achieve a complete metabolic response (CMR) based on a negative 18F-fluorodeoxyglucose–positron emission tomography (PET) scan before ASCT.1,4-6 However, 30% to 40% of patients will relapse within 5 years after ASCT and subsequently have a poor prognosis.1,7 Importantly, it has been shown that patients who achieve a CMR before ASCT have a better prognosis with long-term post-ASCT progression-free survival (PFS) of ∼70% to 80%.1,4,8
In the past decade, new targeted treatment options such as brentuximab vedotin (BV) and checkpoint inhibitors have become available for patients with R/R cHL.9-11 BV is an antibody-drug conjugate composed of an anti-CD30 monoclonal antibody with a cytotoxic payload of monomethyl auristatin E.12 In the first-line setting, BV in combination with adriamycin, vinblastine, and dacarbazine (BV-AVD) has been shown to improve PFS and overall survival (OS) in patients with advanced-stage disease compared with standard adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD).13,14 In the R/R setting, several phase 2 single-arm clinical trials have investigated BV in combination with concomitant or sequential chemotherapy followed by ASCT.15-24 These trials showed a high CMR rate before ASCT, and PFS and OS appear to be higher than historical controls.25 However, no randomized controlled trials (RCTs) investigating the addition of BV to salvage chemotherapy compared with chemotherapy alone in R/R cHL have been published to this date. An individual patient data analysis could provide more power for assessing the effect of novel treatments and can also detect interactions between outcome parameters and patient characteristics outcomes, compared with standard meta-analyses.
Therefore, we aimed to perform a large, individual patient data analysis to investigate the effect of BV addition to salvage chemotherapy vs chemotherapy alone on pre-ASCT PET response, PFS, and OS in transplant-eligible patients with R/R cHL.
Methods
Literature search and data collection
We performed a literature search on PubMed and ClinicalTrials.gov to identify clinical trials investigating BV in combination with salvage chemotherapy (BV cohort), or salvage chemotherapy alone (chemotherapy cohort) followed by ASCT in transplant-eligible patients with cHL with a first relapse or primary refractory disease after first-line (primary) treatment (supplemental Extended Methods, available on the Blood website. Ten studies were identified that met our inclusion criteria, the investigators of all 10 studies provided the individual patient data for inclusion in the analysis. Seven studies, published between 2017 and 2021, were included in the BV cohort and 3 studies, published between 2010 and 2016, were included in the chemotherapy cohort (supplemental Figure 1; supplemental Table 1). We gathered pseudonymized individual patient data from case record forms or study databases from clinical trials through the corresponding authors and/or investigators of the studies. For secondary use of data for this analysis, a waiver for informed consent was obtained from the ethics committee of all participating centers.
End points and definitions
The primary end point was the 3-year PFS. A cutoff of 3 years was chosen because most relapses occur within 2 to 3 years, and limited follow-up for several studies.7 Secondary end points included event-free survival (EFS), OS, and pre-ASCT CMR rate. PFS was defined as time from enrollment in the clinical trial to progressive disease (PD) or death from any cause, whichever occurs first. To eliminate bias in PFS occurring because of differences in study protocols, patients with stable disease (SD) after salvage treatment who did not proceed to ASCT were censored at time of going off study. Patients who did not undergo ASCT but received BV monotherapy instead were censored at time of end of salvage chemotherapy. EFS was defined as time from enrollment to PD or death, or until end of salvage therapy if patients could not proceed to ASCT because of toxicity or insufficient response (SD/PD) after salvage therapy. Patients with SD who received additional therapy before ASCT were counted as “event.” OS was defined as time from enrollment to death from any cause.
CMR was defined as Deauville score (DS) of 1 to 3 according to the 2014 Lugano criteria.26 A partial metabolic response (PMR) was defined as DS of 4 to 5 without progression or development of new lesions. In the ifosfamide, carboplatin, and etoposide (ICE)–gemcitabine, vinorelbine, and docxorubicin (GVD) study of Moskowitz et al, the pre-ASCT PET scans in the chemotherapy cohort were evaluated according to the international working group criteria, in which a positive scan was defined as uptake greater than the mediastinal or abdominal aortic blood pool (comparable with DS ≥3).4,27 To harmonize response assessment, all positive PET scans from the ICE-GVD study were re-assessed according to the Lugano criteria by a nuclear medicine physician (H.S.).26
The definition of primary refractory disease varied among studies, and not all collected relapse interval data. We defined primary refractory disease as “not having achieved a complete response on first-line treatment,” encompassing partial response, SD, and PD, irrespective of relapse interval. Bulky disease was defined as a tumor bulk of ≥5 cm. Early relapse was defined as relapse interval of <1 year. Stage was defined according to the Ann Arbor criteria. In the study of Santoro et al5 (n = 59 patients), stage was not collected but information about the number of lymphatic and extralymphatic sites allowed the identification of patients with stage I (1 lymphatic site) or stage IV disease (≥1 lymphatic and ≥1 extralymphatic site; the investigators confirmed that there were no patients with stage IE/IIE disease). However, stage II and III were combined for n = 24 patients because the infradiaphragmatic or supradiaphragmatic distribution was unknown. Primary treatment was categorized into ABVD; escalated bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine and prednisone (escBEACOPP); or other therapies. Patients initially treated with ABVD and later escalated to escBEACOPP were categorized under escBEACOPP.
Statistical analysis
Pearson χ2 or Fisher exact tests were used to compare categorical variables and Kruskal-Wallis rank-sum test for assessing continuous variables. Survival outcomes were analyzed using the Kaplan-Meier method and pairwise log-rank tests. Univariable and multivariable Cox regression analyses were performed to assess the association between baseline characteristics and survival outcomes. Logistic regression was used to assess the association between baseline characteristics and binary response outcomes. Patients with missing data were only excluded from analyses when the missing variable was required for the specific analysis.
A 1:1 propensity score matching analysis was performed to adjust for the effects of unbalanced covariates between the BV and chemotherapy cohorts.28 We conducted matching based on baseline patient characteristics significantly associated with PFS. To ensure a robust distribution of patients within the matched data set, we repeated the matching process 2000 times as part of internal crossvalidation. More detailed information about the matching procedure is provided in the supplemental Extended Methods.
Statistical analysis was performed using R software version 4.0.3. A P value of <.05 was considered statistically significant.
Results
Patient characteristics
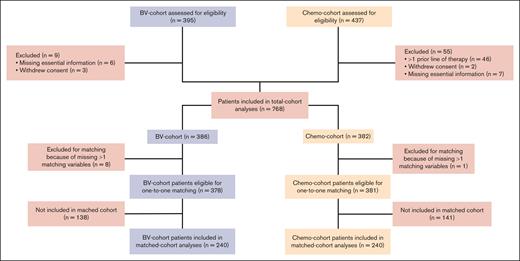
Individual patient data of 10 clinical trials with a total of 832 transplant-eligible patients were collected.4-6,15-21 Sixty-four patients were excluded (mainly because they had received >1 line of therapy). In total, 768 patients were included, with 386 in the BV cohort (BV with or without salvage chemotherapy) and 382 in the chemotherapy cohort (salvage chemotherapy only; Figure 1 and Table 1). There was an imbalance in primary refractory cases (55% vs 20% for the BV and chemotherapy cohort, respectively) because of a substantial number of patients enrolled in the study of Josting et al6 (225 of 382; 59%) that specifically excluded patients with primary refractory disease. Moreover, this study included more patients who were treated with escBEACOPP as primary treatment. An overview of study information including treatment regimens and summarized patient characteristics can be found in supplemental Tables 1 and 2.
CONSORT diagram. Chemo-cohort, chemotherapy cohort; n, number of patients.
Baseline patient characteristics in the whole data set
| Patient characteristics (n; %) . | BV cohort (n = 386) . | Chemotherapy cohort (n = 382) . | P value . |
|---|---|---|---|
| Female sex | 202 (52%) | 168 (44%) | .021 |
| Age, y, median (range) | 31 (5-68) | 34 (18-72) | .031 |
| WHO PS | < .001 | ||
| 0 | 158 (64%) | 256 (70%) | .1030 |
| 1 | 85 (34%) | 79 (22%) | .0008 |
| 2 | 5 (2%) | 29 (8%) | .0029 |
| Unknown | 138 | 18 | |
| Ann Arbor stage | < .001 | ||
| I | 29 (9%) | 43 (11%) | .3589 |
| II | 132 (41%) | 135 (36%) | .1861 |
| III | 53 (16%) | 59 (16%) | .8534 |
| II or III∗ | 0 (0%) | 24 (6%) | NA |
| IV | 109 (34%) | 117 (31%) | .4791 |
| Unknown | 63 | 4 | |
| B symptoms | 107 (28%) | 74 (23%) | .133 |
| Unknown | 2 | 59 | |
| Extranodal disease | 142 (38%) | 134 (35%) | .493 |
| Unknown | 8 | 1 | |
| Bulky disease† | 128 (37%) | 101 (31%) | .126 |
| Unknown | 40 | 60 | |
| Primary refractory‡ | 213 (55%) | 78 (20%) | < .001 |
| Relapse interval in days, median (range) | 147 (0-4883) | 250 (0-5258) | .123 |
| Unknown | 212 | 6 | |
| Early relapse <1 year | 259 (76%) | 230 (61%) | < .001 |
| Unknown | 43 | 5 | |
| Response to primary treatment | < .001 | ||
| Complete response | 173 (59%) | 304 (89%) | < .001 |
| Partial response | 55 (19%) | 21 (6%) | < .001 |
| Stable disease | 18 (6%) | 2 (1%) | < .001 |
| Progressive disease | 46 (16%) | 14 (4%) | < .001 |
| Unknown | 94 | 41 | |
| Primary treatment | < .001 | ||
| ABVD | 254 (90%) | 259 (71%) | < .001 |
| BEACOPP | 16 (6%) | 79 (22%) | < .001 |
| Other | 11 (4%) | 25 (7%) | .1455 |
| Unknown | 105 | 19 | |
| BV maintenance after ASCT | 87 (24%) | NA | NA |
| Patient characteristics (n; %) . | BV cohort (n = 386) . | Chemotherapy cohort (n = 382) . | P value . |
|---|---|---|---|
| Female sex | 202 (52%) | 168 (44%) | .021 |
| Age, y, median (range) | 31 (5-68) | 34 (18-72) | .031 |
| WHO PS | < .001 | ||
| 0 | 158 (64%) | 256 (70%) | .1030 |
| 1 | 85 (34%) | 79 (22%) | .0008 |
| 2 | 5 (2%) | 29 (8%) | .0029 |
| Unknown | 138 | 18 | |
| Ann Arbor stage | < .001 | ||
| I | 29 (9%) | 43 (11%) | .3589 |
| II | 132 (41%) | 135 (36%) | .1861 |
| III | 53 (16%) | 59 (16%) | .8534 |
| II or III∗ | 0 (0%) | 24 (6%) | NA |
| IV | 109 (34%) | 117 (31%) | .4791 |
| Unknown | 63 | 4 | |
| B symptoms | 107 (28%) | 74 (23%) | .133 |
| Unknown | 2 | 59 | |
| Extranodal disease | 142 (38%) | 134 (35%) | .493 |
| Unknown | 8 | 1 | |
| Bulky disease† | 128 (37%) | 101 (31%) | .126 |
| Unknown | 40 | 60 | |
| Primary refractory‡ | 213 (55%) | 78 (20%) | < .001 |
| Relapse interval in days, median (range) | 147 (0-4883) | 250 (0-5258) | .123 |
| Unknown | 212 | 6 | |
| Early relapse <1 year | 259 (76%) | 230 (61%) | < .001 |
| Unknown | 43 | 5 | |
| Response to primary treatment | < .001 | ||
| Complete response | 173 (59%) | 304 (89%) | < .001 |
| Partial response | 55 (19%) | 21 (6%) | < .001 |
| Stable disease | 18 (6%) | 2 (1%) | < .001 |
| Progressive disease | 46 (16%) | 14 (4%) | < .001 |
| Unknown | 94 | 41 | |
| Primary treatment | < .001 | ||
| ABVD | 254 (90%) | 259 (71%) | < .001 |
| BEACOPP | 16 (6%) | 79 (22%) | < .001 |
| Other | 11 (4%) | 25 (7%) | .1455 |
| Unknown | 105 | 19 | |
| BV maintenance after ASCT | 87 (24%) | NA | NA |
Patient characteristics are measured at time of enrollment in the studies, that is, at time of relapse or primary refractory disease, unless indicated otherwise.
NA, not applicable; WHO PS, World Health Organization performance status.
For 24 patients in the chemotherapy cohort from the trial by Santoro et al, stage at relapse was not recorded but stage I and IV were deducted from the amount of involved lymph node sites, extranodal sites, and bone marrow involvement. It was not possible to distinguish between stage II and III disease because no data were available on the spatial distribution of nodal sites (ie, infradiaphragmatic and/or supradiaphragmatic location).
Bulky disease was defined as a single tumor bulk larger than 5 cm.
Primary refractory disease was defined as not having achieved a CR on primary treatment, ie, patients who had a PR, SD, or progressive disease (PD) on primary treatment were considered primary refractory independent of the relapse interval.
Survival outcomes in the whole cohort
The median follow-up time was 38 months (interquartile range [IQR], 24-50) for the BV cohort, and 47 months (IQR, 31-68) for the chemotherapy cohort. Of 242 patients with PD, only 17 (7%) progressed beyond 3 years, supporting the 3-year cutoff for survival analysis (supplemental Table 3). The 3-year PFS, without matching for baseline characteristics, was not significantly different between the BV and chemotherapy cohorts: 66.7% (95% confidence interval [CI], 62-72) vs 67.4% (95% CI, 63-72; P = .61), respectively, and EFS was comparable with PFS (supplemental Figure 2). In the BV cohort, 40 (10.4%) patients died, of whom 9 patients died without having PD (n = 2 toxicity, n = 3 infection, n = 1 other cause, n = 3 unknown). In the chemotherapy cohort, a total of 76 (19.9%) patients died, of whom 14 patients died without PD (toxicity, n = 7; infection, n = 1; other cause, n = 3; unknown, n = 3). Three-year OS was significantly higher for the BV cohort than for the chemotherapy cohort: 91.0% (95% CI, 88-94) vs 80.4% (95% CI, 76-85; P = .002; supplemental Figures 2 and 3).
Survival outcomes in the matched data set
The following variables were significantly related to PFS and were used for propensity score matching: R/R status, bulky disease, extranodal disease, stage IV, B symptoms (at time of enrollment in the studies), and primary treatment with escBEACOPP (supplemental Extended Methods Table 2). The matched data set consists of a total of 480 patients with 240 patients each in the BV and chemotherapy cohort in which the patient characteristics are now equally distributed, except for World Health Organization performance status 2, but this was not significantly related to PFS (P = .6) or OS (P = .6; Table 2; supplemental Extended Methods Table 2).
Patient characteristics in the matched data set
| . | BV cohort (n = 240) . | Chemotherapy cohort (n = 240) . | P value . |
|---|---|---|---|
| Female sex | 132 (55%) | 130 (54%) | .855 |
| Age, y, median (range) | 30 (11-66) | 33 (18-72) | .118 |
| Primary refractory | 78 (32%) | 78 (32%) | 1.000 |
| B symptoms | 70 (29%) | 42 (23%) | .163 |
| Unknown | 1 | 59 | |
| Stage | |||
| I | 16 (8%) | 23 (10%) | .627 |
| II | 77 (38%) | 84 (35%) | .631 |
| II or III∗ | 0 (0%) | 24 (10%) | NA |
| III | 37 (18%) | 27 (11%) | .112 |
| IV | 72 (36%) | 79 (33%) | .612 |
| Unknown | 38 | 3 | |
| Extranodal disease | 102 (42%) | 94 (39%) | .458 |
| Bulky disease† | 89 (41%) | 71 (39%) | .689 |
| Unknown | 24 | 59 | |
| Primary treatment with escBEACOPP | 14 (8%) | 17 (7%) | .985 |
| Early relapse <1 year | 129 (65%) | 162 (68%) | .480 |
| Unknown | 42 | 3 | |
| WHO PS | |||
| 0 | 98 (66%) | 158 (70%) | .505 |
| 1 | 49 (33%) | 48 (21%) | 1.000 |
| 2 | 2 (1%) | 21 (9%) | .0036 |
| Unknown | 91 | 13 | |
| Response to primary treatment = PD | 14 (7%) | 14 (7%) | .414 |
| Unknown | 38 | 41 |
| . | BV cohort (n = 240) . | Chemotherapy cohort (n = 240) . | P value . |
|---|---|---|---|
| Female sex | 132 (55%) | 130 (54%) | .855 |
| Age, y, median (range) | 30 (11-66) | 33 (18-72) | .118 |
| Primary refractory | 78 (32%) | 78 (32%) | 1.000 |
| B symptoms | 70 (29%) | 42 (23%) | .163 |
| Unknown | 1 | 59 | |
| Stage | |||
| I | 16 (8%) | 23 (10%) | .627 |
| II | 77 (38%) | 84 (35%) | .631 |
| II or III∗ | 0 (0%) | 24 (10%) | NA |
| III | 37 (18%) | 27 (11%) | .112 |
| IV | 72 (36%) | 79 (33%) | .612 |
| Unknown | 38 | 3 | |
| Extranodal disease | 102 (42%) | 94 (39%) | .458 |
| Bulky disease† | 89 (41%) | 71 (39%) | .689 |
| Unknown | 24 | 59 | |
| Primary treatment with escBEACOPP | 14 (8%) | 17 (7%) | .985 |
| Early relapse <1 year | 129 (65%) | 162 (68%) | .480 |
| Unknown | 42 | 3 | |
| WHO PS | |||
| 0 | 98 (66%) | 158 (70%) | .505 |
| 1 | 49 (33%) | 48 (21%) | 1.000 |
| 2 | 2 (1%) | 21 (9%) | .0036 |
| Unknown | 91 | 13 | |
| Response to primary treatment = PD | 14 (7%) | 14 (7%) | .414 |
| Unknown | 38 | 41 |
NA, not applicable; WHO PS, World Health Organization performance status.
For 24 patients in the chemotherapy cohort from the trial by Santoro et al, stage at relapse was not recorded but stage I and IV were deducted from the amount of involved lymph node sites, extranodal sites, and bone marrow involvement. It was not possible to distinguish between stage II and III disease because no data were available on the spatial distribution of nodal sites (ie, infradiaphragmatic and/or supradiaphragmatic).
Bulky disease was defined as a single tumor bulk larger than 5 cm.
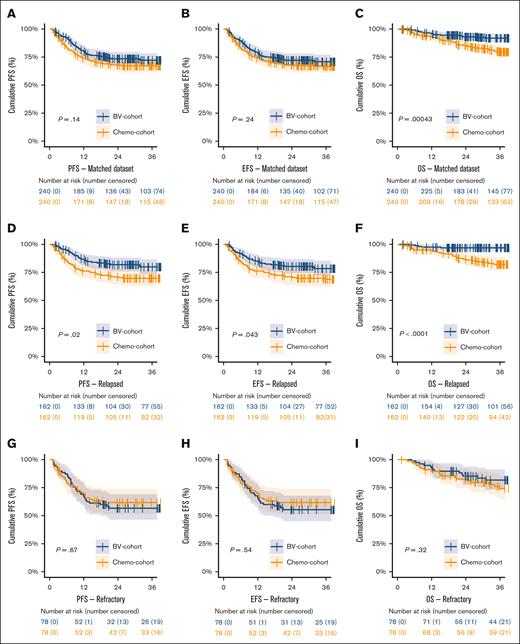
In the matched data set, 3-year PFS did not significantly differ between the BV and chemotherapy cohort, with a 3-year PFS of 72.2% (95% CI, 67-78) vs 67.1% (95% CI, 61-73; P = .14), respectively (Figure 2A; supplemental Table 4). The EFS was similar to PFS. However, there was a significant higher 3-year OS for patients treated within the BV cohort of 91.9% (95% CI, 88-96) vs 79.5% (95% CI, 74-85) for the chemotherapy cohort, P = .00043 (Figure 2C). In patients with PD, significantly more patients died in the chemotherapy cohort (31 of 72; 43%) than in the BV cohort (19 of 65; 29%; P = .0011), whereas in patients without PD there was no significant difference in the number of deaths between the BV cohort (5 of 175; 3%) vs the chemotherapy cohort (8 of 168; 5%; P = .4), suggesting that advances in later lines of therapy are most likely the cause of improved OS in the BV cohort.
Kaplan-Meier survival analyses on the matched cohort. Kaplan-Meier curves showing the PFS, EFS, and OS in the BV and chemotherapy cohort in the matched data set (panels A, B, and C), and corresponding analyses stratified for patients with relapsed (panels D, E, and F) or primary refractory disease (panels G, H, and I). PR, partial response.
Kaplan-Meier survival analyses on the matched cohort. Kaplan-Meier curves showing the PFS, EFS, and OS in the BV and chemotherapy cohort in the matched data set (panels A, B, and C), and corresponding analyses stratified for patients with relapsed (panels D, E, and F) or primary refractory disease (panels G, H, and I). PR, partial response.
In patients with relapsed disease, the BV cohort showed a significantly better 3-year PFS than the chemotherapy cohort of 79.9% (95% CI, 74-87) vs 69.7% (95% CI, 63-77), respectively (P = .02; Figure 2D). The EFS and OS for patients with relapsed disease were also significantly better in the BV cohort (P = .043 and P < .0001, respectively). However, for patients with primary refractory disease, there were no significant differences in 3-year PFS (P = .67), EFS (P = .54), and OS (P = .32) between the BV and chemotherapy cohorts (Figure 2G-I).
In the BV cohort, 216 (90%) patients underwent ASCT compared with 199 (83%) patients in the chemotherapy cohort (P = .023; Table 3). Post-ASCT survival outcomes were comparable between the BV and chemotherapy cohorts (supplemental Figure 4). In patients with relapsed disease who underwent ASCT, the 3-year PFS (P = .32) and EFS (P = .32) were not significantly different, but the OS was significantly better for the BV cohort (P = .0097). Again, for patients with primary refractory disease there was no difference in PFS (P = .18), EFS (P = .22), and OS (P = .48; supplemental Table 5; supplemental Figure 4).
Pre-ASCT response rates and patients who underwent ASCT
| Outcome . | Data set . | BV cohort . | Chemotherapy cohort . | P (χ2 test)∗ . | P (multivar)† . | ||||
|---|---|---|---|---|---|---|---|---|---|
| n . | Total . | % . | n . | Total . | % . | ||||
| Underwent ASCT | Whole | 335 | 386 | 87% | 324 | 382 | 85% | .38 | .064 |
| Underwent ASCT | PET3 | 335 | 386 | 87% | 130 | 157 | 83% | .20 | .23 |
| Underwent ASCT | Matched | 216 | 240 | 90% | 199 | 240 | 83% | .023 | .020 |
| Underwent ASCT | Whole, relapsed | 156 | 173 | 90% | 262 | 304 | 86% | .20 | .012 |
| Underwent ASCT | Whole, refractory | 179 | 213 | 84% | 62 | 78 | 79% | .32 | .40 |
| Underwent ASCT | Whole, stage IV | 92 | 109 | 84% | 91 | 117 | 78% | .15 | .29 |
| CMR | PET | 292 | 386 | 76% | 126 | 157 | 80% | .30 | .23 |
| CMR | Matched‡ | 193 | 240 | 80% | 108 | 137 | 79% | .69 | .28 |
| CMR | PET, relapsed | 148 | 173 | 86% | 78 | 90 | 87% | .72 | .75 |
| CMR | PET, refractory | 144 | 213 | 68% | 48 | 67 | 72% | .67 | .11 |
| CMR | PET, stage IV | 74 | 109 | 68% | 46 | 60 | 77% | .22 | .42 |
| ORR (PET) | PET | 343 | 386 | 89% | 136 | 157 | 87% | .46 | .51 |
| ORR (PET) | PET, relapsed | 164 | 173 | 95% | 81 | 90 | 90% | .14 | .11 |
| ORR (PET) | PET, refractory | 179 | 213 | 84% | 55 | 67 | 82% | .71 | .43 |
| ORR (PET) | PET, stage IV | 90 | 109 | 83% | 50 | 60 | 83% | .90 | .97 |
| ORR (CT) | Whole | 343 | 386 | 89% | 300 | 382 | 79% | < .001 | < .001 |
| ORR (CT) | Whole, relapsed | 164 | 173 | 95% | 238 | 304 | 78% | < .001 | < .001 |
| ORR (CT) | Whole, refractory | 179 | 213 | 84% | 62 | 78 | 79% | .36 | .84 |
| ORR (CT) | Whole, stage IV | 90 | 109 | 83% | 88 | 117 | 75% | .18 | .020 |
| CMR ICE/BeGEV§ | PET | 292 | 386 | 76% | 105 | 157 | 67% | .025 | .0017 |
| CMR ICE/BeGEV | Matched‡ | 193 | 240 | 80% | 93 | 137 | 68% | .005 | .0040 |
| CMR ICE/BeGEV | PET, relapsed | 148 | 173 | 86% | 67 | 90 | 74% | .030 | .007 |
| CMR ICE/BeGEV | PET, refractory | 144 | 213 | 68% | 38 | 67 | 57% | .067 | .15 |
| CMR ICE/BeGEV | PET, stage IV | 74 | 109 | 68% | 39 | 60 | 65% | .69 | .11 |
| Outcome . | Data set . | BV cohort . | Chemotherapy cohort . | P (χ2 test)∗ . | P (multivar)† . | ||||
|---|---|---|---|---|---|---|---|---|---|
| n . | Total . | % . | n . | Total . | % . | ||||
| Underwent ASCT | Whole | 335 | 386 | 87% | 324 | 382 | 85% | .38 | .064 |
| Underwent ASCT | PET3 | 335 | 386 | 87% | 130 | 157 | 83% | .20 | .23 |
| Underwent ASCT | Matched | 216 | 240 | 90% | 199 | 240 | 83% | .023 | .020 |
| Underwent ASCT | Whole, relapsed | 156 | 173 | 90% | 262 | 304 | 86% | .20 | .012 |
| Underwent ASCT | Whole, refractory | 179 | 213 | 84% | 62 | 78 | 79% | .32 | .40 |
| Underwent ASCT | Whole, stage IV | 92 | 109 | 84% | 91 | 117 | 78% | .15 | .29 |
| CMR | PET | 292 | 386 | 76% | 126 | 157 | 80% | .30 | .23 |
| CMR | Matched‡ | 193 | 240 | 80% | 108 | 137 | 79% | .69 | .28 |
| CMR | PET, relapsed | 148 | 173 | 86% | 78 | 90 | 87% | .72 | .75 |
| CMR | PET, refractory | 144 | 213 | 68% | 48 | 67 | 72% | .67 | .11 |
| CMR | PET, stage IV | 74 | 109 | 68% | 46 | 60 | 77% | .22 | .42 |
| ORR (PET) | PET | 343 | 386 | 89% | 136 | 157 | 87% | .46 | .51 |
| ORR (PET) | PET, relapsed | 164 | 173 | 95% | 81 | 90 | 90% | .14 | .11 |
| ORR (PET) | PET, refractory | 179 | 213 | 84% | 55 | 67 | 82% | .71 | .43 |
| ORR (PET) | PET, stage IV | 90 | 109 | 83% | 50 | 60 | 83% | .90 | .97 |
| ORR (CT) | Whole | 343 | 386 | 89% | 300 | 382 | 79% | < .001 | < .001 |
| ORR (CT) | Whole, relapsed | 164 | 173 | 95% | 238 | 304 | 78% | < .001 | < .001 |
| ORR (CT) | Whole, refractory | 179 | 213 | 84% | 62 | 78 | 79% | .36 | .84 |
| ORR (CT) | Whole, stage IV | 90 | 109 | 83% | 88 | 117 | 75% | .18 | .020 |
| CMR ICE/BeGEV§ | PET | 292 | 386 | 76% | 105 | 157 | 67% | .025 | .0017 |
| CMR ICE/BeGEV | Matched‡ | 193 | 240 | 80% | 93 | 137 | 68% | .005 | .0040 |
| CMR ICE/BeGEV | PET, relapsed | 148 | 173 | 86% | 67 | 90 | 74% | .030 | .007 |
| CMR ICE/BeGEV | PET, refractory | 144 | 213 | 68% | 38 | 67 | 57% | .067 | .15 |
| CMR ICE/BeGEV | PET, stage IV | 74 | 109 | 68% | 39 | 60 | 65% | .69 | .11 |
3The PET dataset is the whole data set excluding patients from the study of Josting et al, in which response assessment was done by conventional CT scan only.
BeGEV, bendamustine, gemcitabine, etoposide, and vinorelbine; multivar, multivariable logistic regression analysis.
P values from χ2 comparison of BV vs chemotherapy cohorts.
P values from multivariable logistic regression comparing BV vs chemotherapy cohorts corrected for baseline characteristics: R/R status, stage, B symptoms, extranodal disease, bulky disease, and primary treatment with escBEACOPP.
For CMR calculations in the matched data set, patients from the study of Josting et al have been removed from the chemotherapy cohort, resulting in a smaller chemotherapy cohort of n = 137 patients instead of n = 240.
Comparison of pre-ASCT CMR rates measured after first sequential chemotherapy only. In the study of Moskowitz et al patients received sequential ICE and GVD chemotherapy in case of no CMR. In this comparison the response after ICE only is used in the chemotherapy cohort.
Subgroup analysis for survival between BV and chemotherapy cohorts
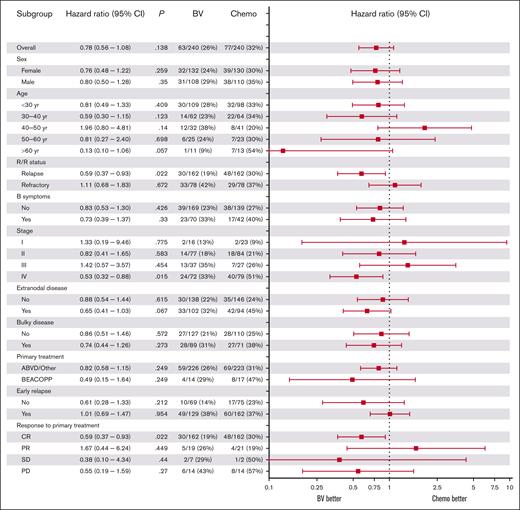
In the matched data set, we tested differences in 3-year PFS between the BV and chemotherapy cohorts for specific subgroups using univariable Cox regression (Figure 3). Patients with relapsed disease in the BV-cohort had a significantly lower risk of PD than those in the chemotherapy cohort (hazard ratio [HR], 0.59; 95% CI, 0.37-0.93; P = .022). Similarly, patients with stage IV disease had significantly lower risk of PD in the BV cohort (HR, 0.53, 95% CI, 0.32-0.88; P = .015). Patients with extranodal disease showed a trend for better PFS in the BV cohort with a HR of 0.65 (95% CI, 0.41-1.03; P = .067) but this was not significant. Exploratory multivariable subgroup analysis of R/R status and stage IV showed a trend for better PFS in the BV cohort for patients who had both stage IV and relapsed disease (n = 97; HR, 0.50; 95% CI, 0.25-1.02; P = .058).
Forest plot of the association between baseline characteristics and differences in PFS between the BV and chemotherapy cohorts. HRs are shown for univariable Cox regression on subgroup analyses of baseline characteristics for PFS comparing the BV and chemotherapy cohorts. A HR of <1 corresponds to a higher PFS in the BV cohort compared with the chemotherapy cohort. CR, complete response; PR, partial response; yr, year.
Forest plot of the association between baseline characteristics and differences in PFS between the BV and chemotherapy cohorts. HRs are shown for univariable Cox regression on subgroup analyses of baseline characteristics for PFS comparing the BV and chemotherapy cohorts. A HR of <1 corresponds to a higher PFS in the BV cohort compared with the chemotherapy cohort. CR, complete response; PR, partial response; yr, year.
Pre-ASCT PET responses in the whole cohort
Of 10 studies, 9 had PET–computed tomography (CT) data available. Overall, N = 225 patients from the study of Josting et al were excluded from the chemotherapy cohort because responses were assessed using conventional CT scan. Consequently, the chemotherapy cohort comprised 157 patients with available PET data. The CMR rate in the whole BV cohort was 76% vs 80% in the chemotherapy cohort (P = .30; Table 3). The overall response rates (ORRs) based on PET were not significantly different between the BV and chemotherapy cohorts. However, when including patients from the study of Josting et al in which the ORR was based on conventional CT, the BV cohort displayed a significantly higher ORR of 89%, compared with 79% in the chemotherapy cohort (P < .001; Table 3).
In subgroup analysis, patients with relapsed disease exhibited higher CMR rates compared with patients with primary refractory disease. However, no significant differences in CMR or ORR rates were observed between the BV and chemotherapy cohorts within these subgroups (Table 3).
In the study of Moskowitz et al within the chemotherapy cohort, patients with a PMR or SD after ICE treatment underwent sequential GVD treatment. This sequential therapy resulted in a conversion from PMR/SD to a CMR in 21 patients (of whom 15 were included in the matched cohort). To ensure a comprehensive assessment, we recalculated the CMR rate after ICE-only, excluding these patients from the CMR count. This adjustment yielded a CMR rate of 67% for the total matched chemotherapy cohort. Upon comparing the CMR rate of 76% in the BV cohort with the CMR rate of 67% after ICE-only in the chemotherapy cohort, a notable significance emerged in both univariable (P = .025) and multivariable analysis (P = .0017; Table 3). This distinction was particularly pronounced among patients with relapsed disease, because in this subgroup the CMR rate was significantly higher in the BV-cohort compared with the chemotherapy cohort. Conversely, in primary refractory patients, no significant differences in CMR rates were observed between the 2 cohorts (Table 3).
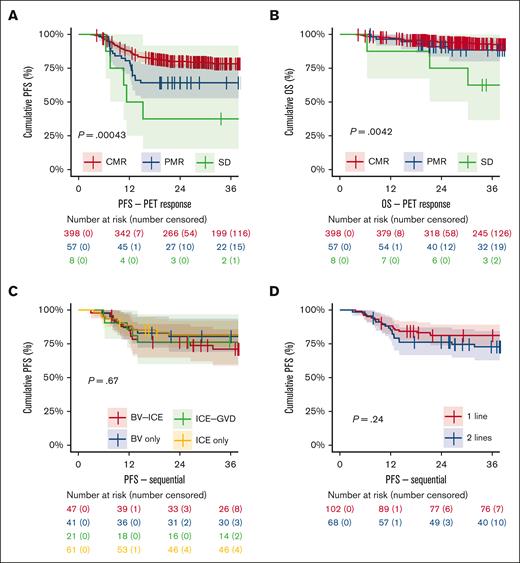
Slightly more patients underwent ASCT in the BV cohort (335 of 386; 87%) vs the chemotherapy cohort (324 of 382; 85%), but this was not significant in univariable (P = .38) or multivariable analysis adjusted for baseline characteristics (P = .06). For relapsed patients, a significant higher percentage of patients underwent ASCT in the BV cohort than in the chemotherapy cohort (90% vs 86%; P = .012 multivariate; Table 3). Among patients who underwent ASCT, those achieving a CMR (n = 398) before ASCT had a 3-year PFS of 78.3% (95% CI, 74-83), which was significantly higher than those who underwent ASCT after a PMR (n = 57) with a 3-year PFS of 64.2% (95% CI, 53%-78%; P = .01), or SD (n = 8) with a 3-year PFS of 37.5% (95% CI, 15-92; P = .0004; Figure 4A). In all patients who received transplantation while having obtained a CMR, there was no difference in 3-year PFS between the BV and chemotherapy cohorts (P = .92; data not shown). Notably, after ASCT, there was a significantly lower OS for patients with SD than those with a CMR (P = .0042), whereas no difference in OS was observed for patients with a PMR vs CMR (P = .286; Figure 4B).
Kaplan-Meier subgroup survival analyses on the whole data set. (A) PFS and (B) OS in patients who underwent ASCT stratified for pre-ASCT PET response in the whole data set. (C-D) PFS for patients who were treated in studies with a sequential approach and achieved a CMR after 1 line of salvage treatment (BV or ICE only) vs patients who initially had no CMR but converted to a CMR after 2 lines of sequential treatment with additional chemotherapy (BV-ICE or ICE-GVD).
Kaplan-Meier subgroup survival analyses on the whole data set. (A) PFS and (B) OS in patients who underwent ASCT stratified for pre-ASCT PET response in the whole data set. (C-D) PFS for patients who were treated in studies with a sequential approach and achieved a CMR after 1 line of salvage treatment (BV or ICE only) vs patients who initially had no CMR but converted to a CMR after 2 lines of sequential treatment with additional chemotherapy (BV-ICE or ICE-GVD).
Influence of BV dose and salvage chemotherapy schedule
Within the whole BV cohort (unmatched data set; BV cohort, n = 386), subgroup analysis shows a nonsignificant trend for a higher PFS (HR, 0.72; 95% CI; 0.50-1.04; P = .079) in studies that used BV with a combination of chemotherapeutic agents, for example, dexamethasone, high-dose cytarabine, and cisplatin, ICE, or etoposide, methylprednisolone, cisplatin and cytarabine (ESHAP), vs a single agent, for example, bendamustine or gemcitabine (supplemental Table 6).16,17,21,24 The use of a sequential schedule (ie, BV monotherapy followed by chemotherapy), the number of BV cycles, and the cumulative BV dose did not have an impact on 3-year PFS or pre-ASCT CMR rate between studies in the BV cohort. This suggests that more cycles of BV does not improve CMR rates or PFS. Two studies applied BV maintenance after ASCT (11% of total number of patients).17,19 However, not all patients received BV maintenance and many patients received less than the intended number of maintenance cycles because of toxicity or other reasons, which limits an analysis to assess the effect of BV maintenance (supplemental Table 2).17,19
Outcomes of sequential treatment
Three studies followed a sequential approach: 2 studies in the BV cohort used 2 to 4 cycles of BV monotherapy, allowing patients with a CMR to proceed directly to ASCT whereas patients with positive PET scans received additional ICE salvage chemotherapy before ASCT, and 1 study in the chemotherapy cohort used 2 cycles of ICE and patients without CMR received additional GVD chemotherapy before ASCT.4,21,24 Subgroup analysis showed no significant differences in 3-year PFS between patients achieving CMR with 1 line of therapy (BV monotherapy or ICE only) and those requiring 2 lines of therapy (BV-ICE or ICE-GVD) to achieve a CMR (P = .24; Figure 4C-D). OS also showed no significant differences between these groups (P = .62; supplemental Table 7).
Discussion
In this matched analysis of individual patient data from prospective single-arm clinical trials, we investigated the effect of BV addition to salvage chemotherapy followed by ASCT in transplant-eligible patients with R/R cHL. We found no statistically significant differences in PFS, EFS, and pre-ASCT CMR rate for patients treated with BV with or without chemotherapy compared with patients treated with salvage chemotherapy only. However, with relapsed disease and those with stage IV disease had a significantly better PFS and EFS when adding BV to the salvage treatment. Although OS was significantly better in the BV cohort, this may be influenced by the time in which the BV studies were conducted (2015-2021) compared with chemotherapy cohort studies (2010-2016). A recent retrospective study in patients with R/R cHL who underwent ASCT showed an OS improvement over time, corresponding to the increased usage of immune checkpoint inhibitors and BV.29 Therefore, the observed OS difference in the BV cohort is probably driven by the availability of checkpoint inhibitors for patients who fail salvage therapy or relapse after ASCT.9-11,30
The disparity in survival outcomes between patients with primary refractory disease and those with relapsed disease could potentially be explained by the antitumor mechanism of action of BV. BV elicits its antitumor effect through the cytotoxic warhead monomethyl auristatin E, a substrate for the multidrug resistance pump P-glycoprotein.31 It has been shown that BV-resistant cell lines have elevated pump P-glycoprotein, which is known to also occur after exposure to other cytotoxic agents such as doxorubicin.32,33 Thus, tumor cells that are able to resist first-line chemotherapy might use the same mechanism to convey resistance to BV. Because patients with primary refractory disease are more likely to be resistant to chemotherapy, this might explain why they could also be resistant to BV. Therefore, in patients with primary refractory disease there is still an unmet need to improve outcomes, and other nonchemotherapeutic therapies such as immune checkpoint inhibitors should be considered.34-36
Patients with stage IV disease had improved PFS in the BV cohort vs the chemotherapy cohort. This may be attributed to a larger total tumor volume, necessitating intensified treatment, which could be achieved by augmenting standard chemotherapy with BV. In subgroup analyses of the Echelon-1 trial, stage IV was also associated with better PFS in patients treated with BV-AVD compared with ABVD, suggesting a similar effect in the R/R setting.13,14
We showed that patients who were treated with a sequential approach who achieved a PMR after BV or ICE only, yet converting to a CMR after salvage chemotherapy with ICE (after BV) or GVD (after ICE) exhibited comparable survival outcomes for those directly achieving CMR. This highlights the feasibility of a sequential approach, potentially sparing chemotherapy in rapid responders. Emphasizing the significance of attaining CMR before ASCT, our study suggests that improving survival in patient with PMR could be accomplished by inducing CMR through additional salvage chemotherapy or immunotherapy before ASCT.4,21,24
Our analysis is limited by missing variables in certain studies, partially mitigated by our matching method. Consequently, not all patients could be included in specific (multivariable) analyses. Although our analysis approach addresses inherent differences in trial populations and design as much as possible, it is essential to emphasize several significant distinctions in design: a large portion of patients in the chemotherapy cohort lacked response assessment using PET, restricting the comparison of pre-ASCT CMR rates between the BV and chemotherapy cohorts. Unfortunately, we could not evaluate the impact of BV maintenance in our analysis because only a limited number of patients received BV maintenance in our cohort, and the number of BV maintenance cycles differed widely across patients because of various reasons, limiting a proper analysis. Additionally, assessing the impact of radiotherapy was hindered by varying protocols among the studies. Although some universally applied pre-ASCT radiotherapy to patients with extranodal and bulky disease, others selectively used it on residual lesions either before or after ASCT.4,16,24
Generally, the PFS, OS, and CMR rates in the chemotherapy cohort appear favorable compared with real-world data.7,37 However, the studies in our analysis only included transplant-eligible patients, known for better outcomes compared with patients who are older or unfit. Furthermore, the study of Josting et al specifically excluded patients with primary refractory disease. Although our analysis minimizes bias through matching and inclusion of prospective trials, caution is warranted in generalizing to real-world scenarios. Therefore, the observed results of our analysis should be interpreted with caution and cannot replace an RCT. Nonetheless, at the moment this, to our knowledge, is the largest matched analysis based on individual patient data in R/R cHL, incorporating recent clinical trial data. Therefore, it serves as a benchmark for future (single-arm) studies exploring novel therapies or regimens that aim to replace high-dose chemotherapy/ASCT with novel drugs.
Preliminary results of an ongoing phase 2b RCT, comparing BV-ESHAP to ESHAP alone in a cohort of 150 patients, indicate a higher CMR rate in the BV-ESHAP group.38 However, the limited sample size of the study may impede subgroup analyses for risk factors. In addition, this study evaluates the substitution of ASCT by BV maintenance therapy in patients with a CMR after salvage treatment. Although this investigation could provide valuable insights into the potential replacement of ASCT with maintenance therapy, it may complicate the direct comparison of long-term outcomes between the BV-ESHAP and ESHAP arms.
Emerging novel therapies, including immune-checkpoint inhibitors, are gaining attention in the relapsed/refractory setting. In a phase 3 head-to-head comparison, single-agent pembrolizumab demonstrated superior median PFS and lower toxicity to BV.39 Checkpoint inhibition, either alone or in combination with BV or chemotherapy, has proven effective in single-arm studies.34-36 Exploring a similar individual patient data analysis for studies combining chemotherapy with checkpoint inhibitors vs BV + chemotherapy or chemotherapy alone could offer valuable insights. The evolving landscape, in which BV is increasingly being used in newly diagnosed patients, raises questions about its retreatment efficacy in the salvage setting.13 However, retreatment with BV in patients with multiple relapses showed persistent efficacy.40 Preliminary findings from an extensive ongoing RCT comparing nivolumab-AVD with BV-AVD demonstrated favorable outcomes for the nivolumab-AVD arm.41 This outcome might potentially prompt a shift toward integrating checkpoint inhibitors as a first-line treatment, thereby reinstating the use of BV in the salvage setting. Consequently, our results remain pertinent for future treatment contexts. As novel therapeutic options shift to earlier lines of therapy, such as the use of checkpoint inhibitors in the first or second line, studying the sequencing effects of these agents becomes increasingly crucial, ideally through prospective clinical trials. However, it is essential to acknowledge the lack of universal global access to these novel (and often expensive) agents, a consideration that should also be addressed in guidelines outlining the optimal treatment for patients with R/R cHL.
In summary, our study indicates that the addition of BV to chemotherapy did not enhance CMR rates or PFS in the overall population of patients with R/R cHL compared with standard salvage chemotherapy. However, notable PFS improvements were observed in patients with relapsed or stage IV disease undergoing salvage treatment that includes BV. Moreover, a sequential approach involving BV monotherapy followed by salvage chemotherapy is both viable and has the potential to reduce the need for salvage chemotherapy in certain patients. In the absence of RCTs, this propensity score–matched analysis on individual patient data offers valuable insights in the treatment landscape for patients with R/R cHL.
Acknowledgments
The authors thank the patients and collaborating investigators who kindly supplied their data.
This work was financially supported by SHOW (Dutch Foundation of hemato-oncological research http://www.steunhematologie.nl/), which is a nonprofit donation fund of Amsterdam UMC. There is no financial support for this work that could have influenced the outcomes described in the manuscript.
For the study of Cole et al20 the following grants apply: NCTN Operations Center grant U10CA180886 and NCTN Statistics & Data Center grant U10CA180899. These grants are not applicable to the current analysis.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: J.D. and M.J.K. designed the study; F.d.W. and J.D. performed the database harmonization and drafted the manuscript with contributions from all authors; H.S. performed the PET revision; J.D. performed the statistical analysis under supervision of B.A.H.; and all authors collected and interpreted the data, and read, commented on, and approved the final version of the manuscript.
Conflict-of-interest disclosure: A.F.H. received research funding from Bristol Myers Squibb, Genentech, Merck, Seattle Genetics, Kite Pharma, Gilead Sciences, AstraZeneca, and ADC Therapeutics, and consulted for Bristol Myers Squibb, Genentech, Merck, Seattle Genetics, AstraZeneca, Karyopharm, ADC Therapeutics, Takeda, Tubulis, Regeneron, Genmab, Pfizer, Caribou Biosciences, Adicet Bio, AbbVie, and Allogene Therapeutics. P.L.Z. consulted for Merck & Co, Inc, Eusapharma, and Novartis; served on the advisory boards of Secura Bio and ADC Therapeutics; and served on the speakers' bureau and advisory boards of Celltrion, Bilead, Janssen-Cilag, Bristol Myers Squibb, Servier, Merck & Co, Inc, AstraZeneca, Takeda, Roche, Eusapharma, Kyowa Kirin, Novartis, Incyte, and BeiGene. A.S.L. consulted for Seagen and Kite Pharma, and served on the speaker's bureau of Research to Practice. C.H.M. received research support from Seattle Genetics. M.F. received honoraria from Celgene, Bristol Myers Squibb, Takeda, Affimed, Lukon, and Janssen. P.B. received honoraria from Bristol Myers Squibb/Celgene, Gilead Sciences, Janssen, Miltenyi Biotech, and Novartis. J.M.Z. received research funding from Takeda and Roche, and consulted for Karyopharm. A.J.M. consulted for Takeda, Imbrium Therapeutics, Janpix, Merck, and Seatle Genetics, and received research funding from Incyte, Merck, Seattle Genetics, ADC Therapeutics, BeiGene, Miragen, and Bristol Myers Squibb. M.J.K. consulted for Bristol Myers Squibb/Celgene, Kite/Gilead, Miltenyi Biotech, Novartis, and Takeda; recievd honoraria from Kite/Gilead, Novartis, and Roche; and received research funding from Kite/Gilead and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Marie José Kersten, Department of Hematology, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; email: m.j.kersten@amsterdamumc.nl; and Julia Driessen, Department of Hematology, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; email: j.driessen@amsterdamumc.nl.
References
Author notes
Presented, in part, at the American Society of Hematology annual meeting, Atlanta, GA, 13 December 2021; and the 12th International Symposium on Hodgkin Lymphoma, Cologne, Germany, 22-24 October 2022. This manuscript contains updated data and inclusion of 1 extra clinical trial in the chemotherapy cohort.
Researchers may request access to certain deidentified data and related study documents by contacting the corresponding authors, Marie José Kersten (m.j.kersten@amsterdamumc.nl) and Julia Driessen (j.driessen@amsterdamumc.nl).
The full-text version of this article contains a data supplement.