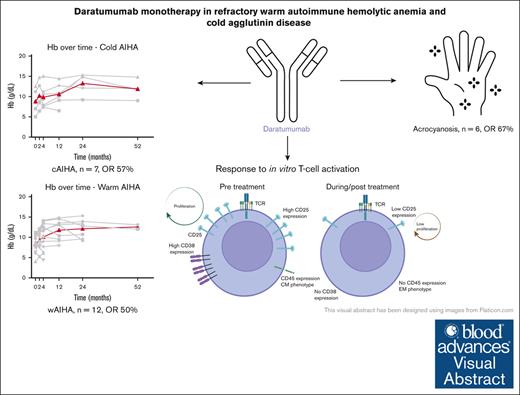
Daratumumab monotherapy may be an effective and well-tolerated treatment in more than half of patients with refractory AIHA and cold agglutinin disease.
Daratumumab can be effective on both the hemolysis and cold agglutinin–induced circulatory symptoms.
Visual Abstract
Autoimmune hemolytic anemia (AIHA) is a rare autoantibody-mediated disease. For steroid and/or rituximab-refractory AIHA, there is no consensus on optimal treatment. Daratumumab, a monoclonal antibody targeting CD38, could be beneficial by suppression of CD38+ plasma cells and thus autoantibody secretion. In addition, because CD38 is also expressed by activated T cells, daratumumab may also act via immunomodulatory effects. We evaluated the efficacy and safety of daratumumab monotherapy in an international retrospective study including 19 adult patients with heavily pretreated refractory AIHA. In warm AIHA (wAIHA, n = 12), overall response was 50% with a median response duration of 5.5 months (range, 2-12), including ongoing response in 2 patients after 6 and 12 months. Of 6 nonresponders, 4 had Evans syndrome. In cold AIHA (cAIHA, n = 7) overall hemoglobin (Hb) response was 57%, with ongoing response in 3 of 7 patients. One additional patient with nonanemic cAIHA was treated for severe acrocyanosis and reached a clinical acrocyanosis response as well as a Hb increase. Of 6 patients with cAIHA with acrocyanosis, 4 had improved symptoms after daratumumab treatment. In 2 patients with wAIHA treated with daratumumab, in whom we prospectively collected blood samples, we found complete CD38+ T-cell depletion after daratumumab, as well as altered T-cell subset differentiation and a severely diminished capacity for cell activation and proliferation. Reappearance of CD38+ T cells coincided with disease relapse in 1 patient. In conclusion, our data show that daratumumab therapy may be a treatment option for refractory AIHA. The observed immunomodulatory effects that may contribute to the clinical response deserve further exploration.
Introduction
Autoimmune hemolytic anemia (AIHA) is a rare and heterogeneous antibody-mediated disease in which red blood cell (RBC) autoantibodies lead to the accelerated destruction of RBCs. Depending on the optimal binding temperature of the autoantibodies in vitro, AIHA can be classified as warm, cold, or mixed (both) AIHA.1 In warm AIHA (wAIHA), polyclonal autoantibodies, immunoglobulin G (IgG) and/or IgA and rarely IgM isotypes, bind to the RBC antigens at an optimum temperature of 37°C. Based on the presence of an underlying disorder (such as a malignancy or other autoimmune disorders such as systemic lupus erythematosus [SLE]), wAIHA can be subdivided in primary or secondary wAIHA. In cold AIHA (cAIHA), RBC autoantibodies lead to complement-mediated hemolysis mostly via extravascular hemolysis in the liver. cAIHA is often due to cold agglutinin disease (CAD), a lymphoproliferative disorder (LPD) with an IgM kappa paraproteinemia, which is called CAD.2,3 Cold agglutinins secondary to other conditions such as overt malignancy or infections are classified as cold agglutinin syndrome. At least 50% of patients with cAIHA have symptoms of acrocyanosis after exposure to low temperatures, causing skin discoloration with or without numbness and tingling. Cold associated symptoms adversely affect quality of life.4-6 The cornerstone of treatment for wAIHA are corticosteroids followed by rituximab in case of steroid refractoriness or dependency. As with immune thrombocytopenia, there is evidence suggesting that a lack of response to rituximab could be due to the expansion of autoreactive long-lived plasma cells lacking CD20 expression.7 For patients with CAD requiring therapy, rituximab alone or combined with bendamustine may be used, however, long-term response to rituximab is much lower than that in wAIHA, and chemotherapy-based regimens may not be suitable for older patients with comorbidities. Additionally, the recently approved complement inhibitor sutimlimab is able to improve hemolysis and anemia in CAD but has no effect on peripheral acrocyanosis symptoms.8 For relapsed/refractory wAIHA/CAD there is little evidence on optimal treatment, and this population represents a pressing unmet need.
Daratumumab is a humanized monoclonal antibody that targets the CD38 glycoprotein highly expressed on plasma cells and is widely used for its registered application in the treatment of patients with multiple myeloma, in whom it shows a favorable toxicity profile.9-11 We and others hypothesize that daratumumab might suppress the secretion of autoantibodies by long-lived plasma cells (and any remaining CD38+ B cells) in patients with AIHA.12,13 In addition, due to the expression of CD38 glycoproteins on other immune cells such as natural killer cells, monocytes, B cells, and T cells, daratumumab has an additional immunomodulatory effect.9-11,14,15 Recent literature describes the successful off-label use of daratumumab in patients with autoimmune-mediated cytopenias.12,13,16-21 These retrospective series mainly involve children and immune-mediated cytopenias in the post–hematopoietic stem cell transplantation setting. Only small numbers of successful AIHA treatments with daratumumab monotherapy in adult patients have been published.12,13,17,19-22 We aimed to evaluate the efficacy and safety of daratumumab monotherapy in a larger series of adult patients with both warm and cold antibody-mediated refractory AIHA. Furthermore, the immunomodulatory effects of daratumumab treatment in patients with AIHA with regard to the T-cell compartment were of special interest. The pathophysiology of especially warm AIHA is complex and not fully elucidated, however, it is clear that there is immune dysregulation including at the T-cell level.23 The production of autoantibodies by B cells from patients with AIHA is thought to be mediated by CD4+ T cells, previously shown to be in a hyperactive state in AIHA in vitro.24 In addition, the presence of sufficient regulatory T cells (Treg) was critical for protecting against AIHA development in a murine model.24-26 We, therefore, characterized immune cell composition, specifically T-cell subsets, numbers, and function in longitudinally collected samples in 2 patients with wAIHA before, during, and after daratumumab therapy.
Patients and methods
We undertook a multinational retrospective observational study of patients with wAIHA and/or cAIHA treated with daratumumab monotherapy. Inclusion criteria were AIHA with or without immune thrombocytopenia (Evans syndrome),27 diagnosed as a combination of anemia, hemolysis (defined by increased reticulocytes, lactate dehydrogenase, and bilirubin with a decreased haptoglobin) and a positive direct antiglobulin test (DAT) for IgG and/or IgA and/or IgM and/or strongly positive for complement deposition. AIHA was classified according to recent international consensus.1 Patients with AIHA after hematopoietic stem cell transplantation were excluded. To ensure the presence of an adequate bone marrow response, patients with reticulocytopenia were excluded. Each investigator was asked to report all consecutive patients with AIHA who received at least 1 dose of daratumumab therapy. Laboratory and clinical data were retrospectively collected regarding underlying disease, bone marrow pathology, hemolytic parameters, and grading of patient-reported acrocyanosis (scored as stable, better, or resolved) at diagnosis, 2 weeks and 1, 3, 6, and 12 months,28 and last date of follow-up. Patients were included in 10 different centers in 5 countries (Austria, Italy, France, The Netherlands, and United Kingdom). Hemoglobin (Hb) response was considered none, partial (PR; Hb, 10-12 g/dL or >2 g/dL increase), or complete (CR; Hb > 12 g/dL), in the absence of recent RBC transfusion. Patients who were not anemic at baseline (Hb > 12 g/dL) were not evaluated for Hb response. Distribution of data regarding continuous variables was described in terms of a median and ranges. The initial overall response (OR) is expressed as the percentage of patients with CR and PR of the total number of patients. All patients were followed until the last available data or loss of response. Adverse events were graded according to the Common Terminology Criteria, version 5.0 (2017). All patients gave informed consent based on local legislation; patients providing material for translational studies signed an additional informed consent. The data of 2 deceased patients were used according to local legislation.
Immune cell phenotyping
Peripheral blood mononuclear cells (PBMCs) were isolated from prospectively collected blood samples from 2 patients with wAIHA (patients 1 and 3), taken at several time points: pretreatment, before cycle 5, 1 week after cycle 8, and 3 months after the end of treatment; and they were cryopreserved as described previously.2 PBMCs were stained with the multifluorochrome antibody panels (details in supplemental Methods; supplemental Table 2). T cells were analyzed either directly ex vivo or stimulated using CD3 (clone1XE) and CD28 (clone15E8) for 2 or 5 days. For assessment of proliferation, PBMCs were stained using CellTrace Violet (C34557; ThermoFisher Scientific) according to manufacturer’s description before stimulation. Cells were acquired on a LSR Fortessa (BD Biosciences), and data were analyzed using FlowJo 10.5.3. Analysis of proliferation was performed using the FlowJo proliferation tool.
All patients gave written informed consent for the retrospective data analysis concerning their treatment of autoimmune hemolytic anemia. Retrospective data analysis was approved by the Ethics committee of the Amsterdam University Medical center. Patients providing material for translational studies signed an additional informed consent and were included in the Data Registry of AutoImmune Hemolytic Anemia study, an observational cohort study, which is approved by the Medical Ethics Committee Leiden The Hague Delft.
Results
Nineteen patients with wAIHA and cAIHA were included in our study. Of these patients, 4 were previously published,12,13,22 and we here update the clinical response data of 2. Patient baseline characteristics are summarized in Table 1. Nine patients were diagnosed with primary wAIHA, including 5 patients with Evans syndrome and 3 patients with secondary wAIHA (1 with myasthenia gravis; 2 with SLE). Of 7 patients with cAIHA, 2 were diagnosed with lymphoplasmacytic lymphoma and 5 with primary CAD. Patients had received a median number of treatments of 5 (range, 2-10). Most of the patients received ongoing immune suppressive treatments at the start of daratumumab therapy (13/19). Median Hb at the start of daratumumab was 8.6 g/dL (range, 4-12.5). At baseline, 9 of 19 patients were transfusion dependent and received a median of 10 RBC units (range, 1-36) during the month before starting daratumumab. In 1 patient with CAD, treatment indication was refractory acrocyanosis only, without significant hemolytic anemia. Six of 7 patients with cAIHA reported symptoms of acrocyanosis. Individual treatment and response data are summarized in Table 2. Of 12 patients with wAIHA, 9 received a fixed duration of daratumumab therapy with either 4 or 8 weekly doses, and 3 patients received a median of 11 doses of daratumumab (range, 11-13). Of the 7 patients with cAIHA, 3 patients received a fixed duration of 8 weekly doses of daratumumab, whereas 4 patients received daratumumab maintenance treatment for a median duration of 15.5 months (range, 11-40). For all patients with AIHA, the median duration of follow-up was 5 months (range, 2-40). Two patients (1 wAIHA and 1 cAIHA) died 3 months after the start of daratumumab, due to uncontrolled severe hemolytic anemia. No patients were lost to follow-up.
Baseline characteristics
| . | Age∗/ sex . | AIHA, DAT results . | Associated condition . | Acrocyanosis . | Previous AIHA treatments . | Ongoing immune suppressive treatments at start daratumumab . | Hb, g/dL, at start daratumumab . | Bilirubin total, μmmol/L, at start daratumumab . | LDH, U/L, at start daratumumab . | RBC transfusion in month before start daratumumab . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 37/F | wAIHA DAT >2+ IgG | - | - | Steroids, rituximab splenectomy, azathioprine, cyclophosphamide bortezomib, mycophenolate, sirolimus, cyclosporin | Steroids | 11.2 | 53 | 425 | - |
| 2. | 54/F | wAIHA, DAT IgG>2+ and IgA>2+ | - | - | Steroids, rituximab EPO, mycophenolate, cyclosporin | Steroids | 9.7 | 32 | 584 | - |
| 3 | 53/M | wAIHA, DAT IgG>2+ | Myasthenia gravis | - | Steroids, rituximab, EPO, azathioprine, mycophenolate, cyclosporin, bortezomib | Steroids | 10 | 74 | 914 | - |
| 4 | 56/F | wAIHA DAT>2+ IgG | SLE | - | Steroids, mycophenolate, rituximab, IVIG EPO, cyclophosphamide,bortezomib | Steroids, hydroxychloro-quine EPO | 10.5 | 11 | 288 | - |
| 5 | 53/F | wAIHA DAT IgG>2+ and C>2+ | - | - | Steroids, rituximab splenectomy IVIG, EPO, azathioprine, bortezomib, sirolimus, cyclosporin, tacrolimus | Steroids, IVIG maintenance (monthly) | 8.8 | 86 | 242 | - |
| 6† | 55/F | wAIHA DAT IgG>2+ and C<2+ | Evans syndrome | - | Steroids, rituximab, splenectomy, azathioprine, bortezomib, everolimus, cyclosporin | - | 8.2 | 31 | 1100 | 10 |
| 7† | 55/F | wAIHA DAT IgG>2+ and C<2+ | Evans syndrome | - | Steroids, rituximab | Steroids | 10.7 | 24 | 335 | - |
| 8 | 42/M | wAIHA DAT IgG>2+ and C<2+ | Evans syndrome, MGUS IgG kappa Nonclonal CD8 T-cell proliferation, T-LGL suspect | - | Steroids, rituximab , splenectomy, plasmapheresis, cyclophosphamide, sirolimus, cyclosporin, danazole, methotrexate | Steroids Sirolimus Plasmapheresis | 4 | 140 | 4800 | 30 |
| 9 | 25/F | wAIHA DAT IgG>2+ | Evans syndrome | - | Steroids, rituximab, IVIG, bortezomib, sirolimus, danazol, EPO | Danazol Sirolimus | 6.3 | 19 | 421 | - |
| 10 | 59/M | wAIHA DAT>2+ IgG | IgG MGUS | - | Steroids, rituximab IVIG, EPO, splenectomy | Steroids Rituximab, IVIG, EPO, splenectomy | 6.1 | 80 | 1609 | 36 |
| 11 | 36/F | wAIHA DAT<2+ IgG | SLE | - | Steroids, rituximab, Obinutuzumab, plasmapheresis, azathioprine, ibrutinib | Steroids IVIG | 7.8 | 41 | 282 | 10 |
| 12 | 64/F | wAIHA DAT IgG <2+ | Evans syndrome, IgG kappa and lambda MGUS | - | Steroid, rituximab, IVIG | Steroids | 7.3 | 61.2 | 671 | 1 |
| 13† | 59/M | cAIHA, DAT negative Low titer cold agglutinin present | CAD IgG kappa MGUS, HBV | Yes | Steroids, rituximab EPO, bortezomib | - | 7 | 67 | 518 | 2 |
| 14 | 62/M | cAIHA DAT C>2+ | CAD | - | Steroids, rituximab, IVIG, plasmapheresis, eculizumab | Steroids Eculizumab | 8.8 | 73 | 480 | 20 |
| 15† | 56/M | cAIHA DAT IgG<2+, IgM>2+ | LPL | Yes | Steroids, rituximab, cyclophosphamide, bortezomib, lenalidomide | - | 12.5‡ | 10 | 351 | - |
| 16 | 74/M | cAIHA DAT C<2+ | CAD | Yes | Rituximab, plasmapheresis | - | 11.2 | 12 | - | - |
| 17 | 80/F | cAIHA DAT C>2+ | LPL | Yes | Steroids, rituximab, cyclophosphamide, mycophenolate, ibrutinib | Steroids | 8.5 | 46 | 972 | - |
| 18 | 75/F | cAIHA | CAD | Yes | Steroids, rituximab, mycophenolate, azathioprine | - | 8.6§ | 57 | 809 | 1 |
| 19 | 70/M | cAIHA | CAD, type 1 cryoglobuli-naemia | Yes | Steroids, plasmapheresis 9 doses of pegcetacoplan/ placebo‖ 1 dose of BIVV020¶ | - | 5 | 30 | 1330 | 4 |
| Median (range) | 56 (25-80) | 5 (2-10) | 8.6 (4-12.5) | 46 (10-140) | 584 (242-4800) | 10 (1-36) |
| . | Age∗/ sex . | AIHA, DAT results . | Associated condition . | Acrocyanosis . | Previous AIHA treatments . | Ongoing immune suppressive treatments at start daratumumab . | Hb, g/dL, at start daratumumab . | Bilirubin total, μmmol/L, at start daratumumab . | LDH, U/L, at start daratumumab . | RBC transfusion in month before start daratumumab . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 37/F | wAIHA DAT >2+ IgG | - | - | Steroids, rituximab splenectomy, azathioprine, cyclophosphamide bortezomib, mycophenolate, sirolimus, cyclosporin | Steroids | 11.2 | 53 | 425 | - |
| 2. | 54/F | wAIHA, DAT IgG>2+ and IgA>2+ | - | - | Steroids, rituximab EPO, mycophenolate, cyclosporin | Steroids | 9.7 | 32 | 584 | - |
| 3 | 53/M | wAIHA, DAT IgG>2+ | Myasthenia gravis | - | Steroids, rituximab, EPO, azathioprine, mycophenolate, cyclosporin, bortezomib | Steroids | 10 | 74 | 914 | - |
| 4 | 56/F | wAIHA DAT>2+ IgG | SLE | - | Steroids, mycophenolate, rituximab, IVIG EPO, cyclophosphamide,bortezomib | Steroids, hydroxychloro-quine EPO | 10.5 | 11 | 288 | - |
| 5 | 53/F | wAIHA DAT IgG>2+ and C>2+ | - | - | Steroids, rituximab splenectomy IVIG, EPO, azathioprine, bortezomib, sirolimus, cyclosporin, tacrolimus | Steroids, IVIG maintenance (monthly) | 8.8 | 86 | 242 | - |
| 6† | 55/F | wAIHA DAT IgG>2+ and C<2+ | Evans syndrome | - | Steroids, rituximab, splenectomy, azathioprine, bortezomib, everolimus, cyclosporin | - | 8.2 | 31 | 1100 | 10 |
| 7† | 55/F | wAIHA DAT IgG>2+ and C<2+ | Evans syndrome | - | Steroids, rituximab | Steroids | 10.7 | 24 | 335 | - |
| 8 | 42/M | wAIHA DAT IgG>2+ and C<2+ | Evans syndrome, MGUS IgG kappa Nonclonal CD8 T-cell proliferation, T-LGL suspect | - | Steroids, rituximab , splenectomy, plasmapheresis, cyclophosphamide, sirolimus, cyclosporin, danazole, methotrexate | Steroids Sirolimus Plasmapheresis | 4 | 140 | 4800 | 30 |
| 9 | 25/F | wAIHA DAT IgG>2+ | Evans syndrome | - | Steroids, rituximab, IVIG, bortezomib, sirolimus, danazol, EPO | Danazol Sirolimus | 6.3 | 19 | 421 | - |
| 10 | 59/M | wAIHA DAT>2+ IgG | IgG MGUS | - | Steroids, rituximab IVIG, EPO, splenectomy | Steroids Rituximab, IVIG, EPO, splenectomy | 6.1 | 80 | 1609 | 36 |
| 11 | 36/F | wAIHA DAT<2+ IgG | SLE | - | Steroids, rituximab, Obinutuzumab, plasmapheresis, azathioprine, ibrutinib | Steroids IVIG | 7.8 | 41 | 282 | 10 |
| 12 | 64/F | wAIHA DAT IgG <2+ | Evans syndrome, IgG kappa and lambda MGUS | - | Steroid, rituximab, IVIG | Steroids | 7.3 | 61.2 | 671 | 1 |
| 13† | 59/M | cAIHA, DAT negative Low titer cold agglutinin present | CAD IgG kappa MGUS, HBV | Yes | Steroids, rituximab EPO, bortezomib | - | 7 | 67 | 518 | 2 |
| 14 | 62/M | cAIHA DAT C>2+ | CAD | - | Steroids, rituximab, IVIG, plasmapheresis, eculizumab | Steroids Eculizumab | 8.8 | 73 | 480 | 20 |
| 15† | 56/M | cAIHA DAT IgG<2+, IgM>2+ | LPL | Yes | Steroids, rituximab, cyclophosphamide, bortezomib, lenalidomide | - | 12.5‡ | 10 | 351 | - |
| 16 | 74/M | cAIHA DAT C<2+ | CAD | Yes | Rituximab, plasmapheresis | - | 11.2 | 12 | - | - |
| 17 | 80/F | cAIHA DAT C>2+ | LPL | Yes | Steroids, rituximab, cyclophosphamide, mycophenolate, ibrutinib | Steroids | 8.5 | 46 | 972 | - |
| 18 | 75/F | cAIHA | CAD | Yes | Steroids, rituximab, mycophenolate, azathioprine | - | 8.6§ | 57 | 809 | 1 |
| 19 | 70/M | cAIHA | CAD, type 1 cryoglobuli-naemia | Yes | Steroids, plasmapheresis 9 doses of pegcetacoplan/ placebo‖ 1 dose of BIVV020¶ | - | 5 | 30 | 1330 | 4 |
| Median (range) | 56 (25-80) | 5 (2-10) | 8.6 (4-12.5) | 46 (10-140) | 584 (242-4800) | 10 (1-36) |
EPO, erythropoietin; HBV, hepatitis B virus; HCV, hepatitis C virus; IVIG, intravenous immune globulin; MGUS, monoclonal gammopathy of unknown significance.
Age in years at start daratumumab.
Previously published.
Treatment indication was severe acrocyanosis.
After RBC transfusion.
Participated in the randomized, placebo-controlled phase 3 trial trial (ClinicalTrials.gov, NCT05096403).
Participated in the PDY16370 study, received 1 dose of BIVV020 (anti-C1s humanized IgG4 monoclonal antibody), but went off study (ClinicalTrials.gov, NCT04269551).
Individual response data
| Case, AIHA type . | Daratumumab treatment schedule . | Time to partial Hb response in wk∗ (Hb, g/dL, Δ) . | Time to complete Hb response in wk∗ (Hb, g/dL, Δ) . | Best Hb response in wk from start daratumumab (Hb, g/dL, Δ) . | Transfusion dependence (if transfusion dependent at baseline) . | Acrocyanosis improved/resolved (for patients with acrocyanosis at baseline) . | Duration of response after the start of daratumumab . |
|---|---|---|---|---|---|---|---|
| 1 wAIHA | 8× weekly sc 1800 mg | - | 4 (12.5, +1.3) | 4 (12.5, +1.3) | - | - | 2 mo |
| 2 wAIHA | 8× weekly sc 1800 mg | 2 (11.9, +2.2) | 4 (13.4, +3.7) | 4 (13.4, +3.7) | - | - | 5 mo |
| 3 wAIHA | 8× weekly sc 1800 mg | 2 (11.0, +1.0) | 9 (12.4, +2.4) | 24 (13.4, +3.4) | - | - | Ongoing at 12 mo |
| 4 wAIHA | 4× weekly sc 1800 mg | - | 8 (12.7, +2.2) | 12 (13.5, +2) | - | - | Ongoing at 4 mo† |
| 5 wAIHA | 8× weekly sc 1800 mg, then 2× 2-weekly sc 1800mg | - | - | - | - | - | No response after 4 mo |
| 6 wAIHA | 8× weekly 16 mg/kg iv, 3 × 2-weekly 16 mg/kg IV | - | - | - | Ongoing | - | No response after 3, 5 mo |
| 7 wAIHA | 4× weekly 16mg/kg IV | 2 (12.1, +1.4) | 12 (14.6, +3.9) | - | - | 9 mo | |
| 8 wAIHA | 8× weekly 16 mg/kg IV | - | - | - | Ongoing | - | No response after 2, 5 mo |
| 9 wAIHA‡ | 4× weekly 16 mg/kg IV | - | - | - | - | - | No response after 2, 5 mo |
| 10 wAIHA | 4× weekly sc 1800 mg | 2 (10.1, + 4.0) | 4 (14.2, +8.1) | 24 (15.3, +9.2) | Transfusion independent after 14 d | - | Ongoing at 6 mo |
| 11 wAIHA | 4× weekly sc 1800 mg | - | - | - | Ongoing | - | No response after 2 mo |
| 12 wAIHA | 2× weekly 16 mg/kg IV 11× 2-weekly sc 1800 mg | - | - | - | Ongoing | - | No response after 6 mo |
| 13 cAIHA | 8× weekly 16 mg/kg IV, 16x 2-weekly 16 mg/kg IV, 9× monthly 16 mg/kg IV | 12 (9.1, +2.1) | - | 40 (10.2, +3.2) | Transfusion independent after 6 mo | Improvement after 3 mo | 19 mo§ |
| 14 cAIHA | 8 × weekly sc 1800 mg | 2 (10.1, +1.3) | 16 (12.0, +3.2) | 24 (14.5, +5.7) | Transfusion independent after 3 mo | - | Ongoing at 8 mo |
| 15 cAIHA | First dose 16 mg/kg IV followed by 8× weekly sc 1800 mg, 8x 2-weekly sc 1800 mg, followed by maintenance 1800 mg sc monthly | - | ‖ | 8 (16.6, +4.1) | - | Improved after 2 wk Resolved after 3 mo | Ongoing at 40 mo§ |
| 16 cAIHA | First dose 16mg/kg IV followed by 8× weekly sc 1800 mg, 8x 2-weekly sc 1800 mg, followed by maintenance 1800 mg sc monthly | - | 2 (12.7, +1.5) | 24 (14.8, +3.6) | - | Improved after 2 wk Resolved after 12 mo | Ongoing at 12 mo§ |
| 17 cAIHA | First dose 16mg/kg IV followed by 8× weekly sc 1800 mg, 8x 2-weekly sc 1800 mg, followed by maintenance 1800 mg sc monthly | 4 (11.2, +2.7) | 12 (12.7, +4.2) | 12 (12.7, +4.2) | - | Improved after 6 mo | Ongoing at 11 mo§ |
| 18 cAIHA | 8× weekly sc 1800 mg | - | - | - | Ongoing | No response | No response after 2 mo |
| 19 cAIHA‡ | 8× weekly sc 1800 mg | - | - | - | Ongoing | No response | No response after 3 mo |
| Median (range) | 2 (2-12 wk) Δ + 2.2 g/dL (1.0-4.0) | 2 (2-16 wk) Δ + 2.3 g/dL (1.3-8.1) | 12 (2-40 wk) Δ + 3.7 g/dL (1.3-9.2) |
| Case, AIHA type . | Daratumumab treatment schedule . | Time to partial Hb response in wk∗ (Hb, g/dL, Δ) . | Time to complete Hb response in wk∗ (Hb, g/dL, Δ) . | Best Hb response in wk from start daratumumab (Hb, g/dL, Δ) . | Transfusion dependence (if transfusion dependent at baseline) . | Acrocyanosis improved/resolved (for patients with acrocyanosis at baseline) . | Duration of response after the start of daratumumab . |
|---|---|---|---|---|---|---|---|
| 1 wAIHA | 8× weekly sc 1800 mg | - | 4 (12.5, +1.3) | 4 (12.5, +1.3) | - | - | 2 mo |
| 2 wAIHA | 8× weekly sc 1800 mg | 2 (11.9, +2.2) | 4 (13.4, +3.7) | 4 (13.4, +3.7) | - | - | 5 mo |
| 3 wAIHA | 8× weekly sc 1800 mg | 2 (11.0, +1.0) | 9 (12.4, +2.4) | 24 (13.4, +3.4) | - | - | Ongoing at 12 mo |
| 4 wAIHA | 4× weekly sc 1800 mg | - | 8 (12.7, +2.2) | 12 (13.5, +2) | - | - | Ongoing at 4 mo† |
| 5 wAIHA | 8× weekly sc 1800 mg, then 2× 2-weekly sc 1800mg | - | - | - | - | - | No response after 4 mo |
| 6 wAIHA | 8× weekly 16 mg/kg iv, 3 × 2-weekly 16 mg/kg IV | - | - | - | Ongoing | - | No response after 3, 5 mo |
| 7 wAIHA | 4× weekly 16mg/kg IV | 2 (12.1, +1.4) | 12 (14.6, +3.9) | - | - | 9 mo | |
| 8 wAIHA | 8× weekly 16 mg/kg IV | - | - | - | Ongoing | - | No response after 2, 5 mo |
| 9 wAIHA‡ | 4× weekly 16 mg/kg IV | - | - | - | - | - | No response after 2, 5 mo |
| 10 wAIHA | 4× weekly sc 1800 mg | 2 (10.1, + 4.0) | 4 (14.2, +8.1) | 24 (15.3, +9.2) | Transfusion independent after 14 d | - | Ongoing at 6 mo |
| 11 wAIHA | 4× weekly sc 1800 mg | - | - | - | Ongoing | - | No response after 2 mo |
| 12 wAIHA | 2× weekly 16 mg/kg IV 11× 2-weekly sc 1800 mg | - | - | - | Ongoing | - | No response after 6 mo |
| 13 cAIHA | 8× weekly 16 mg/kg IV, 16x 2-weekly 16 mg/kg IV, 9× monthly 16 mg/kg IV | 12 (9.1, +2.1) | - | 40 (10.2, +3.2) | Transfusion independent after 6 mo | Improvement after 3 mo | 19 mo§ |
| 14 cAIHA | 8 × weekly sc 1800 mg | 2 (10.1, +1.3) | 16 (12.0, +3.2) | 24 (14.5, +5.7) | Transfusion independent after 3 mo | - | Ongoing at 8 mo |
| 15 cAIHA | First dose 16 mg/kg IV followed by 8× weekly sc 1800 mg, 8x 2-weekly sc 1800 mg, followed by maintenance 1800 mg sc monthly | - | ‖ | 8 (16.6, +4.1) | - | Improved after 2 wk Resolved after 3 mo | Ongoing at 40 mo§ |
| 16 cAIHA | First dose 16mg/kg IV followed by 8× weekly sc 1800 mg, 8x 2-weekly sc 1800 mg, followed by maintenance 1800 mg sc monthly | - | 2 (12.7, +1.5) | 24 (14.8, +3.6) | - | Improved after 2 wk Resolved after 12 mo | Ongoing at 12 mo§ |
| 17 cAIHA | First dose 16mg/kg IV followed by 8× weekly sc 1800 mg, 8x 2-weekly sc 1800 mg, followed by maintenance 1800 mg sc monthly | 4 (11.2, +2.7) | 12 (12.7, +4.2) | 12 (12.7, +4.2) | - | Improved after 6 mo | Ongoing at 11 mo§ |
| 18 cAIHA | 8× weekly sc 1800 mg | - | - | - | Ongoing | No response | No response after 2 mo |
| 19 cAIHA‡ | 8× weekly sc 1800 mg | - | - | - | Ongoing | No response | No response after 3 mo |
| Median (range) | 2 (2-12 wk) Δ + 2.2 g/dL (1.0-4.0) | 2 (2-16 wk) Δ + 2.3 g/dL (1.3-8.1) | 12 (2-40 wk) Δ + 3.7 g/dL (1.3-9.2) |
Δ, delta; NA, not applicable; sc, subcutaneously.
Hb responses were defined as partial (PR, >2 g/dL Hb increase or >10g/dL) or complete (CR, >12g/dL).
Pat 4 has started belimumab for underlying SLE 4 months after the start of daratumumab, Hb response data not analyzed after 4 months. Patient is after 2 years still in CR of AIHA.
Patient died due to uncontrolled hemolytic anemia
Daratumumab maintenance therapy until progression
Treatment indication was severe acrocyanosis, therefore patient was excluded from assessment of Hb response.
Treatment response in wAIHA
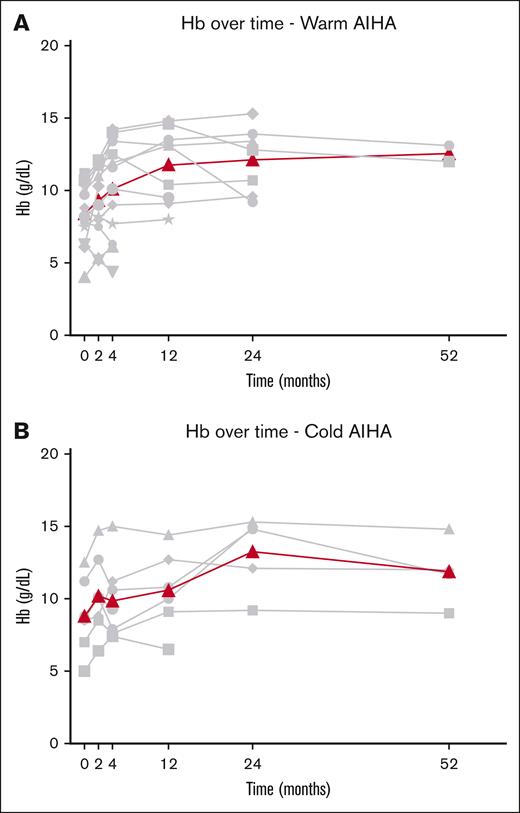
Median Hb at start of daratumumab treatment was 8.5 g/dL (range, 4-11.2). Daratumumab treatment resulted in an overall complete Hb response in 6 of 12 patients with wAIHA (50%), with a median time to OR of 2 weeks (range, 2-8). Complete Hb response was reached after a median of 4 weeks after the start of daratumumab (range, 2-9 weeks), with a median Hb increase of 2.3 g/dL (range, 1.3-8.1 g/dL; Figure 1). Median duration of response was 5.5 months (range, 2-12) after the start of daratumumab. Median duration of therapy was 2 months (range, 1-6), with a median duration of follow-up of 4 months (range, 2-12). At 4 months after the start of daratumumab, 1 patient with wAIHA with a CR from 2 months of daratumumab therapy onward started belimumab, a human monoclonal antibody that is thought to inhibit B-cell survival and thereby production of autoantibodies,29 for underlying SLE. This patient is still in CR after 2 years, however. Data from this patient were censored from the time of start of belimumab.
Hb response after the initiation of daratumumab therapy. (A) Patients with wAIHA. (B) Patients with cAIHA. Gray lines represent individual data of Hb; bold line represents median Hb over time.
Hb response after the initiation of daratumumab therapy. (A) Patients with wAIHA. (B) Patients with cAIHA. Gray lines represent individual data of Hb; bold line represents median Hb over time.
At the time of data collection, 2 patients with heavily pretreated wAIHA, with a median number of previous therapies of 6 (range, 5-7), showed an ongoing complete Hb response at 6 and 12 months after fixed duration daratumumab treatment (4 and 8 doses, respectively). Three additional patients relapsed 2, 5, and 9 months after the start of daratumumab. Six of 12 patients with wAIHA (50%) did not show an improvement of hemolysis after a median of duration of therapy of 2.5 months (range, 1-6) and a median duration of follow-up of 3 months (range, 2-6). These were all patients with a median of 7 previous lines of therapies (range, 3-10), of whom 4 of 6 were with underlying Evans syndrome.
Five patients with wAIHA were heavily RBC transfusion dependent at the start of daratumumab treatment with a median of 20 RBC transfusions per month before starting daratumumab (range, 1-36 units). Four patients had an ongoing need for transfusion after a median duration of therapy of 3 months (range, 1-6). One patient who received 36 units of RBC transfusions in the month before starting daratumumab treatment became transfusion independent after 2 weeks. However, due to the severity of the disease, this patient received more lines of therapy in the month before starting daratumumab treatment, so we cannot exclude the contribution of comedication (Table 1).
Treatment response in cAIHA
Median Hb at start of daratumumab treatment was 8.6 g/dL (range, 5-12.5). Daratumumab treatment resulted in an overall Hb response in 4 of 7 patients (57%) with cAIHA, with a median time to OR of 3 weeks (range, 2-12) (Figure 1). Complete Hb response was reached in 3 patients, with a median Hb increase of 3.2 g/dL (range, 1.5-4.2) 3 months after the start of daratumumab (range, 0.5-4). One patient with cAIHA reached a long-lasting PR with an increase of Hb of 2.1 g/dL after 12 weeks. One patient started daratumumab treatment because of acrocyanosis without significant hemolytic anemia (Hb at start, 12.5 g/L), yet showed a Hb increase of 4.1 g/dL after 2 months, with an ongoing Hb response at 40 months. However, because of our definition of CR of Hb at the start of daratumumab, this patient was excluded from assessment of Hb response.
Median duration of therapy was 11 months (range, 2-40), with a median duration of follow-up of 11 months (range, 2-40). At the time of data collection, 2 of 3 patients with cAIHA on daratumumab monthly maintenance therapy had an ongoing Hb response after a median of 11.5 months (range, 11-12). One patient showed progression of AIHA after 19 months of maintenance therapy. Of the 3 patients with a fixed duration of 2 months of daratumumab therapy, 1 patient showed an ongoing CR of Hb after 8 months. The 2 other patients with a fixed duration of 2 months of daratumumab therapy did not have a response. Four patients were transfusion dependent before the start of daratumumab therapy with a median of 3 RBC transfusions per month (range, 1-20). Two patients became transfusion independent after 3 and 6 months, respectively.
Of the 6 patients with acrocyanosis, 4 patients reported clinical improvement, of whom 2 within 14 days and the other 2 patients after 3 and 6 months, respectively. Complete resolution of acrocyanosis was reached in 2 patients after 3 and 12 months, respectively.
Safety
Three patients had a grade 2 infusion reaction after the first dose of IV daratumumab. One patient suffered from a grade 3 chronic COVID infection requiring hospitalization. There was 1 varicella zoster virus infection grade 1 reported. One patient reported a grade 3 febrile neutropenia and presumed pneumonia requiring hospitalization. One patient had a grade 3 bacterial sepsis and an anal abscess requiring surgery. This patient was heavily pretreated with immunosuppressive drugs and was still on prednisolone and sirolimus therapy when daratumumab treatment was initiated.
Immunomodulatory effects of daratumumab treatment
To characterize the immune cell composition and potential immunomodulatory effects of daratumumab in patients with AIHA, response evaluable PBMC samples prospectively collected from 2 patients with wAIHA (patients 1 and 3; Table 1) were analyzed at baseline (Pre), during treatment (Mid), immediately after treatment cessation (EOT), and 3 months after end of treatment (Post). In addition, for patient 3, a sample taken 6 weeks after the last cycle (6 weeks) was also included. The percentage of B cells, which usually constitutes about 10% of lymphocytes, was relatively low at baseline in the PBMC pool of these patients and declined further over the course of treatment (Figure 2A).30 The preexisting low B-cell abundance could be related to the heavily pretreated status of the studied patients, including immunosuppressive agents as described in Table 1. In the 6 months before the initiation of daratumumab, patient 1 was treated with steroids in combination with first sirolimus followed by cyclosporin because progression of disease. Due to cyclosporin intolerance, steroid monotherapy was continued in the last 3 months before the start of daratumumab. Patient 3 was only treated with steroids in the 6 months before start of daratumumab. Last dose of rituximab was 15 and 2 years before the start of daratumumab for patients 1 and 3, respectively.
Daratumumab treatment reduced the abundance of B cells and affected subset distributions of T cells. PBMCs from 2 patients with wAIHA at different time points: baseline (Pre), during treatment (Mid), immediately after treatment cessation (EOT), 6 weeks after the last cycle (6 weeks), and 3 months after end of treatment (Post) were analyzed by flow cytometry, either right after thawing or after a 2- to 5-day culture-cell stimulation by αCD3/αCD28 antibodies. (A) Frequency and subset distribution of B cells over the course of daratumumab treatment. (B) Analysis of CD3+ and Treg frequency, CD4+: CD8+ ratio, CD4+subset distribution and CD38 expression. (C) Hemoglobin (g/dL) levels of patients over course of treatment. CM, central memory; EM, effector memory; EMRA, effector memory RA+.
Daratumumab treatment reduced the abundance of B cells and affected subset distributions of T cells. PBMCs from 2 patients with wAIHA at different time points: baseline (Pre), during treatment (Mid), immediately after treatment cessation (EOT), 6 weeks after the last cycle (6 weeks), and 3 months after end of treatment (Post) were analyzed by flow cytometry, either right after thawing or after a 2- to 5-day culture-cell stimulation by αCD3/αCD28 antibodies. (A) Frequency and subset distribution of B cells over the course of daratumumab treatment. (B) Analysis of CD3+ and Treg frequency, CD4+: CD8+ ratio, CD4+subset distribution and CD38 expression. (C) Hemoglobin (g/dL) levels of patients over course of treatment. CM, central memory; EM, effector memory; EMRA, effector memory RA+.
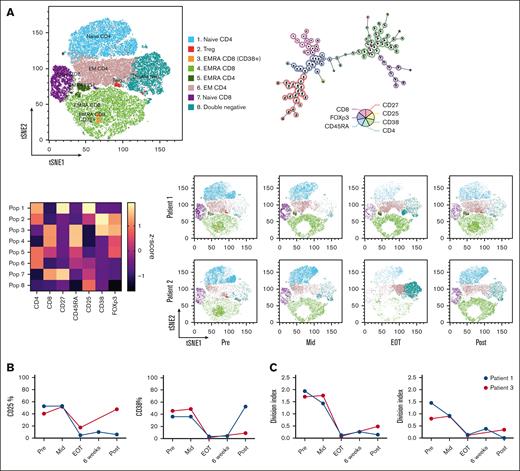
The composition of the B-cell subsets, characterized using CD27 and IgD, did not change during the treatment (Figure 2A; supplemental Figure 1A). We also observed patient-specific changes in the percentage of Treg and in the CD4+: CD8+ ratio but not in the total abundance of T cells at baseline (Figure 2B; supplemental Figure 1B). When characterizing T-cell subsets using CD27 and CD45RA, we identified a reduction in central memory (CM; CD27+CD45RA–) and an increase in effector memory (EM; CD27–CD45RA–) in CD4+ (but not in CD8+) T cells at the EOT time point (Figure 2B). This shift partially normalized at 3 months after end of treatment (Figure 2B; supplemental Figure 1C). As expected, the population of T cells expressing CD38 disappeared during daratumumab treatment. In 1 patient, the CD38+ subset reappeared after treatment cessation, which coincided with a swift disease relapse, shown by corresponding changes in Hb levels (Figure 2B; Figure 2C; supplemental Figure 1C). Next, the unsupervised clustering algorithm FlowSOM31 was applied on these samples. The 8 meta clusters were named, based on the combination of markers expressed (Figure 3A; supplemental Figure 1D). The tSNE maps of the different time points clearly visualized the changes in subset distribution during treatment, which were mostly consistent in these 2 patients. After treatment, the aforementioned reappearance of the CD38+ subset was visible in patient 1 (Figure 3A). To investigate functional responses of T cells at the different time points, we stimulated the patient samples using CD3/CD28 antibodies (supplemental Figure 1E). After stimulation, expression of the activation marker CD25 was reduced on T cells from samples taken at later time points during treatment (Figure 3B; supplemental Figure 1F). However, CD38 is also an activation marker on healthy T cells and daratumumab treatment caused depletion of CD38-expressing T cells (Figure 3B; supplemental Figure 1F). After in vitro T-cell activation, no effects of daratumumab treatment on T-cell frequency or CD4+: CD8+ ratio were seen (supplemental Figure 1F). An important functionality of T cells is proliferation, which was measured after 5 days of stimulation. Our data showed that proliferation capacity in both CD4+ and CD8+ T cells was severely diminished (Figure 3C; supplemental Figure1G). Altogether, these data indicate that in addition to altering immune composition, daratumumab also disrupts normal T-cell function.
T-cell function and populations altered by daratumumab treatment. (A) Pooled tSNE map, minimal spanning tree, and heat map of the 8 FlowSOM metaclusters based on marker intensity, followed by tSNE maps of metaclusters in individual patients over treatment course: baseline (Pre), during treatment (Mid), immediately after treatment cessation (EOT), 6 weeks after the last cycle (6 weeks) and 3 months after end of treatment (Post). (B) Expression of CD25, CD38 on CD4+ T cells measured after a 48-hour T-cell stimulation. (C) Samples were stained with CTV and proliferation was assessed after 5 days of T-cell stimulation. CM, central memory; CTV, cell trace violet; EM, effector memory; EMRA, effector memory RA+; Treg, T-regulatory.
T-cell function and populations altered by daratumumab treatment. (A) Pooled tSNE map, minimal spanning tree, and heat map of the 8 FlowSOM metaclusters based on marker intensity, followed by tSNE maps of metaclusters in individual patients over treatment course: baseline (Pre), during treatment (Mid), immediately after treatment cessation (EOT), 6 weeks after the last cycle (6 weeks) and 3 months after end of treatment (Post). (B) Expression of CD25, CD38 on CD4+ T cells measured after a 48-hour T-cell stimulation. (C) Samples were stained with CTV and proliferation was assessed after 5 days of T-cell stimulation. CM, central memory; CTV, cell trace violet; EM, effector memory; EMRA, effector memory RA+; Treg, T-regulatory.
Discussion
To our knowledge, this is the largest study on daratumumab monotherapy in AIHA, with a total of 19 adult patients with refractory AIHA and a particularly high burden of disease, of whom 9 were transfusion dependent and 5 had Evans syndrome. The effect of daratumumab in wAIHA in our series (50%) is lower than that of previously published retrospective data in which 83% of patients (5/6) showed a Hb response with a median duration of response of 5 months (range, 2-20) after a median of 6 doses of daratumumab (range, 4-12; Table 3).13,17,19,20,32 This may be related to positive reporting bias in previously published case reports and smaller case series.
Review of literature, daratumumab monotherapy in adult patients with primary and secondary AIHA (non–stem cell transplantation setting)
| age/sex . | Disease . | Previous treatments . | Daratumumab schedule . | Best response . | Time to response Hb PR/CR∗ and acrocyanosis . | Duration of response . | Reference . |
|---|---|---|---|---|---|---|---|
| 60, F | wAIHA | Steroids, rituximab azathioprine | 4× 16 mg/kg IV weekly | PR | 10 wk | 5 mo | 19 |
| 44, F | wAIHA | Steroids, rituximab, IVIG, HSA, splenectomy, cylcosporine, mycophenolate | 6× 16 mg/kg IV weekly + 6× 16 mg/kg IV maintenance | CR | na | 5 mo | 17 |
| 55, F | wAIHA | Steroids, rituximab | 6× 16 mg/kg IV weekly | CR | na | 2 mo | 17 |
| 55, F† | wAIHA | Steroids | 6× 16 mg/kg IV weekly | CR | 1 wk | Relapse after 9 mo | 13 |
| 55, F† | wAIHA | Steroids, azathioprine, cyclosporine, everolimus, bortezomib | 8× 16 mg/kg IV weekly + 3× 2-weekly 16mg/kg IV | No response | No response | - | 13 |
| 64,F | wAIHA | Prednison, rituximab, splenectomy | 8× 16 mg/kg IV weekly | CR | Na | Ongoing response after 20 mo | 20 |
| 56, M† | cAIHA | Steroids, rituximab, bortezomib, cyclophosfamide, lenalidomide | 8× weekly 16mg/kg IV + 16× 2-weekly 16mg/kg IV + monthly 16mg/kg IV maintenance | CR Hb PR acrocyanosis | 2 wk 2 wk improvement of acrocyanosis | Ongoing response after 10 mo | 22 |
| 59, M† | cAIHA | Steroids, rituximab, EPO bortezomib | 8× weekly 16mg/kg IV + 16× 2-weekly 16mg/kg IV + monthly 16mg/kg IV maintenance | PR | 12 wk | Ongoing after 16 mo | 12 |
| 73, M | cAIHA | Rituximab, ibrutinib, bendamustine | Na, maintenance therapy ongoing at 15 mo | PR | na | Ongoing response after 15 mo | 20 |
| 64, F | cAIHA | Rituximab | 8× weekly 16mg/kg IV + 8× 2-weekly 16mg/kg IV + monthly 16mg/kg IV maintenance Combination with HSA | CR | 5 mo PR 6 mo CR | Ongoing response after 8 mo | 21 |
| age/sex . | Disease . | Previous treatments . | Daratumumab schedule . | Best response . | Time to response Hb PR/CR∗ and acrocyanosis . | Duration of response . | Reference . |
|---|---|---|---|---|---|---|---|
| 60, F | wAIHA | Steroids, rituximab azathioprine | 4× 16 mg/kg IV weekly | PR | 10 wk | 5 mo | 19 |
| 44, F | wAIHA | Steroids, rituximab, IVIG, HSA, splenectomy, cylcosporine, mycophenolate | 6× 16 mg/kg IV weekly + 6× 16 mg/kg IV maintenance | CR | na | 5 mo | 17 |
| 55, F | wAIHA | Steroids, rituximab | 6× 16 mg/kg IV weekly | CR | na | 2 mo | 17 |
| 55, F† | wAIHA | Steroids | 6× 16 mg/kg IV weekly | CR | 1 wk | Relapse after 9 mo | 13 |
| 55, F† | wAIHA | Steroids, azathioprine, cyclosporine, everolimus, bortezomib | 8× 16 mg/kg IV weekly + 3× 2-weekly 16mg/kg IV | No response | No response | - | 13 |
| 64,F | wAIHA | Prednison, rituximab, splenectomy | 8× 16 mg/kg IV weekly | CR | Na | Ongoing response after 20 mo | 20 |
| 56, M† | cAIHA | Steroids, rituximab, bortezomib, cyclophosfamide, lenalidomide | 8× weekly 16mg/kg IV + 16× 2-weekly 16mg/kg IV + monthly 16mg/kg IV maintenance | CR Hb PR acrocyanosis | 2 wk 2 wk improvement of acrocyanosis | Ongoing response after 10 mo | 22 |
| 59, M† | cAIHA | Steroids, rituximab, EPO bortezomib | 8× weekly 16mg/kg IV + 16× 2-weekly 16mg/kg IV + monthly 16mg/kg IV maintenance | PR | 12 wk | Ongoing after 16 mo | 12 |
| 73, M | cAIHA | Rituximab, ibrutinib, bendamustine | Na, maintenance therapy ongoing at 15 mo | PR | na | Ongoing response after 15 mo | 20 |
| 64, F | cAIHA | Rituximab | 8× weekly 16mg/kg IV + 8× 2-weekly 16mg/kg IV + monthly 16mg/kg IV maintenance Combination with HSA | CR | 5 mo PR 6 mo CR | Ongoing response after 8 mo | 21 |
CR, complete response; F, female; HSA, haematopoiesis-stimulating agents; M, male; PR, partial response;
Hemoglobin (Hb) response was considered partial (PR; Hb, 10-12 g/dL) or complete (CR, Hb > 12 g/dL).
Case included and updated in our case series.
Still, all patients with wAIHA in our study had severe refractory and heavily pretreated AIHA, with median previous lines of therapy of 6, in which little treatment options are available. Furthermore, the time to response (median of 2 weeks) is short, which is very relevant in the setting of severe hemolytic disease. Interestingly, most nonresponders with wAIHA had Evans syndrome, which may suggest that anti-CD38 therapy is less promising in this setting. Indeed, Evans syndrome is associated with a more aggressive course and is more treatment resistant, suggesting another mechanism of disease compared with normal wAIHA.27
In cAIHA, daratumumab therapy was effective in more than half of patients with cAIHA, with an overall Hb response of 57% and a clinical improvement of acrocyanosis in 67% of the patients. One patient with nonresponding cAIHA had no detectible M-protein and no evidence of a lymphoproliferative disorder in the bone marrow. It is thought that in classic CAD-LPD and lymphoplasmacytic lymphoma, monoclonal autoantibodies are produced by B cells and long-lived plasma cells, which may explain the sensitivity to daratumumab treatment. Consequently, the mechanism of disease might have been different from this nonresponding patient without underlying LPD and thus less sensitive to daratumumab.33,34
All patients with cAIHA receiving maintenance daratumumab treatment showed long-lasting responses, with 1 patient showing a relapse of AIHA after 19 months of daratumumab treatment. These results suggest that there might be a role for maintenance daratumumab therapy in a responsive patients. This is in line with previously published retrospective data on 4 patients with primary cAIHA, of whom we could update the response data of 2 patients in our study, treated with daratumumab maintenance therapy. All 4 patients with cAIHA had an ongoing Hb response after a median of 12.5 months of maintenance therapy (range, 8-16) (Table 3).
Prospective studies should confirm the efficacy of antiCD38 therapy in both wAIHA as well as cAIHA and explore whether maintenance therapy with daratumumab might be beneficial in patients who show an initial response. A phase 1b/2 trial with the anti-CD38 antibody isatuximab in patients with wAIHA was initiated but terminated prematurely due to strategic sponsor decisions (NCT04661033). We are not aware of any other ongoing clinical studies.
Importantly, after the initiation of daratumumab therapy, acrocyanosis symptoms improved in 4 of 6 patients with cAIHA, with complete resolution in 2. This is a notable finding, because acrocyanosis can significantly affect the quality of life in a large proportion of patients with cAIHA. In patients with disabling acrocyanosis, daratumumab therapy or other clone-directed therapy might therefore be preferred over the novel complement inhibitors now being approved for CAD, because cold autoantibody–induced circulatory symptoms are not complement mediated and symptoms might even worsen after the start of complement inhibitors.5,28,35
In our cohort, infections were reported in 4 of 19 patients (21%), including 3 of grade 3 and 1 of grade 1. Due to the retrospective nature of this study and missing data on immunoglobulin titers, we could not assess to what extent these infections were causally related to the initiation of daratumumab in addition to the patients’ heavy pretreatments with other immunosuppressive drugs. By comparison, in patients with heavily pretreated myeloma, infection-related serious adverse events occurred in 10% to 17% of the patients treated with daratumumab monotherapy.36 The potential for hypogammaglobulinemia and infections should be kept in mind when considering daratumumab treatment.
Whether daratumumab treatment has additional immunomodulatory effects on the active T-cell involvement in AIHA has not been investigated. In 2 patients with wAIHA (patients 1 and 3), we showed alterations in T-cell skewing toward an EM phenotype and a severely dampened capacity for T-cell activation and proliferation during exposure to daratumumab, all indicators of immunomodulation. We did not, however, observe the reduced abundance of Treg that was described earlier in patients with daratumumab-treated myeloma2 and thus found no evidence that daratumumab provides additional control of AIHA by modulating Treg presence.
Strikingly, we observed a clear difference in the restoration of a CD38-expressing T-cell population between the 2 patients. In particular, the rapid recurrence in patient 1 at the same time of AIHA relapse shortly after treatment cessation warrants further attention. CD38 is a T-cell surface marker that is upregulated upon activation. It is involved in susceptibility to (viral) infections and the activation of cytotoxic T cells upon exposure to a pathogen by promoting intracellular calcium release.37 The depletion of CD38+ T cells likely leads to a broad reduction of activated T cells, which usually coexpress markers such as CD25 (interleukin-2 receptor), CD69, and/or CD44.38 The disappearance of these (activated) T cells could therefore hamper T-cell support of autoantibody-producing B cells. Additionally, the early recurrence of CD38+ T cells and AIHA disease relapse shortly after in 1 patient suggest that CD38 expression on T cells could be an early biomarker of (auto)immune reactivation and relapse after daratumumab treatment. These preliminary findings deserve further study in a larger cohort. Daratumumab has been used off label at a small scale in patients suffering from autoimmune disease such as SLE and (refractory) immune thrombocytopenia.13 We hypothesize that the immunomodulatory effect of daratumumab on CD38-expressing T cells contributes to the responses achieved in these patients, as has been described in patients with SLE who have been shown to portray altered CD38 expression in their T-cell compartments.39 Furthermore, this may have relevance for the potential of anti-CD38 antibodies in other autoimmune diseases characterized by increased T-cell activation, such as inflammatory bowel diseases and multiple sclerosis.39
Limitations of this study are related to its retrospective design and the low incidence of refractory AIHA that both leads to the potential for selection bias as well as incompleteness of data; that is regarding adverse events, the small number of cases, the variable dosing schedules, and the lack of a control group. Furthermore, we cannot exclude late responses to the comedication given before initiation of daratumumab treatment.
In conclusion, daratumumab monotherapy may be an effective and well-tolerated treatment option associated with rapid responses for a proportion of refractory patients with AIHA, with an effect on both the hemolytic anemia as well as the cold-induced circulatory symptoms. Prospective studies should confirm these findings including the role of daratumumab maintenance therapy in patients showing an initial response. Furthermore, it would be relevant to understand better which subpopulations of patients with AIHA might be more likely to respond. The immunomodulatory effects that we observed may provide additional mechanisms via which daratumumab leads to response in patients with refractory wAIHA.
Authorship
Contribution: All authors were involved in data collection and the development of the manuscript; M.J., and J.M.I.V. designed the study and analyzed the data; and all authors critically reviewed the manuscript.
Conflict-of-interest disclosure: B.F. received consultancy honoraria from Alexion, Novartis, Janssen, and Sobi. M.M. received consultancy fees and/or speakers fees received from Novartis, Alexion, Sanofi, Union Chimique Belge, Argenx, and Sobi. E.C. received honoraria (advisory boards, speaker’s fees) from Novartis, Union Chimique Belge, Amgen, and Sanofi. Q.A.H. received speaker honoraria from Grifols and Novartis; consultancy from Amgen, Argenx, Gliknik, Incyte, Immunovant, Janssen, Novartis, Sanofi, and Sobi. U.J. received honoraria from Sanofi, Roche, Novartis, Incyte, Janssen, and Bristol Myers Squibb; advisory role fees from Sanofi, Roche, and Novartis. A.K. received research funding from AbbVie, AstraZeneca, Bristol Myers Squibb, Janssen, and Roche/Genentech; received patent royalties from Janssen and LAVA; and served on the board of directors or advisory committees for AstraZeneca, BMS, Roche/Genentech, Janssen, AbbVie, and LAVA; and received speaker’s fees from AbbVie, AstraZeneca, and Janssen. S.D.S. received research funding from BeiGene and Janssen; advisory board fees from BeiGene, Sanofi, Janssen and Cellectar; speakers bureau fees from Janssen and BeiGene. J.M.I.V. received consultancy and advisory board honoraria from Sanofi and Janssen; research support from BeiGene and AbbVie/Genmab; and participated in the speakers’ bureau for Bristol Myers Squibb, Sanofi, and Amgen. All honoraria received are directed to the institute. The remaining authors declare no competing financial interests.
Correspondence: Marit Jalink, Department of Hematology, Leiden University Medical Center, Postbus 9600, 2300 RC Leiden, The Netherlands; email: m.jalink@lumc.nl.
References
Author notes
The authors confirm that the data supporting the findings of this study are available within the article and its supplemental Figure. Additional raw data supporting the findings of this study are available on request from the corresponding author, Marit Jalink (m.jalink@lumc.nl).
The full-text version of this article contains a data supplement.