Key Points
Brentuximab vedotin plus AD without radiation is an effective therapy for nonbulky limited-stage HL, with a favorable toxicity profile.
Risk of neutropenia, neutropenic fever, and grade ≥2 peripheral neuropathy appear significantly reduced compared with BV-AVD.
Abstract
ABVD (Adriamycin, bleomycin, vinblastine, dacarbazine) with or without radiation has been the standard treatment for limited-stage Hodgkin lymphoma (HL) but carries risks of bleomycin lung injury and radiation toxicity. Brentuximab vedotin (BV) is approved with AVD for stage III-IV HL, but carries increased risks of peripheral neuropathy (PN) and neutropenic fever, likely due to overlapping toxicity between BV and vinblastine. We therefore evaluated BV in combination with AD for 4 or 6 cycles based on interim positron emission tomography response. Thirty-four patients with nonbulky stage I-II HL were enrolled. Risk was early favorable in 53% and unfavorable in 47%. The overall and complete response rates (CRRs) were 100% and 97%, respectively, with a 5-year progression-free survival (PFS) of 91%. No differences in outcome were observed based on stage (I vs II) or risk status (early favorable vs unfavorable). The most common adverse events were nausea (85%), peripheral sensory neuropathy (59%), and fatigue (56%). There were no cases of grade-4 neutropenia or neutropenic fever, and no patient received granulocyte-colony stimulating factor. Most cases of PN were grade 1, and no patient experienced grade ≥3 PN. BV-AD produced a high CRR and durable PFS with most patients requiring 4 cycles of therapy. Compared with BV-AVD, the toxicity profile appeared improved, with predominantly grade 1 reversible PN and no case of grade 4 neutropenia or neutropenic fever. This regimen warrants further study in HL and may serve as a backbone for the addition of novel agents. This trial is registered on clinicaltrials.gov (NCT02505269).
Introduction
Limited-stage classical Hodgkin lymphoma (cHL) is a highly curable disease with >85% of patients being cured with ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine), with or without radiation.1-5 Combined modality therapy (CMT) had been the historic standard of care for limited-stage disease, but chemotherapy alone has emerged as a preferred approach in patients without bulky disease with the goal of avoiding the long-term risks of radiation therapy, most notably secondary malignancies and heart and lung disease.6-11 ABVD also carries risks of serious toxicities, most notably bleomycin lung injury, which can rarely be fatal, prompting consideration of non–bleomycin-containing approaches for patients with cHL.12 Regimens that reduce or eliminate bleomycin entirely have emerged as standard therapies in the management of advanced-stage HL,13,14 but bleomycin remains a component of all current standard regimens in the treatment of patients with nonbulky limited-stage disease. A study by the German Hodgkin Lymphoma study group evaluated the omission of bleomycin, dacarbazine, or both from the ABVD regimen in patients receiving CMT for limited-stage cHL, but elimination of either dacarbazine (ABV), bleomycin (AVD), or both (AV) were each associated with inferior progression-free survival (PFS), with omission of dacarbazine being the most detrimental.15 One appealing approach would be to substitute a highly active targeted therapy for bleomycin in the ABVD regimen. Brentuximab vedotin (BV) is an anti-CD30 antibody-drug conjugate, US Food and Drug Administration approved as a single agent for relapsed cHL and in combination with AVD as initial therapy for stage III to IV cHL. We previously conducted a phase 2 trial of BV in combination with AVD for 4 to 6 cycles without bleomycin and radiation therapy for patients with nonbulky limited-stage cHL and observed encouraging efficacy with a complete response rate (CRR) of 100% and 3-year PFS of 94%16 but with elevated rates of peripheral neuropathy (PN) and neutropenic fever, the latter prompting an amendment to the study to include primary prophylaxis with granulocyte-colony stimulating factor (G-CSF). This observation was echoed in the ECHELON-1 randomized trial of BV-AVD vs ABVD in advanced-stage cHL, which resulted in improvement in both PFS and overall survival (OS) but at the cost of significant increase in PN and neutropenic fever, also resulting in the mandated inclusion of prophylactic white blood cell growth factor.14,17,18
We hypothesized that the excess toxicity associated with the BV-AVD regimen results from the overlapping mechanisms of action for BV and vinblastine, both of which target the microtubule and are known to be associated with neuropathy and myelosuppression. We therefore conducted a phase 2 trial, evaluating BV plus AD without bleomycin, vinblastine, or planned consolidative radiation therapy in patients with nonbulky limited-stage cHL.
Patients and methods
Eligibility criteria
Patients aged ≥18 years with previously untreated cHL were eligible for this study. Patients had to have nonbulky stage I or II disease (A or B), with bulk defined as any site >10 cm in maximal dimension as measured on computed tomography (CT) scan. Laboratory parameters included absolute neutrophil count ≥ 1000/μL, platelet count ≥ 100 000/μL, creatinine ≤ 2 mg/dL, total bilirubin ≤ 2 mg/dL, and aspartate aminotransferase/alanine aminotransferase ≤ 2.5-times institutional upper limit of normal. Additional eligibility criteria included Eastern Cooperative Oncology Group performance status of ≤2 and left ventricular ejection fraction within the institutional normal limits.
Study design and treatment
In this multicenter, open label phase 2 study, all patients were treated with BV 1.2 mg/kg IV plus doxorubicin 25 mg/m2 IV and dacarbazine 375 mg/m2 IV, all administered on days 1 and 15 of each 28-day cycle. Patients received a total of 4 or 6 cycles based on interim positron emission tomography (PET)/CT response. No prophylactic G-CSF or pegylated G-CSF was included as primary prophylaxis but was allowed at investigator discretion if a patient developed neutropenic fever. Brentuximab dose was reduced to 0.8 mg/kg for grade ≥2 PN and further reduced to 0.6 mg/kg for persistent toxicity, if needed. Interim PET/CT was performed between days 8 and 14 of cycle 2 (PET-2). Responding patients with an interim PET complete response (CR) defined as Deauville score 1 to 3 received 2 additional cycles to complete 4 total cycles of BV-AD; patients positive for PET-2, defined as Deauville 4 to 5, received 4 additional cycles to complete 6 total cycles. End-of-treatment PET/CT was performed 6 weeks after completion of therapy. All PET/CT scans were centrally reviewed. No radiation therapy was planned for patients in CR at the end of treatment. Following completion of therapy, patients had clinical and laboratory evaluations every 3 months and CT scans of chest, abdomen, and pelvis every 6 months for 2 years. After 2 years, clinical evaluations were performed every 6 months through year 5 and then once annually, and imaging was performed at the discretion of the treating investigator. The primary objective was to investigate the clinical activity of BV plus AD in nonbulky limited-stage cHL. The secondary objective was to evaluate the safety of the combination. The study was approved by the institutional review boards of all participating institutions, was conducted in accord with the Declaration of Helsinki, and registered on clinicaltrials.gov (NCT02505269).
Statistics
The primary end point of this study was CRR as defined by the revised International Working Group Criteria19 with a calculated sample size of 34 patients. The regimen would be considered worthy of further study if at least 31 (91%) of evaluable patients achieve CR. The study has 91% power and 0.09 1-sided type I error to test a CRR of 95% vs a CRR of 81%. With 34 eligible patients, the 90% confidence interval (CI) for the true response rate will be no wider than 30%. Secondary end points include overall response rate (ORR), PFS, and OS. OS is measured from date of study entry to date of death from any cause. PFS is measured from date of study entry to date of progression or death from any cause. All patients are considered evaluable for toxicity and efficacy from the time of their first treatment. We report responses to study treatment as proportions with 90% exact binomial CIs. Continuous measures summarized as median and range, and categorical variables are summarized as proportions. Response outcomes and other categorical variables were tested for association with continuous and other categorical variables using Wilcoxon rank-sum (or Kruskal-Wallis for 3 or more groups) or Fisher exact tests, respectively. Time-to-event end points were estimated using the method of Kaplan and Meier with 95% CIs calculated using method of variance estimation by Greenwood. Median follow-up is calculated using the reverse Kaplan-Meier method. Statistical analyses were performed using R, version 4.1.2 (2021-11-01).
Results
Patients
Thirty-four patients were enrolled with a median age of 36 years (range 18-63 years). Approximately two-thirds of the patients were female, and the most common stage was IIA in 29 patients (85%), followed by IA in 3 patients (9%) and IB and IIB in 1 patient each. Patients were classified as early favorable or early unfavorable in 18 (53%) and 16 (47%) cases, respectively, with unfavorable defined as the presence of any of the following risk factors: involvement of ≥3 nodal regions, extranodal disease, or elevated erythrocyte sedimentation rate (≥50 mm/h without B symptoms or ≥30 mm/h with B symptoms). Histologic subtypes included nodular sclerosis (n = 17, 50%), lymphocytic rich (n = 3, 9%), mixed cellularity (n = 1, 3%), and cHL not otherwise specified (n = 13, 38%). Baseline characteristics are summarized in Tables 1 and 2.
Patient characteristics (N = 34)
| Characteristic . | Participants . |
|---|---|
| Age, median (range), y | 36 (18-63) |
| Age ≥60, n (%), y | 2 (6) |
| Female/male | 23/11 |
| Stage, n (%) | |
| IA | 3 (9) |
| IB | 1 (3) |
| IIA | 29 (85) |
| IIB | 1 (3) |
| ECOG PS, n (%) | |
| 0 | 30 (88) |
| 1 | 4 (12) |
| Risk, n (%) | |
| Early favorable | 18 (53) |
| Early unfavorable | 16 (47) |
| Histology, n (%) | |
| Nodular sclerosis | 17 (50) |
| Lymphocyte rich | 3 (9) |
| Mixed cellularity | 1 (3) |
| Classical NOS | 13 (38) |
| Characteristic . | Participants . |
|---|---|
| Age, median (range), y | 36 (18-63) |
| Age ≥60, n (%), y | 2 (6) |
| Female/male | 23/11 |
| Stage, n (%) | |
| IA | 3 (9) |
| IB | 1 (3) |
| IIA | 29 (85) |
| IIB | 1 (3) |
| ECOG PS, n (%) | |
| 0 | 30 (88) |
| 1 | 4 (12) |
| Risk, n (%) | |
| Early favorable | 18 (53) |
| Early unfavorable | 16 (47) |
| Histology, n (%) | |
| Nodular sclerosis | 17 (50) |
| Lymphocyte rich | 3 (9) |
| Mixed cellularity | 1 (3) |
| Classical NOS | 13 (38) |
ECOG, Eastern Cooperative Oncology Group; NOS, not otherwise specified; PS, performance status.
Response (N = 34)
| . | Cycle 2 . | End of treatment . |
|---|---|---|
| Overall response, N (%; 95% CI) | 34 (100%; 92%-100%) | 34 (100%; 76.3%-98.1%) |
| CR, N (%; 95% CI) | 32 (94%; 83%-99%) | 33 (97%; 87%-100%) |
| PR, N (%; 95% CI) | 2 (6%; 1%-17%) | 1 (3%; 0%-13%) |
| . | Cycle 2 . | End of treatment . |
|---|---|---|
| Overall response, N (%; 95% CI) | 34 (100%; 92%-100%) | 34 (100%; 76.3%-98.1%) |
| CR, N (%; 95% CI) | 32 (94%; 83%-99%) | 33 (97%; 87%-100%) |
| PR, N (%; 95% CI) | 2 (6%; 1%-17%) | 1 (3%; 0%-13%) |
Efficacy
All 34 patients completed the protocol therapy and were evaluable for response. After 2 cycles of brentuximab-AD, the ORR was 100% (34/34), and the CRR was 94% (32/34). Thirty-two patients (94%) who achieved PET-2 CR received 4 total cycles of therapy, whereas the 2 patients (6%) with interim partial response (PR) received 6 total cycles according to the protocol. Both PET-2 PRs had a Deauville score of 4. At the end of treatment, the ORR was 100%, and 33 patients were in CR (97%). Of the 2 patients with interim PR, 1 completed 6 total cycles and achieved CR, which has been durable, whereas the other patient completed 6 cycles and was in PR at end of treatment and subsequently progressed.
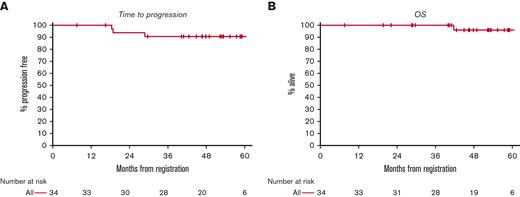
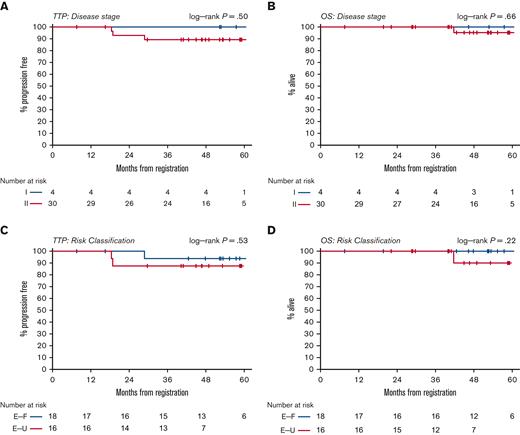
With a median follow-up of 53 months, the median PFS and OS have not been reached (Figure 1). The estimated 5-year PFS is 91% (95% CI, 73-97). No PFS differences were observed among patients in stage I and II or between patients with early favorable and unfavorable risk disease (Figure 2). The estimated 5-year OS was 96% (95% CI, 75-99). There was 1 death in a patient who achieved interim and end-of-treatment CR and subsequently relapsed but died following autologous stem cell transplant due to cardiac cause attributed as unrelated to lymphoma or the study treatment.
PFS (A) and OS (B) for all patients. The median length of follow-up is 53 months. The median PFS and OS have not been reached.
PFS (A) and OS (B) for all patients. The median length of follow-up is 53 months. The median PFS and OS have not been reached.
PFS and OS by stage (panels A and B) and risk group (panels C and D). TTP, time to progression; E-F, early favorable; E-U, early unfavorable.
PFS and OS by stage (panels A and B) and risk group (panels C and D). TTP, time to progression; E-F, early favorable; E-U, early unfavorable.
Safety
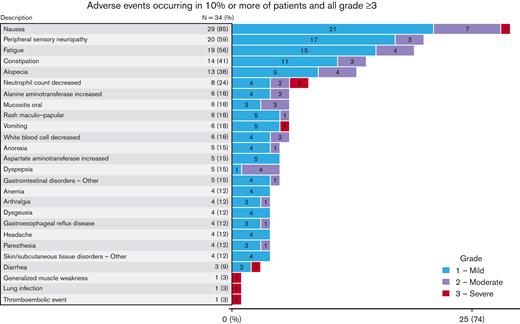
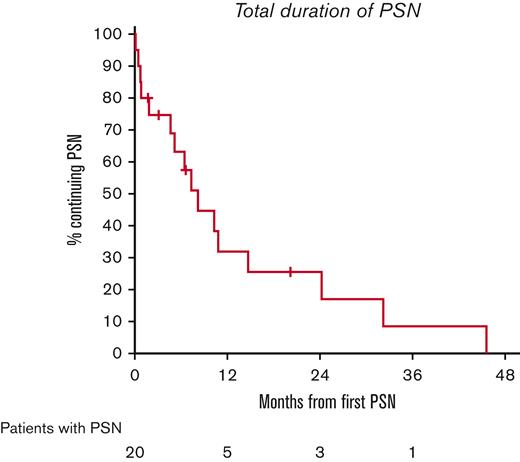
All 34 patients were evaluable for toxicity. The highest grade of toxicity was grade 1 in 11 patients (32%) and grade 2 in 17 patients (50%). Grade 3 was the highest grade of toxicity in only 5 patients (15%) and no grade 4 or grade 5 toxicities were observed. Adverse events are summarized in Figure 3. The most common toxicities of any grade were nausea (29/34, 85%), PN (20/34, 59%), fatigue (19/34, 56%), constipation (14/34, 41%), alopecia (13/34, 38%), and neutropenia (8/34, 24%). Severe toxicities were rare and included diarrhea, generalized muscle weakness, lung infection, nausea, vomiting, and thromboembolic event, all in 1 patient each. There was no grade 4 neutropenia, no case of neutropenic fever, and no patient received G-CSF support. Most PN was grade 1 (n = 17, 85%), with the remaining cases being grade 2 (n = 3, 15%). No grade 3 or 4 PN was observed. For all cases of PN, the median time to resolution was 6.4 months (95% CI, 0.8-10.8), and only 1 patient had persistent neuropathy at last follow-up (grade 1, Figure 4). Three patients required dose reductions of BV. All reductions were due to grade 2 PN in cycle 4; there were no dose reduction of the other agents.
There was a total of 6 serious adverse events reported as possibly related to study treatment, occurring in 4 discrete patients. These were 1 event each of arthralgia, upper respiratory tract infection, nausea, vomiting, diarrhea, and pulmonary embolism.
Discussion
Nonbulky limited-stage cHL is a highly curable disease, but the historic standard of CMT confers short- and long-term risks, including bleomycin lung injury and late complications of radiation therapy such as secondary malignancies and heart disease. Chemotherapy alone has emerged as an acceptable alternative to chemoradiation in nonbulky limited-stage disease based on multiple prospective clinical trials demonstrating favorable outcomes with ABVD alone.3,8,20,21 ABVD, however, continues to expose patients with low-risk cHL to the potential morbidity and mortality of bleomycin lung toxicity. Compared with ABVD in advanced-stage disease, substitution of bleomycin with the anti-CD30 antibody-drug conjugate BV demonstrated superior PFS and OS14,18 and demonstrated encouraging efficacy in a phase 2 trial in nonbulky limited-stage disease16 but at the cost of significantly increased PN, neutropenia, and neutropenic fever. The BV-AVD regimen requires routine prophylactic growth factor support and has been associated with ongoing PN in nearly 1 in 5 patients with 5 years of follow-up.18 Given that the excess neuropathy and hematologic toxicity of the BV-AVD regimen likely derives from the overlapping mechanism of action and toxicity profile of BV and vinblastine, both of which target the microtubule, we evaluated the BV-AD regimen, which omits both bleomycin and vinblastine, in patients with nonbulky limited-stage cHL. The study met its primary end point for efficacy with a CRR of 97% and estimated PFS of 94% and 91% at 2 and 5 years, respectively, with similar efficacy among patients in stage I and II, as well as in patients with both early favorable and unfavorable risk disease. The toxicity profile was also encouraging with only 15% of the patients having grade 3 toxicities of any kind and no grade 4 or 5 adverse events. The risks of neutropenia, neutropenic fever, and grade ≥2 PN were markedly reduced compared with what has been observed in prospective trials of BV-AVD. In our study, no patient received white blood cell growth factor and the overall incidence of neutropenia was low at 24% for any grade, with only 2 patients having grade 3 neutropenia and no grade 4 events. There were also no cases of neutropenic fever. Regarding PN, 59% of the patients reported any grade of PN, which was predominantly grade 1, and there were no grade 3 or 4 cases reported; all but 1 patient has had complete resolution at last follow-up, showing this to be a manageable and reversible toxicity associated with BV-AD. These results suggest slightly higher incidence of PN with BV-AD than would be expected with standard ABVD, which is reported to cause PN in 43% of the patients.18 However, our results compare quite favorably with our prior trial of BV-AVD in the same patient population where PN of any grade occurred in 79% of the patients, and was grade ≥2 in 32% of the patients. Neutropenic fever was also eliminated compared with 35% with BV-AVD.16 These toxicity data also compare favorably with BV-AVD in the ECHELON-1 trial, where any-grade PN occurred in 67% of the patients and was grade ≥2 in 8% of the patients.17,18 Dose-level reductions in our study were slightly different than used in ECHELON-1. In the BV-AD regimen, BV was reduced from 1.2 to 0.9 mg/kg and further reduced to 0.6 mg/kg if needed due to PN. In ECHELON-1, only 1 dose reduction to 0.9 mg/kg was allowed. The incidence of neutropenic fever on BV-AVD was 19% in ECHELON-1, compared with 0% in our study of BV-AD. Interim results of a PET-adapted randomized trial evaluating BV-AVD vs ABVD in early unfavorable cHL also reported excess toxicity in the BV-AVD arm with 74% of the patients on BV-AVD experiencing grade ≥3 adverse events after only 2 cycles of therapy, compared with 56% in the ABVD arm.22 In our study of BV-AD, only 15% of the patients had grade ≥3 adverse events after completing all cycles of therapy.
The 5-year PFS for BV-AD in our trial appears comparable with larger studies of ABVD alone in nonbulky limited-stage cHL, which have consistently demonstrated durable PFS rates of 87% to 91%.5,8,20,21,23 Modestly improved PFS has been demonstrated in these trials for inclusion of consolidative radiation, even in patients who are interim PET-negative, but without improvement in OS, and therefore, chemotherapy alone is often favored in this population in order to reduce the risk of late radiation-associated toxicities. It is important to recognize, however, that modern radiation fields are smaller than used in historic studies of CMT, and follow-up of contemporary studies using CMT is too short to see the true incidence of secondary malignancies, which is almost certainly improved relative to historic radiation approaches.
The primary limitation of our study is the small sample size of 34 patients and the noncomparative nature of this phase 2 trial. We did include similar numbers of early favorable and unfavorable patients, although only 1 patient had stage IIB disease, and no patient had bulky mediastinal disease due to the eligibility criteria; therefore, our data in this subset cannot be directly compared with other studies which included patients with all early unfavorable risk characteristics. Our study also included very few older patients with HL. A larger study would therefore be required to understand generalizability of our results. Nonetheless, this study does demonstrate encouraging efficacy and safety of BV-AD in nonbulky limited-stage cHL and allows omission of historic and potentially toxic standards of bleomycin, vinblastine, and radiation therapy. These data also question the role of vinblastine in the BV-AVD regimen, in which dual targeting of the microtubule results in excess neuropathy and neutropenia, which appears to be mitigated by eliminating vinblastine in favor of BV. With a 5-year PFS of ∼91% for this and other radiation-sparing approaches, there is still room to optimize efficacy of initial therapy in patients with nonbulky limited-stage cHL.
We believe that these data identify BV-AD as an appealing regimen worthy of further study in a larger trial in patients with limited-stage cHL, as well as potentially in patients with low-risk advanced-stage disease, in which the overlapping toxicities of BV and vinblastine can be significant. BV-AD may also serve as an appealing backbone for the addition of targeted therapies in future trials of initial therapy in cHL, with one such study already underway (NCT03646123).
Acknowledgments
The authors thank the participating patients and their families as well as the dedicated research nurses, coordinators, and data managers who assisted with this study.
Authorship
Contribution: J.S.A., E.B., R.R., J.A.B., T.T., L.S., F.L., P.A., B.S., E.J., R.M., E.T., S.M., V.P., A.S.L., and C.M.B. contributed to the study design, patient recruitment, data collection, and data analysis; J.S.A. wrote the manuscript; and all authors commented on and revised the manuscript.
Conflict-of-interest disclosure: Funding support and investigational product were supplied by Seagen, Inc. J.S.A. reports research funding from Bristol Myers Squibb (BMS) and Seagen and consultancy for AbbVie, AstraZeneca, BeiGene, BMS, Century Therapeutics, Epizyme, Genentech, Genmab, Incyte, Janssen, Kite Pharma, Kymera, Lilly, MorphoSys, Mustang Bio, Ono Pharma, and Regeneron. L.S. reports research funding from EUSA Pharmaceuticals and Kyowa Kirin and consultancy for Daiichi Sankyo, Dren Bio, EUSA Pharmaceuticals, Kyowa Kirin, and Secura Bio. P.A. reports research funding from Merck, BMS, Affimed, Adaptive, Roche, Tensha, Otsuka, Sigma Tau, Genentech, IGM, and Kite Pharma; consultancy for Merck, BMS, Pfizer, Affimed, Adaptive, Infinity, ADC Therapeutics, Celgene, MorphoSys, Daiichi Sankyo, Miltenyi, Tessa, Genmab, C4, Enterome, Regeneron, Epizyme, AstraZeneca, Genentech, and Xencor; and honoraria from Merck and BMS. C.M.B reports consultancy for Seagen. The remaining authors declare no competing financial interests.
Correspondence: Jeremy S. Abramson, Massachusetts General Hospital Cancer Center, 55 Fruit St, Boston, MA 02114; e-mail: jabramson@mgh.harvard.edu.
References
Author notes
Data are available on request from the corresponding author, Jeremy S. Abramson (jabramson@mgh.harvard.edu).