TO THE EDITOR:
B-cell maturation antigen (BCMA) is a novel target for immune-oncology therapies because of its preferential expression on the surface of plasma cells and overexpression and activation in multiple myeloma (MM).1 BCMA is expressed on the cell surface as a 184-amino acid single-transmembrane protein and undergoes proteolytic shedding into a truncated, soluble form (sBCMA; supplemental Figure 1). Although BCMA is a validated therapeutic target in MM, sBCMA may be a significant covariate affecting the pharmacokinetics (PK) of some BCMA-targeted therapies, such as belantamab mafodotin, by reducing binding of these agents to membrane-bound BCMA and, in turn, reducing their efficacy.2 It is unclear if this effect will broadly alter the efficacy of all BCMA-targeting agents. Additionally, it is unclear whether sBCMA levels are associated with baseline tumor burden and treatment response independent of therapeutic target.
The use of bispecific antibody constructs is emerging as an immunotherapy-based approach for MM.1 Bispecific antibodies have dual-antigen specificity and a demonstrated ability to redirect the T-cell response to tumor surface antigens, resulting in cytotoxicity of malignant cells.3 G protein–coupled receptor family C group 5 member D (GPRC5D), which is expressed on malignant plasma cells in patients with MM,4 is another potential therapeutic target. Levels of GPRC5D expression are correlated with plasma cell burden and high-risk genetic aberrations.5 Teclistamab (JNJ-64007957) and talquetamab (JNJ-64407564) are CD3-bispecific antibodies that recruit CD3+ T cells to BCMA+ and GPRC5D+ MM cells, respectively. Both antibodies are under investigation as monotherapies and in combination with other agents.
We evaluated sBCMA levels in patients with RRMM from the first-in-human, phase 1, open-label, multicenter studies of teclistamab (MajesTEC-1; NCT03145181) and talquetamab (MonumenTAL-1; NCT03399799).6,7 Study designs are available in supplemental Methods. Both studies were conducted in accordance with the principles that originate in the Declaration of Helsinki and Good Clinical Practice guidelines of the International Conference on Harmonisation. All patients provided written informed consent, and all relevant study documents (eg, protocols and amendments and informed consent forms) were approved by the independent ethics committee or review board at each study site.
sBCMA data were quantitatively analyzed relative to patient response to therapy, percentage of bone marrow plasma cells (BMPCs), cytogenetic risk, and PK data. Patient response was determined by the International Myeloma Working Group response criteria.8-10 For these analyses, 147 patients (MajesTEC-1) and 153 patients (MonumenTAL-1) had evaluable sBCMA baseline data. Of those, 96 and 99 patients treated with teclistamab and talquetamab, respectively, had evaluable data on cycle 3, day 1 (C3D1). The most active doses of teclistamab were weekly IV doses of 0.27 and 0.720 mg/kg and weekly subcutaneous doses of 0.72, 1.5, and 3.0 mg/kg; the recommended phase 2 dose (RP2D) was identified as weekly subcutaneous 1.5 mg/kg teclistamab following 0.06 and 0.3 mg/kg step-up doses.6 The most active doses of talquetamab were weekly IV doses of 60 and 180 μg/kg and weekly subcutaneous doses of 405 and 800 μg/kg; the first RP2D was identified as weekly subcutaneous 405 μg/kg talquetamab following 10 and 60 μg/kg step-up doses.7
Most patients who responded to teclistamab (88% [50 of 57]) or talquetamab (98% [49 of 50]) had a reduction in sBCMA from baseline to C3D1 (supplemental Figure 2). The degree of sBCMA reduction corresponded to the depth of response, with the greatest reductions from baseline observed for patients who achieved a complete response (CR) or stringent complete response (sCR) for teclistamab (median [min; max] −92.8% [−95.2; −66.9] for CR and −94.5% [−98.6; −90.4] for sCR) and a very good partial response (VGPR) or partial response (PR) for talquetamab (median [min; max] −92.3% [−98.7; −48.1] for VGPR and −89.7% [−99.3; 37.3] for PR) (Figure 1). Conversely, among nonresponders, 80% (33 of 41) of those who received teclistamab and 49% (24 of 49) of those who received talquetamab had increased sBCMA levels from baseline to C3D1. Among teclistamab nonresponders, 1 patient with minimal response (MR), 14 of 22 patients with stable disease (SD), and all 18 patients with progressive disease (PD) had increased sBCMA levels (median [min; max] 17.7% [−81.3; 2620] for SD and 138% [10.5; 374] for PD). Among talquetamab nonresponders, 1 of 5 patients with MR, 12 of 31 patients with SD, and 11 of 13 patients with PD had increased sBCMA levels (median [min; max] −7.85% [−99.1; 167] for SD and 32.4% [−12.6; 176] for PD). Some nonresponders may have responded to teclistamab or talquetamab after the analysis cutoff date.
Change in sBCMA levels on cycle 3, day 1 vs baseline according to depth of response to bispecific treatment. (A) sBCMA levels relative to depth of response for teclistamab. Patients received weekly intravenous (0.0003-0.72 mg/kg) or subcutaneous (80-3.0 mg/kg) doses of teclistamab. Cycle 3, day 8 data were used for 3 patients who had missing cycle 3, day 1 data. (B) sBCMA levels relative to depth of response for talquetamab. Cycle 3, day 8 data were used for 2 patients who had missing cycle 3, day 1 data. Data are not shown for 3 patients with sBCMA change >500% (508% [SD], 1201% [SD], and 2620% [SD]). Median percentage sBCMA change (minimum; maximum) shown. IV, intravenous; MR, minimal response; N/A, not applicable; PD, progressive disease; SC, subcutaneous; SD, stable disease.
Change in sBCMA levels on cycle 3, day 1 vs baseline according to depth of response to bispecific treatment. (A) sBCMA levels relative to depth of response for teclistamab. Patients received weekly intravenous (0.0003-0.72 mg/kg) or subcutaneous (80-3.0 mg/kg) doses of teclistamab. Cycle 3, day 8 data were used for 3 patients who had missing cycle 3, day 1 data. (B) sBCMA levels relative to depth of response for talquetamab. Cycle 3, day 8 data were used for 2 patients who had missing cycle 3, day 1 data. Data are not shown for 3 patients with sBCMA change >500% (508% [SD], 1201% [SD], and 2620% [SD]). Median percentage sBCMA change (minimum; maximum) shown. IV, intravenous; MR, minimal response; N/A, not applicable; PD, progressive disease; SC, subcutaneous; SD, stable disease.
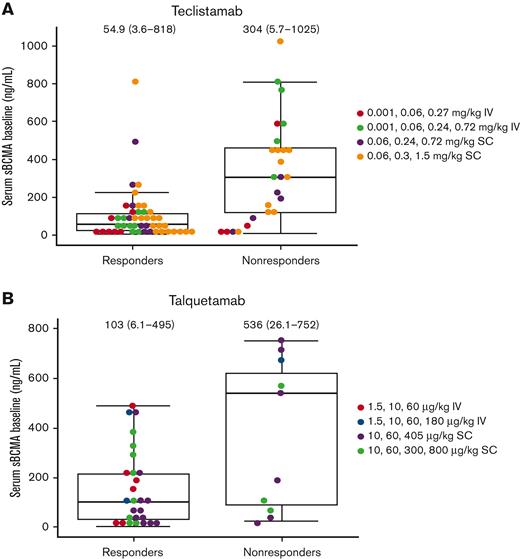
Patients with baseline sBCMA ≥200 ng/mL appeared to respond to active doses of teclistamab or talquetamab (Figure 2). One patient with a high baseline level of sBCMA (∼800 ng/mL), and extramedullary disease responded to teclistamab at the RP2D.
Baseline sBCMA and response to treatment. (A) Teclistamab. (B) Talquetamab. Data cutoff dates for response were 24 December 2020 (MajesTEC-1) and 2 January 2021 (MonumenTAL-1); median (range) shown. Patients were categorized as responders or nonresponders based on their best responses. IV, intravenous; SC, subcutaneous.
Baseline sBCMA and response to treatment. (A) Teclistamab. (B) Talquetamab. Data cutoff dates for response were 24 December 2020 (MajesTEC-1) and 2 January 2021 (MonumenTAL-1); median (range) shown. Patients were categorized as responders or nonresponders based on their best responses. IV, intravenous; SC, subcutaneous.
To assess the correlation between sBCMA and tumor burden, sBCMA levels were combined across both studies. Baseline sBCMA levels correlated with the percentage of BMPC (supplemental Figure 3). In addition, patients with extramedullary plasmacytomas with low levels of BMPCs (<10%) tended to have high levels of sBCMA (≥400 ng/mL).
In both MajesTEC-1 and MonumenTAL-1, baseline sBCMA levels were similar in patients with high-risk cytogenetics (median [range]: 100 [8.1-600] and 226 [21.0-1259] ng/mL, respectively) and in those with standard-risk cytogenetics (median [range]: 79.6 [3.6-1025] and 127 [4.5-2586] ng/mL, respectively) (supplemental Figure 4). By C3D1, teclistamab and talquetamab modulated sBCMA levels in patients with standard-risk cytogenetics (median [min; max] percent change from baseline: 65.4% [−100; 2620] for teclistamab and 94.5% [−98.9; 167] for talquetamab) and in patients with high-risk cytogenetics (median [min; max]: 54.7% [−100; 354] for teclistamab and 83.5% [−99.0; −41.6] for talquetamab) (supplemental Figure 4). No statistically significant differences were observed in sBCMA levels between cytogenetic risk groups at either time point for teclistamab or talquetamab.
In a population PK analysis, baseline sBCMA was not identified as a significant covariate affecting teclistamab exposure. Analysis of teclistamab trough levels following the first treatment dose and multiple dosing (C3D1) indicates that sBCMA levels did not alter teclistamab exposure (supplemental Figure 5). Additionally, binding data indicate that teclistamab binds to full-length BCMA (FC-BCMA Kd: 150-200 pM) with an affinity approximately fivefold to 10-fold higher than sBCMA (sBCMA Kd: 930-1350 pM) (supplemental Figure 6).
Although BCMA-targeted therapies have been approved, some studies have indicated that elevated sBCMA levels may interfere with the activity of these agents.11,12 We found that baseline sBCMA was not a significant covariate affecting teclistamab exposure, suggesting that sBCMA may not act as a sink for teclistamab, and high levels of sBCMA are unlikely to diminish the clinical activity of teclistamab. Preclinical studies have also indicated that incubation with sBCMA (up to 166 nM) had minimal effect on the cytotoxicity potential of teclistamab.13
The value of sBCMA as a marker of treatment response seems to be increasing. In this analysis, most patients who responded to teclistamab or talquetamab had a reduction in sBCMA levels. The degree of reduction corresponded to the depth of response; patients who achieved ≥CR following treatment demonstrated nearly 100% reductions in sBCMA levels by C3D1. These findings are consistent with studies of BCMA-targeted chimeric antigen receptor T-cell therapies in which greater declines in sBCMA levels were associated with greater treatment response.14-16 Similar findings were observed at the RP2D in MajesTEC-1.17 sBCMA also seemed to be a marker of responses to talquetamab, suggesting that it is a useful potential surrogate marker of response irrespective of the target.
Our findings suggest that sBCMA might provide a comprehensive marker for tumor burden, as evidenced by the finding that baseline sBCMA levels correlated with the percentage of BMPC and with extramedullary plasmacytomas. These findings support not only preclinical data that suggest that BCMA is primarily expressed on plasmablasts and plasma cells and enhances the survival of these cells18-20 but also analyses of patients with MM that showed that higher sBCMA levels were associated with poorer clinical outcomes.21,22
Acknowledgments: The authors thank the patients who participated in the study and their families and caregivers, the physicians and nurses who cared for patients and supported this clinical trial, the staff members at the study sites, and the staff members involved in data collection and analyses.
These studies were funded by Janssen Research & Development, LLC. Medical writing support was provided by Valerie Kinchen and Joanna Bloom of Eloquent Scientific Solutions and funded by Janssen Global Services, LLC.
Contribution: S.G. conceived and designed the study and analyzed and interpreted data; S.X.W.L. designed, analyzed, and interpreted data; K.P., D.V., T.C., and D.F. performed experiments and contributed to data generation and interpretation; X.M. and Y.C. analyzed data and contributed to data interpretation; R.V. conceived and contributed to the project design; J.I., Y.E., J.S., and J.D.G. designed the clinical studies and provided study oversight; J.D.G., T.S., A.B., B.W.H., and J.R. managed the clinical study and interpreted results; and all authors reviewed and revised the manuscript, approved the final version, and agreed to submit the manuscript for publication.
Conflict-of-interest disclosure: D.F., Y.C., and J.R. are former employees of Janssen Research & Development and may own stock/stock options in Johnson & Johnson. The remaining authors are currently employees of Janssen Research & Development and may own stock/stock options in Johnson & Johnson.
Correspondence: Suzette Girgis, Janssen Research & Development, 920 Route 202, Raritan, NJ 08869; e-mail: sgirgis@its.jnj.com.
References
Author notes
Data from the analyses reported herein were previously presented at the Annual Meeting of the American Society of Clinical Oncology (ASCO), 4-8 June 2021.
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
The full-text version of this article contains a data supplement.

![Change in sBCMA levels on cycle 3, day 1 vs baseline according to depth of response to bispecific treatment. (A) sBCMA levels relative to depth of response for teclistamab. Patients received weekly intravenous (0.0003-0.72 mg/kg) or subcutaneous (80-3.0 mg/kg) doses of teclistamab. Cycle 3, day 8 data were used for 3 patients who had missing cycle 3, day 1 data. (B) sBCMA levels relative to depth of response for talquetamab. Cycle 3, day 8 data were used for 2 patients who had missing cycle 3, day 1 data. Data are not shown for 3 patients with sBCMA change >500% (508% [SD], 1201% [SD], and 2620% [SD]). Median percentage sBCMA change (minimum; maximum) shown. IV, intravenous; MR, minimal response; N/A, not applicable; PD, progressive disease; SC, subcutaneous; SD, stable disease.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/4/10.1182_bloodadvances.2022007625/7/m_blooda_adv-2022-007625-gr1.jpeg?Expires=1769097019&Signature=wLseONxci1BvT4fa73vrZACg2Kmk20F-P-p9H9U-f0DzPguf9ScPaC6xvgNbjx5i3Y7rHmviM2B4-lxUufD~Lov23vHSet93OWRzNKXg20HNEnGzN7nP2Ae49BWXFLQydlYOdPXJVubzDEFIfa5qR6XmmvlWHJWwRiip~E4csTjzM~CejQIEq7t-uvQgvmEmbNPT8wK9h~XbbccaB~cKTxuwdx20FVkGBh-s-4vHXDeKwcVmXwRg~2IhHAxpiBHLOGKWv525Jm4rllCce~NLyUt8K4lPUG9lDgOrcQLEnGxqM-F8xVP-4XOusvSqZKpbX~HGYER4~PrpWsyKeF6xRQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)