TO THE EDITOR:
Sickle cell anemia (SCA) is a hemoglobinopathy that causes major red blood cell (RBC) dysfunction; however, studies have also highlighted the significant role of polymorphonuclear cells in SCA pathophysiology.1-3 Patients with SCA have an increased leukocyte count at steady state and exhibit neutrophil activation markers,4 including increased expression of adhesion molecules5 such as the integrins Mac-1 (CD18/CD11b) and LFA-1 (CD18/CD11a),6 increased expressions of activation proteins such as CD64,7 and increased expression of aging markers such as CXCR4high/CD62Llow.8 Hydroxyurea (HU) may also benefit patients with SCA by suppressing neutrophil activation and correcting the dysregulated expression of adhesion markers on these cells.9
One of the most severe complications of SCA is cerebral vasculopathy (CV) as a result of large cerebral artery occlusion. In 1998, the Stroke Prevention (STOP) trial demonstrated that monthly blood transfusions could reduce the risk of stroke by 90% in children with SCA who have CV.10 However, there is great heterogeneity in the course of CV in patients who receive chronic transfusion therapy,11-13 even though they display a percentage of hemoglobin S (HbS) that is permanently below 40%. To decipher the reason for the persistence of CV in the majority of patients despite years of exchange transfusions (ETs), we investigated the impact of transfusion programs on neutrophil count, activation, and adhesion and compared these parameters in nontransfused patients with SCA. Finally, we investigated the effect of treatment with HU (when it is associated with transfusion programs) on the neutrophil profile.
We included patients with SCA age 2 to 18 years old with no acute complications (infection, vaso-occlusive crises) on the day of blood collection. Consent was obtained from all participants before inclusion. Patients had been included in the ET program because of CV for at least 12 months and received ETs either by erythrocytapheresis or by manual ET. Immunophenotypic characterization was performed by flow cytometry. Flow adhesion experiments were carried out on human endothelial cell monolayers, as previously described.14 Plasma levels of neutrophil elastase were estimated by using enzyme-linked immunosorbent assays, and inflammatory mediator quantification was performed by using the Luminex technique. Detailed methodology is described in supplementary Data.
Eighty-eight children with SCA undergoing treatment with ETs were included in the study, and their demographic and clinical characteristics are described in Table 1. No major adverse effects of the ET program were observed in this cohort, and good control of patients’ iron overload was maintained (median ferritinemia, 352 μg/L [IQR, 75-1061 μg/L]). For children receiving ET to prevent primary strokes (ie, patients with abnormal transcranial Doppler ultrasound with or without arterial stenosis on cerebral magnetic resonance angiography but without history of stroke), the evolution of CV under the ET program was favorable for 25%, whereas 67% had stabilized vascular lesions and 8% demonstrated progression of their CV, with an extension of stenosis and/or the occurrence of new arterial lesions. For those receiving ET to prevent secondary strokes (ie, patients with history of stroke), the evolution of CV under the ET program was favorable for only 5% (with a progressive resolution of the arterial stenosis and no relapse of stroke), whereas 71% had stabilized vascular lesions and no relapse of ischemic stroke and 24% had CV that progressed, with an extension of stenosis and/or the occurrence of new arterial lesions and/or the relapse of ischemic stroke (Table 1).
Characteristics of the cohort and evolution of clinical and biological parameters under the ET program
| . | Patients on the ET program . | Patients on the ET program who received HU therapy . | ||
|---|---|---|---|---|
| N (%) . | Median (IQR) . | N (%) . | Median (IQR) . | |
| No. of patients | 48 | 40 | ||
| Age (y) | 11.67 (8.13-16.64) | 9.48 (6.63-13.54)†† | ||
| Duration of the ET program (y) | 4.81 (1.96-8.99) (ns) | 3.72 (1.84-6.17) (ns) | ||
| Characteristics of CV before the start of the ET program | ||||
| Abnormal TCD and normal cerebral MRA without stroke | 2 (4.2) | 10 (25) | ||
| Stenosis of intracranial arteries on MRA without stroke | 25 (52) | 16 (40) | ||
| Stenosis of cervical arteries on MRA without stroke | 7 (14.6) | 7 (17.5) | ||
| Initial stroke | 14 (29.2) | 7 (17.5) | ||
| Evolution of CV for patients without initial stroke (primary prevention) | ||||
| Improvement | 11 (32) | 6 (18) | ||
| Stagnation | 22 (65) | 23 (70) | ||
| Worsening | 1 (3) | 4 (12) | ||
| Evolution of CV for patients with history of stroke (secondary prevention) | ||||
| Improvement | 1 (7) | 0 (0) | ||
| Stagnation | 10 (72) | 5 (71) | ||
| Worsening | 3 (21) | 2 (29) | ||
| Hemoglobin level (g/dL) | 9.1 (8.7-9.5) | 8.6 (8.4-9.2) (ns) | ||
| Median hemoglobin S (%) | ||||
| Before the ET session | 35.3 (30.4-41.2) | 35 (29-38.4) | ||
| After the ET session | 13 (11.7-15.5) | 14 (11.8-16) | ||
| Leukocyte count (109/L) | ||||
| Before ET program | 12.03 (10.09-14.47)** | 12.31 (10.85-15.10)*** | ||
| During ET program | 11.36 (9.32-12.38) | 9.46 (7.24-11.42)† | ||
| Neutrophil count (109/L) | ||||
| Before ET program | 4.98 (3.69-6.52) (ns) | 5.40 (4.00-7.44)** | ||
| During ET program | 6.19 (4.66-7.71) | 4.86 (361-6.94)†† | ||
| Monocyte count (109/L) | ||||
| Before ET program | 10.8 (5.77-1.43) (ns) | 1.10 (7.66-1.61)* | ||
| During ET program | 1.24 (8.94-1.59) | 9.25 (6.74-1.12)††† | ||
| Lymphocyte count (109/L) | ||||
| Before ET program | 5.16 (4.01-8.21)*** | 5.19 (4.18-5.94)** | ||
| During ET program | 2.96 (2.12-7.51) | 2.71 (2.19-3.58)† | ||
| Platelet count (109/L) | ||||
| Before ET program | 363.0 (312.5-469.5) (ns) | 426.0 (325.2-506.0) (ns) | ||
| During ET program | 397.5 (281.2-483.2) | 361.0 (325.0-503.0) | ||
| Lactate dehydrogenase level (U/L) | ||||
| Before ET program | 950 (590-1209)*** | 1081 (625-1423)*** | ||
| During ET program | 531 (400-718) | 519 (408-612) | ||
| Bilirubin (indirect) (μmol/L) | ||||
| Before ET program | 37 (26-52) (ns) | 41 (33-48) (ns) | ||
| During ET program | 32 (21-52) | 35 (23-55) | ||
| Reticulocyte count (109/L) | ||||
| Before ET program | 313 (236-34 1)* | 312 (232-415) (ns) | ||
| During ET program | 337 (262-458) | 286 (221-376) | ||
| . | Patients on the ET program . | Patients on the ET program who received HU therapy . | ||
|---|---|---|---|---|
| N (%) . | Median (IQR) . | N (%) . | Median (IQR) . | |
| No. of patients | 48 | 40 | ||
| Age (y) | 11.67 (8.13-16.64) | 9.48 (6.63-13.54)†† | ||
| Duration of the ET program (y) | 4.81 (1.96-8.99) (ns) | 3.72 (1.84-6.17) (ns) | ||
| Characteristics of CV before the start of the ET program | ||||
| Abnormal TCD and normal cerebral MRA without stroke | 2 (4.2) | 10 (25) | ||
| Stenosis of intracranial arteries on MRA without stroke | 25 (52) | 16 (40) | ||
| Stenosis of cervical arteries on MRA without stroke | 7 (14.6) | 7 (17.5) | ||
| Initial stroke | 14 (29.2) | 7 (17.5) | ||
| Evolution of CV for patients without initial stroke (primary prevention) | ||||
| Improvement | 11 (32) | 6 (18) | ||
| Stagnation | 22 (65) | 23 (70) | ||
| Worsening | 1 (3) | 4 (12) | ||
| Evolution of CV for patients with history of stroke (secondary prevention) | ||||
| Improvement | 1 (7) | 0 (0) | ||
| Stagnation | 10 (72) | 5 (71) | ||
| Worsening | 3 (21) | 2 (29) | ||
| Hemoglobin level (g/dL) | 9.1 (8.7-9.5) | 8.6 (8.4-9.2) (ns) | ||
| Median hemoglobin S (%) | ||||
| Before the ET session | 35.3 (30.4-41.2) | 35 (29-38.4) | ||
| After the ET session | 13 (11.7-15.5) | 14 (11.8-16) | ||
| Leukocyte count (109/L) | ||||
| Before ET program | 12.03 (10.09-14.47)** | 12.31 (10.85-15.10)*** | ||
| During ET program | 11.36 (9.32-12.38) | 9.46 (7.24-11.42)† | ||
| Neutrophil count (109/L) | ||||
| Before ET program | 4.98 (3.69-6.52) (ns) | 5.40 (4.00-7.44)** | ||
| During ET program | 6.19 (4.66-7.71) | 4.86 (361-6.94)†† | ||
| Monocyte count (109/L) | ||||
| Before ET program | 10.8 (5.77-1.43) (ns) | 1.10 (7.66-1.61)* | ||
| During ET program | 1.24 (8.94-1.59) | 9.25 (6.74-1.12)††† | ||
| Lymphocyte count (109/L) | ||||
| Before ET program | 5.16 (4.01-8.21)*** | 5.19 (4.18-5.94)** | ||
| During ET program | 2.96 (2.12-7.51) | 2.71 (2.19-3.58)† | ||
| Platelet count (109/L) | ||||
| Before ET program | 363.0 (312.5-469.5) (ns) | 426.0 (325.2-506.0) (ns) | ||
| During ET program | 397.5 (281.2-483.2) | 361.0 (325.0-503.0) | ||
| Lactate dehydrogenase level (U/L) | ||||
| Before ET program | 950 (590-1209)*** | 1081 (625-1423)*** | ||
| During ET program | 531 (400-718) | 519 (408-612) | ||
| Bilirubin (indirect) (μmol/L) | ||||
| Before ET program | 37 (26-52) (ns) | 41 (33-48) (ns) | ||
| During ET program | 32 (21-52) | 35 (23-55) | ||
| Reticulocyte count (109/L) | ||||
| Before ET program | 313 (236-34 1)* | 312 (232-415) (ns) | ||
| During ET program | 337 (262-458) | 286 (221-376) | ||
Improvement of CV: a decrease in the percentage of arterial stenosis in at least 1 cerebral artery on cerebral magnetic resonance angiography (MRA) and/or a decrease in the cerebral blood flow velocities in at least 1 cerebral artery (below 200 cm/s) on transcranial Doppler (TCD) ultrasound. Stabilization of CV: persistence of stable arterial stenosis on cerebral MRA and/or persistence of cerebral blood flow velocities upon 200 cm/s on TCD. Worsening of CV: an increase in the percentage of arterial stenosis in at least 1 cerebral artery and/or the appearance of a new arterial stenosis on cerebral MRA and/or the appearance of new abnormal cerebral blood flow velocities in at least 1 cerebral artery (200 cm/s) on TCD and/or the occurrence of 1 or several new strokes. The results are presented as medians (interquartile range). Statistical comparisons within biological parameters before and during the ET program for the same patients was determined using a nonparametric paired t test (Wilcoxon rank test) and expressed as ns(P > .05), *(P < .05), **(P < .01), ***(P < .001). Statistical comparison within biological parameters from patients on the ET program without hydroxyurea (HU treatment) compared to patients on both the ET program and on HU therapy was determined using a nonparametric unpaired t test (Mann–Whitney t test) and expressed as ns(P > .05), †(P < .05), ††(P < .01), †††(P < .001).
Patients’ neutrophil, monocyte, and platelet counts remained unchanged between baseline (before the ET program was started) and during the ET program (Table 1). Nevertheless, hemolysis was partially controlled, as demonstrated by the decrease in lactate dehydrogenase levels under ET (Table 1). To explore the phenotype of the polymorphonuclear leukocytes in patients with SCA in the ET program, we compared the expression of adhesion molecules (Mac-1 and LFA-1 integrins, CD66a, and PSLG-1) and the proportion of aged neutrophils in healthy controls (AA; n = 10), age-matched patients with SCA who were not on the ET program or receiving HU treatment (SS; n = 12), and in a random sample of 20 patients with SCA from the ET program group(SS-ET).
As expected, neutrophils from patients with SCA exhibited high expression of the adhesion molecules Mac-1, LFA-1, CD66a, and PSLG-1 and a high proportion of aged neutrophils compared with healthy controls. Patients with untreated SCA also had a higher level of elastase in the plasma, indicating exacerbated production of neutrophils extracellular traps (NETosis) and increased plasma levels of tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and IL-8 compared with controls, as previously described.6
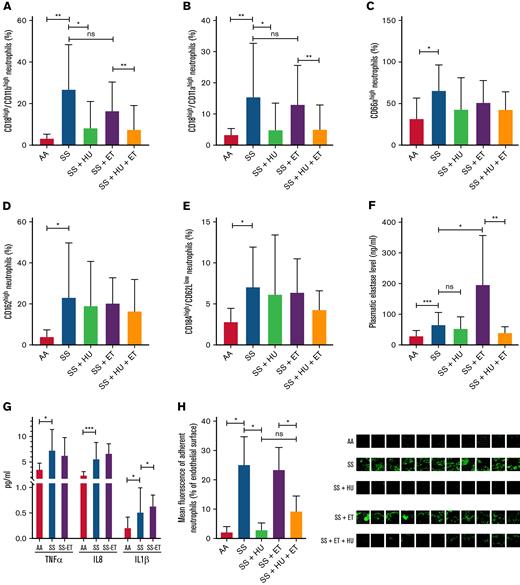
Surprisingly, the neutrophils from patients on the ET program exhibited exactly the same activated and aged phenotype as patients with untreated SCA, with a high expression of adhesion molecules and a high proportion of aged neutrophils (Figure 1A-E). With regard to NETosis, the elastase level was as high in the plasma from patients in the manual ET program as it was in untreated patients, and it was even higher in the plasma from patients with SCA undergoing erythrocytapheresis (Figure 1F). The plasma levels of proinflammatory cytokines were comparable to or even higher in the plasma from patients in the ET program compared with plasma from untreated patients (Figure 1G).
Characteristics of neutrophils from healthy controls, untreated patients with SCA, patients with SCA treated with HU, patients with SCA in the ET program, and patients with SCA treated with both HU and ET therapy. Expression of (A) Mac-1 integrin (CD18/CD11b), (B) LFA-1 integrin (CD18/CD11a), (C) CD66a, and (D) CD162 adhesion molecules. (E) Population of aged CD62low/CD184high neutrophils. Plasma level of (F) elastase, (G) TNF-α, IL-8, and IL-1β in healthy controls (AA, n = 8), untreated patients with SCA (SS, n = 8), and patients with SCA in the ET program (SS-ET, n = 8). (H) Percentage of endothelial surface covered by adherent neutrophils after flow adhesion experiments performed on whole blood from healthy controls (AA, n = 3), untreated patients with SCA (SS, n = 3), patients with SCA treated with HU (SS + HU, n = 3), patients with SCA undergoing erythrocytapheresis (SS + ET, n = 3), and patients with SCA in the ET program and treated with HU (SS + ET + HU, n = 3). Representative images of the flow adhesion assay of whole blood on endothelial cells with fluorescent labeling of neutrophils (in green). Data for patients in panels A-H are from healthy controls (AA, n = 10), untreated patients with SCA (SS, n = 12), patients with SCA treated with HU (SS + HU, n = 12), patients with SCA on the ET program (SS + ET, n = 20), and patients with SCA treated with HU and on the ET program (SS + HU + ET, n = 20). The Mann-Whitney t test was used for the statistical comparison within 2 groups. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ns, not significant (P > .05).
Characteristics of neutrophils from healthy controls, untreated patients with SCA, patients with SCA treated with HU, patients with SCA in the ET program, and patients with SCA treated with both HU and ET therapy. Expression of (A) Mac-1 integrin (CD18/CD11b), (B) LFA-1 integrin (CD18/CD11a), (C) CD66a, and (D) CD162 adhesion molecules. (E) Population of aged CD62low/CD184high neutrophils. Plasma level of (F) elastase, (G) TNF-α, IL-8, and IL-1β in healthy controls (AA, n = 8), untreated patients with SCA (SS, n = 8), and patients with SCA in the ET program (SS-ET, n = 8). (H) Percentage of endothelial surface covered by adherent neutrophils after flow adhesion experiments performed on whole blood from healthy controls (AA, n = 3), untreated patients with SCA (SS, n = 3), patients with SCA treated with HU (SS + HU, n = 3), patients with SCA undergoing erythrocytapheresis (SS + ET, n = 3), and patients with SCA in the ET program and treated with HU (SS + ET + HU, n = 3). Representative images of the flow adhesion assay of whole blood on endothelial cells with fluorescent labeling of neutrophils (in green). Data for patients in panels A-H are from healthy controls (AA, n = 10), untreated patients with SCA (SS, n = 12), patients with SCA treated with HU (SS + HU, n = 12), patients with SCA on the ET program (SS + ET, n = 20), and patients with SCA treated with HU and on the ET program (SS + HU + ET, n = 20). The Mann-Whitney t test was used for the statistical comparison within 2 groups. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ns, not significant (P > .05).
Finally, to investigate the functional impact of this activated and aged phenotype on neutrophil adhesion, we performed flow adhesion experiments on activated human mammary endothelial cells with whole blood from healthy controls (n = 3), patients with untreated SCA (n = 3), and patients with SCA who were in the ET program (n = 3). As we previously reported,14 neutrophils from patients with untreated SCA adhered far more to activated endothelial cells than neutrophils from healthy controls did (Figure 1H). Once again, neutrophils from patients with SCA undergoing monthly erythrocytapheresis presented the same level of adhesion as those from patients with untreated SCA.
Among the cohort of 88 patients with SCA on the ET program because of their CV, 40 had also been treated with HU for at least 6 months for various reasons (described in supplemental Methods). As expected, these patients had lower leukocyte, neutrophil, monocyte, and reticulocyte counts than patients on the ET program without HU treatment (Table 1). Administration of HU to these patients was associated with a partial reversal of the inflammatory phenotype with a significant decrease in the expression of Mac-1 and LFA-1 on neutrophils (Figure 1A-B), a decrease in the plasma elastase levels (Figure 1F), and a decrease in neutrophil adhesion during flow experiments (Figure 1G-H). However, HU treatment had no impact on plasma TNF-α, IL-1, and IL-8 levels in patients (data not shown). These effects were comparable to those observed in patients with SCA who were treated with HU but did not have CV or ET therapy (SS-HU group) compared with untreated patients (SS group) (Figure 1).
Taken together, after several years of ET therapy and a constant low HbS rate, patients with SCA maintained an activated neutrophil profile, leading to significant adhesion of neutrophils to endothelial cells, high NETosis activity, and high plasma levels of inflammatory cytokines, which could be partially reversed by administering HU.
Pathological phenomena responsible for CV are complex and interrelated, and they involve abnormal blood viscosity, blood cell aggregation, shear stress, local decreases in nitric oxide availability incurred by hemolysis, local hypoxia, and inflammation.3,15-17 We found that children with SCA who received chronic transfusions sustain chronic inflammation and an abnormal neutrophil phenotype, which may contribute to vascular damage. The persistence and even the increased NETosis in this population are particularly worrisome. Neutrophil extracellular traps (NETs) are extracellular DNA-scaffolded structures that anchor granule components with potent antipathogen properties, which allow for more effective antimicrobial activity. These structures can induce coagulation and even trigger vascular injury; thus, they may contribute to vasculopathy in SCA.18-20
In conclusion, our study shows, for the first time, that replacing sickle RBCs with healthy RBCs is not sufficient for treating the inflammatory profile of SCA. This raises the question of systematically combining HU treatment with an ET program to improve its efficiency in curing CV. A prospective longitudinal study that compares the effect of ET alone with ET in association with HU in patients with SCA who have a recent diagnosis of CV and at the onset of the transfusion program may be necessary.
Acknowledgments: The authors thank the patients and their families for their participation in the study and all members of the Sickle Cell Disease Center at Robert Debré Hospital for managing blood samples, the Assistance Publique-Hopitaux de Paris for promoting the study, and Nicola Conran for her careful proofreading.
This work was supported by a grant from l’Association Recherche et Transfusion and the Laboratoire d’Excellence du Globule Rouge (ANR-11-LABX-0051). Laboratoire d’Excellence du Globule Rouge is funded by a grant from the “Investissements d’Avenir” program of the French National Research Agency (ANR-11-IDEX-0005-02).
Contribution: A.K.D. and P.H. performed the experiments and analyzed the data; F.M., E.L., L.H., V.B., G.I., M.-H.O., M.B., and B.K. were in charge of the patients and contributed to recruitment for the study; C.L.V.K. helped supervise the project and revise the manuscript; B.K. designed the study, supervised the experiments, analyzed the data, and wrote the manuscript; and all authors discussed the results and contributed to the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Berengere Koehl, Centre de la Drépanocytose, Hopital Robert Debré, 48 Boul Sérurier, 75019 Paris, France; e-mail: berengere.koehl@aphp.fr.
References
Author notes
For data sharing, please contact the corresponding author, Berengere Koehl (berengere.koehl@aphp.fr).
The full-text version of this article contains a data supplement.