TO THE EDITOR:
Renal impairment (RI) is a frequent complication of multiple myeloma (MM), affecting 20% to 40% of patients with newly diagnosed MM (NDMM), and it is considered to be independently associated with adverse survival outcomes for patients with myeloma.1-3 Because renal recovery could be associated with improved survival,4,5 more effective antimyeloma treatments that could also improve renal function are urgently needed for such patients. Although the combination of bortezomib-lenalidomide-dexamethasone (VRD) was recommended as frontline therapy for most patients with NDMM,6 it is not adequate as frontline therapy for patients with MM-RI because lenalidomide should be given with dose reduction that might result in underdosing.7,8 Other bortezomib-based triplet regimens (doxorubicin, cyclophosphamide, or thalidomide with dexamethasone) have been recommended for MM with RI by the International Myeloma Working Group (IMWG).9 However, standard recommendation is still lacking. Pomalidomide has been proven to be tolerable and effective in patients with severe RI and is not required for dose adjustment.10 The pomalidomide-bortezomib-dexamethasone (PVD) combination has been approved in relapsed/refractory MM (R/R MM) based on the results of phase 3 OPTIMISMM trial11 and has also shown high efficacy in patients with NDMM.12 However, prospective data on PVD in patients with RI are still lacking. This study was aimed to evaluate the renal response of PVD in NDMM with RI.
In this prospective, open-label, multicenter phase 2 study, eligible patients with NDMM with myeloma-related RI (estimated glomerular filtration rate [eGFR] < 40 mL/min, determined using the Modification of Diet in Renal Disease equation9) were aged 18 to 80 years with measurable disease (serum M-component ≥ 10 g/L; 24-h urine M-component ≥200 mg; and/or involved free light chain [FLC] level ≥ 100 mg/L plus abnormal serum FLC ratio). The type of RI should be restricted to cast nephropathy, which could be confirmed by biopsy or clinical judgment by the investigators according to light chain proteinuria. If the proportion of urine albumin exceeded 30% of the urinary total protein, renal biopsy was required to confirm cast nephropathy. The diagnosis and response assessment of MM were according to the IMWG criteria.13,14 Enrolled patients received continuous PVD therapy up to 9 cycles, including the first two 21-day cycles of pomalidomide (4 mg oral on days 1-14), bortezomib (1.3 mg/m2 subcutaneous on days 1, 4, 8, and 11), and dexamethasone (20 mg oral or IV on days 1, 2, 4, 5, 8, 9, 11, and 12), which was followed by 35-day cycles with weekly bortezomib and 21-day pomalidomide. Dosage modifications and interruptions were implemented when grade ≥3 toxicity occurred. Autologous stem cell transplantation (ASCT) could be implemented after 3 to 6 PVD cycles for patients eligible for transplantation. The primary end point was 3-month renal overall response (renal-ORR), including renal minor response (renal-MR; eGFR from <15 mL/min to 15-29 mL/min or from 15-29 mL/min to 30-59 mL/min), renal partial response (renal-PR; eGFR from <15 mL/min to 30-59 mL/min), and renal complete response (renal-CR; eGFR from <50 mL/min to ≥60 mL/min) according to IMWG criteria.9 Assessment of adverse events (AEs) was conducted in alignment with National Cancer Institute Common Terminology Criteria for Adverse Events 5.0.
Statistical analysis was performed by SPSS v24.0. Survival curves were plotted by Kaplan-Meier method, whereas the differences between curves were compared by log-rank test. Factors associated with 3-month renal recovery were analyzed by logistic regression model. Statistical significance was considered if P value was <.05. The protocol had been approved by Beijing Chaoyang Hospital Ethics Committee. All patients had written informed consents before treatment.
From 28 February 2021 to 21 January 2022, a total of 61 patients were enrolled in the intention-to-treat population from 28 hospitals in China (supplemental Figure 1). Baseline characteristics are listed in supplemental Table 1. Enrolled patients were of a median age of 61 years (range, 38-79 years); among them, 56.9% were at revised International Staging System stage III, and 24.6% carried high-risk cytogenetics (defined as t(14;16), t(4;14) and/or 17p deletion). Median serum creatine was 349 μmol/L (range, 155-1718 μmol/L), median eGFR was 12.9 mL/min (range, 2.5-36.3 mL/min), and median difference between involved and uninvolved FLC levels was 4347.3 mg/L (range, 34.2-91 986.4 mg/L). Thirty-two patients (52.5%) presented with severe renal failure (eGFR < 15 mL/min), of whom 12 (19.7%) required dialysis before PVD treatment. Patients received a median of 5 PVD cycles (range, 1-9). A total of 18 patients had stem cell collection after 3 or 4 PVD cycles, with a median stem cell yield of 7.65 × 106 CD34+ per kg (range, 1.42-16.58). ASCT was implemented in 13 patients (21.3%).
At 3 months, 46 patients (75.4%) achieved renal response, including 29.5% renal-CR, 9.8% renal-PR, and 36.1% renal-MR. The best renal-ORR further improved to 78.7% (renal-CR, 41.0%; renal-PR, 9.8%; and renal-MR, 27.9%) (Table 1). The median time to achieve renal response was 21 days (range, 7-228 days). Regarding the 12 patients undergoing dialysis, 8 (66.7%) had become dialysis independent at a median of 26 days (range, 15-90 days) after PVD treatment. Logistic regression analysis revealed that early rapid reduction of FLC (>82% at day 22) was significantly associated with 3-month renal-ORR in multivariate analysis (odds ratio, 6.503; 95% confidence interval, 1.038-40.722; P =.045) (supplemental Table 2).
Renal response and hematological response rates in patients receiving PVD regimen (n = 61)
| . | Response . | At 3 mo, n (%) . | Best response, n (%) . |
|---|---|---|---|
| Renal response | CR | 18 (29.5) | 25 (41.0) |
| PR | 6 (9.8) | 6 (9.8) | |
| MR | 22 (36.1) | 17 (27.9) | |
| NR | 15 (24.6) | 13 (21.3) | |
| ORR | 46 (75.4) | 48 (78.7) | |
| MRR | 24 (39.3) | 31 (50.8) | |
| Hematological response | sCR | 8 (13.1) | 27 (44.3) |
| CR | 7 (11.5) | 6 (9.8) | |
| VGPR | 24 (39.3) | 14 (23.0) | |
| PR | 14 (23.0) | 9 (14.8) | |
| MR | 5 (8.2) | 2 (3.3) | |
| SD | 1 (1.6) | 1 (1.6) | |
| Missing | 2 (3.3) | 2 (3.3) | |
| ORR | 53 (86.9) | 56 (91.8) | |
| ≥VGPR | 39 (63.9) | 47 (77.0) |
| . | Response . | At 3 mo, n (%) . | Best response, n (%) . |
|---|---|---|---|
| Renal response | CR | 18 (29.5) | 25 (41.0) |
| PR | 6 (9.8) | 6 (9.8) | |
| MR | 22 (36.1) | 17 (27.9) | |
| NR | 15 (24.6) | 13 (21.3) | |
| ORR | 46 (75.4) | 48 (78.7) | |
| MRR | 24 (39.3) | 31 (50.8) | |
| Hematological response | sCR | 8 (13.1) | 27 (44.3) |
| CR | 7 (11.5) | 6 (9.8) | |
| VGPR | 24 (39.3) | 14 (23.0) | |
| PR | 14 (23.0) | 9 (14.8) | |
| MR | 5 (8.2) | 2 (3.3) | |
| SD | 1 (1.6) | 1 (1.6) | |
| Missing | 2 (3.3) | 2 (3.3) | |
| ORR | 53 (86.9) | 56 (91.8) | |
| ≥VGPR | 39 (63.9) | 47 (77.0) |
PR, partial response; MR, minor response; NR, no response; ORR, overall response rate (CR + PR + MR for renal response and sCR + CR + VGPR + PR for hematological response); MRR, major response rate (CR + PR); sCR, stringent complete response.
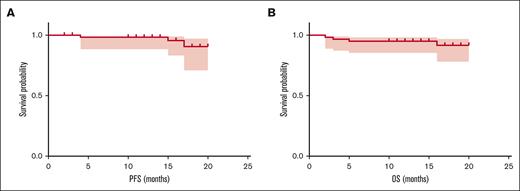
The 3-month hematological ORR was 86.9% (53/61), with 39 of 61 patients (63.9%) achieving at least very good partial response (VGPR) and 15 (24.6%) achieving CR or stringent CR. The best ORR was improved to 91.8%, among which 77.0% achieved at least VGPR and 54.1% achieved CR/stringent CR. Minimal residual disease (MRD) detection by next-generation flow cytometry (with a sensitivity of 10−6) was performed in 21 of 23 patients who had finished 9 PVD cycles or ASCT, among whom 18 (85.7%) had undetectable MRD. The median follow-up of our cohort was 16 months (range, 11-22 months). Three patients had progressive disease. Four patients died. Causes of these deaths were thrombosis (n = 2), pneumonia (n = 1), and myeloma progression (n = 1). The 18-month progression-free survival and overall survival (OS) was 90.6% and 91.7%, respectively, whereas median progression-free survival and OS have not been reached (Figure 1). Patients who achieved renal response had significantly longer OS than those who did not (18-month OS, 97.9% vs 56.4%; P = .002) (supplemental Figure 2).
Survival of patients with NDMM with RI receiving PVD regimen. (A) PFS in all 61 patients who received PVD therapy. (B) OS in all 61 patients who received PVD therapy. PFS, progression-free survival.
Survival of patients with NDMM with RI receiving PVD regimen. (A) PFS in all 61 patients who received PVD therapy. (B) OS in all 61 patients who received PVD therapy. PFS, progression-free survival.
Of all patients receiving PVD treatment, AEs were reported in 44 patients (72.1%). The most common AEs (incidence >10%) at all grades were infection (31.1%), peripheral neuritis (21.3%), myelosuppression (18.0%), and skin rash (11.5%). AEs of grade ≥3 were reported in 24 patients (39.3%), including infection (19.7%), myelosuppression (14.8%), peripheral neuritis (4.9%), rash (3.3%), and shock (3.3%) (supplemental Table 3). Of the whole patient cohort, 1 died of a serious adverse event (pulmonary embolism) during PVD treatment. Another 8 patients prematurely discontinued PVD treatment due to serious adverse events, including 2 cases of severe drug eruptions, 2 of peripheral neuritis, 1 of severe pneumonia, 1 of grade 4 myelosuppression, 1 of thrombosis, and 1 of ventricular arrhythmia.
To the best of our knowledge, this is the first prospective study to evaluate renal response of PVD in NDMM with RI. According to previous prospective studies, renal responses of bortezomib-based triplet combinations in patients with RI ranged from 44% to 62%.15-17 Although subanalysis of HOVON-65/GMMG-HD4 study reported the renal response of 81% of PAD (bortezomib-doxorubicin-dexamethasone) regimen,18 it was designed for ASCT and enrolled patients were aged <65 years. Our study demonstrates PVD regimen could achieve a 3-month renal response of 75.4%, which is higher than most bortezomib-based triplet regimens. High and rapid hematological responses with considerable MRD-negative rates are also exhibited in our study, comparable with previous PVD studies in patients with MM regardless of renal function,11,12 which is essential for achieving renal recovery. Because the efficacy of PVD in patients with R/R MM with RI had been proved in the subanalysis of OPTIMISMM study, with a 54.3% achieving at least VGPR,19 our study provided further evidence in NDMM with RI and showed higher response rates (63.9% of ≥VGPR) than that of patients with R/R MM. Safety profiles in our study are comparable with previous studies.10,16 However, because grade ≥3 AEs were still frequently reported, more attention should be paid to safety during PVD treatment. Because patients with RI were usually less well-tolerated, dose reduction or prolonged intervals due to toxicity should be considered if necessary.
In summary, PVD regimen exhibited favorable efficacy in NDMM with RI, and dose adjustments could be considered. Further evaluation of PVD in randomized trials with other bortezomib-based regimens in such patients could be promising.
Acknowledgments: The authors acknowledge all patients who participated in this study and their families.
This study was supported by Beijing Natural Science Foundation (7212041), Clinical Research Incubation Project (Beijing Chao-Yang Hospital, Capital Medical University, CYFH202208), National Natural Science Foundation of China (81974011), and National High Level Hospital Clinical Research Funding (2022-PUMCH-B-048).
Contribution: W.G. and J.L. designed the study, interpreted the data, and reviewed the manuscript; Y.J., L.C., M.-X.S., J.L., and W.G. interpreted the data and wrote the manuscript. Y.J., L.C., M.-X.S., Y.S., X.-X.C., H.X., W.-R.H., X.-L. Shen, J.M., G.-R.J., Y.-Q.F., Z.-F.X., Y.-H.Z., Y.-P.M., J.X., G.-Y.M., Q.-M.W., L.B., Y.-J.D., H.-B.Z., C.-Y.S., G.-H.S., Y.Y., S.-Y.Q., L.-P.S., J.-N.S., W.-W.T., X.-L.Sun, H.-M.J., D.G., and W.-M.C. recruited patients and collected the data; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jian Li, Department of Hematology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shuaifuyuan No.1, Dongcheng District, Beijing 100005, China; email: lijian@pumch.cn; and Wen Gao, Department of Hematology, Myeloma Research Center of Beijing, Beijing Chao-Yang Hospital, Capital Medical University, Gongtinanlu No. 8, Chaoyang District, Beijing 100020, China; email: gaowenpingping@163.com.
References
Author notes
∗Y.J., L.C., and M.S. have contributed equally to this work and are joint first authors.
Presented as a poster abstract at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 10 to 13 December 2022 [Yuan Jian, et al. Blood (2022) 140 (supplement 1): 10167–10169.]; and as a poster abstract at the EHA2022 Congress, Vienna, Austria, 9 to 17 June 2022 (P909).
Data will be shared upon reasonable request from the corresponding authors, Jian Li (lijian@pumch.cn) and Wen Gao (gaowenpingping@163.com).
The full-text version of this article contains a data supplement.