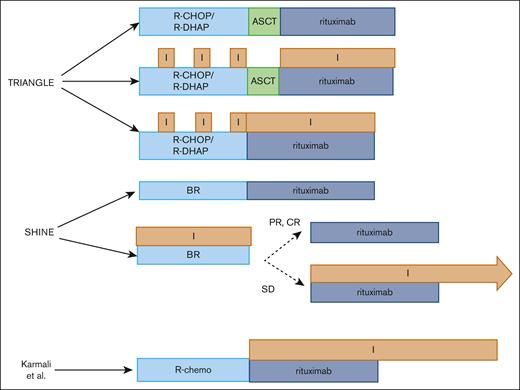
In this issue of Blood Advances, Karmali et al1 conducted a phase 2 study in patients with mantle cell lymphoma (MCL; n = 36) and showed that fixed duration ibrutinib (560 mg daily) for up to 4 years after induction chemotherapy results in impressive 5-year progression-free survival (PFS) and overall survival (OS) rates of 89% and 91%, respectively. This is despite the fact that a majority of the patients (53%) were not able to complete the full 4-year course of ibrutinib as planned. Although the numbers were small, patients who received a prior autologous stem cell transplantation (ASCT; n = 18) had better 5-year PFS rates (100% vs 77%; P = .04) and OS rates (100% vs 83%; P = .07) than those who did not (n = 18). The survival rates seen in patients undergoing ASCT in this study compared favorably with the 4-year PFS and OS rates of patients receiving ASCT followed by those who were randomized to receive rituximab maintenance in the LYMA study (83% and 89%, respectively). The non-ASCT eligible patients compared favorably (median PFS not reached at a median follow-up of 4.6 years in Karmali et al) with patients randomized to rituximab maintenance in the MCL Elderly study in which the median PFS was 5.4 years.2,3 Overall, these results suggest a potential role for the use of Bruton tyrosine kinase inhibitors (BTKi) as maintenance after initial induction therapy to extend the disease-free survival of patients with MCL, however, with the caveat that these deductions were made from cross-trial comparisons.
The advancement of BTKi into the frontline setting to improve upon outcomes in patients with MCL is a highly attractive treatment approach, given the rapid disease progression and poor outcomes after treatment failure in the second-line setting.4 We would like to highlight 2 studies, TRIANGLE and SHINE, that provide insight into the clinical efficacy of upfront BTKi in transplant-eligible and transplant-ineligible patients with MCL (see figure). In the TRIANGLE study, transplant-eligible patients were randomized to either (1) standard (high-dose cytarabine based) induction therapy,5 ASCT consolidation, and maintenance rituximab (cohort A; n = 288); (2) ibrutinib with standard induction therapy followed by ASCT consolidation and 2 years of maintenance rituximab and ibrutinib (cohort B; n = 292); or (3) ibrutinib with standard induction therapy followed by 2 years of maintenance rituximab and ibrutinib (cohort C; n = 290).6 The 3-year FFS and OS rates were 72% and 88% in cohort A, 86% and 86% in cohort B, and 91% and 92% in cohort C, respectively. In the SHINE trial, transplant–ineligible older patients (age ≥65 years) were randomized to BR with (n = 261; BR + I) or without ibrutinib (n = 260; BR + placebo) followed by rituximab maintenance in those who achieved a response (CR or PR) to induction therapy.7 Patients with stable disease continued to receive ibrutinib maintenance with rituximab. Although a PFS benefit was seen in the BR+I arm compared with BR+placebo (median PFS, 80.6 months vs 52.9 months, respectively), OS was similar. The overall response rate was also similar between the 2 groups (89.7% vs 88.5%), but patients on ibrutinib tended to have deeper responses (CR rate, 65.5% vs 57.6%). With the caveat that the study was not powered to evaluate subgroups, the PFS benefit seemed to be limited to better prognostic groups such as those with low or intermediate-risk MIPI and unmutated TP53 status.
Schematic overview of clinical trials incorporating ibrutinib in the frontline setting in MCL.
Schematic overview of clinical trials incorporating ibrutinib in the frontline setting in MCL.
When taking the results of these trials together, there are a few important considerations. In the young fit (transplant eligible) patients, the FFS and PFS benefits seen with the use of ibrutinib in first-line/maintenance setting (TRIANGLE and Karmali et al) strongly suggest that frontline use of BTKi can improve long-term outcomes in transplant-eligible patients. However, a longer follow-up is needed to confirm these findings and evaluate the OS data. The comparable outcomes of patients receiving ibrutinib in first-line/maintenance setting with (cohort B) or without ASCT (cohort C) in the TRIANGLE study, along with the PFS benefit with ibrutinib maintenance after induction therapy in the study by Karmali et al, suggest that BTKi maintenance may be sufficient to consolidate chemotherapy responses in those achieving a response to induction therapy for durable disease control. However, a longer follow-up of the TRIANGLE study is needed to clarify the benefit of ASCT in the presence of ibrutinib treatment.
On the contrary, the utility of frontline BTKi therapy in transplant-ineligible patients with MCL is a little more nuanced. Although the SHINE study showed a PFS benefit (mainly in low- to intermediate-risk patients), this did not translate to an OS benefit and was at the expense of added toxicity. The BTKi maintenance strategy in the SHINE study was distinct from the Karmali study; patients who only achieved stable disease to first-line therapy received maintenance ibrutinib in SHINE (see figure), whereas all patients who achieved a response (CR or PR) to the induction therapy received BTKi maintenance therapy in the study by Karmali et al and had durable disease control. Thus, it appears that a greater degree of clinical benefit (and lesser toxicity) with BTKi in the frontline setting may be achieved by using it as a maintenance therapy in patients achieving a deep response to first-line therapies rather than improving upon suboptimal responses when it is combined with chemotherapy.
Since the initial conceptualization of these trials, there has been an advent of newer BTKi including acalabrutinib, zanubrutinib, and pirtobrutinib. With the voluntary withdrawal of ibrutinib from the MCL space after the results of the SHINE study, future use of BTKi in the frontline setting will rely on these next-generation BTKi. It is likely that more consistent BTK inhibition can now be achieved with these next-generation inhibitors with reduced toxicity, but whether this will translate into improved PFS and OS will require additional evaluation.
Overall, the study by Karmali et al adds further support for the use of BTKi as a part of frontline therapy in MCL. Further maturation of the results of these trials including TRIANGLE and SHINE is eagerly awaited. These trials will set the framework for future frontline trial designs of next-generation BTKi to define the strategy that provides the highest efficacy while limiting toxicity.
Conflict-of-interest disclosure: N.E. reports receiving research funding from BeiGene, speakers bureau for Incyte, Novartis, and BeiGene; and honoraria/consulting/ad board fees from Merck, ADC Therapeutics, Lilly, and Novartis. W.H. declares no competing financial interests.