TO THE EDITOR:
Despite significant improvements in therapy, outcomes for childhood and young persons with T-cell acute lymphoblastic leukemia (T-ALL) remain inferior compared with those with B-cell ALL.1 The aggressive nature of T-ALL is illustrated by the increased incidence of central nervous system (CNS) infiltration at diagnosis, typically identified through the presence of leukemic blasts in the cerebrospinal fluid (CSF), which predicts an increased risk of subsequent CNS relapse.2 Historically, CNS-directed therapy consisted of prophylactic cranial radiotherapy (CRT). However, owing to the significant burden of toxicity, several consortia now either omit CRT altogether or limit its use to specific subgroups, instead using intrathecal chemotherapy.3-5
In a recent issue of Blood, the Children’s Oncology Group reported the impact of CNS status on outcome in an impressive cohort of 2164 patients treated in the AALL0434 and AALL1231 trials, showing a worse outcome for patients with CNS-3 status at diagnosis.6 Importantly, although AALL0434 delivered CRT to >90% of patients, AALL1231 limited it to the 10% of patients with CNS-3 or very high-risk disease. Despite this change in practice for patients with CNS-1 or those with CNS-2, outcomes were comparable across the 2 trials, indicating that omission of CRT had no significant impact on outcome, supporting the decision to remove CRT in these groups.
Notably, other consortia including the United Kingdom, St Jude Children’s Research Hospital, and the Dutch Childhood Oncology Group (COG) have gone a step further, eliminating CRT in the front line treatment of all patients with T-ALL, including those with CNS-3.7-10 Because the COG trials delivered CRT to all patients with CNS-3, the authors were unable to assess the impact of CRT in this group, meaning the benefit of CRT in CNS-3 remains unanswered. To address this, we have reviewed the outcomes of the 665 patients treated for T-ALL on the analogous UK National Cancer Research Institute trials, UKALL2003 and UKALL2011, which ran concurrently with the COG trials and eliminated CRT for all patients including those with CNS-3 disease. UKALL2003 was approved by the Scottish Multi-Centre Research Ethics Committee. UKALL2011 was approved by the North Thames Research Ethics Committee. The studies were conducted according to the Declaration of Helsinki.
Both UKALL2003 and UKALL2011 recruited patients aged from 1 to 24 years and have been previously reported.4,5,11,12 Briefly, treatment comprised a dexamethasone-based backbone that included a 4-drug induction, BFM (Berlin-Franfurt-Münster) consolidation, interim maintenance, delayed intensification, and maintenance therapy. Stratification was based on morphological early response and the end of induction minimal residual disease. UKALL2003 randomizations found improved outcomes for escalated treatments, including Capizzi-style methotrexate (MTX), for patients positive for minimal residual disease5 and found no impact of omitting 1 of the 2 delayed intensification blocks in low-risk patients.4 UKALL2011 randomizations identified no impact of a shorter dexamethasone course in induction,11 the addition of high-dose MTX, or the removal of dexamethasone-vincristine pulses in maintenance.12 Although UKALL2003 initially recommended CRT for patients with CNS-3, this was eliminated from 2009 onward, after which CNS-directed therapy consisted of intrathecal MTX at regular intervals throughout treatment. UKALL2003 recommended additional weekly intrathecal MTX throughout induction for patients with CNS-3, whereas UKALL2011 recommended it for both patients with CNS-2 and those with CNS-3. CNS status was assessed by a combination of cell count (manual or automated) and cytospin using standard definitions (CNS-1, ≤5 white blood cells [WBCs] per μL; CNS-2, ≤5 WBCs per μL with cells present on cytospin; CNS-3, >5 WBCs per μL).
In total, 637 patients with T-ALL had CNS status available. There were 557 patients with CNS-1 (87.4%), 44 with CNS-2 (6.9%), and 36 with CNS-3 (5.7%). Eight patients with CNS-3 recruited before 2009 who received CRT were excluded from further analyses. Although the proportion of patients with CNS-3 is comparable with that of the COG studies (5.7% vs 7.0%), there were significantly fewer patients with CNS-2 (6.9% vs 20.4%) and significantly more patients with CNS-1 (87.4% vs 72.3%) in the UK cohort (P < .001). This is unsurprising given the very wide variation in the rates of CNS-2 reported between different trial groups, which is thought to be because of the variability in methodological and analytical practices rather than a true clinical difference.13
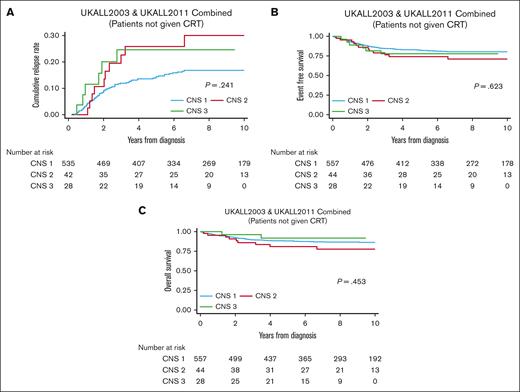
Kaplan-Meier plots showing cumulative incidence of relapse, event-free survival (EFS), and overall survival (OS) for patients treated on UKALL2003 and UKALL2011 split by CNS status are shown in Figure 1. Comparison of the 4-year survival rates based on CNS status are shown for the UKALL and COG cohorts in Table 1. Most importantly, outcomes are not significantly different for the patients with CNS-3, despite omission of CRT in the UK cohort. Although there is a slightly higher relapse rate in UK patients with CNS-3, this did not translate into poorer long-term survival. This is similar to a meta-analysis of >16 000 patients with predominantly B-cell ALL that concluded that CRT reduced the risk of isolated and combined CNS relapse in patients with CNS-3 but had no impact on EFS and OS.14 As with the COG data, patients with CNS-3 in the UKALL trials had an increased risk of isolated CNS relapse (CNS-1, 3%; CNS-2, 9%; CNS-3, 14%; P = .005).
Outcomes of patients with T-ALL treated on UKALL2003 and UKALL2011 divided by CNS status. Kaplan-Meier plots showing (A) CIR, (B) EFS, and (C) OS split by CNS status for patients with T-ALL treated on UKALL2003 and UKALL2011. CIR, cumulative incidence of relapse.
Outcomes of patients with T-ALL treated on UKALL2003 and UKALL2011 divided by CNS status. Kaplan-Meier plots showing (A) CIR, (B) EFS, and (C) OS split by CNS status for patients with T-ALL treated on UKALL2003 and UKALL2011. CIR, cumulative incidence of relapse.
Comparison of outcomes between patients treated in UKALL2003/UKALL2011 and COG AALL0434/AALL1231
| . | . | CNS-1 . | CNS-2 . | CNS-3 . | P value∗ . |
|---|---|---|---|---|---|
| UKALL2003/UKALL2011 | Proportion | 87.4% | 6.9% | 5.7% | |
| 4-y CIR | 13.6% ± 1.7% | 25.9% ± 8.6% | 24.6% ± 10.1% | .241 | |
| 4-y EFS | 82.9% ± 1.6% | 74.1% ± 6.7% | 77.8% ± 8.0% | .623 | |
| 4-y OS | 88.6% ± 1.4% | 80.9% ± 6.1% | 91.8% ± 5.6% | .453 | |
| COG AALL0434/AALL1231 | Proportion | 72.3% | 20.4% | 7.3% | |
| 4-y CIR | 7.6% ± 0.7% | 9.9% ± 1.4% | 17.9% ± 3.1% | .0002 | |
| 4-y EFS | 85.1% ± 1.0% | 83.2 ± 2.0 | 71.8% ± 4.0% | .0004 | |
| 4-y OS | 90.1% ± 0.8% | 90.5% ± 1.6% | 82.7% ± 3.4% | .005 |
| . | . | CNS-1 . | CNS-2 . | CNS-3 . | P value∗ . |
|---|---|---|---|---|---|
| UKALL2003/UKALL2011 | Proportion | 87.4% | 6.9% | 5.7% | |
| 4-y CIR | 13.6% ± 1.7% | 25.9% ± 8.6% | 24.6% ± 10.1% | .241 | |
| 4-y EFS | 82.9% ± 1.6% | 74.1% ± 6.7% | 77.8% ± 8.0% | .623 | |
| 4-y OS | 88.6% ± 1.4% | 80.9% ± 6.1% | 91.8% ± 5.6% | .453 | |
| COG AALL0434/AALL1231 | Proportion | 72.3% | 20.4% | 7.3% | |
| 4-y CIR | 7.6% ± 0.7% | 9.9% ± 1.4% | 17.9% ± 3.1% | .0002 | |
| 4-y EFS | 85.1% ± 1.0% | 83.2 ± 2.0 | 71.8% ± 4.0% | .0004 | |
| 4-y OS | 90.1% ± 0.8% | 90.5% ± 1.6% | 82.7% ± 3.4% | .005 |
Survival rates are presented as rates ± standard errors.
CIR, cumulative incidence of relapse.
1-sided log-rank test.
Outcomes for patients with CNS-1 were comparable across the 2 cohorts. In contrast, outcomes for patients with CNS-2 were worse in the UK cohort with double the relapse rate and lower EFS and OS. This may, in part, be related to the lower proportion of patients with CNS-2 in the UK cohort. It is possible that the methodology used in the United Kingdom is less sensitive than that used by COG, meaning that patients labeled as CNS-2 have a higher burden of disease, more similar to CNS-3 status, whereas patients with lower level disease, who COG would diagnose as CNS-2, are diagnosed as CNS-1 in the United Kingdom. Importantly, this means that the finding of the COG that CNS-2 status does not affect outcome may not be generalizable to other trial groups. Going forward, given the poor reproducibility of microscopy across individual labs and trial consortia, further research is needed to develop more sensitive biomarkers for the accurate detection of CSF disease that can be used to assess initial burden and response to therapy. Recently, multicolor flow cytometric analysis has been shown to provide a more sensitive analysis of CSF-115,16; an international study to assess the clinical utility of routine flow cytometric analysis of CSF samples is currently underway as part of the European ALLTogether Trial (NCT03911128).
Overall, comparison of these cohorts provides a strong indication that CRT provides minimal benefit to patients with CNS-3 disease at diagnosis. Given the high rates of neurocognitive impairment and secondary CNS malignancies,17-19 we believe strong consideration should be given to eliminating CRT in first-line treatment for all patients with T-ALL. We note that COG AALL0434 showed a remarkable benefit for nelarabine in the CNS-3 group with a 4-year DFS (disease-free survival) of 93.1% ± 5.2% with nelarabine vs 70.2% ± 5.8% without.20 Although these patients also received CRT and the numbers are small, the improvement is impressive and raises the question of whether nelarabine would have a similar beneficial effect in the absence of CRT.
Acknowledgments: The study is funded by a Cancer Research UK Clinician Scientist Fellowship (A27177) (to D.O.) and by Blood Cancer UK (15036) (research funding to A.V.M.). The authors thank all the patients who took part in the UKALL trials, as well as their families. UKALL2003 was supported by grants from Blood Cancer UK (previously Leukaemia and Lymphoma Research) and the Medical Research Council. UKALL2011 was funded by Cancer Research UK. Delivery of both trials was supported by the UK National Cancer Research Institute.
Contribution: D.O. and A.V. wrote the first and subsequent drafts of the manuscript; and all authors contributed to the acquisition or analysis of data, critically revised the manuscript, approved the final version for publication, and agreed to be accountable for the results published.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ajay Vora, Department of Haematology, Great Ormond Street Children’s Hospital, Great Ormond St, London WC1N 3JH, United Kingdom; e-mail: ajay.vora@gosh.nhs.uk.
References
Author notes
Data are available upon reasonable request from the author, David O’Connor (david.o'connor@ucl.ac.uk).