Key Points
CMV infection was common after letermovir withdrawal in patients undergoing transplantation with PT-Cy–based GVHD prophylaxis.
Immunoglobulin G reconstitution, but not immune cell subset reconstitution, predicted CMV infection after letermovir withdrawal.
Abstract
Reactivation of latent cytomegalovirus (CMV) is increased in recipients of allogeneic hematopoietic cell transplantation (allo-HCT) with seropositive CMV using posttransplant cyclophosphamide (PT-Cy)–based graft-versus-host disease (GVHD) prophylaxis. Letermovir, a novel DNA terminase complex inhibitor, reduces the incidence of clinically significant CMV infection (csCMVi) in this population; however, parameters that predict csCMVi after letermovir withdrawal are not well described. Here, we examined clinical and immunological parameters in 294 recipients of PT-Cy–based allo-HCT, including 157 patients with CMV, of whom 80 completed letermovir prophylaxis without csCMVi and subsequently stopped letermovir. In this population, the median duration of letermovir exposure was 203 days (interquartile range [IQR], 160-250 days). After letermovir withdrawal, the 90-day cumulative incidence of csCMVi was 23.0% (95% confidence interval, 14.3-32.8). There were no episodes of CMV end-organ disease. Hypogammaglobulinemia before letermovir discontinuation was predictive of csCMVi (hazard ratio, 0.33; 95% confidence interval, 0.12-0.93; P = .03), whereas T-cell and B-cell reconstitution before letermovir withdrawal were not predictive of csCMVi. Higher numbers of natural killer cells were found before letermovir withdrawal in patients who experienced csCMVi (median, 202 vs 160; P = .03). In recipients with seropositive CMV, CD3+CD4–CD8+ T-cell reconstitution was faster in patients with CMV regardless of letermovir exposure. Taken together, these data suggest that csCMVi after letermovir withdrawal was frequent in patients treated with PT-Cy, despite prolonged exposure. Strategies to boost CMV-specific adaptive immunity in patients with persistent hypogammaglobulinemia is a logical pathway to reduce csCMVi after letermovir withdrawal.
Introduction
Reactivation of latent cytomegalovirus (CMV) is a common and serious infection that occurs in roughly 40% to 70% of recipients with seropositive CMV of allogeneic hematopoietic cell transplantation (allo-HCT).1-3 In particular, early reactivation of CMV is associated with an increased risk of nonrelapse mortality and lower overall survival (OS).3 Antiviral medications with activity against CMV are associated with significant toxicity and thus unsuitable for primary prophylaxis. Instead, a preemptive strategy is typically implemented in patients with CMV, whereby serial monitoring of blood CMV viral titers triggers the initiation of CMV-specific antiviral therapy before the emergence of end-organ manifestations.4 More recently, the novel terminase inhibitor letermovir was approved by the US Food and Drug Administration for primary prophylaxis of CMV infection in adult patients undergoing allo-HCT.5 Letermovir prevents CMV virion assembly within infected cells via inhibition the CMV-terminase–associated pUL56 and pUL89 proteins.6 In a randomized, phase 3 study, a 14-week course of letermovir resulted in a reduction in clinically significant CMV infection (csCMVi) in allo-HCT recipients.5 All-cause mortality at week 24 was reduced in recipients of letermovir; however, this reduction did not reach statistical significance at week 48 after allo-HCT. Importantly, late csCMVi events occurred after letermovir withdrawal, which may in part abrogate the clinical benefit of letermovir exposure on survival seen at week 24. The drug sponsor completed a randomized study examining extended course letermovir in allo-HCT recipients (NCT03930615). Here, the cumulative incidence of csCMVi at 200 days after allo-HCT was 2.8% in patients treated with extended letermovir vs 18.9% in placebo-treated patients (P = .0005). Importantly, the week 38 cumulative incidence of csCMVi was similar between the 2 groups: 14.6% in letermovir-treated patients vs 20.3% in placebo-treated patients (P = .159), suggesting that late csCMVi occurred after letermovir withdrawal.
Patients at higher risk of primary CMV infection, such as recipients of umbilical cord blood allo-HCT or T-cell depleted allografts, have increased risk of csCMVi after letermovir withdrawal.7-9 Similarly, recipients of allo-HCT using posttransplant cyclophosphamide (PT-Cy)–based graft-versus-host disease (GVHD) prophylaxis have increased burden of CMV infection relative to recipients of unmodified allograft in the absence of letermovir exposure.10-12 We and others have demonstrated that the use of letermovir is effective in recipients of PT-Cy; however, late csCMVi is a frequent complication that can occur after letermovir withdrawal. Whether specific subpopulations within PT-Cy–treated allo-HCT recipients are at greater risk is not known. Furthermore, whether extended course letermovir can improve outcomes in these patients is also not described.
Here, we sought to determine the probability of csCMVi after a letermovir withdrawal in patients who received PT-Cy–based allo-HCT and whether common laboratory or immunological parameters obtained at the time of letermovir withdrawal are predictive biomarkers for subsequent csCMVi. The underlying goal of the study is to refine csCMVi risk appraisal in this selected population. We conducted a retrospective cohort study of patients treated at our center with PT-Cy–based allo-HCT, with a specific focus on patients who underwent voluntary letermovir withdrawal.
Methods
Patient population, clinical CMV monitoring, and letermovir prophylaxis
Patients included in the analysis were adult allo-HCT recipients treated at Memorial Sloan Kettering Cancer center between 2015 and 2022 who received an adult donor allograft using PT-Cy. HLA-matched siblings and unrelated donors matched at HLA-A, -B, -C, and -DRB1 (8/8) were considered matched, whereas related haploidentical and unrelated donors matched at ≤7 of 8 loci were considered HLA mismatched. Selection criteria for inclusion in the analysis cohort were as follows: patients were excluded from the analysis of csCMVi after letermovir withdrawal if they discontinued letermovir before 12 weeks of therapy after allo-HCT, developed prior csCMVi on letermovir, or died from any cause within 45 days of letermovir discontinuation. All recipients received GVHD prophylaxis with PT-Cy as previously described.13 Letermovir was only administered as prophylaxis to recipients with seropositive CMV, regardless of donor CMV serostatus, according to the product labeling guidelines. The study was reviewed and approved by the Institutional Review Board and Privacy Board.
CMV viremia and disease were assigned according to standard guidelines. Plasma CMV viral load (VL) was monitored by quantitative CMV polymerase chain reaction (PCR) (COBAS AmpliPrep/COBAS TaqMan CMV Test, Roche Diagnostics) according to the manufacturer’s guidelines.14 CMV blood PCR was monitored on day +5 and continued weekly until day +100, then every 1 to 2 weeks for 3 to 6 months after allo-HCT. The limit of detection of this assay was 137 IU/mL. Primary letermovir prophylaxis was instituted at 7 to 21 days after allo-HCT unless there were extenuating clinical circumstances. Patients who discontinued letermovir underwent blood CMV PCR evaluation every 14 days for 42 days after letermovir withdrawal, then as clinically indicated per the treating physician thereafter. Duration of letermovir exposure was not standardized at our institution during this study period. Anti-CMV therapy was instituted when there were 2 consecutive values of CMV VL >300 IU/mL or a single CMV VL >1000 IU/mL. Standard supportive care measures included chemoprophylaxis for herpes simplex virus, Pneumocystis jirovecii, and fungi, instituted per standard guidelines.
Statistical considerations and study end points
The primary outcome was the proportion of patients who developed csCMVi within 90 days of letermovir discontinuation. Clinically significant CMVi was defined as CMV viremia or end-organ disease requiring systemic antiviral therapy. Prolonged CMVi was defined as CMV viremia or end-organ disease that persisted for >28 days despite optimal therapy. Hypogammaglobulinemia was defined as serum immunoglobulin G (IgG) level below the lower limit of the normal range in the commercial assay used at our center (<650 mg/dL). Death without csCMVi was considered a competing event, whereas disease relapse was not. Incidence of csCMVi was compared using Gray test. Event-free survival was either relapse/progression of malignancy or death from any cause after allo-HCT. OS was determined using the Kaplan-Meier method and compared using the log-rank test. Secondary end points included cumulative incidences of grades 2 to 4 acute GVHD (with competing event of death without GVHD), nonrelapse mortality, and relapse. Absolute lymphocyte (ALC) recovery and CD3+CD8+ T-cell reconstitution were compared using the Wilcoxon rank-sum test. Parametric and nonparametric hypothesis testing were used when appropriate for descriptive statistics. Analyses were performed using R version 4.0.3.
All patients signed informed consent for clinical research.
Results
Patient cohort and baseline characteristics at letermovir discontinuation
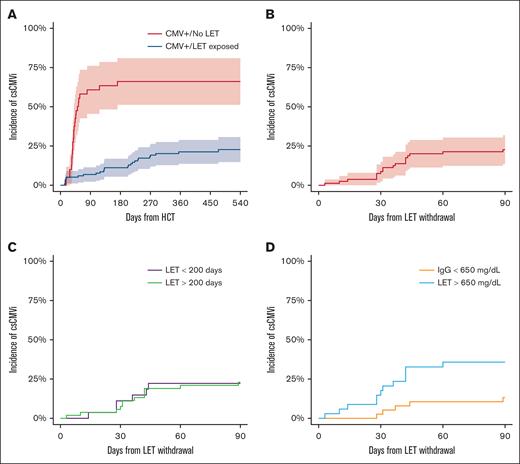
We evaluated 294 recipients of PT-Cy–based HLA-matched or -mismatched donor allo-HCT, among whom 157 patients were CMV seropositive (Table 1). Other baseline transplant characteristics are outlined in Table 1. Among patients with CMV, 117 received letermovir, whereas 40 patients with CMV subjects underwent transplantation before letermovir approval. The 6- and 12-month cumulative incidence of csCMVi in patients with CMV who were treated with letermovir (n = 117) was 11% (95% confidence interval [CI], 5-17) and 21% (95% CI, 14-29) (Figure 1A). Among the patients with CMV/exposed to letermovir, 80 completed at least 12 weeks of letermovir therapy without csCMVi and discontinued letermovir with at least 45-days of follow-up thereafter. Among the 37 patients with CMV/exposed to letermovir not in this cohort, 8 received letermovir therapy as secondary prophylaxis after early csCMVi, 11 were continuously on letermovir therapy at the time of data acquisition and analysis, and 18 discontinued letermovir as part of a planned hospice intervention. Eight patients had breakthrough csCMVi on letermovir and were not analyzed. Among these patients, breakthrough csCMVi was due to medication noncompliance in 3 patients, and 2 patients were on concomitant steroids for acute GVHD. The remaining 3 patients who developed csCMVi on letermovir therapy had a prior history of autologous HCT or chimeric antigen receptor T-cell therapy for lymphoma.
Patient demographics
| Characteristic . | Recipient without CMV n = 137 . | Recipient with CMV n = 157 . |
|---|---|---|
| Donor CMV+, n (%) | 49 (36%) | 73 (46%) |
| Conditioning intensity, n (%) | ||
| Ablative | 44 (32%) | 63 (40%) |
| Nonmyeloablative | 40 (29%) | 46 (29%) |
| Reduced intensity | 53 (39%) | 48 (31%) |
| Disease histology, n (%) | ||
| ALL | 6 (4.4%) | 11 (7.0%) |
| AML | 39 (28%) | 44 (28%) |
| CLL | 5 (3.6%) | 5 (3.2%) |
| HL | 5 (3.6%) | 7 (4.5%) |
| MDS/MPN | 45 (33%) | 46 (29%) |
| MM | 4 (2.9%) | 5 (3.2%) |
| NHL | 26 (19%) | 32 (20%) |
| Non-malignant disorder | 6 (4.4%) | 7 (4.5%) |
| Other | 1 (0.7%) | 0 (0%) |
| Sex, female, n (%) | 44 (32%) | 63 (40%) |
| Age, median (IQR) | 64 (56, 69) | 62 (53, 69) |
| Conditioning regimen, n (%) | ||
| Busulfan based | 16 (12%) | 10 (6.4%) |
| Fludarabine/cyclophosphamide/TBI | 45 (33%) | 54 (34%) |
| Melphalan based | 76 (55%) | 93 (59%) |
| Graft source, n (%) | ||
| Bone marrow | 37 (27%) | 47 (30%) |
| Mobilized blood | 100 (73%) | 110 (70%) |
| Donor relatedness, n (%) | ||
| HLA-matched related | 11 (8.0%) | 17 (11%) |
| HLA-mismatched related | 50 (36%) | 87 (55%) |
| HLA-matched unrelated | 31 (23%) | 19 (12%) |
| HLA-mismatched unrelated | 44 (32%) | 34 (22%) |
| Characteristic . | Recipient without CMV n = 137 . | Recipient with CMV n = 157 . |
|---|---|---|
| Donor CMV+, n (%) | 49 (36%) | 73 (46%) |
| Conditioning intensity, n (%) | ||
| Ablative | 44 (32%) | 63 (40%) |
| Nonmyeloablative | 40 (29%) | 46 (29%) |
| Reduced intensity | 53 (39%) | 48 (31%) |
| Disease histology, n (%) | ||
| ALL | 6 (4.4%) | 11 (7.0%) |
| AML | 39 (28%) | 44 (28%) |
| CLL | 5 (3.6%) | 5 (3.2%) |
| HL | 5 (3.6%) | 7 (4.5%) |
| MDS/MPN | 45 (33%) | 46 (29%) |
| MM | 4 (2.9%) | 5 (3.2%) |
| NHL | 26 (19%) | 32 (20%) |
| Non-malignant disorder | 6 (4.4%) | 7 (4.5%) |
| Other | 1 (0.7%) | 0 (0%) |
| Sex, female, n (%) | 44 (32%) | 63 (40%) |
| Age, median (IQR) | 64 (56, 69) | 62 (53, 69) |
| Conditioning regimen, n (%) | ||
| Busulfan based | 16 (12%) | 10 (6.4%) |
| Fludarabine/cyclophosphamide/TBI | 45 (33%) | 54 (34%) |
| Melphalan based | 76 (55%) | 93 (59%) |
| Graft source, n (%) | ||
| Bone marrow | 37 (27%) | 47 (30%) |
| Mobilized blood | 100 (73%) | 110 (70%) |
| Donor relatedness, n (%) | ||
| HLA-matched related | 11 (8.0%) | 17 (11%) |
| HLA-mismatched related | 50 (36%) | 87 (55%) |
| HLA-matched unrelated | 31 (23%) | 19 (12%) |
| HLA-mismatched unrelated | 44 (32%) | 34 (22%) |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; HL, Hodgkin lymphoma; MDS/MPN, myelodysplastic syndrome/myeloproliferative neoplasm; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; TBI, total body irradiation.
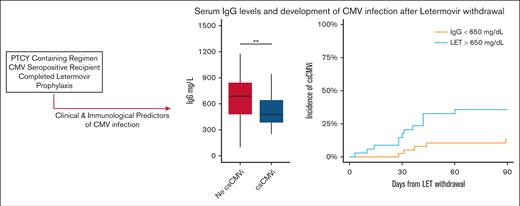
Risk of CMV reactivation based on clinical and laboratory parameters. (A) Major lymphocyte subsets in patients treated with letermovir after allo-HCT: ALC, CD3+CD4+CD8− T cells (CD4), CD3+CD4−CD8+ T-cells (CD8), CD3−CD56+ NK cells, CD3−CD19+ B cells (CD19) are shown. There were no statistically significant differences between patients who experienced or those who did not experience csCMVi. (B) Pre-letermovir discontinuation IgG levels. ∗P < .05; ∗∗P < .005 (Wilcoxon rank-sum test).
Risk of CMV reactivation based on clinical and laboratory parameters. (A) Major lymphocyte subsets in patients treated with letermovir after allo-HCT: ALC, CD3+CD4+CD8− T cells (CD4), CD3+CD4−CD8+ T-cells (CD8), CD3−CD56+ NK cells, CD3−CD19+ B cells (CD19) are shown. There were no statistically significant differences between patients who experienced or those who did not experience csCMVi. (B) Pre-letermovir discontinuation IgG levels. ∗P < .05; ∗∗P < .005 (Wilcoxon rank-sum test).
Among the 80 patients who withdrew letermovir after a completed course of the drug and were considered evaluable, the median time of letermovir discontinuation relative to allo-HCT was 210 days (interquartile range [IQR], 166-257 days). The median duration of follow-up after letermovir discontinuation in the study cohort was 209 days (IQR, 56-374 days). Among evaluable patients with CMV/exposed to letermovir, 22% received high-dose glucocorticoids during the follow-up period, and 68% remained on systemic immune suppression at the time of letermovir discontinuation. The 90-day cumulative incidence of csCMVi was 23.0% (95% CI, 14.3-32.8) in the cohort of recipients with seropositive CMV receiving primary prophylaxis with letermovir (Figure 1B). The median time to development of csCMVi in patients who did develop infection was 36 days (IQR, 28-42.5 days). Late csCMVi was seen in 1 patient at day 342. All other csCMVi events were noted before day 90. No patient developed prolonged CMV viremia (>28 days despite optimal therapy) and no patient developed CMV visceral organ disease in the study cohort. letermovir was used for secondary prophylaxis in 13 patients (68%) who developed csCMVi.
Clinical and immunological parameters predictive of csCMVi after letermovir withdrawal
We explored common clinical parameters to determine association with csCMVi after letermovir withdrawal. No patient treated with nonmyeloablative conditioning intensity developed csCMVi (0/23), whereas the 90-day cumulative incidence of csCMVi in recipients of reduced intensity or myeloablative conditioning was 32% (95% CI, 20-45). Increasing patient age was marginally associated with risk of csCMVi (hazard ratio [HR], 1.04; 95% CI, 1.00-1.09; P = .05) on univariate analysis. Donor CMV serostatus was not associated with csCMVi (HR, 0.7; 95% CI, 0.3-1.7; P = .44). In patients who discontinued letermovir before day 200, the 90-day cumulative incidence of csCMVi was 30% (16%-45%) vs 17% (8%-31%; P = .13) in patients who completed ≥200 days of letermovir.
Quantitative immune subsets and blood IgG levels at the time of or before letermovir withdrawal were evaluated to determine whether these parameters were predictive of csCMVi. Quantitative immune cell subsets obtained before letermovir discontinuation are given in Figure 2. The median ALC count was 733 cells per mcL (IQR, 500-1342 cells per mcL) and the median CD3+CD4+CD8− cell count was 177 cells per mcL (116-279 cells per mcL) in the entire cohort. Patients who experienced csCMVi had slightly higher natural killer (NK) cell subsets before letermovir withdrawal than patients without csCMVi (median, 202 cells per mcL [178-291] vs 160 cells per mcL [112-247]; P = .034). Other cell subsets were statistically similar between patients who experienced csCMVi vs those who did not. There was no difference in quantitative immune cell subsets based on donor CMV serostatus (Figure 2). Pre-letermovir discontinuation levels of serum IgG differed between patients who experienced csCMVi vs patients who did not experience csCMVi (mean, 517 vs 774 mg/dL in patients with csCMVi and those without csCMVi, respectively) (P = .002; Figure 2B). Hypogammaglobulinemia (<650 mg/dL) at the time of letermovir discontinuation was associated with increased incidence of csCMVi: 37% (20%-54%) compared with 14% (3%-25%; P = .02) with normal IgG levels in univariable analysis. In cause-specific, multivariable competing risk regression, pre-letermovir discontinuation IgG levels remained associated with csCMVi (HR, 0.33; 95% CI, 0.12-0.93; P = .03). Among recipients with seropositive CMV, 25 of 79 (31%) received supplemental IVIG during the first year after allo-HCT. Four patients received IVIG for hypogammaglobulinemia within 30 days of letermovir withdrawal, of whom 2 developed csCMVi after letermovir withdrawal and 2 did not.
Outcomes in CMV+ treated with letermovir. (A) Cumulative incidence in csCMVi landmarked from the date of transplantation in patients with seropositive CMV treated with letermovir and not treated with letermovir. CMV reactivation was not seen in patients without CMV in this cohort. (B-D) Cumulative incidence of csCMVi in patients treated with letermovir ≥14 weeks (n = 80) landmarked from the time of letermovir withdrawal: (B) entire cohort; (C) patients are grouped based on time of discontinuation of letermovir; and (D) patients are group based on IgG level before letermovir discontinuation (<650 vs ≥650 mg/dL).
Outcomes in CMV+ treated with letermovir. (A) Cumulative incidence in csCMVi landmarked from the date of transplantation in patients with seropositive CMV treated with letermovir and not treated with letermovir. CMV reactivation was not seen in patients without CMV in this cohort. (B-D) Cumulative incidence of csCMVi in patients treated with letermovir ≥14 weeks (n = 80) landmarked from the time of letermovir withdrawal: (B) entire cohort; (C) patients are grouped based on time of discontinuation of letermovir; and (D) patients are group based on IgG level before letermovir discontinuation (<650 vs ≥650 mg/dL).
Immune reconstitution in CMV+ patients treated with letermovir
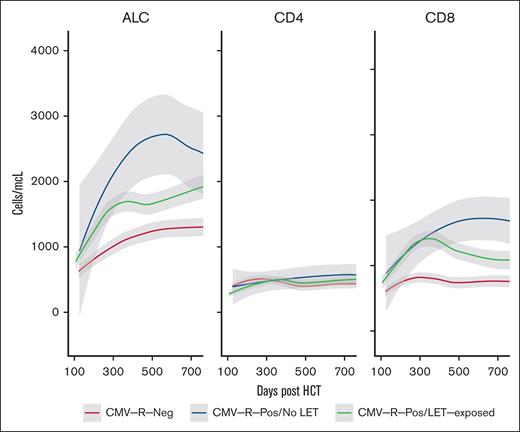
Quantitative total lymphocyte and T-cell reconstitution after transplantation are enumerated in Figure 3. ALC recovery was more rapid in patients with seropositive CMV regardless of letermovir exposure. The 6-month mean (±standard error) ALC was 1129 (88) in patients with CMV regardless of letermovir therapy, whereas the 6-month ALC was significantly decreased in recipients without CMV (780 ± 69 cells per μL; P = .004). Similarly, the 6-month mean (±standard error) CD3+CD8+ T-cell count was greater in patients with CMV regardless of letermovir therapy (730 ± 79) than recipients without CMV (597 ± 83). Interestingly, CD3+CD8+ T-cell reconstitution was sustained in patients with CMV not treated with letermovir compared with letermovir-treated patients (Figure 3). We hypothesize that this may be due to the absolute degree of CMV exposure, because most patients with csCMVi after letermovir withdrawal were treated with a second course of letermovir after CMV-specific therapy was completed. As we previously described,15 prolonged CMVi occurred in 31.2% of patients who were not treated with letermovir, whereas prolonged CMV infection was not observed in patients who developed csCMVi after completing a standard course of letermovir prophylaxis. There were no differences in CD3+CD4− T-cell recovery between the patient groups (Figure 3). We observe similar results if letermovir-treated patients are filtered to include only patients who never experienced csCMVi (supplemental Figure).
Immune reconstitution in treated patients. (A) Loess regression curves demonstrating T-cell subset reconstitution in patients with seropositive CMV (green) and those who were treated with letermovir (letermovir, red) or were not treated with letermovir (blue); (B) CD3+CD4−CD8+ specific immune reconstitution in patients with seropositive CMV treated with letermovir who had csCMVi after letermovir withdrawal vs those who did not have csCMVi after letermovir withdrawal.
Immune reconstitution in treated patients. (A) Loess regression curves demonstrating T-cell subset reconstitution in patients with seropositive CMV (green) and those who were treated with letermovir (letermovir, red) or were not treated with letermovir (blue); (B) CD3+CD4−CD8+ specific immune reconstitution in patients with seropositive CMV treated with letermovir who had csCMVi after letermovir withdrawal vs those who did not have csCMVi after letermovir withdrawal.
Clinical outcomes relative to HCT recipients with seronegative CMV
We sought to determine the clinical outcomes in CMV seropositive recipients receiving letermovir, including patients who received letermovir but were excluded from the post-letermovir outcomes analysis, compared with recipients with seropositive CMV who underwent allo-HCT at our center during the same time. Recipients with and those without CMV were matched with respect to key transplant parameters and demographics (Table 1). Outcomes based on recipient CMV serostatus and letermovir exposure are given in Table 2. Previous studies demonstrated an association between GVHD and letermovir exposure. Here, we found similar incidence of grade 2 to 4 acute GVHD in patients without CMV (day-100 cumulative incidence, 49%; 40%-57%), patients with CMV who did not receive letermovir (48%; 31%-62%), and those who received letermovir (53%; 43%-62%). One patient developed moderate to severe chronic GVHD before letermovir withdrawal. This patient developed csCMVi at 42 days after letermovir withdrawal. The 1-year cumulative incidence of relapse and event-free survival were not different based on CMV serostatus and letermovir exposure in this cohort (Table 2). OS was reduced in recipients with CMV who did not receive letermovir but was statistically similar to those with CMV who received letermovir (Table 2).
Cumulative incidence (95% CI) of acute (grade 2-4) based on CMV serostatus and letermovir exposure
| Outcome . | CMV− . | CMV+/letermovir . | CMV+/no letermovir . | P value . |
|---|---|---|---|---|
| 1-y OS | 75% (68%, 83%) | 72% (64%, 81%) | 64% (50%, 81%) | .5 |
| 1-y EFS | 63% (55%, 72%) | 65% (57%, 75%) | 56% (43%, 74%) | .8 |
| 100-d grade 2-4 acute GVHD | 49% (40%, 57%) | 53% (43%, 62%) | 48% (31%, 62%) | .8 |
| Outcome . | CMV− . | CMV+/letermovir . | CMV+/no letermovir . | P value . |
|---|---|---|---|---|
| 1-y OS | 75% (68%, 83%) | 72% (64%, 81%) | 64% (50%, 81%) | .5 |
| 1-y EFS | 63% (55%, 72%) | 65% (57%, 75%) | 56% (43%, 74%) | .8 |
| 100-d grade 2-4 acute GVHD | 49% (40%, 57%) | 53% (43%, 62%) | 48% (31%, 62%) | .8 |
EFS, event-free survival.
Discussion
In this study, we link real-world evidence of letermovir efficacy in patients treated with PT-Cy–based GVHD prophylaxis with immunological and clinical parameters. The underlying goal of the study was first to describe outcomes in this population, including the incidence of csCMVi after letermovir withdrawal; and second, to determine what parameters were prognostic for csCMVi after letermovir withdrawal to identify patients who may benefit from increased duration of letermovir therapy. We note that csCMVi was a frequent clinical event after letermovir withdrawal in this cohort. The main findings of this study suggest that patients treated with lower-intensity conditioning programs and those with more rapid reconstitution of circulating IgG have lower incidence csCMVi after PT-Cy–based GVHD prophylaxis. Recognizing an underlying treatment selection bias, the duration of letermovir exposure was not strongly linked with csCMVi.
Importantly, quantitative immune subsets at the time of letermovir discontinuation were largely uninformative to risk of csCMVi after letermovir withdrawal. Further examination demonstrated that CD8+ T-cell reconstitution was similarly robust in patients treated with letermovir who did not develop CMV vs untreated patients or those who were treated and did develop csCMVi. These findings support a proposed a hypothesis that endogenous CMV protein replication may trigger adaptive immune response in the absence of clinically evident viral infection.6,16 Counter to this, Zamora et al found that letermovir exposure after allo-HCT reduced reconstitution of CMV-specific T-cell immunity measured by polyfunctional assays.17 Here, we found that increasing numbers of NK cells were evident before letermovir withdrawal in patients who developed csCMVi. Adaptive NK cells are recognized to undergo clonal expansion in individuals with CMV and can form a long-lasting reservoir of CMV-specific immune cells.18-20 Exploration of phenotype or transcriptionally defined subpopulations of NK cells before letermovir withdrawal may expand on our current ability to predict later csCMVi.
These findings confirm risk associations determined in other retrospective series examining risk of csCMVi after allo-HCT.9,21-24 Donor HLA matching was not found to predict csCMVi here, a finding that is consistent with results from a large, retrospective series examined by the Center for International Blood & Marrow Transplant Research.21 Furthermore, the association between IgG and CMV outcomes is previously described in other retrospective series.22 We extend these results to demonstrate that these risk factors remain predictive of csCMVi after letermovir prophylaxis, suggesting that letermovir exposure does not abrogate underlying risk of CMVi.
The principal limitation of this study is bias in letermovir exposure. At our center, we recommended 6 months of letermovir exposure for patients treated with PT-Cy based on clinical observations that extended duration of letermovir was beneficial. This practice was later supported by the prospective, industry-sponsored study examining 100 vs 200 days of letermovir exposure. Here, unstructured use of letermovir will result in bias in the overall exposure of the drug based on clinician perception of patient-specific risk. For example, no patient who was on glucocorticoid therapy for GVHD was purposefully withdrawn from letermovir in this study. Therefore, these results should be interpreted with caution in patients on continued steroid therapy after HCT. We do note that prior exposure to steroid at the time of letermovir withdrawal was not associated with a change in csCMVi in this study, suggesting that there were not long-lasting impacts on CMV-specific immunity from peri-HCT steroid exposure. Other limitations of note are that chronic GVHD was too infrequent in this cohort to examine as a relevant clinical variable. There was significant heterogeneity in the follow-up of these patients, with censoring events due to relapse that may contribute further to the burden of CMV in this population. Another important consideration is the lack of end-organ CMV disease seen in patients who developed csCMVi after letermovir withdrawal. The canonical end point of “clinically significant” CMV includes both end-organ and blood viremia as a composite end point; however, the former is a more robust predictor of mortality.3 Novel end points to assess the impact of CMV infection given the use of letermovir are warranted to more accurately appraise the burden of CMV illness in transplant recipients.
Taken together, these results provide a novel framework to assess the risk of csCMVi and provide insights into the impact of letermovir exposure on immune reconstitution in allo-HCT recipients. Future studies will focus on leveraging high-throughput assays to query blood and tissue lymphocyte transcriptional and phenotypic parameters that may associate with protection from csCMVi.
Acknowledgments
The authors acknowledge the following sources of funding for this project: National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute grant K23 HL140134 03 (B.C.S.). This research was supported in part by NIH award P01 CA23766 and NIH/National Cancer Institute Cancer Center support grant P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authorship
Contribution: A.L. performed data collection and analysis, wrote and reviewed the manuscript, and designed the study; S.B. performed data analysis and interpretation, wrote and reviewed the manuscript, and designed the study; S.C., Y.J.L., and S.K.S. collected data; D.M.P., Z.S., S.G., G.A.P., and M.-A.P. designed the study and wrote and reviewed the manuscript; and B.C.S. designed the study, collected and analyzed data, and wrote and reviewed the manuscript.
Conflict-of-interest disclosure: A.L. has performed consulting for Incyte, Medexus, and EUSA Pharma. Y.J.L. has served as an investigator for Karius, AiCuris, and Scynexis, and received research grant support from Merck Inc. S.K.S. reports research support from Merck Inc. D.M.P. serves as consultant to Kadmon/Sanofi Corporation, CareDx, Incyte, and Ceramedix, and receives research funding from Incyte. S.G. receives research funding from Miltenyi Biotec, Takeda Pharmaceutical, Celgene, Amgen, Sanofi, Johnson & Johnson, Actinium Pharmaceuticals, and Omeros, and is a member on the advisory boards for Kite Pharma, Celgene, Sanofi, Novartis, Johnson & Johnson, Amgen, Takeda Pharmaceutical, Jazz Pharmaceuticals, Janssen, Actinium Pharmaceuticals, and Spectrum Pharma. G.A.P. has received research support from Merck and Shire Pharmaceutical (now known as Takeda), and consulting/other fees from AlloVir, Amplyx, Cidara, Merck & Co, Octapharma, SLC Behring, Takeda, Symbio, and Vera, all outside the submitted work. M.-A.P. reports honoraria from Adicet, Allogene, AlloVir, Caribou Biosciences, Celgene, Bristol Myers Squibb, Equilium, Exevir, ImmPACT Bio, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Syncopation, VectivBio AG, and Vor Biopharma; serves on data and safety monitoring boards for Cidara Therapeutics, Medigene, and Sellas Life Sciences; serves on the scientific advisory board of NexImmune; has ownership interests in NexImmune, Omeros, and OrcaBio; has received institutional research support for clinical trials from Allogene, Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis; serves in a volunteer capacity as a member of the board of directors of the American Society for Transplantation and Cellular Therapy and on the CIBMTR Cellular Immunotherapy Data Resource Executive Committee; and previously served on the board of directors of Be the Match (National Marrow Donor Program). B.C.S. reports consulting fee from Hansa Biopharma and Gamida Cell. The remaining authors declare no competing financial interests.
Correspondence: Brian C. Shaffer, Adult BMT Service, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: shaffeb1@mskcc.org.
References
Author notes
Data are available upon reasonable request from the corresponding author, Brian C. Shaffer (shaffeb1@mskcc.org).
The full-text version of this article contains a data supplement.