Hereditary and acquired thrombophilia are risk factors for venous thromboembolism (VTE). Whether testing helps guide management decisions is controversial.
These evidence-based guidelines from the American Society of Hematology (ASH) intend to support decision making about thrombophilia testing.
ASH formed a multidisciplinary guideline panel covering clinical and methodological expertise and minimizing bias from conflicts of interest. The McMaster University GRADE Centre provided logistical support, performed systematic reviews, and created evidence profiles and evidence-to-decision tables. The Grading of Recommendations Assessment, Development, and Evaluation approach (GRADE) was used. Recommendations were subject to public comment.
The panel agreed on 23 recommendations regarding thrombophilia testing and associated management. Nearly all recommendations are based on very low certainty in the evidence due to modeling assumptions.
The panel issued a strong recommendation against testing the general population before starting combined oral contraceptives (COCs) and conditional recommendations for thrombophilia testing in the following scenarios: (a) patients with VTE associated with nonsurgical major transient or hormonal risk factors; (b) patients with cerebral or splanchnic venous thrombosis, in settings where anticoagulation would otherwise be discontinued; (c) individuals with a family history of antithrombin, protein C, or protein S deficiency when considering thromboprophylaxis for minor provoking risk factors and for guidance to avoid COCs/hormone replacement therapy; (d) pregnant women with a family history of high-risk thrombophilia types; and (e) patients with cancer at low or intermediate risk of thrombosis and with a family history of VTE. For all other questions, the panel provided conditional recommendations against testing for thrombophilia.
Summary of recommendations
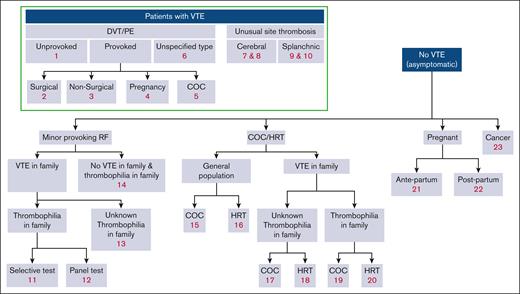
For each of the clinical questions for patients with venous thromboembolism (VTE), the panel compared 2 scenarios: (a) thrombophilia testing and subsequent indefinite anticoagulation of only the individuals found to have the thrombophilia and (b) no thrombophilia testing and indefinite anticoagulation for all or none of the individuals, depending on the standard of care. For scenario “b” of not testing for thrombophilia, the recommendations provided by other ASH VTE guidelines were considered the standard of care.1 Other clinical scenarios considered thromboprophylaxis during risk episodes for VTE (ie, minor transient risk factors, pregnancy or the postpartum period, or cancer) or avoiding hormone treatment based on the outcome of thrombophilia testing. The comparison of testing vs not testing for thrombophilia included balancing the risk for first or recurrent VTE events, bleeding events, the cost and burden associated with both testing and anticoagulant treatment or thromboprophylaxis, and patient preferences. When the recommendation is to prolong treatment or provide thromboprophylaxis based on the outcome of thrombophilia testing, the user will refer to the recommendations of the other ASH VTE guidelines for treating and preventing VTE for specific details. The guideline panel considered the effect of performing a full thrombophilia panel (consisting of simultaneously testing for factor V Leiden [FVL], prothrombin 20210A gene mutation [PGM], deficiencies of antithrombin, protein C, or protein S, and antiphospholipid antibodies [APLAs]) compatible with antiphospholipid syndrome (APS). When considering family testing, the panel only considered hereditary defects. Further details of the approach taken to balance events, costs, patient preferences, and other relevant considerations can be found in “Methods.” Figure 1 provides a visual overview of all guideline questions that are covered in this guideline, and Table 1 provides a synopsis of all resulting recommendations.
Overview of guideline questions. Minor provoking risk factors: circumstances that generally do not require prophylaxis, such as immobility or minor injury, illness, or infection. RF, risk factor.
Overview of guideline questions. Minor provoking risk factors: circumstances that generally do not require prophylaxis, such as immobility or minor injury, illness, or infection. RF, risk factor.
Synopsis of the recommendations
| Recommendation (R) no. . | Population . | Recommendation . | Strength, certainty in evidence∗ . |
|---|---|---|---|
| Patients with symptomatic VTE | |||
| R1 | Unprovoked VTE | Do not test for thrombophilia | Conditional, ⊕○○○ |
| R2 | VTE provoked by surgery | Do not test for thrombophilia | Conditional, ⊕○○○ |
| R3 | VTE provoked by nonsurgical major transient risk factor | Test for thrombophilia, and indefinite anticoagulant treatment for patients with thrombophilia | Conditional, ⊕○○○ |
| R4 | VTE provoked by pregnancy or postpartum | Test for thrombophilia, and indefinite anticoagulant treatment for patients with thrombophilia | Conditional, ⊕○○○ |
| R5 | VTE associated with use of COC | Test for thrombophilia, and indefinite anticoagulant treatment for patients with thrombophilia | Conditional, ⊕○○○ |
| R6 | An unspecified type of VTE (ie, not specified as provoked or unprovoked VTE) | Do not test for thrombophilia | Conditional, ⊕○○○ |
| Patients with symptomatic VTE in unusual sites | |||
| There is no unanimous approach to the optimal duration of anticoagulation treatment of CVT and splanchnic venous thromboses, with some providers and settings adopting long- and other short-term anticoagulation, and others deciding based on the clinical presentation. The panel issued 2 recommendations for each clinical scenario, separately for (a) settings where the standard of care would be stopping treatment in most patients after primary treatment of 3-6 months and (b) for settings where the standard of care would be treating most patients with indefinite anticoagulation. | |||
| R7 | CVT | (a) In settings when anticoagulation would otherwise be discontinued after primary short-term treatment: test for thrombophilia, and indefinite anticoagulant treatment for patients with thrombophilia | Conditional, ⊕○○○ |
| R8 | (b) In settings when anticoagulation would otherwise be continued indefinitely: do not test for thrombophilia | Conditional, ⊕○○○ | |
| R9 | Splanchnic venous thrombosis | (a) In settings when anticoagulation would otherwise be discontinued after primary short-term treatment: test for thrombophilia, and indefinite anticoagulant treatment for patients with thrombophilia | Conditional, ⊕○○○ |
| R10 | (b) In settings when anticoagulation would otherwise be continued indefinitely: do not test for thrombophilia | Conditional, ⊕○○○ | |
| Asymptomatic individuals with a family history of VTE and/or thrombophilia | |||
| Individuals with a minor transient risk factor for VTE | |||
| The panel considered the scenario where an individual with a family history of VTE and/or thrombophilia was presenting with a minor transient risk factor for VTE. The clinical question was if testing and providing pharmacological prophylaxis to individuals with thrombophilia would be beneficial. Two testing strategies were separately considered: (a) doing a thrombophilia panel (ie, testing for all hereditary thrombophilias) and (b) selective testing for the thrombophilia known in the family. | |||
| R11 | Individuals with a family history of VTE and known thrombophilia | Strategy #1: selective testing for the thrombophilia known in the family | |
| Heterozygous FVL or heterozygous PGM | Do not test for thrombophilia | Conditional, ⊕○○○ | |
| Protein C, S, or antithrombin deficiency | Test for the thrombophilia known in the family and use thromboprophylaxis in individuals with thrombophilia | Conditional, ⊕○○○ | |
| R12 | Individuals with a family history of VTE and known thrombophilia | Strategy #2: doing a thrombophilia panel | |
| Heterozygous FVL or heterozygous PGM | Do not test for a panel of hereditary thrombophilias (panel) | Conditional, ⊕○○○ | |
| Protein C, S, or antithrombin deficiency | Test for all hereditary thrombophilia (panel) and use thromboprophylaxis in individuals with thrombophilia | Conditional, ⊕○○○ | |
| R13 | Individuals with a family history of VTE and unknown thrombophilia status | Do not test for thrombophilia | Conditional, ⊕○○○ |
| R14 | Individuals with a family history of thrombophilia but no VTE | ||
| Heterozygous FVL or heterozygous PGM | Do not test for thrombophilia | Conditional, ⊕○○○ | |
| Protein C, S, or antithrombin deficiency in first-degree relatives | Test for the thrombophilia known in the family and use thromboprophylaxis in individuals with thrombophilia | Conditional, ⊕○○○ | |
| Protein C, S, or antithrombin deficiency in second-degree relatives | Either test or do not test for the thrombophilia known in the family to guide thromboprophylaxis | Conditional, ⊕○○○ | |
| Women considering using COC or HRT | |||
| The panel considered the scenario where a woman, either from the general population or with a family history of VTE and/or thrombophilia, considers using hormones that increase VTE risk, that is, COCs or HRT. The clinical question was if it would be beneficial to test and avoid these hormones in women with thrombophilia. Two testing strategies were separately considered: (a) doing a thrombophilia panel (ie, testing for all hereditary thrombophilias) and (b) selective testing for the thrombophilia known in the family. | |||
| R15 | Women from the general population considering COCs | Do not test for thrombophilia | Strong, ⊕⊕○○ |
| R16 | Women from the general population considering HRT | Do not test for thrombophilia | Conditional, ⊕⊕○○ |
| R17 | Women with a family history of VTE and unknown thrombophilia in the family considering COCs | Do not test for thrombophilia | Conditional, ⊕○○○ |
| R18 | Women with a family history of VTE and unknown thrombophilia in the family considering HRT | Do not test for thrombophilia | Conditional, ⊕○○○ |
| R19 | Women with a family history of VTE and thrombophilia considering COCs | Strategy: selective testing for the thrombophilia known in the family | |
| FVL or PGM | Do not test for thrombophilia | Conditional, ⊕○○○ | |
| Protein C, S, or antithrombin deficiency | Test for thrombophilia and avoid COCs in women with thrombophilia | Conditional, ⊕○○○ | |
| R20 | Women with a family history of VTE and thrombophilia considering HRT | Strategy: selective testing for the thrombophilia known in the family | |
| FVL or PGM | Do not test for thrombophilia | Conditional, ⊕○○○ | |
| Protein C, S, or antithrombin deficiency | Test for thrombophilia and avoid HRT in women with thrombophilia | Conditional, ⊕○○○ | |
| Women who are planning pregnancy | |||
| The panel considered the scenario where a woman with a family history of VTE and thrombophilia is planning a pregnancy. The clinical question was if testing and using antepartum and/or postpartum thromboprophylaxis in women with thrombophilia would be beneficial. Only the strategy of selective testing for the thrombophilia known in the family was considered. Recommendations on antepartum and postpartum prophylaxis in women with thrombophilia are already given in the ASH guidelines on the management of VTE in the context of pregnancy.27 Hence, the panel did not review the evidence for women with heterozygous FVL or heterozygous PGM, as the ASH guidelines on the management of VTE in the context of pregnancy already suggest not to prescribe thromboprophylaxis in these women. | |||
| Antepartum prophylaxis | |||
| R21 | Women with a family history of VTE and thrombophilia | Strategy: selective testing for the thrombophilia known in the family | |
| Known homozygous FVL, combination of FVL and PGM, or antithrombin deficiency | Test for the thrombophilia known in the family and use antepartum thromboprophylaxis in women with thrombophilia | Conditional, ⊕○○○ | |
| Known protein C or protein S deficiency in the family | Either test or do not test for the thrombophilia known in the family to guide antepartum thromboprophylaxis | Conditional, ⊕○○○ | |
| Postpartum prophylaxis | |||
| R22 | Women with a family history of VTE and thrombophilia | Strategy: selective testing for the thrombophilia known in the family | |
| Known homozygous FVL, combination of FVL and PGM, or antithrombin, protein C, or protein S deficiency | Test for the thrombophilia known in the family and use postpartum thromboprophylaxis in women with thrombophilia | Conditional, ⊕○○○ | |
| Known combination of FVL and PGM, or antithrombin deficiency in second-degree relatives | Test for the thrombophilia known in the family and use postpartum thromboprophylaxis in women with thrombophilia | Conditional, ⊕○○○ | |
| Known protein C or protein S deficiency in the family | Either test or do not test for the thrombophilia known in the family to guide postpartum thromboprophylaxis | Conditional, ⊕○○○ | |
| Patients with cancer | |||
| The panel only addressed patients with cancer who are classified to be at low or moderate risk of VTE, as the ASH VTE guidelines on prevention and treatment for patients with cancer already suggest using DOAC prophylaxis in all ambulatory patients with cancer at high risk of VTE. | |||
| R23 | Ambulatory patients with cancer who are classified to be at low or intermediate risk for VTE, who have a family history of VTE in first-degree relatives | Strategy: doing a thrombophilia panel Test for all hereditary thrombophilia (panel) and use thromboprophylaxis in individuals with thrombophilia | Conditional, ⊕○○○ |
| Recommendation (R) no. . | Population . | Recommendation . | Strength, certainty in evidence∗ . |
|---|---|---|---|
| Patients with symptomatic VTE | |||
| R1 | Unprovoked VTE | Do not test for thrombophilia | Conditional, ⊕○○○ |
| R2 | VTE provoked by surgery | Do not test for thrombophilia | Conditional, ⊕○○○ |
| R3 | VTE provoked by nonsurgical major transient risk factor | Test for thrombophilia, and indefinite anticoagulant treatment for patients with thrombophilia | Conditional, ⊕○○○ |
| R4 | VTE provoked by pregnancy or postpartum | Test for thrombophilia, and indefinite anticoagulant treatment for patients with thrombophilia | Conditional, ⊕○○○ |
| R5 | VTE associated with use of COC | Test for thrombophilia, and indefinite anticoagulant treatment for patients with thrombophilia | Conditional, ⊕○○○ |
| R6 | An unspecified type of VTE (ie, not specified as provoked or unprovoked VTE) | Do not test for thrombophilia | Conditional, ⊕○○○ |
| Patients with symptomatic VTE in unusual sites | |||
| There is no unanimous approach to the optimal duration of anticoagulation treatment of CVT and splanchnic venous thromboses, with some providers and settings adopting long- and other short-term anticoagulation, and others deciding based on the clinical presentation. The panel issued 2 recommendations for each clinical scenario, separately for (a) settings where the standard of care would be stopping treatment in most patients after primary treatment of 3-6 months and (b) for settings where the standard of care would be treating most patients with indefinite anticoagulation. | |||
| R7 | CVT | (a) In settings when anticoagulation would otherwise be discontinued after primary short-term treatment: test for thrombophilia, and indefinite anticoagulant treatment for patients with thrombophilia | Conditional, ⊕○○○ |
| R8 | (b) In settings when anticoagulation would otherwise be continued indefinitely: do not test for thrombophilia | Conditional, ⊕○○○ | |
| R9 | Splanchnic venous thrombosis | (a) In settings when anticoagulation would otherwise be discontinued after primary short-term treatment: test for thrombophilia, and indefinite anticoagulant treatment for patients with thrombophilia | Conditional, ⊕○○○ |
| R10 | (b) In settings when anticoagulation would otherwise be continued indefinitely: do not test for thrombophilia | Conditional, ⊕○○○ | |
| Asymptomatic individuals with a family history of VTE and/or thrombophilia | |||
| Individuals with a minor transient risk factor for VTE | |||
| The panel considered the scenario where an individual with a family history of VTE and/or thrombophilia was presenting with a minor transient risk factor for VTE. The clinical question was if testing and providing pharmacological prophylaxis to individuals with thrombophilia would be beneficial. Two testing strategies were separately considered: (a) doing a thrombophilia panel (ie, testing for all hereditary thrombophilias) and (b) selective testing for the thrombophilia known in the family. | |||
| R11 | Individuals with a family history of VTE and known thrombophilia | Strategy #1: selective testing for the thrombophilia known in the family | |
| Heterozygous FVL or heterozygous PGM | Do not test for thrombophilia | Conditional, ⊕○○○ | |
| Protein C, S, or antithrombin deficiency | Test for the thrombophilia known in the family and use thromboprophylaxis in individuals with thrombophilia | Conditional, ⊕○○○ | |
| R12 | Individuals with a family history of VTE and known thrombophilia | Strategy #2: doing a thrombophilia panel | |
| Heterozygous FVL or heterozygous PGM | Do not test for a panel of hereditary thrombophilias (panel) | Conditional, ⊕○○○ | |
| Protein C, S, or antithrombin deficiency | Test for all hereditary thrombophilia (panel) and use thromboprophylaxis in individuals with thrombophilia | Conditional, ⊕○○○ | |
| R13 | Individuals with a family history of VTE and unknown thrombophilia status | Do not test for thrombophilia | Conditional, ⊕○○○ |
| R14 | Individuals with a family history of thrombophilia but no VTE | ||
| Heterozygous FVL or heterozygous PGM | Do not test for thrombophilia | Conditional, ⊕○○○ | |
| Protein C, S, or antithrombin deficiency in first-degree relatives | Test for the thrombophilia known in the family and use thromboprophylaxis in individuals with thrombophilia | Conditional, ⊕○○○ | |
| Protein C, S, or antithrombin deficiency in second-degree relatives | Either test or do not test for the thrombophilia known in the family to guide thromboprophylaxis | Conditional, ⊕○○○ | |
| Women considering using COC or HRT | |||
| The panel considered the scenario where a woman, either from the general population or with a family history of VTE and/or thrombophilia, considers using hormones that increase VTE risk, that is, COCs or HRT. The clinical question was if it would be beneficial to test and avoid these hormones in women with thrombophilia. Two testing strategies were separately considered: (a) doing a thrombophilia panel (ie, testing for all hereditary thrombophilias) and (b) selective testing for the thrombophilia known in the family. | |||
| R15 | Women from the general population considering COCs | Do not test for thrombophilia | Strong, ⊕⊕○○ |
| R16 | Women from the general population considering HRT | Do not test for thrombophilia | Conditional, ⊕⊕○○ |
| R17 | Women with a family history of VTE and unknown thrombophilia in the family considering COCs | Do not test for thrombophilia | Conditional, ⊕○○○ |
| R18 | Women with a family history of VTE and unknown thrombophilia in the family considering HRT | Do not test for thrombophilia | Conditional, ⊕○○○ |
| R19 | Women with a family history of VTE and thrombophilia considering COCs | Strategy: selective testing for the thrombophilia known in the family | |
| FVL or PGM | Do not test for thrombophilia | Conditional, ⊕○○○ | |
| Protein C, S, or antithrombin deficiency | Test for thrombophilia and avoid COCs in women with thrombophilia | Conditional, ⊕○○○ | |
| R20 | Women with a family history of VTE and thrombophilia considering HRT | Strategy: selective testing for the thrombophilia known in the family | |
| FVL or PGM | Do not test for thrombophilia | Conditional, ⊕○○○ | |
| Protein C, S, or antithrombin deficiency | Test for thrombophilia and avoid HRT in women with thrombophilia | Conditional, ⊕○○○ | |
| Women who are planning pregnancy | |||
| The panel considered the scenario where a woman with a family history of VTE and thrombophilia is planning a pregnancy. The clinical question was if testing and using antepartum and/or postpartum thromboprophylaxis in women with thrombophilia would be beneficial. Only the strategy of selective testing for the thrombophilia known in the family was considered. Recommendations on antepartum and postpartum prophylaxis in women with thrombophilia are already given in the ASH guidelines on the management of VTE in the context of pregnancy.27 Hence, the panel did not review the evidence for women with heterozygous FVL or heterozygous PGM, as the ASH guidelines on the management of VTE in the context of pregnancy already suggest not to prescribe thromboprophylaxis in these women. | |||
| Antepartum prophylaxis | |||
| R21 | Women with a family history of VTE and thrombophilia | Strategy: selective testing for the thrombophilia known in the family | |
| Known homozygous FVL, combination of FVL and PGM, or antithrombin deficiency | Test for the thrombophilia known in the family and use antepartum thromboprophylaxis in women with thrombophilia | Conditional, ⊕○○○ | |
| Known protein C or protein S deficiency in the family | Either test or do not test for the thrombophilia known in the family to guide antepartum thromboprophylaxis | Conditional, ⊕○○○ | |
| Postpartum prophylaxis | |||
| R22 | Women with a family history of VTE and thrombophilia | Strategy: selective testing for the thrombophilia known in the family | |
| Known homozygous FVL, combination of FVL and PGM, or antithrombin, protein C, or protein S deficiency | Test for the thrombophilia known in the family and use postpartum thromboprophylaxis in women with thrombophilia | Conditional, ⊕○○○ | |
| Known combination of FVL and PGM, or antithrombin deficiency in second-degree relatives | Test for the thrombophilia known in the family and use postpartum thromboprophylaxis in women with thrombophilia | Conditional, ⊕○○○ | |
| Known protein C or protein S deficiency in the family | Either test or do not test for the thrombophilia known in the family to guide postpartum thromboprophylaxis | Conditional, ⊕○○○ | |
| Patients with cancer | |||
| The panel only addressed patients with cancer who are classified to be at low or moderate risk of VTE, as the ASH VTE guidelines on prevention and treatment for patients with cancer already suggest using DOAC prophylaxis in all ambulatory patients with cancer at high risk of VTE. | |||
| R23 | Ambulatory patients with cancer who are classified to be at low or intermediate risk for VTE, who have a family history of VTE in first-degree relatives | Strategy: doing a thrombophilia panel Test for all hereditary thrombophilia (panel) and use thromboprophylaxis in individuals with thrombophilia | Conditional, ⊕○○○ |
For an explanation of conditional and strong recommendations, see Table 2.
These American Society of Hematology (ASH) guidelines are based on ad hoc or updated systematic reviews of evidence conducted under the direction of the McMaster University GRADE Centre. The panel followed best practices for guideline development recommended by the US National Academy of Medicine and the Guidelines International Network (GIN).2-5 The panel used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach6,7 to assess the certainty in the evidence and formulate recommendations.
Introduction
Aims of this guideline and specific objectives
Thrombophilia, either acquired or hereditary, can be identified in many patients presenting with VTE.
The currently most commonly tested hereditary thrombophilias include deficiencies of antithrombin, protein C, or protein S and the gain-of-function mutations FVL and PGM. Lupus anticoagulants, anticardiolipin antibodies, and anti–β2-glycoprotein 1 antibodies, which are laboratory features of the acquired thrombophilic APS, are also generally included in a thrombophilia testing panel. These types of thrombophilias are rational components of a thrombophilia testing panel, as these are consistently found to be associated with VTE. This guideline refrains from providing guidance on other tests that in some laboratories are being included in thrombophilia test panels because these have been shown not to be associated with VTE (methylenetetrahydrofolate reductase polymorphisms [MTHFRs] 677C→T, 1298A→C), or have not been conclusively associated with VTE (eg, factor VIII, factor IX, and factor XI activity, plasminogen activator inhibitor type 1 (PAI-1), and the 4G/5G PAI-1 promoter polymorphism).8 It is important to note that results of thrombophilia tests should be interpreted with knowledge of clinical pitfalls in laboratory testing, most notably the possibility of finding acquired rather than inherited deficiencies of antithrombin, protein C, or protein S with comorbidities or hormone exposure, as well as intraindividual fluctuations of anticoagulant proteins and the far from perfect diagnostic test characteristics of coagulation tests in general.
Thrombophilia testing is often performed in patients with VTE, particularly if they are young, have recurrent episodes, have thrombosis at unusual sites, or have a positive family history of the disease. Testing patients with VTE or relatives of patients with VTE and thrombophilia has a moderate to high chance of finding a positive test result, suggesting that the incremental value of knowing about the presence or absence of thrombophilia may be low. Thrombophilia testing can lead to overdiagnosis, defined as the labeling of a person with a disease or abnormal condition that would not have caused the person clinical harm if left undiscovered, although they may experience physical, psychological, or financial harm if the condition is discovered. The purpose of these guidelines is to provide evidence-based recommendations about whether thrombophilia testing and tailoring management based on the test result would improve patient-important outcomes.
Because no randomized controlled trials (RCTs) have directly addressed these questions, we performed modeling using observational evidence for the prevalence of thrombophilia and associated risk of VTE events with and without thrombophilia and RCT-based evidence for the risk reduction related to anticoagulation, a different duration of anticoagulation for prevention of VTE or VTE recurrence, or for women, choices regarding the use of hormones that increase the risk of VTE.
The target audience includes hematologists, internists, general practitioners, hospitalists, obstetricians and gynecologists, clinical laboratory physicians, other clinicians (eg, emergency medicine or critical care physicians), decision makers, and patients. Policy-makers interested in these guidelines include those involved in developing local, national, or international programs aiming to safely reduce the incidence of VTE and/or to evaluate direct and indirect harms and costs related to VTE and its prevention. This document may also serve as the basis for adaptation by local, regional, or national guideline panels.
Description of the health problem(s)
Thrombophilia is a generic term used for several acquired or hereditary conditions that indicate that a patient has a higher-than-normal risk of VTE. Acquired thrombophilia, that is, APS, also increases the risk of pregnancy complications. The heritability of VTE, that is, the proportion of variance attributable to genetic effects, is estimated to be as high as 60%.9 There are several known genetically determined defects associated with thrombophilia, collectively linked to at least a third of cases of VTE. This guideline is focused on the most common hereditary thrombophilias, which include the gain-of-function mutations in factor Va, that is, the FVL mutation (FVL), and the G20210A mutation of the prothrombin gene (PGM), as well as deficiencies of antithrombin, protein C, and protein S. Among the acquired thrombophilias, we focus on APS (defined as 1 or more of lupus anticoagulants, anticardiolipin antibodies, and anti–β2-glycoprotein 1 antibodies combined with clinical criteria).10 This guideline refrains from providing guidance on tests that have been shown not to be associated with VTE or have not been conclusively associated with VTE.
Because in many clinical settings thrombophilia is tested as a panel, we will generally consider the scenario of “testing for any thrombophilia.” Selective testing is the term used for “testing for a specific thrombophilia defect,” which is of interest in families with known carriers of a specific defect. Details on background pathophysiology and genetics of thrombophilia can be found in other reviews.11-13 It is important to note that the results of thrombophilia tests should be interpreted with knowledge of the clinical pitfalls in laboratory testing.
Methods
The guideline panel developed and graded the recommendations and assessed the certainty in the supporting evidence following the GRADE approach.6,7,14-18 The overall guideline development process, including funding of the work, panel formation, management of conflicts of interest, internal and external review, and organizational approval, was guided by ASH policies and procedures derived from the GIN-McMaster Guideline Development Checklist (https://cebgrade.mcmaster.ca/guidecheck.html) and was intended to meet recommendations for trustworthy guidelines by the Institute of Medicine and GIN.2-5 Further details about the specific GRADE methodology and operational protocols specific to the ASH guideline projects were published separately.19 The modeling framework adopted for the specific management strategy (test and treat according to the risk level associated with the test results) is described below and was built using a previously published method20 and online calculator (https://hiru.mcmaster.ca/AbsoluteRiskCalculator/).
Organization, panel composition, planning, and coordination
The work of this panel was coordinated with 9 other guideline panels (addressing other aspects of VTE management) by ASH and the McMaster GRADE Centre (funded by ASH). Project oversight was initially provided by a coordination panel, which reported to the ASH Committee on Quality, then by the coordination panel chair (Adam Cuker) and vice-chair (H.J.S.).
In 2015, ASH vetted and appointed 8 individuals to the guideline panel. During the guideline development process, 4 of these individuals stopped participating: 2 in 2015, 1 in 2018, and 1 in 2019. In 2018, ASH vetted and appointed 6 new individuals to the guideline panel. Most panelists discontinued because of lack of time to continue on this panel. The final panel of 10 individuals included physicians with clinical and research expertise on the guideline topic (n = 8) and patient representatives (n = 2). One of these panel members stopped participating in April 2020. The physicians included hematologists, internists, an emergency care physician, an intensivist, and an obstetrician. The panel also included methodologists with expertise in evidence appraisal and guideline development. The panel chair was a content expert. The vice-chair was a content expert with specialized expertise in guideline development.
The McMaster GRADE Centre vetted and retained researchers to conduct systematic reviews of evidence and coordinate the guideline development process, including the use of the GRADE approach.
The membership of the panel and the GRADE Centre team is described in Supplement 1.
In addition to synthesizing evidence systematically, the McMaster GRADE Centre supported the guideline development process, including determining methods, preparing agendas and meeting materials, and facilitating panel discussions. The panel’s work was done using web-based tools (www.surveymonkey.com, www.gradepro.org) and face-to-face and online meetings (gotomeeting.com and zoom.us).
Guideline funding and management of conflicts of interest
The development of these guidelines was wholly funded by ASH, a nonprofit medical specialty society that represents hematologists. Some members of the guideline panel were members of ASH. ASH staff supported panel appointments and coordinated meetings but had no role in choosing the guideline questions or determining the recommendations.
Members of the guideline panel received travel reimbursement for attendance at in-person meetings. Through the McMaster GRADE Centre, some researchers who contributed to the systematic evidence reviews received salary or grant support. Other researchers participated to fulfill the requirements of an academic degree or program.
Conflicts of interest of all participants were managed according to ASH policies approved in 2015 based on recommendations of the Institute of Medicine21 and GIN.4 During the development of these guidelines, a majority of the guideline panel, including the chair and the vice-chair, had no conflicts of interest as defined and judged by ASH staff and oversight ASH members, that is, no current material interest in any commercial entity with a product that could be directly affected by the guidelines. Some individuals on the guideline panel reported indirect financial relationships with commercial entities that could be indirectly affected by these guidelines, for example, research funding supported by companies that market anticoagulant drugs. ASH staff and oversight ASH members did not judge these relationships to be a material conflict of interest.
Before appointment to the panel, individuals disclosed both financial and nonfinancial interests. Disclosures were updated throughout the guideline development process. Supplement 2 provides the complete “Disclosure of Interests” forms of the 10 individuals who continued on the panel through finalization of the guidelines in 2022, that is, the 10 panelists who are listed as authors of this report (S. Middeldorp, D.B., L.B.K., M.C., D.H., A.J., E.L., S. Moll, T.M., and A.I.). The forms also describe ASH judgments and management decisions. The forms also show that 1 reported COI for 1 panel member (S. Moll) started after finalization of all recommendations; in the period after the COI started, the direction and strength of the recommendations did not change, and the panel member contributed to the tailoring of the wording for recommendations and the manuscript.
None of the McMaster University–affiliated researchers who contributed to the systematic evidence reviews or who supported the guideline development process had any current material interest in a commercial entity with any product that could be affected by the guidelines. Supplement 3 provides the complete “Disclosure of Interest” forms of the researchers who made substantial contributions to these guidelines, that is, the 8 researchers who are listed as authors of this report (R.N., M.B., C.C.-A., L.E.C.-L., S.G.K., H.J.S., W.W., and Y.Z.).
Formulating specific clinical questions and determining outcomes of interest
The panel used the GRADEpro Guideline Development Tool (www.gradepro.org) and SurveyMonkey (surveymonkey.com) to scope and then prioritize the questions described in supplemental Appendix D. Two questions on testing for APS in women with previous placenta-mediated complications or recurrent miscarriage were dropped at the final online panel meetings because of resource constraints.
The panel selected outcomes of interest for each question a priori, following an approach described in detail elsewhere.22 In brief, the panel first brainstormed all possible outcomes before rating the relative importance of each outcome for decision making. During this rating process, the panel used definitions of the outcomes (“marker states”) that were developed for these guidelines. The panel rated the following outcomes as critical for clinical decision making across questions: mortality, pulmonary embolism (PE), deep vein thrombosis (DVT), and major bleeding. The panel did not distinguish different clinical severities of locations of DVT and PE, and major bleeding definitions varied across clinical studies.
The panel adopted a threshold-based approach to judging the size of outcome effects and continuously verified during the process the consistency of judgments, noting when exceptions were made (eg, based on the median age of the population of interest). In general, the following thresholds were used to judge the reduction in VTE (first-time or recurrence): trivial: ≤5 events per 1000 patient-years; small: 5 to 20 per 1000; moderate: 20 to 50 per 1000. Whenever a different threshold was used, the rationale is reported in the discussion of the specific recommendation.
Evidence review and development of recommendations
Evidence elements, retrieval, extraction, and appraisal
For each guideline question, the McMaster GRADE Centre retrieved and summarized evidence for each population of interest for the following domains using separate systematic reviews: (a) thrombophilia prevalence; (b) measure of association between thrombophilia and outcomes of interest; (c) effect sizes of indefinite anticoagulant treatment after primary treatment (ie, 3-6 months of anticoagulant treatment) for VTE, thromboprophylaxis, or avoidance of oral contraceptives or hormone replacement therapy (HRT) for the beneficial and harmful effects. For each domain, well-done and recent systematic reviews of appropriate study designs were searched first and updated if necessary. In the absence of suitable systematic reviews, individual studies with appropriate study designs were retrieved and appraised. The most recent search dates for the different domains were run between 26 January 2018 and 12 June 2018. Published systematic reviews were searched from 2006. Original studies were searched from 1996 or from the final search date of an eligible, well-done systematic review that needed updating.
For thrombophilia prevalence, cohort studies were considered and appraised following the GRADE guidance for overall prognosis.23,24 Prevalence was extracted as cases/patients at risk for specific thrombophilia and any thrombophilia as reported. The prevalence figure for any type of thrombophilia was also calculated by cumulating individual defects when appropriate.
For the risk association between thrombophilia and the outcomes of interest (first VTE, VTE recurrence, or major bleeding), preference was given to studies reporting the absolute risk of events in people with and without thrombophilia, followed by cohort studies reporting relative measures of risk (relative risk [RR], hazard ratio) and by case-control studies (odds ratio, hazard ratio). The studies were appraised using the GRADE guidance for prognostic factors.25 The risk association was extracted as reported in the source papers.
The effect size for the intervention of interest was sought in the companion ASH guidelines for the treatment of VTE, prevention of VTE in the surgical and medical (nonsurgical) hospital setting, primary prevention of VTE in pregnancy, and ambulatory patients with cancer.26-28 From such guidelines, 2 relevant pieces of information were extracted: (a) the recommended duration of anticoagulation treatment for the specific clinical setting (of interest in the field of thrombophilia is indefinite vs stopping after primary VTE treatment) and (b) the effect size for the recommended treatment. Whenever possible, the effect size adopted by the companion guideline was used; when needed, effect sizes were recalculated after excluding/regrouping studies as appropriate for this guideline. Details will be provided with each specific recommendation as necessary. The effect size for the VTE risk associated with combined oral contraceptives (COCs) or HRT was estimated with a specific systematic review performed ad hoc, as it was not covered by any other ASH companion guideline.
In addition to conducting systematic reviews of the different components to calculate the effect of a thrombophilia testing strategy, the researchers searched for values, preferences, costs, equity, acceptability, and feasibility of thrombophilia testing and summarized findings within the evidence-to-decision (EtD) frameworks.14,15,18 Subsequently, the certainty in the body of evidence (also known as quality of the evidence or confidence in the estimated effects) was assessed for each effect estimate of the outcomes of interest following the GRADE approach based on the following domains: risk of bias, precision, consistency, directness of the evidence, publication bias, presence of large effects, dose-response relationship, and an assessment of the effect of residual, opposing confounding. The certainty was categorized into 4 levels, ranging from very low to high, per outcome as well as for the overall body of evidence for a recommendation.6,16
Modeling
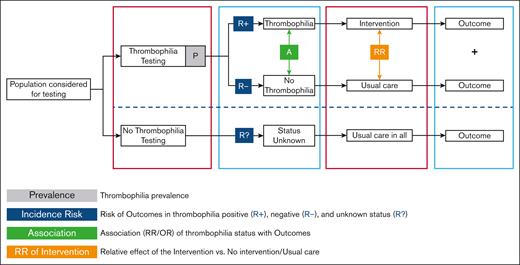
For each specific guideline question, prevalence and risk association data were used to calculate the absolute risk of events in people with and without thrombophilia using the approach previously published.20 For each absolute risk, we calculated the lowest and highest boundary by combining minimum and maximum prevalence and 95% confidence interval 95% (CI) boundaries for the risk association of thrombophilia with first-time or recurrent VTE (no such association was assumed for the outcome of major bleeding). Finally, we calculated the absolute number of events in the comparator group (no thrombophilia testing) and the intervention group (thrombophilia testing) by simulating the proportion of people with a positive result for thrombophilia (a function of the prevalence of thrombophilia), the expected event rate before treatment in people with or without thrombophilia (a function of the risk associated with thrombophilia), and the reduction (or increase) of outcomes produced by the intervention. In other words, the panel judged the appropriateness of the management strategy (test and treat accordingly) by considering the VTE prevented/tolerated and the bleeds prevented/tolerated by continuing or stopping treatment based on the results of thrombophilia testing out of 1000 patients tested and the specific proportion treated. The cost incurred (or saved) by recommending tests and whether to treat specific subgroups of patients was considered as requested by the standard guideline process. Details about the modeling approach are provided in Figure 2. ASH aims to develop a thrombophilia-specific online calculator.
Modeling approach for determining the effect of thrombophilia testing. Population considered for testing: Figure 1 with the guideline flowchart for the different populations for which a recommendation regarding thrombophilia testing was provided. Thrombophilia: any type of thrombophilia or a specific type, depending on whether the recommendation question addresses panel testing or testing for a known specific type in the family. Intervention: course of action other than “usual care.” Depending on the specific question, this means prescribing thromboprophylaxis, withholding thromboprophylaxis, extending thromboprophylaxis, stopping thromboprophylaxis, withholding COCs, or withholding HRT. Usual care: for populations where “usual care” was ambiguous, 2 scenarios were modeled, and separate recommendations were provided (see recommendations 7-10).
Modeling approach for determining the effect of thrombophilia testing. Population considered for testing: Figure 1 with the guideline flowchart for the different populations for which a recommendation regarding thrombophilia testing was provided. Thrombophilia: any type of thrombophilia or a specific type, depending on whether the recommendation question addresses panel testing or testing for a known specific type in the family. Intervention: course of action other than “usual care.” Depending on the specific question, this means prescribing thromboprophylaxis, withholding thromboprophylaxis, extending thromboprophylaxis, stopping thromboprophylaxis, withholding COCs, or withholding HRT. Usual care: for populations where “usual care” was ambiguous, 2 scenarios were modeled, and separate recommendations were provided (see recommendations 7-10).
Ad hoc evidence profiles were developed to make the modeling results available to the panel and were included in the EtD. For each guideline question, the McMaster GRADE Centre prepared a GRADE EtD framework, using the GRADEpro Guideline Development Tool (www.gradepro.org).14,15,18 The EtD table summarized the results of systematic reviews of the literature that were updated or performed for this guideline as well as the modeling data. The EtD table addressed effects of interventions, values, and preferences (relative importance of outcomes), resource use (cost-effectiveness), equity, acceptability, and feasibility.
Panel evidence review and deliberation process
The panel members reviewed the evidence at various stages during the process. They first reviewed the source evidence (prevalence, risk association, and treatment effect) and commented on its completeness and directness. They subsequently reviewed the modeling results and absolute effects in evidence profiles and finally reviewed the EtD frameworks.
During the in-person or online meetings, the panel developed clinical recommendations based on the evidence summarized in the EtD tables. For each recommendation, the panel took a population perspective and came to consensus on the following: the certainty in the evidence, the balance of benefits and harms of the compared management options, and the assumptions about the values and preferences associated with the decision. The guideline panel considered the extent of resource use associated with alternative management options. The panel agreed on the recommendations (including direction and strength), remarks, and qualifications by consensus or, in rare instances, by voting (an 80% majority was required for a strong recommendation), based on the balance of all desirable and undesirable consequences. The final guidelines, including recommendations, were reviewed and approved by all members of the panel.
As described above and in Supplement 1, before the recommendations were finalized, 4 individuals stopped participating and 6 individuals were added to the guideline panel. These guidelines represent the consensus of the 10 individuals described in Supplement 1, whose participation continued through 2022.
Interpretation of strong and conditional recommendations
The recommendations are labeled as either “strong” or “conditional” according to the GRADE approach. The words “the guideline panel recommends” are used for strong recommendations and “the guideline panel suggests” for conditional recommendations. Table 2 provides the suggested interpretation of strong and conditional recommendations by patients, clinicians, and health care policymakers.
Interpretation of strong and conditional recommendations
| Implications for: . | Strong recommendation . | Conditional recommendation . |
|---|---|---|
| Patients | Most individuals in this situation would want the recommended course of action, and only a small proportion would not. | Most individuals in this situation would want the suggested course of action, but many would not. Decision aids may be useful in helping patients to make decisions consistent with their individual risks, values, and preferences. |
| Clinicians | Most individuals should follow the recommended course of action. Formal decision aids are not likely to be needed to help individual patients make decisions consistent with their values and preferences. | Different choices will be appropriate for individual patients; clinicians must help each patient arrive at a management decision consistent with the patient’s values and preferences. Decision aids may be useful in helping individuals to make decisions consistent with their individual risks, values, and preferences. |
| Policymakers | The recommendation can be adopted as policy in most situations. Adherence to this recommendation according to the guideline could be used as a quality criterion or performance indicator. | Policymaking will require substantial debate and involvement of various stakeholders. Performance measures should assess if decision making is appropriate. |
| Researchers | The recommendation is supported by credible research or other convincing judgments that make additional research unlikely to alter the recommendation. On occasion, a strong recommendation is based on low or very low certainty in the evidence. In such instances, further research may provide important information that alters the recommendations. | The recommendation is likely to be strengthened (for future updates or adaptation) by additional research. An evaluation of the conditions and criteria (and the related judgments, research evidence, and additional considerations) that determined the conditional (rather than strong) recommendation will help identify possible research gaps. |
| Implications for: . | Strong recommendation . | Conditional recommendation . |
|---|---|---|
| Patients | Most individuals in this situation would want the recommended course of action, and only a small proportion would not. | Most individuals in this situation would want the suggested course of action, but many would not. Decision aids may be useful in helping patients to make decisions consistent with their individual risks, values, and preferences. |
| Clinicians | Most individuals should follow the recommended course of action. Formal decision aids are not likely to be needed to help individual patients make decisions consistent with their values and preferences. | Different choices will be appropriate for individual patients; clinicians must help each patient arrive at a management decision consistent with the patient’s values and preferences. Decision aids may be useful in helping individuals to make decisions consistent with their individual risks, values, and preferences. |
| Policymakers | The recommendation can be adopted as policy in most situations. Adherence to this recommendation according to the guideline could be used as a quality criterion or performance indicator. | Policymaking will require substantial debate and involvement of various stakeholders. Performance measures should assess if decision making is appropriate. |
| Researchers | The recommendation is supported by credible research or other convincing judgments that make additional research unlikely to alter the recommendation. On occasion, a strong recommendation is based on low or very low certainty in the evidence. In such instances, further research may provide important information that alters the recommendations. | The recommendation is likely to be strengthened (for future updates or adaptation) by additional research. An evaluation of the conditions and criteria (and the related judgments, research evidence, and additional considerations) that determined the conditional (rather than strong) recommendation will help identify possible research gaps. |
Document review
In July 2021, the draft recommendations were made available on the ASH website for external review by stakeholders, including allied organizations, other medical professionals, patients, and the public. The content was published within a PDF file and within an online survey that included structured questions and fields for open comment. The survey was viewed 594 times and completed by 41 individuals. Three letters (or emails) were also received, including 1 letter signed by 75 individuals. The panel did not change the direction or strength of the recommendations; however, the panel revised supporting remarks and discussion. The panel then developed this guideline report, which was reviewed by the ASH Guideline Oversight Subcommittee in January 2023, approved by the Committee on Quality on 22 February 2023, and by the ASH officers on 1 March 2023, and then subjected to peer review.
How to use these guidelines
ASH guidelines are primarily intended to help clinicians make decisions about diagnostic strategies and associated management. Other purposes are to inform policy, education, and advocacy and to state future research needs. They may also be used by patients. These guidelines are not intended to serve or be construed as a standard of care. Clinicians must make decisions based on the clinical presentation of each individual patient, ideally through a shared process that considers the patient’s values and preferences with respect to the anticipated outcomes of the chosen option. Decisions may be constrained by the realities of a specific clinical setting and local resources, including but not limited to institutional policies, time limitations, and the availability of treatments. These guidelines may not include all appropriate methods of care for the clinical scenarios described. As science advances and new evidence becomes available, recommendations may become outdated. Following these guidelines cannot guarantee successful outcomes. ASH does not warrant or guarantee any products described in these guidelines.
Statements about the underlying values and preferences as well as qualifying remarks accompanying each recommendation are its integral parts and serve to facilitate a more accurate interpretation. They should never be omitted when recommendations from these guidelines are quoted or translated. Implementation of the guidelines may be facilitated by clinical decision support tools available from ASH. The use of these guidelines is also facilitated by the links to the EtD frameworks and interactive summary-of-findings tables in each section.
Recommendations
Thrombophilia testing for patients with symptomatic VTE
For patients with unprovoked VTE, should thrombophilia testing be performed to guide treatment duration?
Recommendation 1
For patients with unprovoked VTE who have completed primary short-term treatment, the ASH guideline panel suggests not to perform thrombophilia testing to guide the duration of anticoagulant treatment (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
Remarks:
In the ASH VTE treatment guideline,1 indefinite antithrombotic therapy is suggested for most patients with unprovoked VTE (recommendation 19).
A strategy with testing for thrombophilia would mean that patients with thrombophilia would receive indefinite anticoagulant treatment, and patients without thrombophilia would stop anticoagulant treatment.
This recommendation refers to testing for hereditary and acquired types of thrombophilia.
Summary of the evidence
We did not identify studies directly answering this question. The estimates of thrombophilia prevalence, the RR of VTE recurrence in patients with thrombophilia vs patients without thrombophilia and the effect of indefinite anticoagulant treatment are reported in Table 3. We identified 20 studies to assess the prevalence of any thrombophilia, 6 studies to estimate the risk association for recurrent VTE for patients with thrombophilia vs patients without thrombophilia, 4 RCTs to assess the effect of indefinite anticoagulation on VTE recurrence, and 11 RCTs to assess the effect of indefinite anticoagulation on major bleeding. We used 1 systematic review to estimate the overall risk for VTE recurrence for patients with any VTE when stopping anticoagulant therapy after completion of primary treatment. See the online evidence profile for study references.
Estimates used to calculate the effect of thrombophilia testing for patients with VTE
| . | Prevalence, median % (min-max) . | RR for VTE recurrence, positive vs negative (95% CI) . | Treatment effect for VTE recurrence, RR (95% CI) . | Treatment effect major bleeding, RR (95% CI) . |
|---|---|---|---|---|
| Any thrombophilia | 38.0 (21.6-59.5) | 1.65 (1.28-2.47) | 0.15 (0.10-0.23) | 2.17 (1.40-3.35) |
| FVL homozygous | 1.5 (0.3-3.1) | 2.10 (1.09-4.06) | ||
| FVL heterozygous | 17.5 (4.1-34.8) | 1.36 (1.19-1.57) | ||
| PGM | 6.1 (1.4-16.3) | 1.34 (1.05-1.71) | ||
| Antithrombin (AT) deficiency | 2.2 (0.2-8.7) | 2.07 (1.50-2.87) | ||
| Protein C (PC) deficiency | 2.5 (0.7-8.6) | 2.13 (1.26-3.59) | ||
| Protein S (PS) deficiency | 2.3 (0.7-7.3) | 1.30 (0.87-1.94) | ||
| AT, PC, or PS deficiency | 7.0 (2.5-18.4) | 1.62 (1.17-2.23) | ||
| APLA | 9.7 (1.9-19.4) | 1.92 (0.99-3.72) |
| . | Prevalence, median % (min-max) . | RR for VTE recurrence, positive vs negative (95% CI) . | Treatment effect for VTE recurrence, RR (95% CI) . | Treatment effect major bleeding, RR (95% CI) . |
|---|---|---|---|---|
| Any thrombophilia | 38.0 (21.6-59.5) | 1.65 (1.28-2.47) | 0.15 (0.10-0.23) | 2.17 (1.40-3.35) |
| FVL homozygous | 1.5 (0.3-3.1) | 2.10 (1.09-4.06) | ||
| FVL heterozygous | 17.5 (4.1-34.8) | 1.36 (1.19-1.57) | ||
| PGM | 6.1 (1.4-16.3) | 1.34 (1.05-1.71) | ||
| Antithrombin (AT) deficiency | 2.2 (0.2-8.7) | 2.07 (1.50-2.87) | ||
| Protein C (PC) deficiency | 2.5 (0.7-8.6) | 2.13 (1.26-3.59) | ||
| Protein S (PS) deficiency | 2.3 (0.7-7.3) | 1.30 (0.87-1.94) | ||
| AT, PC, or PS deficiency | 7.0 (2.5-18.4) | 1.62 (1.17-2.23) | ||
| APLA | 9.7 (1.9-19.4) | 1.92 (0.99-3.72) |
APLA, antiphospholipid antibody (including lupus anticoagulant).
Table 3 summarizes the assumptions on thrombophilia prevalence, the RR of recurrent VTE for thrombophilia vs no thrombophilia, and the effects of indefinite anticoagulant treatment on the risk of recurrent VTE and major bleeding. These estimates are used for all questions on symptomatic VTE at usual sites (recommendations 1-6).
The median prevalence of any hereditary thrombophilia (ie, heterozygous FVL, homozygous FVL, heterozygous PGM, antithrombin deficiency, protein C deficiency, or protein S deficiency) was 28.3%, and the median prevalence for antiphospholipid antibodies or lupus anticoagulants was 9.7%. Hence, the median prevalence of any thrombophilia, assuming no overlap, was 38.0% (minimum 21.6%; maximum 59.5%). The prevalence of all aforementioned individual effects was added up, and therefore, combinations of thrombophilia types are indirectly considered (probably overestimating their effect). For this reason and because of their estimated very low prevalence, homozygous PGM or the combination of heterozygous FVL and PGM are not specifically included.
The risk for recurrent VTE in patients with thrombophilia vs patients without thrombophilia was assessed for any hereditary thrombophilia (RR, 1.56; 95% CI, 1.31-1.86) and for APLAs/lupus anticoagulants (RR, 1.92; 95% CI, 0.99-3.72), which were then pooled in a weighted manner based on their prevalence (RR, 1.65; 95% CI, 1.28-2.47). Although for this question and recommendation, we focus on any thrombophilia, the RRs for specific thrombophilia types are also provided in Table 3 and range from 1.30 (95% CI, 0.87-1.94) for protein S deficiency to 2.13 (95% CI, 1.26-3.59) for protein C deficiency.
For the effect of indefinite anticoagulant treatment compared with stopping anticoagulant treatment after completion of primary treatment for VTE, we used the RR of recurrent VTE of 0.15 (95% CI, 0.10-0.23) as reported in the ASH guideline on the treatment of DVT or PE for the use of direct oral anticoagulant (DOAC). The RR of major bleeding with indefinite anticoagulant treatment was 2.17 (95% CI, 1.40-3.35), also based on included trials from the ASH guideline on the treatment of DVT or PE but excluding 1 trial assessing the effect of aspirin.
Specifically in patients not continuing anticoagulant therapy indefinitely, we estimated that the overall risk for VTE recurrence after unprovoked VTE was 100 per 1000 patients in the first year, based on 1 systematic review. We estimated the risk of major bleeding at 5 per 1000 patients at low risk and 15 per 1000 patients at high risk of bleeding per year, based on the lowest and highest observed rates among 11 RCTs.
The evidence profile and EtD framework are shown online at:
Benefits
We considered as comparator management strategy no thrombophilia testing and indefinite anticoagulant treatment for all patients with unprovoked symptomatic VTE as recommended by ASH. Therefore, the potential benefits of thrombophilia testing and only treating patients with thrombophilia would consist of treating fewer patients with indefinite anticoagulation and, thereby, preventing major bleeding. The calculations based on a total of 31 studies showed that a strategy of thrombophilia testing followed by indefinite anticoagulant treatment for patients with thrombophilia and stopping anticoagulant treatment for patients without thrombophilia would lead to 4 fewer major bleeds per 1000 patients at low risk of bleeding (95% CI, from 1 to 9 fewer) and 11 fewer major bleeds per 1000 patients at high risk of major bleeding (ranging from 2 to 28 fewer) per year.
Harms and burden
Under the assumption of indefinite anticoagulant treatment for all patients with unprovoked symptomatic VTE as a comparison, potential harms and burden of thrombophilia testing and only treating patients with thrombophilia would consist of treating fewer patients with indefinite anticoagulation, with a subsequent increase in the risk of recurrent VTE in those stopping anticoagulation after completion of primary treatment. The calculations based on a total of 24 studies showed that a strategy of thrombophilia testing followed by indefinite anticoagulant treatment for patients with thrombophilia and stopping anticoagulant treatment for patients without thrombophilia would lead to 42 more VTE recurrences per 1000 patients per year (ranging from 17 to 67 more).
Certainty in the evidence of effects
We rated the overall certainty in the evidence of effects as very low because our estimates were based on calculations with serious indirectness and imprecision of the estimates.
Other EtD criteria and considerations
The panel determined that on balance, with small desirable effects (preventing major bleeding) and moderate undesirable effects (more recurrent VTE), a strategy of not testing for thrombophilia and treating all patients with unprovoked VTE with indefinite anticoagulant treatment would probably be favored. The panel did not consider potential moderate savings of the intervention through the reduction of treatment costs.
Conclusions and research needs for this recommendation
The guideline panel acknowledges that some patients with unprovoked VTE may discontinue anticoagulant treatment after primary treatment of 3 months, whereas the assumptions of benefits and harms were made as if the entire population would continue anticoagulation indefinitely, as suggested in the 2020 ASH guidelines for the management of VTE.1
As a general conclusion, the guideline panel acknowledges that our recommendation is based on calculations with prevalence and RR estimates for recurrent VTE for any type of thrombophilia. Although specific high-risk thrombophilia types carry higher risks for recurrent VTE, their low prevalence will result in a small absolute effect on the entire population. In addition, the panel realizes that the prevalence of hereditary thrombophilia differs geographically. The information with median prevalence and ranges of prevalence provided in Table 3 can be used to estimate the effect in a specific (geographic) population as well as for specific thrombophilia defects.20
The panel determined that it would be valuable to have direct evidence from high-quality studies comparing these interventions, but no such study has been performed thus far.30
For patients with VTE provoked by surgery, should thrombophilia testing be performed to guide treatment duration?
Recommendation 2
For patients with VTE provoked by surgery who have completed primary short-term treatment, the ASH guideline panel suggests not to perform thrombophilia testing to determine the duration of anticoagulant treatment (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
Remarks:
According to the ASH VTE treatment guideline,1 most patients with VTE provoked by temporary risk factors will discontinue anticoagulant therapy after completion of the primary treatment.
A strategy with testing for thrombophilia would mean that patients with thrombophilia would receive indefinite anticoagulant treatment, and patients without thrombophilia would stop anticoagulant treatment after completion of primary short-term treatment.
This recommendation refers to testing for hereditary and acquired types of thrombophilia.
Summary of the evidence
We did not identify studies directly answering this question. For thrombophilia prevalence, the RR of patients with thrombophilia vs patients without thrombophilia, and the effect of indefinite anticoagulant treatment on VTE and major bleeding, the same estimates were used as in recommendation 1 (Table 3). See the online evidence profile for study references.
Without continuing anticoagulant therapy indefinitely, we estimated that the overall risk for VTE recurrence after VTE provoked by a surgical risk factor was 10 per 1000 in the first year, based on 1 systematic review. We estimated the risk of major bleeding at 5 per 1000 patients at low risk and 15 per 1000 patients at high risk of bleeding per year, based on the lowest and highest observed rates among 11 RCTs.
The evidence profile and EtD framework are shown online at:
Benefits
We considered as comparator management strategy no thrombophilia testing and stopping anticoagulant treatment after completion of primary treatment for all patients with symptomatic VTE provoked by surgery, as recommended by ASH. Therefore, potential benefits of thrombophilia testing and treating patients with thrombophilia with indefinite anticoagulation would be to reduce recurrent VTE. The calculations based on a total of 31 studies showed that a strategy of thrombophilia testing followed by indefinite anticoagulant treatment for patients with thrombophilia and stopping anticoagulant treatment for patients without thrombophilia would result in 4 fewer VTE recurrences per 1000 patients per year (ranging from 2 to 7 fewer).
Harms and burden
Under the assumption of stopping treatment in all patients as a comparator, the potential harms and burden of thrombophilia testing and treating patients with thrombophilia with indefinite anticoagulation consist of an increase in major bleeding. The calculations based on a total of 31 observational studies showed that a strategy of thrombophilia testing followed by indefinite anticoagulant treatment for patients with thrombophilia and stopping anticoagulant treatment for patients without thrombophilia would lead to 2 more major bleeds per 1000 patients at low risk of bleeding (ranging from 0 to 7 more) and 7 more major bleeds per 1000 patients at high risk of bleeding (ranging from 1 to 21 more) per year.
Certainty in the evidence of effects
We rated the overall certainty in the evidence of effects as very low because our estimates were based on calculations with serious indirectness and imprecision of the estimates.
Other EtD criteria and considerations
The panel determined that on balance, with trivial desirable effects (preventing recurrent VTE) and small undesirable effects (more major bleeding), neither testing for thrombophilia and treating patients with thrombophilia with symptomatic VTE provoked by a surgical risk factor with indefinite anticoagulation, nor no thrombophilia testing and stopping anticoagulant treatment in all, would be favored. The panel considered the potential moderate costs of the intervention by testing for thrombophilia and the subsequent treatment costs.
Conclusions and research needs for this recommendation
The guideline panel acknowledges the fact that some patients with provoked VTE may continue anticoagulant treatment after 3 to 6 months, whereas the assumptions of benefits and harms were made as if the entire population would discontinue anticoagulation, as suggested in the 2020 ASH guidelines for the management of VTE.1
Similar general conclusions as for recommendation 1 are valid for this recommendation. The information with median prevalence and ranges of prevalence provided in Table 3 can be used to estimate the effect in a specific (geographic) population as well as for specific thrombophilia defects.20
For patients with VTE provoked by a nonsurgical major transient risk factor, should thrombophilia testing be performed to guide treatment duration?
Recommendation 3
For patients with VTE provoked by a nonsurgical major transient risk factor who have completed primary short-term treatment, the ASH guideline panel suggests testing for thrombophilia to guide anticoagulant treatment duration. The panel suggests indefinite anticoagulant treatment for patients with thrombophilia and stopping anticoagulant treatment for patients without thrombophilia (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
Remarks:
According to the ASH VTE treatment guideline,1 most patients with VTE provoked by temporary risk factors will discontinue anticoagulant therapy after completion of the primary treatment.
Nonsurgical major transient risk factors: for example, confinement to bed in hospital for at least 3 days with an acute illness (“bathroom privileges only”), or a combination of minor transient risk factors such as admission to hospital for less than 3 days with an acute illness, confinement to bed out of hospital for at least 3 days with an acute illness, or leg injury associated with decreased mobility for at least 3 days. (Table 3 in the ASH 2020 VTE guidelines for treatment of DVT and PE1).
A strategy with testing for thrombophilia would mean that patients with thrombophilia would receive indefinite anticoagulant treatment, and patients without thrombophilia would stop anticoagulant treatment.
This recommendation refers to testing for hereditary and acquired types of thrombophilia.
For women with VTE provoked by pregnancy or postpartum, should thrombophilia testing be performed to guide treatment duration?
Recommendation 4
For women with VTE provoked by pregnancy or postpartum who have completed primary treatment, the ASH guideline panel suggests thrombophilia testing to guide anticoagulant treatment duration. The panel suggests indefinite anticoagulant treatment for women with thrombophilia and stopping anticoagulant treatment for women without thrombophilia (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
Remarks:
According to the ASH VTE treatment guideline,1 most patients with VTE provoked by temporary risk factors will discontinue anticoagulant therapy after completion of the primary treatment.
A strategy with testing for thrombophilia would mean that women with thrombophilia would receive indefinite anticoagulant treatment, and women without thrombophilia would stop anticoagulant treatment.
This recommendation refers to testing for hereditary and acquired types of thrombophilia.
For women with VTE associated with COCs, should thrombophilia testing be performed to guide treatment duration?
Recommendation 5
For women with VTE associated with COCs who have completed primary short-term treatment, the ASH guideline panel suggests testing for thrombophilia to guide anticoagulant treatment duration. The panel suggests indefinite anticoagulant treatment for women with thrombophilia and stopping anticoagulant treatment for women without thrombophilia (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
Remarks:
According to the ASH VTE treatment guideline,1 most patients with VTE provoked by temporary risk factors will discontinue anticoagulant therapy after completion of the primary treatment.
A strategy with testing for thrombophilia would mean that women with thrombophilia would receive indefinite anticoagulant treatment, and women without thrombophilia would stop anticoagulant treatment.
This recommendation refers to testing for hereditary and acquired types of thrombophilia.
Summary of the evidence
We did not identify direct studies to answer these questions. Here, we clustered the description of the evidence for the questions of (a) VTE provoked by a nonsurgical major transient risk factor, (b) VTE provoked by pregnancy or the postpartum period, and (c) VTE associated with use of COCs, as the same indirect evidence was used for all.
For thrombophilia prevalence, the RR of patients with thrombophilia vs patients without thrombophilia, and the effect of indefinite anticoagulant treatment, the same estimates were used as in recommendation 1 (Table 3). See the online evidence profiles for study references.
The overall risk for VTE recurrence after VTE provoked by a nonsurgical major transient risk factor, pregnancy or postpartum, or associated with COCs was estimated at 50 per 1000 in the first year after acute VTE, based on 1 systematic review. We estimated the risk of major bleeding at 5 per 1000 in patients at low risk and 15 per 1000 in patients at high risk of bleeding, based on the lowest and highest observed rates among 11 RCTs.
The evidence profiles and EtD frameworks are shown online at:
Recommendation 3
Recommendation 4
Recommendation 5
Benefits
We considered as comparator management strategy no thrombophilia testing and stopping anticoagulant treatment after primary treatment for all patients with symptomatic VTE provoked by a nonsurgical major transient risk factor, pregnancy or postpartum, or associated with COCs. Therefore, the potential benefits of thrombophilia testing and treating patients with thrombophilia with indefinite anticoagulation would be to reduce recurrent VTE. The calculations based on a total of 24 studies showed that a strategy of thrombophilia testing followed by indefinite anticoagulant treatment for patients with thrombophilia and stopping anticoagulant treatment for patients without thrombophilia would result in 21 fewer VTE recurrences per 1000 patients per year (ranging from 10 to 35 fewer). Of the 21 of 1000 VTE recurrences that would be prevented, 13 of 1000 would be prevented by treating those who have FVL or PTM.
Harms and burden
Potential harms and burden of thrombophilia testing and treating patients with thrombophilia with indefinite anticoagulation include an increase in major bleeding. The calculations based on a total of 31 studies showed that a strategy of thrombophilia testing followed by indefinite anticoagulant treatment for patients with thrombophilia and stopping anticoagulant treatment for patients without thrombophilia would lead to 2 more major bleeds per 1000 patients at low risk of bleeding (ranging from 0 to 7 more) and 7 more major bleeds per 1000 patients at high risk of bleeding (ranging from 1 to 21 more) per year.
Certainty in the evidence of effects
We rated the overall certainty in the evidence of effects as very low because our estimates were based on calculations, with serious indirectness and imprecision of the estimates.
Other EtD criteria and considerations
The panel determined that on balance, with small desirable effects (preventing recurrent VTE) and trivial (for pregnancy- or postpartum- or COC-associated VTE) to small (for nonsurgical provoked VTE) undesirable effects (more major bleeding), a strategy of testing for thrombophilia and treating patients with thrombophilia with symptomatic VTE provoked by a nonhormonal risk factor, pregnancy or postpartum, or associated with COCs with indefinite anticoagulation would probably be favored. The panel did consider the potential moderate costs of the intervention by testing for thrombophilia and the subsequent treatment costs. For women with thrombophilia with symptomatic VTE provoked by pregnancy or postpartum, the panel did not consider the impact of the choice of anticoagulant regimen while breastfeeding. The intervention of thrombophilia testing was considered acceptable by patients and health care providers and probably feasible, although several studies have described inappropriate and inadequate implementation of thrombophilia testing at the local level.
Conclusions and research needs for this recommendation
For the recommendations on thrombophilia testing for VTE provoked by a nonsurgical major transient risk factor, pregnancy, postpartum, or oral contraceptives, the evidence on the absolute risk of recurrent VTE was based on meta-analyses of observational evidence that clustered various types of such provoking risk factors, whereas there may be heterogeneity between the impact of these types of provoking risk factors on recurrent VTE.
The guideline panel also acknowledges the fact that some patients with VTE provoked by a nonsurgical major transient risk factor, pregnancy, postpartum, or oral contraceptives may continue anticoagulant treatment after 3 to 6 months, whereas the assumptions of benefits and harms were made as if the entire population would discontinue anticoagulation, as suggested in the 2020 ASH guidelines for the management of VTE.1
Similar general conclusions as for recommendation 1 are valid for this recommendation. The information with median prevalence and ranges of prevalence provided in Table 3 can be used to estimate the effect in a specific (geographic) population as well as for specific thrombophilia defects.20
Should thrombophilia testing be performed for patients with an unspecified type of VTE to guide treatment duration?
Recommendation 6
For patients with an unspecified type of VTE who have completed primary short-term treatment, the ASH guideline panel suggests not performing thrombophilia testing to guide anticoagulant treatment duration (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
Remarks:
Whenever anticoagulant treatment decisions are being made without considering whether the VTE is provoked or unprovoked, it is advisable not to test for thrombophilia, to start treatment, and to refer the patient to an expert for further decision making.
Thrombosis experts would consider the population “with an unspecified type of VTE” (ie, without reference to provoked or unprovoked) as theoretical because determining if a clot is provoked or unprovoked is a standard way to stratify the risk of VTE recurrence and hence guide treatment decisions. However, in general clinical practice, which is the setting where thrombophilia testing is frequently performed, VTE is often managed regardless of circumstances qualifying the VTE as provoked or unprovoked (an unspecified type of VTE), and for this reason, the panel decided to address this question.
A strategy with testing for thrombophilia would mean that patients with thrombophilia would receive indefinite anticoagulant treatment, and patients without thrombophilia would stop anticoagulant treatment.
This recommendation refers to testing for hereditary and acquired types of thrombophilia.
Summary of the evidence
We did not identify direct studies to answer this question. For thrombophilia prevalence, the RR of patients with thrombophilia vs patients without thrombophilia, and the effect of indefinite anticoagulant treatment, the same estimates were used as in recommendation 1 (Table 3). See the online evidence profile for study references.
Without continuing anticoagulant therapy indefinitely, we estimated that the overall risk for VTE recurrence after any VTE was 75 per 1000 patients in the first year, based on 1 systematic review. We estimated the risk of major bleeding at 5 per 1000 patients at low risk and 15 per 1000 patients at high risk of bleeding in the first year, based on the lowest and highest observed rates among 11 RCTs.
The evidence profile and EtD framework are shown online at:
Benefits
We used a strategy of no thrombophilia testing and indefinite anticoagulant treatment for all patients with any symptomatic VTE as the comparison. Therefore, the potential benefits of thrombophilia testing and only treating patients with thrombophilia would consist of treating fewer patients with indefinite anticoagulation and preventing major bleeding. The calculations based on a total of 31 studies showed that a strategy of thrombophilia testing followed by indefinite anticoagulant treatment for patients with thrombophilia and stopping anticoagulant treatment for patients without thrombophilia would lead to 4 fewer major bleeds per 1000 patients at low risk of bleeding (ranging from 1 to 9 fewer), and to 11 fewer major bleeds per 1000 patients at high risk of major bleeding (ranging from 2 to 28 fewer) per year.
Harms and burden
We used a strategy of no thrombophilia testing and indefinite anticoagulant treatment for all patients with any symptomatic VTE as the comparison. Therefore, the potential harms and burden of thrombophilia testing and only treating patients with thrombophilia would consist of treating fewer patients with indefinite anticoagulation, with a subsequent increase in the risk of recurrent VTE in those stopping anticoagulation after completion of primary treatment. The calculations based on a total of 24 studies showed that a strategy of thrombophilia testing followed by indefinite anticoagulant treatment for patients with thrombophilia and stopping anticoagulant treatment for patients without thrombophilia would lead to 32 more recurrent VTE per 1000 patients per year (ranging from 12 to 50 more).
Certainty in the evidence of effects
We rated the overall certainty in the evidence of effects as very low because our estimates were based on modeling of several data points and their dispersion, with serious indirectness and imprecision of the estimates.
Other EtD criteria and considerations
The panel determined that on balance, with small desirable effects (preventing major bleeding) and moderate undesirable effects (more recurrent VTE), a strategy of not testing for thrombophilia and treating all patients with an unspecified type of symptomatic VTE with indefinite anticoagulant treatment would probably be favored. The panel did not consider the potential moderate savings of the intervention through the reduction of treatment costs.
The guideline panel acknowledges that “an unspecified type of VTE” may be theoretical rather than real and that the assumed comparison (no thrombophilia testing and indefinite anticoagulant treatment in all patients) may also be theoretical and may be an overestimation because patients with a provoked first VTE will generally discontinue anticoagulant treatment after 3 to 6 months. However, recommending not to test for thrombophilia when the VTE is yet unclassified was judged to be important by the panel. Indeed, it is to be hoped that the patient will be referred at some point to a specialist for assessing the optimal duration of anticoagulation; the decision about the appropriateness of testing for thrombophilia would be better delayed until then. In other words, the present recommendations should be read as follows: whenever anticoagulant treatment decisions are being made without considering whether the VTE is provoked or unprovoked, it is advisable not to test for thrombophilia, to start treatment, and to refer the patient to an expert for further decision making.
Conclusions and research needs for this recommendation
The guideline panel acknowledges the fact that most patients with provoked VTE and some patients with unprovoked VTE may discontinue anticoagulant treatment after 3 to 6 months, whereas the assumptions of benefits and harms were made as if the entire population would continue anticoagulation indefinitely.
Patients with symptomatic VTE at unusual sites
Unusual site thrombosis is a rare and serious event that often triggers thrombophilia testing. For this guideline, we considered acute cerebral venous thrombosis as well as acute splanchnic venous thrombosis in the absence of liver cirrhosis. Because guidelines are indecisive regarding the optimal duration of anticoagulant therapy after such events, we used 2 scenarios as a comparison: either stopping anticoagulation after completion of primary treatment of thrombosis in all patients or indefinite duration anticoagulation in all patients.31,32 As continuing or discontinuing anticoagulant treatment varies with the local standard and is often individualized based on risk, the panel explored what the contribution of testing for thrombophilia could be in this setting. For the sake of clarity, the panel issued 2 recommendations for both cerebral venous thrombosis (CVT) and splanchnic venous thrombosis. Please note that the apparent discordances between recommendations 7 and 8 and between recommendations 9 and 10 are due to the different comparator being used, which relates to the overall uncertainty about how to treat these rare conditions. Finally, note that the panel did not consider thrombophilic conditions outside the context of this article, such as the JAK2 V617F mutation or paroxysmal nocturnal hemoglobinuria, which are sometimes considered in these specific settings.
For patients with CVT planning to discontinue anticoagulation, should thrombophilia testing be performed to guide treatment duration?
Recommendation 7
For patients with CVT who have completed primary treatment in a setting where anticoagulation would be discontinued, the ASH guideline panel suggests thrombophilia testing to guide anticoagulant treatment duration. The panel suggests indefinite anticoagulation for patients with thrombophilia (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
Remarks:
A strategy with testing for thrombophilia would mean that patients with thrombophilia would receive indefinite anticoagulant treatment, and patients without thrombophilia would stop anticoagulant treatment.
This recommendation refers to testing for hereditary and acquired types of thrombophilia.
This recommendation addresses settings where the standard of care for patients with CVT is stopping anticoagulant treatment; the ASH guideline panel provides a separate recommendation for settings where the standard of care is indefinite anticoagulant treatment (recommendation 8).
For patients with CVT planning to continue anticoagulation indefinitely, should thrombophilia testing be performed to guide treatment duration?
Recommendation 8
For patients with CVT who have completed primary treatment in a setting where anticoagulation would be continued indefinitely, the ASH guideline panel suggests not to perform thrombophilia testing to guide anticoagulant treatment duration (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
Remarks:
A strategy with testing for thrombophilia would mean that patients with thrombophilia would receive indefinite anticoagulant treatment, and patients without thrombophilia would stop anticoagulant treatment.
This recommendation refers to testing for hereditary and acquired types of thrombophilia.
This recommendation addresses settings where the standard of care for patients with CVT is indefinite anticoagulant treatment; the ASH guideline panel provides a separate recommendation for settings where the standard of care is stopping anticoagulant treatment (recommendation 7).
Summary of the evidence
We did not identify direct studies to answer the question of the benefit of thrombophilia testing for patients with CVT. For patients with CVT, we were uncertain if the comparison should be limited duration of anticoagulant therapy or indefinite duration of anticoagulant therapy in all patients. The 2017 European Stroke Organization guideline for the diagnosis and treatment of CVT suggests “using oral anticoagulants (vitamin K antagonists) for a variable period (3-12 months) after CVT to prevent recurrent CVT and other venous thromboembolic events,” as a weak recommendation based on very low-quality evidence.32 As a remark, the guideline also states that “patients with recurrent venous thrombosis or with an associated prothrombotic condition with a high thrombotic risk may need permanent anticoagulation.” We therefore chose to answer the question using 2 scenarios: a strategy of thrombophilia testing compared with stopping anticoagulant treatment after completion of primary treatment in all patients, and a strategy of thrombophilia testing compared with indefinite anticoagulation in all patients. The effects of thrombophilia testing and a subsequent strategy of stopping anticoagulant treatment for patients without thrombophilia only, or indefinite anticoagulant treatment for patients with thrombophilia only, were indirectly calculated using 3 observational studies for thrombophilia prevalence unique to patients with CVT, RR of recurrent VTE in those with positive vs negative results for thrombophilia, and the effect of indefinite treatment, as detailed in Table 3. See the online evidence profiles for study references.
The overall risk for VTE recurrence after CVT was estimated at 38 per 1000 in the first year, based on 4 observational studies. We estimated the risk of major bleeding at 5 per 1000 in patients at low risk and 15 per 1000 in patients at high risk of bleeding, based on the lowest and highest observed rates among 11 RCTs.
The evidence profiles and EtD frameworks are shown online at:
Recommendation 7
Recommendation 8
Benefits
The unifying concept of benefits underlying recommendations 7 and 8 is that the impact of recurrent events for patients with CVT and thrombophilia is higher than we would normally accept. Consequently, when using a strategy of no thrombophilia testing and stopping anticoagulant treatment after completion of primary treatment for all patients with CVT as the comparison, the potential benefits of thrombophilia testing and treating patients with thrombophilia with indefinite anticoagulation would be to reduce recurrent VTE. The calculations based on a total of 17 studies showed that a strategy of thrombophilia testing followed by indefinite anticoagulant treatment for patients with thrombophilia and stopping anticoagulant treatment for patients without thrombophilia would result in 18 per 1000 fewer recurrent VTE (ranging from 14 to 23 fewer) per year compared with a no-testing strategy.
In contrast, when using a strategy of no thrombophilia testing and indefinite anticoagulant treatment for all patients with CVT as the comparison, the potential benefits of thrombophilia testing and only treating patients with thrombophilia would be less major bleeding because fewer patients would be treated with indefinite anticoagulation. The calculations based on a total of 15 studies showed that a strategy of thrombophilia testing followed by indefinite anticoagulant treatment for patients with thrombophilia and stopping anticoagulant treatment for patients without thrombophilia would lead to 3 per 1000 fewer major bleeds in patients at low risk of bleeding (ranging from 1 to 7 fewer) and to 10 fewer major bleeds in patients at high risk of major bleeding (ranging from 3 to 20 fewer) per year compared with a no-testing strategy.
Harms and burden
Under the assumption of no thrombophilia testing and stopping anticoagulant treatment after completion of primary treatment for all patients with CVT as the comparison, the potential harms and burden of thrombophilia testing and continuing anticoagulant treatment for patients with thrombophilia are an increase in major bleeding. The calculations based on a total of 15 studies showed that a strategy of thrombophilia testing followed by indefinite anticoagulant treatment for patients with thrombophilia and stopping anticoagulant treatment for patients without thrombophilia would lead to 3 per 1000 more major bleeds in patients at low risk (ranging from 1 to 5 fewer) and 8 per 1000 more in patients at high risk of bleeding (ranging from 3 to 16 fewer) per year compared with a no-testing strategy.
In contrast, under the assumption of no thrombophilia testing and indefinite anticoagulant treatment for all patients with CVT as the comparison, the potential harms and burden of thrombophilia testing and only treating patients with thrombophilia would consist of treating fewer patients with indefinite anticoagulation, with a subsequent increase in the risk of recurrent VTE in those stopping anticoagulation. The calculations based on a total of 17 studies showed that a strategy of thrombophilia testing followed by indefinite anticoagulant treatment for patients with thrombophilia and stopping anticoagulant treatment for patients without thrombophilia would lead to 14 per 1000 more recurrent VTE (ranging from 10 to 18 more) per year compared with a no-testing strategy.
Certainty in the evidence of effects
We rated the overall certainty in the evidence of effects as very low because our estimates were based on calculations with serious indirectness and imprecision of the estimates.
Other EtD criteria and considerations
When balancing risk and benefits, costs and burden of care, and patient preferences under the assumption of stopping anticoagulant treatment after completion of primary treatment for all patients with CVT, the panel determined that the balance of small desirable effects (preventing recurrent VTE) and trivial undesirable effects (more major bleeding) would probably favor a strategy of testing for thrombophilia and treating patients with thrombophilia with indefinite anticoagulation and stopping for patients who are negative for thrombophilia. The panel did not consider the potential moderate costs of the intervention by an increase in testing and treatment costs.
In contrast, when balancing risk and benefits, costs and burden of care, and patient preferences under the assumption of indefinite anticoagulation in all patients with CVT, the panel determined that the balance of trivial desirable effects (preventing major bleeding) and small undesirable effects (more recurrent VTE) would probably favor a strategy of not testing for thrombophilia and treating all patients with CVT with indefinite anticoagulation. The panel did not consider the potential moderate savings of the intervention through the reduction of treatment costs.
The panel put a large weight on patient preference to warrant optimal treatment for the patient with thrombophilia, which would require testing in a setting where the standard of care would be a discrete treatment period and would not require testing in a setting where every patient would be offered indefinite treatment.
Conclusions and research needs for this recommendation
The absolute risk of recurrent VTE after CVT is uncertain, and the panel used the best available indirect evidence. More research into the risk of recurrent VTE and its association with prognostic variables, as well as the optimal duration of anticoagulant therapy after acute CVT, is needed.
Similar general conclusions as for recommendation 1 are valid for this recommendation. The information with median prevalence and ranges of prevalence provided in Table 3 can be used to estimate the effect in a specific (geographic) population as well as for specific thrombophilia defects.20
For patients with splanchnic venous thrombosis without cirrhosis planning to discontinue anticoagulation, should thrombophilia testing be performed to guide treatment duration?
Recommendation 9
For patients with splanchnic venous thrombosis who have completed primary treatment in a setting where anticoagulation would be discontinued, the ASH guideline panel suggests thrombophilia testing to guide anticoagulant treatment duration. The panel suggests indefinite anticoagulation for patients with thrombophilia (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
Remarks:
A strategy with testing for thrombophilia would mean that patients with thrombophilia would receive indefinite anticoagulant treatment, and patients without thrombophilia would stop anticoagulant treatment.
This recommendation refers to testing for hereditary and acquired types of thrombophilia.
This recommendation addresses settings where the standard of care for patients with splanchnic venous thrombosis is stopping anticoagulant treatment; the ASH guideline panel provides a separate recommendation for settings where the standard of care is indefinite anticoagulant treatment (recommendation 10).
For patients with splanchnic venous thrombosis without cirrhosis planning to continue anticoagulation indefinitely, should thrombophilia testing be performed to guide treatment duration?
Recommendation 10
For patients with splanchnic venous thrombosis who have completed primary treatment in a setting where anticoagulation would be continued indefinitely, the ASH guideline panel suggests not performing thrombophilia testing to guide anticoagulant treatment duration (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
Remarks:
A strategy with testing for thrombophilia would mean that patients with thrombophilia would receive indefinite anticoagulant treatment, and patients without thrombophilia would stop anticoagulant treatment.
This recommendation refers to testing for hereditary and acquired types of thrombophilia.
This recommendation addresses settings where the standard of care for patients with splanchnic venous thrombosis is indefinite anticoagulant treatment; the ASH guideline panel provides a separate recommendation for settings where the standard of care is stopping anticoagulant treatment (recommendation 9).
Summary of the evidence
We did not identify direct studies to answer the question of the benefit of thrombophilia testing for patients with acute splanchnic venous thrombosis. For patients with acute splanchnic venous thrombosis, we were uncertain about the appropriate comparison, that is, limited duration of anticoagulant therapy or indefinite duration of anticoagulant therapy in all patients. The 2020 ISTH SSC Subcommittee Control of Anticoagulation Guidance on Antithrombotic therapy for splanchnic venous thrombosis recommends “anticoagulant therapy for at least 3 to 6 months, irrespective of thrombosis extension and underlying risk factors,” and “longer courses of anticoagulation or indefinite anticoagulant treatment for patients with thrombosis progression or recurrence after treatment discontinuation, unprovoked splanchnic venous thrombosis, or persistent risk factors,” without providing the formal strength of the recommendation.31 We chose to answer the question using 2 scenarios: a strategy of thrombophilia testing compared with stopping anticoagulant treatment after completion of primary treatment in all patients, and a strategy of thrombophilia testing compared with indefinite duration anticoagulation in all patients. The effects of thrombophilia testing and a subsequent strategy of stopping anticoagulant treatment for patients without thrombophilia only or indefinite anticoagulant treatment for patients with thrombophilia only were indirectly calculated using 3 observational studies for thrombophilia prevalence unique to patients with splanchnic venous thrombosis, RR of recurrent VTE in those with a positive vs negative result for thrombophilia, and the effect of indefinite treatment, as detailed in Table 3. See the online evidence profiles for study references.
The overall risk for VTE recurrence after splanchnic venous thrombosis was estimated at 27 per 1000 in the first year, based on 2 observational studies. We estimated the risk of major bleeding at 5 per 1000 in patients at low risk and 15 per 1000 in patients at high risk of bleeding, based on the lowest and highest observed rates among 11 RCTs.
The evidence profiles and EtD frameworks are shown online at:
Recommendation 9
Recommendation 10
Benefits
The unifying concept of the benefits underlying recommendations 9 and 10 is that the impact of recurrent events for patients with splanchnic venous thrombosis and thrombophilia is higher than we would normally accept. Therefore, when using a strategy of no thrombophilia testing and stopping anticoagulant treatment after completion of primary treatment for all patients with acute splanchnic venous thrombosis as the comparison, the potential benefits of thrombophilia testing and treating patients with thrombophilia with indefinite anticoagulation would be reducing recurrent VTE. The calculations based on a total of 18 studies showed that a strategy of thrombophilia testing followed by indefinite anticoagulant treatment for patients with thrombophilia and stopping anticoagulant treatment for patients without thrombophilia would result in 23 per 1000 fewer recurrent VTE (ranging from 14 to 36 fewer) per year compared with a no-testing strategy.
In contrast, when using a strategy of no thrombophilia testing and indefinite anticoagulant treatment for all patients with acute splanchnic venous thrombosis as the comparison, the potential benefits of thrombophilia testing and only treating patients with thrombophilia would consist of treating fewer patients with indefinite anticoagulation and preventing major bleeding. The calculations based on a total of 18 studies showed that a strategy of thrombophilia testing followed by indefinite anticoagulant treatment for patients with thrombophilia and stopping anticoagulant treatment for patients without thrombophilia would lead to 3 per 1000 fewer major bleeds for patients at low risk of bleeding (ranging from 1 to 8 fewer) and to 10 fewer major bleeds for patients at high risk of major bleeding (ranging from 2 to 24 fewer) per year compared with a no-testing strategy.
Harms and burden
Under the assumption of a strategy of no thrombophilia testing and stopping anticoagulant treatment after completion of primary treatment for all patients with acute splanchnic venous thrombosis as the comparison, the potential harms and burden of thrombophilia testing and indefinite anticoagulant treatment for patients with thrombophilia are an increase in major bleeding. The calculations based on a total of 18 studies showed that a strategy of thrombophilia testing followed by indefinite anticoagulant treatment for patients with thrombophilia and stopping anticoagulant treatment for patients without thrombophilia would lead to 2 per 1000 more major bleeds in patients at low risk (ranging from 1 to 7 more) and 7 per 1000 more in patients at high risk of bleeding (ranging from 2 to 22 more) per year compared with a no-testing strategy.
In contrast, under the assumption of no thrombophilia testing and indefinite anticoagulant treatment for all patients with acute splanchnic venous thrombosis as the comparison, the potential harms and burden of thrombophilia testing and only treating patients with thrombophilia would consist of treating fewer patients with indefinite anticoagulation, with a subsequent increase in the risk of recurrent VTE in those stopping anticoagulation. The calculations based on a total of 18 studies showed that a strategy of thrombophilia testing followed by indefinite anticoagulant treatment for patients with thrombophilia and stopping anticoagulant treatment for patients without thrombophilia would lead to 20 more recurrent VTE per 1000 patients (ranging from 8 to 29 more) per year compared with a no-testing strategy.
Certainty in the evidence of effects
We rated the overall certainty in the evidence of effects as very low because our estimates were based on calculations with serious indirectness and imprecision of the estimates.
Other EtD criteria and considerations
When balancing risk and benefits, costs and burden of care, and patient preferences under the assumption of stopping anticoagulant treatment after completion of primary treatment for all patients with acute splanchnic venous thrombosis, the panel determined that the balance of small desirable effects (preventing recurrent VTE) and trivial undesirable effects (more major bleeding) would probably favor a strategy of testing for thrombophilia and treating patients with thrombophilia with indefinite anticoagulation and stopping for patients with a negative result for thrombophilia. The panel did not consider the potential moderate costs of the intervention by an increase in testing and treatment costs.
In contrast, when balancing risk and benefits, costs and burden of care, and patient preferences under the assumption of indefinite anticoagulation in all patients with acute splanchnic venous thrombosis, the panel determined that the balance of small desirable effects (preventing major bleeding) and moderate undesirable effects (more recurrent VTE) would probably favor a strategy of not testing for thrombophilia and treating all patients with acute splanchnic venous thrombosis with indefinite anticoagulation. The panel did not consider the potential moderate savings of the intervention through the reduction of treatment costs.
The panel put a large weight on the patient preference to warrant optimal treatment for the patient with thrombophilia, which would require testing in a setting where the standard of care would be a discrete treatment period and would not require testing in a setting where every patient would be offered indefinite treatment.
Conclusions and research needs for this recommendation
The absolute risk of recurrent VTE after acute splanchnic venous thrombosis is uncertain, and the panel used the best indirect evidence available. More research into the risk of recurrent VTE and its association with prognostic variables, as well as the optimal duration of anticoagulant therapy after acute splanchnic venous thrombosis is much needed.
Thrombophilia testing for individuals with a family history of VTE and/or thrombophilia
Introduction
In families of patients with VTE, people often ask whether it is useful to test asymptomatic relatives for thrombophilia. As discussed in the “Introduction,” the relevant question and aim of the current guideline is to assess whether thrombophilia testing and tailoring management to the test result would improve patient-important outcomes. For instance, should an asymptomatic relative with thrombophilia receive thromboprophylaxis during minor VTE-provoking risk episodes that generally do not require prophylaxis, such as immobility or minor injury, illness, or infection? For women, should their thrombophilia status affect choices about hormonal contraception or dictate a need for prophylaxis around pregnancy and the postpartum period? In addition, sometimes hereditary thrombophilia is known in a family without anyone having experienced VTE. Examples of such clinical scenarios are testing for thrombophilia in women with recurrent miscarriage or other pregnancy complications, in young patients with arterial thrombosis, or population testing. The question is whether asymptomatic relatives of someone known to have thrombophilia would benefit from thrombophilia testing.
The panel took the perspective that risk changed by testing for thrombophilia and tailoring management matters more than the absolute risk of events associated with thrombophilia.
Regarding the testing strategy, the panel modeled 2 scenarios: whether the patient with VTE (referred to as proband) is known to have a specific thrombophilia or whether the thrombophilia status of the proband is unknown. If a specific thrombophilia is known in the proband, the question arises if the relative should be tested for the specific defect only (selective testing) or tested for all hereditary thrombophilias. These questions are all closely related, and in general, similar evidence is used for all these questions. However, the results in terms of the number needed to test and treat to prevent 1 VTE will differ, as thrombophilias have different prevalence in the population and are associated with a different risk of VTE. Furthermore, the prevalence of having any thrombophilia is constant in the population, but the prevalence of having the specific thrombophilia defect running in a family varies with the degree of the relation (ie, there is a Mendelian prevalence of 50% in first-degree relatives [parents, offspring, and siblings] and 25% in second-degree relatives [grandparents and grandchildren, half-siblings, aunts/uncles, and nieces/nephews] of a proband with a known defect; there are obvious exceptions for homozygous probands or those carrying multiple defects).
In this guideline, relatives in this scenario are referred to as individuals with a positive family history of VTE and/or thrombophilia.
Several clinical scenarios are possible including the need for pharmacological thromboprophylaxis during minor VTE risk–provoking factors, such as immobility, minor injury, illness, or infection (recommendations 11-14), avoidance of hormones for women intending to use hormones (recommendations 15-20), the need for pharmacological thromboprophylaxis during pregnancy or postpartum (recommendations 21 and 22), or for patients with cancer who would otherwise not qualify for thromboprophylaxis (recommendation 23).
Thrombophilia testing for individuals with a family history of VTE and/or family history of thrombophilia to prevent VTE associated with exposure to minor risk factors
For individuals with a family history of VTE and thrombophilia, should selective thrombophilia testing be performed to guide the use of thromboprophylaxis for a minor provoking risk factor?
Recommendation 11
For individuals with a family history of VTE and known FVL or PGM (low-risk thrombophilia) who have a minor provoking risk factor for VTE (eg, immobility or minor injury, illness, or infection), the ASH guideline panel suggests not testing for the known familial thrombophilia to guide thromboprophylaxis (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
For individuals with a family history of VTE and known antithrombin, protein C, or protein S deficiency (high-risk thrombophilia) who have a minor provoking risk factor for VTE, the ASH guideline panel suggests testing for the known familial thrombophilia. The panel suggests thromboprophylaxis in individuals with thrombophilia and no thromboprophylaxis in individuals without thrombophilia (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
Remarks:
A strategy with selective testing for the known familial thrombophilia type would mean that individuals with thrombophilia would receive thromboprophylaxis for a minor provoking risk factor, and individuals without thrombophilia would receive no thromboprophylaxis.
A positive family history is defined as having a first- or second-degree relative with VTE or thrombophilia.
These recommendations do not address homozygous defects or combinations of thrombophilia types.
This recommendation does not consider the time it takes to perform the test and is based on the assumption that thrombophilia test results are available at the time the individual is at risk for VTE because of a minor provoking risk factor.
These recommendations refer to selective testing for the known familial thrombophilia type. A separate question in this guideline addressed testing for all hereditary thrombophilias (using a panel of tests) in this population (recommendation 12), and the resulting recommendations are the same. It is most sensible to selectively test for the known familial thrombophilia (recommendation 11) rather than test for the entire panel (recommendation 12), because of the trivial additional number of VTE episodes prevented and major bleeds caused by a strategy of panel testing for all hereditary thrombophilias.
For individuals with a family history of VTE and thrombophilia, should thrombophilia testing (using a panel of tests) be performed to guide the use of thromboprophylaxis for a minor provoking risk factor?
Recommendation 12
For individuals with a family history of VTE and known FVL or PGM (low-risk thrombophilia) who have a minor provoking risk factor for VTE (eg, immobility or minor injury, illness, or infection), the ASH guideline panel suggests not testing for all hereditary thrombophilias to guide thromboprophylaxis (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
For individuals with a family history of VTE and known antithrombin, protein C, or protein S deficiency (high-risk thrombophilia) who have a minor provoking risk factor for VTE, the ASH guideline panel suggests testing for all hereditary thrombophilias (using a panel of tests). The panel suggests thromboprophylaxis in individuals with thrombophilia and no thromboprophylaxis for a minor provoking risk factor in individuals without thrombophilia (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
Remarks:
A strategy with testing for hereditary thrombophilia (using a panel of tests) would mean that individuals with thrombophilia receive thromboprophylaxis or a minor provoking risk factor, and individuals without thrombophilia would receive no thromboprophylaxis.
A positive family history is defined as having a first- or second-degree relative with VTE or thrombophilia.
These recommendations do not address homozygous defects or combinations of thrombophilia types.
This recommendation does not consider the time it takes to perform the test and is based on the assumption that thrombophilia test results are available at the time the individual is at risk for VTE because of a minor provoking risk factor.
These recommendations refer to testing for all hereditary thrombophilias using a panel of tests. A separate question in this guideline addressed selective testing only for the known familial thrombophilia type in this population (recommendation 11), and the resulting recommendations are the same.
It is most sensible to selectively test for the known familial thrombophilia (recommendation 11) rather than test for the entire panel (recommendation 12) because of the trivial additional number of VTE episodes prevented and major bleeds caused by a strategy of panel testing for all hereditary thrombophilias.
Summary of the evidence
We did not identify direct studies to answer these questions. The effect of selective thrombophilia testing (recommendation 11) and a subsequent strategy of providing thromboprophylaxis to individuals with thrombophilia and not to individuals without thrombophilia during risk situations was indirectly calculated using the known thrombophilia prevalence in families (ie, 50% in individuals with a first-degree family history of VTE, and 25% in individuals with a second-degree family history of VTE), RRs for a first VTE event in individuals with thrombophilia vs individuals without thrombophilia based on 4 to 9 observational studies (depending on the thrombophilia type), and the effect of thromboprophylaxis on VTE and major bleeding based on 4 RCTs (summary in Table 4). We did not provide formal recommendations for individuals with a family history of VTE and known homozygous FVL or a combination of hereditary thrombophilia types. For individuals with a homozygous first-degree relative, the prevalence of thrombophilia would be 100%. This prevalence would be lower for second-degree relatives and for individuals with first-degree relatives with varying combinations of thrombophilia types. Furthermore, there is a lack of evidence regarding VTE risk with various combinations of hereditary thrombophilia. Therefore, we were unable to perform adequate modeling and calculations.
Estimates used to calculate effect of thrombophilia testing in individuals with a family history of VTE
| Thrombophilia defect in the family . | RR for first VTE, positive vs negative (95% CI) . | Treatment effect for VTE occurrence, RR (95% CI)∗ . | Treatment effect major bleeding, RR (95% CI)∗ . |
|---|---|---|---|
| FVL (FVL) | 2.71 (2.06-3.56) | 0.54 (0.32-0.91) | 2.09 (1.33-3.27) |
| Prothrombin (PT) mutation | 2.35 (1.46-3.78) | ||
| Antithrombin (AT) deficiency | 12.17 (5.45-27.17) | ||
| Protein C (PC) deficiency | 7.47 (2.81-19.81) | ||
| Protein S (PS) deficiency | 5.98 (2.45-14.57) |
| Thrombophilia defect in the family . | RR for first VTE, positive vs negative (95% CI) . | Treatment effect for VTE occurrence, RR (95% CI)∗ . | Treatment effect major bleeding, RR (95% CI)∗ . |
|---|---|---|---|
| FVL (FVL) | 2.71 (2.06-3.56) | 0.54 (0.32-0.91) | 2.09 (1.33-3.27) |
| Prothrombin (PT) mutation | 2.35 (1.46-3.78) | ||
| Antithrombin (AT) deficiency | 12.17 (5.45-27.17) | ||
| Protein C (PC) deficiency | 7.47 (2.81-19.81) | ||
| Protein S (PS) deficiency | 5.98 (2.45-14.57) |
Estimates taken from ASH Medical Prophylaxis guideline-medical outpatients with minor provoking risk factors for VTE.
For individuals with a first-degree family history of VTE and a specific thrombophilia, the risk for a first VTE during minor risk episodes was estimated at 15 per 1000 for a family history of FVL or the PGM and 50 per 1000 for a family history of antithrombin deficiency, protein C deficiency, or protein S deficiency, based on 6 observational studies. We estimated the overall risk of major bleeding at 4 per 1000 patients, based on the estimates from the ASH VTE guidelines recommendation on prophylaxis in medical outpatients with minor provoking risk factors for VTE (eg, immobility, minor injury, illness, infection).29
For recommendation 12, the data and assumptions were the same, with the assumption that additional hereditary thrombophilia types would be identified with the same frequency as in the general population.
The evidence profiles and EtD frameworks are shown online at:
Recommendation 11
Recommendation 12
Benefits
We used a strategy of no thrombophilia testing and no thromboprophylaxis during minor VTE-provoking risk factors as the comparison. Therefore, potential benefits of thrombophilia testing and providing thromboprophylaxis to individuals with thrombophilia would be reducing VTE. For selective testing (recommendation 11), the calculations based on a total of 12 to 16 observational studies (depending on the specific thrombophilia type) showed that a strategy of selective thrombophilia testing in individuals with a first-degree family history of VTE and thrombophilia, followed by thromboprophylaxis in individuals with thrombophilia and not providing thromboprophylaxis to individuals without thrombophilia would result in 5.04 (0.91-7.96) fewer VTE events per 1000 risk episodes in individuals with a family history of VTE and FVL, 4.84 (0.80-8.07) fewer VTE events per 1000 risk episodes with VTE and the PGM; 21.25 (3.80-32.79) fewer VTE events per 1000 risk episodes with VTE and antithrombin deficiency; 20.28 (3.32-32.37) fewer VTE events per 1000 risk episodes with VTE and protein C deficiency; and 19.70 (3.20-31.82) fewer VTE events per 1000 risk episodes with VTE and protein S deficiency. Because individuals with a second-degree family history of VTE and thrombophilia have a 25% prevalence of the thrombophilia known in the family, the number of VTE episodes prevented is half of that estimated in individuals with a first-degree family history.
For panel testing (recommendation 12), the calculations resulted in minimal differences compared with recommendation 11 because some additional family members would be identified as thrombophilia positive.
Harms and burden
Potential harms and burden of selective thrombophilia testing and providing thromboprophylaxis to patients with thrombophilia would be an increase in major bleeding. The calculations based on a total of 4 RCTs showed that a strategy of thrombophilia testing followed by thromboprophylaxis in individuals with thrombophilia and not providing thromboprophylaxis in individuals without thrombophilia would result in 2.18 (0.66-4.54) more major bleeds per 1000 risk episodes. This effect did not differ between the various thrombophilia types in the family, as with selective testing, always 50% of first-degree and 25% of second-degree family members would be treated with thromboprophylaxis.
For panel testing (recommendation 12), the calculations resulted in minimal differences compared with recommendation 11 because some additional family members would be identified as thrombophilia positive.
Certainty in the evidence of effects
We rated the overall certainty in the evidence of effects as very low for both recommendations because our estimates for the prevention of VTE were based on calculations with serious indirectness and imprecision in the estimates.
Other EtD criteria and considerations
The panel determined that on balance, with trivial desirable effects (preventing VTE during minor risk episodes) and trivial undesirable effects (more major bleeding) for individuals with a first- or second-degree family history of VTE and with FVL or the PGM as the intervention, a strategy of testing for thrombophilia and thromboprophylaxis in individuals with thrombophilia would not be favored. For individuals with a first- and second-degree family history of VTE and antithrombin, protein C, or protein S deficiency, however, the panel determined that on balance, with small desirable effects (preventing VTE during minor risk episodes) and trivial undesirable effects (more major bleeding), a strategy of testing for thrombophilia and thromboprophylaxis in individuals with thrombophilia would probably be favored.
The panel did consider the potential moderate costs of the intervention by testing for thrombophilia and the subsequent prophylaxis costs. The intervention of thrombophilia testing was considered acceptable by patients and health care providers and probably feasible, although several studies have described inappropriate and inadequate thrombophilia testing.
Conclusions and research needs for this recommendation
When considering both recommendations (recommendations 11 and 12) to test individuals with a first- or second-degree family history of VTE and known antithrombin, protein C, or protein S deficiency (high-risk thrombophilias), it is most sensible to selectively test for the known familial thrombophilia (recommendation 11) rather than testing for the entire panel (recommendation 12). This is because of the trivial additional number of VTE episodes prevented and major bleeds caused by a strategy of panel testing for all hereditary thrombophilias. This is not obvious when recommendation 12 is read in isolation from recommendation 11.
As general conclusions, the absolute risk estimates of VTE during minor provoking risk factors in individuals with a family history of VTE and thrombophilia in the absence of thromboprophylaxis are based on retrospective cohort studies with their inherent biases, and the panel used the best available evidence.
The guideline panel acknowledges the fact that our recommendations were based on risk increases for a first VTE for the various specific thrombophilia types. The panel realizes that the prevalence of hereditary thrombophilia differs geographically. It is therefore the aim of ASH to provide an online calculator to make calculations for specific thrombophilia defects and allow for the input of localized prevalence values.
The panel determined that it would be valuable to have direct evidence from high-quality studies comparing these interventions, but no such study has been performed.
For individuals with a family history of VTE and unknown thrombophilia status, should thrombophilia testing (using a panel of tests) be performed to guide the use of thromboprophylaxis for a minor provoking risk factor?
Recommendation 13
For individuals with a family history of VTE and unknown thrombophilia status in the family who have a minor provoking risk factor for VTE (eg, immobility or minor injury, illness, or infection), the ASH guideline panel suggests not testing for all hereditary thrombophilias (using a panel of tests) to guide thromboprophylaxis (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
Remarks:
Thrombophilia testing may be considered if individuals have multiple family members with VTE, if the family member with VTE was young, with patient preference, and in settings where testing incurs a low cost.
A positive family history is defined as having a first- or second-degree relative with VTE.
A strategy with testing for hereditary thrombophilia (using a panel of tests) would mean that individuals with thrombophilia receive thromboprophylaxis for a minor provoking risk factor, and individuals without thrombophilia would receive no thromboprophylaxis.
These recommendations have not considered the possibility of finding homozygous defects or combinations of thrombophilia types in an individual with a positive family history of VTE and an unknown thrombophilia status.
Summary of the evidence
We did not identify direct studies to answer this question. The effect of thrombophilia testing and a subsequent strategy of providing thromboprophylaxis to individuals with thrombophilia and not to individuals without thrombophilia during risk situations was indirectly calculated using separate observational studies for thrombophilia prevalence in patients with VTE and unknown thrombophilia status (Table 3), and subsequently dividing this prevalence depending on the relationship to the proband (ie, 50% in individuals with a first-degree family history and 25% in individuals with a second-degree family history). We used RRs for a first event in individuals with thrombophilia vs individuals without thrombophilia and RCT evidence for the effect of thromboprophylaxis, as detailed in Table 4.
The risk for a first VTE during minor risk episodes was estimated at 12 per 1000, based on 6 observational studies. We estimated the overall risk of major bleeding at 4 per 1000 based on the estimates from the VTE prophylaxis in medical outpatients with minor provoking risk factors for VTE (eg, immobility, minor injury, illness, infection).29
The evidence profile and EtD framework are shown online at:
Benefits
We used a strategy of no thrombophilia testing and no thromboprophylaxis during minor VTE-provoking risk factors as the comparison. Therefore, the potential benefits of thrombophilia testing and providing thromboprophylaxis to individuals with thrombophilia would be reducing VTE. The calculations based on a total of 29 studies showed that a strategy of thrombophilia testing in individuals with a first-degree family history of VTE for all known hereditary defects, followed by thromboprophylaxis in individuals with thrombophilia and not providing thromboprophylaxis to individuals without thrombophilia, would result in 2.16 (from 0.02-5.66) fewer VTE events per 1000 risk episodes in individuals with a family history of VTE in whom the thrombophilia status in the family is unknown.
Harms and burden
Potential harms and burden of thrombophilia testing and providing thromboprophylaxis to patients with thrombophilia would be an increase in major bleeding. The calculations based on a total of 24 studies showed that a strategy of thrombophilia testing followed by thromboprophylaxis in individuals with thrombophilia and not providing thromboprophylaxis to individuals without thrombophilia would result in ∼0.62 (from 0.13-1.82) more major bleeds per 1000 risk episodes.
Certainty in the evidence of effects
We rated the overall certainty in the evidence of effects as very low because our estimates were based on calculations using observational studies and RCTs, hence rating down for the use of observational studies and the serious indirectness of the estimates.
Other EtD criteria and considerations
The panel determined that on balance, with trivial desirable effects (preventing VTE during minor risk episodes) and trivial undesirable effects (more major bleeding) for individuals with a first- or second-degree family history of VTE and unknown thrombophilia status in the family, a strategy of testing for thrombophilia and thromboprophylaxis in individuals with thrombophilia would not be favored.
The panel did consider the potential moderate costs of the intervention by testing for thrombophilia and the subsequent prophylaxis costs. The intervention of thrombophilia testing was considered acceptable by patients and health care providers and probably feasible, although several studies have described inappropriate and inadequate thrombophilia testing.
Conclusions and research needs for this recommendation
General conclusions and research needs as stated with recommendations 11 and 12 are also valid here.
For individuals with a family history of thrombophilia but no VTE should selective thrombophilia testing be performed to guide the use of thromboprophylaxis for a minor provoking risk factor?
Recommendation 14
For individuals with a family history of FVL or PGM (low-risk thrombophilia) but no family history of VTE who have a minor provoking risk factor for VTE (eg, immobility or minor injury, illness, or infection), the ASH guideline panel suggests not testing for the known thrombophilia to guide thromboprophylaxis (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○)
For individuals with a first-degree family history of antithrombin, protein C, or protein S deficiency (high-risk thrombophilia) but no family history of VTE who have a minor provoking risk factor for VTE, the ASH guideline panel suggests testing for the known thrombophilia. The panel suggests thromboprophylaxis in individuals with thrombophilia and no thromboprophylaxis in individuals without thrombophilia (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
For individuals with a second-degree family history of antithrombin, protein C, or protein S deficiency (high-risk thrombophilia) but no family history of VTE who have a minor provoking risk factor for VTE, the ASH guideline panel suggests either testing for the known thrombophilia or not testing for thrombophilia to guide the use of thromboprophylaxis (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
Remarks:
A strategy with selective testing for the known familial thrombophilia type would mean that individuals with thrombophilia would receive thromboprophylaxis for a minor provoking risk factor, and individuals without thrombophilia would receive no thromboprophylaxis.
A positive family history is defined as having a first- or second-degree relative with VTE, unless otherwise specified.
These recommendations do not address homozygous defects or combinations of thrombophilia types.
Summary of the evidence
Here, we question whether an individual with a first- or second-degree family history of thrombophilia but no family history of VTE (ie, testing has been performed for other reasons) benefits from selective testing for hereditary thrombophilia to provide thromboprophylaxis during minor VTE-provoking risk factors.
We did not identify direct studies to answer this question. The approach was similar to recommendation 11, where probands had had VTE and were known to have a specific thrombophilia. The only difference for the current recommendation 14 is that probands did not experience VTE, and the overall risk for first-time VTE in their relatives was assumed to be half as high as in recommendation 11.33
We used RRs for a first event in individuals with thrombophilia vs individuals without thrombophilia and RCT evidence for the effect of thromboprophylaxis, as detailed in Table 4.
The risk for a first VTE during minor risk episodes was estimated at 7.5 per 1000 for individuals with a first-degree family history of FVL or the PGM and 25 per 1000 for individuals with a family history of antithrombin deficiency, protein C deficiency, or protein S deficiency, based on 6 observational studies. We estimated the overall risk of major bleeding at 4 per 1000, based on the estimates from the ASH VTE guidelines recommendation on prophylaxis in medical outpatients with minor provoking risk factors for VTE (eg, immobility, minor injury, illness, infection).29
The evidence profile and EtD framework are shown online at:
Benefits
We used a strategy of no thrombophilia testing and no thromboprophylaxis during minor VTE-provoking risk factors as the comparison. Therefore, the potential benefits of thrombophilia testing and providing thromboprophylaxis to individuals with thrombophilia would be reducing VTE. The calculations based on a total of 12 to 16 studies (depending on the type of thrombophilia) showed that a strategy of selective thrombophilia testing in individuals with a first-degree family history of thrombophilia, followed by thromboprophylaxis in individuals with thrombophilia and not providing thromboprophylaxis to individuals without thrombophilia would result in 2.52 (ranging from 0.45 to 3.98) fewer VTE events per 1000 risk episodes in individuals with a family history of FVL; 2.42 (0.40-4.03) fewer VTE events per 1000 risk episodes with the PGM; 10.63 (ranging from 1.90-16.40) fewer VTE events per 1000 risk episodes with antithrombin deficiency; 10.14 (ranging from 1.66-16.18) fewer VTE events per 1000 risk episodes protein C deficiency; and 9.85 (ranging from 1.60-15.91) fewer VTE events per 1000 risk episodes with protein S deficiency. As individuals with a second-degree family history of VTE have a 25% prevalence of the thrombophilia known in the family, the number of VTE episodes prevented is half of that estimated in individuals with a first-degree family history.
Harms and burden
Potential harms and burden of thrombophilia testing and providing thromboprophylaxis to individuals with thrombophilia would be an increase in major bleeding. The calculations based on a total of 4 RCTs showed that a strategy of thrombophilia testing followed by thromboprophylaxis in individuals with thrombophilia and not providing thromboprophylaxis in individuals without thrombophilia would result in ∼2.18 (ranging from 0.66-4.54) more major bleeds per 1000 risk episodes.
Certainty in the evidence of effects
We rated the overall certainty in the evidence of effects as very low because our estimates were based on calculations with serious indirectness and imprecision of the estimates.
Other EtD criteria and considerations
The panel determined that on balance, with trivial desirable effects (preventing VTE during minor risk episodes) and trivial undesirable effects (more major bleeding) for individuals with a first- or second-degree family history of FVL or the PGM, the intervention, a strategy of testing for thrombophilia and thromboprophylaxis in individuals with thrombophilia, would not be favored. For antithrombin, protein C, or protein S deficiency, however, the panel determined that on balance, with small desirable effects (preventing VTE during minor risk episodes) and trivial undesirable effects (more major bleeding) for individuals with a first-degree family history of VTE in probands with any of these thrombophilias, a strategy of testing for thrombophilia and thromboprophylaxis for patients with thrombophilia would probably be favored. For individuals with a second-degree family history of antithrombin, protein C, or protein S deficiency, the panel decided that the balance between benefits and harms did not favor either selective testing or no testing.
The panel did consider the potential moderate costs of the intervention by testing for thrombophilia and the subsequent prophylaxis costs. The intervention of thrombophilia testing was considered acceptable by patients and health care providers and probably feasible, although several studies have described inappropriate and inadequate thrombophilia testing.
Conclusions and research needs for this recommendation
General conclusions and research needs as stated with recommendations 11 and 12 are also valid here.
Thrombophilia testing for women with a family history of VTE and/or thrombophilia to prevent VTE associated with hormone use
For women from the general population, should thrombophilia testing be performed to guide the use of oral contraceptives (COCs)?
Recommendation 15
For women from the general population who are considering using COCs, the ASH guideline panel recommends not performing thrombophilia testing to guide the use of COC (strong recommendation based on low certainty in the evidence about effects ⊕⊕○○).
Remarks:
Women with risk factors for VTE, such as a family history of VTE and/or a family history of thrombophilia, are at higher risk of VTE. Other recommendations in this guideline address thrombophilia testing in these populations (recommendations 17 and 19).
A strategy with testing for thrombophilia (using a panel of tests) would mean that women with thrombophilia would not use COCs, and women without thrombophilia would use COCs.
Summary of the evidence
We did not identify direct studies to answer this question. The effect of thrombophilia testing and a subsequent strategy to avoid COCs in women with thrombophilia was indirectly calculated using separate studies for overall risk, thrombophilia prevalence, RR of a first episode of VTE in women with thrombophilia vs women without thrombophilia, and effect of COCs on VTE risk.
We identified 3 observational studies for the overall risk of VTE, 5 observational studies to assess the prevalence of any thrombophilia in the general population, 1 systematic review to estimate the risk association for VTE for women with thrombophilia vs women without thrombophilia, and 1 systematic review to assess the effect of COC on the risk of VTE. See the online evidence profile for study references.
The median prevalence of any hereditary thrombophilia (ie, heterozygous FVL, homozygous FVL, heterozygous PGM, antithrombin deficiency, protein C deficiency, or protein S deficiency) was 6.85% (minimum 3.43%; maximum 13.70%).
The risk for VTE in women with thrombophilia vs women without thrombophilia was assessed for any hereditary thrombophilia (RR, 5.89; 95% CI, 4.21-8.23), based on 1 systematic review. The effect of COC use was estimated at RR 3.5 (95% CI, 2.9-4.3), based on 1 systematic review. The overall risk of VTE for women who are candidates for COCs was estimated at 0.35 per 1000.
The evidence profile and EtD framework are shown online at:
Benefits
We used a strategy of no thrombophilia testing and the use of COCs in all women from the general population as the comparison. Therefore, the potential benefits of thrombophilia testing and avoiding COCs in women with thrombophilia would consist of fewer VTE. The calculations based on a total of 10 studies showed that a strategy of thrombophilia testing followed by avoidance of COCs in women with thrombophilia would lead to 0.26 fewer VTE events per 1000 women (ranging from 0.09 to 0.65 fewer) per year.
Harms and burden
Potential harms and burden of thrombophilia testing and avoidance of COCs in women with thrombophilia are intangible, as they fall into a wider scope than VTE. The ASH guideline panel considered unwanted pregnancies, labeling women as thrombophilia positive, and other potential consequences of testing, without calculating the effects on VTE from these potential harms. We were unable to attribute these harms to any specific effect size (eg, trivial, small, moderate, or large), but we felt that in the presence of trivial benefits and a large cost, the effort required to quantify the size of the harmful effect would have been disproportionate to the gain.
Certainty in the evidence of effects
We rated the overall certainty in the evidence of effects as very low, even with a supporting systematic review of large trials, because our estimates were based on modeling with serious indirectness and imprecision of the estimates.
Other EtD criteria and considerations
The panel determined that on balance, with trivial desirable effects (preventing VTE) and intangible undesirable effects (including unwanted pregnancies and other consequences of avoiding COCs), a strategy of not testing for thrombophilia and using COCs in women from the general population should be favored. The panel considered that there is important variability, as younger women may value a different trade-off between benefits and risk than older women who are candidates for COCs. However, the panel considered the large costs of thrombophilia testing for women intending to use COCs in the general population and decided to issue a strong recommendation for large anticipated costs against a trivial benefit.
Conclusions and research needs for this recommendation
The guideline panel acknowledges the fact that our recommendation is based on modeling with prevalence and RR estimates for VTE for any type of thrombophilia. The ASH panel recommendation is in line with previously published cost-effectiveness analyses (see EtD). It is unlikely that further research will alter the recommendations on this specific question.
For women from the general population, should thrombophilia testing be performed to guide the use of HRT?
Recommendation 16
For women from the general population who are considering using HRT, the ASH guideline panel suggests not performing thrombophilia testing to guide the use of HRT (conditional recommendation based on low certainty in the evidence about effects ⊕⊕○○).
Remarks:
Women with risk factors for VTE, such as a family history of VTE and/or thrombophilia, are at higher risk of VTE. Other recommendations in this guideline address thrombophilia testing in these populations (recommendations 18 and 20).
A strategy with testing for thrombophilia (using a panel of tests) would mean that women with thrombophilia would not use HRT, and women without thrombophilia would use HRT.
Summary of the evidence
We did not identify direct studies to answer this question. The effect of thrombophilia testing and a subsequent strategy to avoid HRT in women with thrombophilia was indirectly calculated using separate studies for overall risk in postmenopausal women, thrombophilia prevalence, RR of a first episode of VTE in women with thrombophilia vs women without thrombophilia, and effect of HRT on VTE risk.
We identified 1 observational study for the overall risk of VTE, 5 observational studies to assess the prevalence of any thrombophilia in the general population, 2 observational studies to estimate the risk association for VTE for women with thrombophilia vs women without thrombophilia, and 1 systematic review to assess the effect of estrogen-only HRT and combined HRT on the risk of VTE. See the online evidence profile for study references.
The median prevalence of any hereditary thrombophilia (ie, heterozygous FVL, homozygous FVL, heterozygous PGM, antithrombin deficiency, protein C deficiency, or protein S deficiency) was 6.85% (minimum 3.43%; maximum 13.70%).
The risk for VTE in women with thrombophilia vs women without thrombophilia was assessed for hereditary thrombophilia (RR, 1.8; 95% CI, 0.8-2.6). The effect of HRT use was estimated at 2.22 (95% CI, 1.12-4.39) for estrogen-only HRT and 4.28 (95% CI, 2.49-7.34) for combined HRT. The overall risk of VTE in postmenopausal women was estimated at 2 per 1000.
The evidence profile and EtD framework are shown online at:
Benefits
We used a strategy of no thrombophilia testing and the use of HRT in all postmenopausal women from the general population as the comparison. Therefore, the potential benefits of thrombophilia testing and avoiding HRT in women with thrombophilia would consist of fewer VTE. The calculations based on a total of 9 studies showed that a strategy of thrombophilia testing followed by avoidance of estrogen-only HRT for women with thrombophilia would lead to 0.29 fewer VTE events per 1000 women (ranging from 0.01 to 1.98 fewer) per year, whereas testing followed by avoidance of combined HRT would lead to 0.77 fewer VTE events per 1000 women (ranging from 0.08 to 3.70 fewer) per year. In line with the panel’s judgment across this set of guidelines, this benefit was defined as trivial.
Harms and burden
Potential harms and burden of thrombophilia testing and avoidance of HRT in women with thrombophilia are intangible, as they fall into a wider scope than VTE. The ASH guideline panel considered labeling women as thrombophilia positive and potential other consequences of testing without calculating the effects on VTE from these potential harms. As for COCs, we felt that in the presence of trivial benefits and a large cost, the effort required to quantify the size of the harmful effect would have been disproportionate to the gain.
Certainty in the evidence of effects
We rated the overall certainty in the evidence of effects as very low because our estimates were based on modeling with serious indirectness and imprecision of the estimates.
Other EtD criteria and considerations
The panel determined that on balance, with trivial desirable effects (preventing VTE) and intangible undesirable effects, a strategy of not testing for thrombophilia and using HRT in all women from the general population would probably be favored. The panel considered the lack of benefit, unknown harmful effects, and large costs involved in testing all women who are considering HRT.
Conclusions and research needs for this recommendation
The guideline panel acknowledges the fact that our recommendation is based on calculations with prevalence and RR estimates for VTE for any type of thrombophilia. The ASH panel recommendation is in line with previously published cost-effectiveness analyses (see EtD). It is unlikely that further research will alter the recommendations on this specific question.
For women with a family history of VTE and unknown thrombophilia status, should thrombophilia testing (using a panel of tests) be performed to guide the use of COCs?
Recommendation 17
For women with a family history of VTE and unknown thrombophilia status in the family who are considering using COCs, the ASH guideline panel suggests not testing for hereditary thrombophilia (using a panel of tests) to guide the use of COC (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
Remarks:
Women with a family history of VTE and a known thrombophilia in the family are at higher risk of testing positive for thrombophilia and are therefore at higher risk for VTE. Another recommendation in this guideline addresses thrombophilia testing in this population (recommendation 19).
A strategy with testing for hereditary thrombophilia (using a panel of tests) would mean that women with thrombophilia would not use COCs, and women without thrombophilia would use COCs.
A positive family history is defined as having a first- or second-degree relative with VTE.
Summary of the evidence
We did not identify direct studies to answer this question. The effect of thrombophilia testing and a subsequent strategy to avoid COCs in women with thrombophilia was indirectly calculated using separate studies for overall risk, thrombophilia prevalence, RR of a first episode of VTE in women with thrombophilia vs women without thrombophilia, and effect of COCs on VTE risk.
We identified 1 observational study for the overall risk of VTE, 20 studies to assess the prevalence of any thrombophilia in patients with VTE, 14 studies to estimate the risk association for VTE for women with thrombophilia vs women without thrombophilia, 1 systematic review to assess the effect of COCs on the risk of VTE, and 1 study for the overall risk of VTE in this specific population. See the online evidence profile for study references.
The median prevalence of any hereditary thrombophilia (ie, heterozygous FVL, homozygous FVL, heterozygous PGM, antithrombin deficiency, protein C deficiency, or protein S deficiency) was 14.15% (minimum 9.85%; maximum 20.05%).
The risk for VTE in women with thrombophilia vs women without thrombophilia was assessed for hereditary thrombophilia (RR, 3.89; 95% CI, 2.15-9.01). The effect of COC use was estimated at 3.5 (2.9-4.3). The overall risk for women with a family history of VTE who are candidates for COCs was estimated at 12 per 1000.
The evidence profile and EtD framework are shown online at:
Benefits
We used a strategy of no thrombophilia testing and the use of COCs in all women from families with VTE and unknown thrombophilia status as the comparison. Therefore, the potential benefits of thrombophilia testing and avoiding COCs in women with thrombophilia would consist of fewer VTE. The calculations based on a total of 36 studies showed that a strategy of thrombophilia testing followed by avoidance of COCs in women with thrombophilia would lead to 1.17 fewer VTE events per 1000 women (ranging from 0.06 to 1.55 fewer) per year.
Harms and burden
Potential harms and burden of thrombophilia testing and avoidance of COCs in women with thrombophilia are intangible, as they fall into a wider scope than VTE. The ASH guideline panel considered unwanted pregnancies, labeling women as thrombophilia positive, and potentially other consequences of testing, without calculating the effects on VTE from these potential harms. As for the general population, we felt that in the presence of trivial benefits and a moderate cost, the effort required to quantify the size of the harmful effect would have been disproportionate to the gain.
Certainty in the evidence of effects
We rated the overall certainty in the evidence of effects as very low because our estimates were based on calculations with serious indirectness and imprecision of the estimates.
Other EtD criteria and considerations
The panel determined that on balance, with trivial desirable effects (preventing VTE) and intangible undesirable effects (including unwanted pregnancies and other consequences of avoiding COCs), a strategy of not testing for thrombophilia and using COCs in women with a family history of VTE would probably be favored. The panel considered that there is important variability, as younger women may value a different trade-off than older women who are candidates for COCs. The panel considered the moderate costs of thrombophilia testing for women with a family history of VTE intending to use COCs.
Conclusions and research needs for this recommendation
A family history of VTE increases the risk of VTE by twofold regardless of the presence of thrombophilia,33 and as such, may lead to a cautious prescription of COCs in this population.
The guideline panel acknowledges the fact that our recommendation is based on calculations with prevalence of any type of hereditary thrombophilia, which may vary geographically, and RR estimates of thrombophilia for VTE.
For women with a family history of VTE and unknown thrombophilia status, should thrombophilia testing (using a panel of tests) be performed to guide the use of HRT?
Recommendation 18
For women with a family history of VTE and unknown thrombophilia in the family who are considering using HRT, the ASH guideline panel suggests not performing thrombophilia testing for any hereditary thrombophilia to guide the use of HRT (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
Remarks:
Women with a family history of VTE and a known thrombophilia in the family are at higher risk for testing positive for thrombophilia and are therefore at higher risk for VTE. Another recommendation in this guideline addresses thrombophilia testing in this population (recommendation 20).
A strategy with testing for hereditary thrombophilia (using a panel of tests) would mean that women with thrombophilia would not use HRT, and women without thrombophilia would use HRT.
A positive family history is defined as having a first- or second-degree relative with VTE.
Summary of the evidence
We did not identify direct studies to answer this question. The effect of thrombophilia testing and a subsequent strategy to avoid HRT in women with thrombophilia was indirectly calculated using separate studies for overall risk in postmenopausal women, thrombophilia prevalence, RR of a first episode of VTE in women with thrombophilia vs women without thrombophilia, and effect of HRT on VTE risk.
We identified 1 observational study for the overall risk of VTE, 20 studies assessing the prevalence of any thrombophilia in patients with VTE, 2 observational studies estimating the risk association for VTE for women with thrombophilia vs women without thrombophilia, and 1 systematic review assessing the effect of estrogen-only HRT and combined HRT on the risk of VTE. See the online evidence profile for study references.
The median prevalence of hereditary thrombophilia (ie, heterozygous FVL, homozygous FVL, heterozygous PGM, antithrombin deficiency, protein C deficiency, or protein S deficiency) was 14.15% (minimum 9.85%; maximum 20.05%).
The risk for VTE in women with thrombophilia vs women without thrombophilia was assessed for hereditary thrombophilia (RR, 2.08; 95% CI, 1.02-4.10). The effect of HRT use was estimated at 2.22 (95% CI, 1.12-4.39) for estrogen-only HRT, and 4.28 (95% CI, 2.49-7.34) for combined HRT. The overall risk of VTE in postmenopausal women was estimated at 30 per 1000.
The evidence profile and EtD framework are shown online at:
Benefits
We used a strategy of no thrombophilia testing and the use of HRT in all postmenopausal women with a family history of VTE as the comparison. Therefore, the potential benefits of thrombophilia testing and avoiding HRT in women positive for thrombophilia would consist of fewer VTE. The calculations based on a total of 24 studies showed that a strategy of thrombophilia testing followed by avoidance of estrogen-only HRT in women with thrombophilia would lead to 0.94 fewer VTE events per 1000 women (ranging from 0.01 to 5.16 fewer) per year, whereas testing followed by avoidance of combined HRT would lead to 2.52 fewer VTE events per 1000 women (ranging from 0.07 to 9.65 fewer) per year.
Harms and burden
Potential harms and burden of thrombophilia testing and avoidance of HRT in women with thrombophilia are intangible, as they fall into a wider scope than VTE. The ASH guideline panel considered labeling women as thrombophilia positive and potentially other consequences of testing without calculating the effects on VTE from these potential harms. As for the general population, we felt that in the presence of trivial benefits and a moderate cost, the effort required to quantify the size of the harmful effect would have been disproportionate to the gain.
Certainty in the evidence of effects
We rated the overall certainty in the evidence of effects as very low because our estimates were based on calculations with serious indirectness and imprecision of the estimates.
Other EtD criteria and considerations
The panel determined that on balance, with trivial desirable effects (preventing VTE) and intangible undesirable effects, not testing for thrombophilia and using HRT in all women with a family history of VTE would probably be favored. The panel considered the lack of benefit, unknown harmful effects, and moderate costs involved in testing all women with a family history of VTE who are considering HRT.
Conclusions and research needs for this recommendation
A family history of VTE increases the risk of VTE by twofold regardless of the presence of thrombophilia33 and as such may lead to cautious prescription of HRT in this population.
The guideline panel acknowledges the fact that our recommendation is based on calculations with prevalence of any type of hereditary thrombophilia, which may vary geographically, and RR estimates of thrombophilia for VTE.
For women with a family history of VTE and thrombophilia, should selective thrombophilia testing be performed to guide the use of COCs?
Recommendation 19
For women with a family history of VTE and known FVL or PGM in the family (low-risk thrombophilia), the ASH guideline panel suggests not testing for the known familial thrombophilia to guide the use of COC (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
For women with a family history of VTE and known antithrombin, protein C, or protein S deficiency in the family (high-risk thrombophilia), the ASH guideline panel suggests testing for the known familial thrombophilia. The panel suggests avoidance of COCs for women with high-risk thrombophilia (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
Remarks:
A strategy with selective testing for known familial thrombophilia would mean that women with thrombophilia would avoid COCs, and women without thrombophilia would use COCs.
A positive family history is defined as having a first- or second-degree relative with VTE.
These recommendations do not address homozygous defects or combinations of thrombophilia types.
Summary of the evidence
We did not identify direct studies to answer this question. The effect of thrombophilia testing and a subsequent strategy to avoid COCs in women with thrombophilia was indirectly calculated using separate studies for overall risk, thrombophilia prevalence, RR of a first episode of VTE in women with thrombophilia vs women without thrombophilia, and effect of COCs on VTE risk. We calculated effects for specific hereditary thrombophilia defects separately. Given the autosomal dominant inheritance pattern, the prevalence of thrombophilia was set at 50% in women with a first-degree family history and 25% in women with a second-degree family history. We did not provide formal recommendations for women with a family history of VTE and known homozygous FVL or a combination of hereditary thrombophilia types. For individuals with a homozygous first-degree relative, the prevalence of thrombophilia would be 100%. This prevalence would be lower for second-degree relatives and for individuals with first-degree relatives with varying combinations of thrombophilia types. Furthermore, there is a lack of evidence regarding VTE risk with various combinations of hereditary thrombophilia. Therefore, we were unable to perform adequate modeling and calculations.
We identified 7 observational studies for the overall risk of VTE, 9 observational studies for the RR of a first episode of VTE in women with thrombophilia vs women without thrombophilia, and 1 systematic review to assess the effect of COCs on the risk of VTE. See the online evidence profile for study references.
We used RRs for a first event in women with thrombophilia vs women without thrombophilia, as detailed in Table 4. The effect of COC use was estimated at 3.5 (95% CI, 2.9-4.3). The overall risk for a first VTE was estimated at 2.5 per 1000 for individuals with a first-degree family history of VTE and FVL or the PGM, 8.4 per 1000 for antithrombin deficiency, 6.3 per 1000 for protein C deficiency, and 4.9 per 1000 for protein S deficiency.
The evidence profile and EtD framework are shown online at:
Benefits
We used a strategy of no thrombophilia testing and the use of COCs in all women with a family history of VTE and thrombophilia as the comparison. Therefore, the potential benefits of thrombophilia testing and avoiding COCs in women with thrombophilia would consist of fewer VTE.
For women with a first-degree family history of VTE and FVL, the calculations based on a total of 14 studies showed that a strategy of thrombophilia testing followed by avoidance of COCs in women with FVL would lead to 4.57 fewer VTE events (ranging from 3.71-5.55) per 1000 women per year compared with a no-testing strategy. As women with a second-degree family history have a 25% prevalence of the thrombophilia known in the proband, the number of VTE episodes prevented is half of that estimated in women with a first-degree family history.
For women with a first-degree family history of VTE and the PGM, the calculations based on a total of 10 studies showed that a strategy of thrombophilia testing followed by avoidance of COCs in women with the PGM would lead to 4.38 fewer VTE events (ranging from 3.76-4.90) per 1000 women per year compared with a no-testing strategy. Because women with a second-degree family history have a 25% prevalence of the thrombophilia known in the proband, the number of VTE episodes prevented is half of that estimated in women with a first-degree family history.
For women with a first-degree family history of VTE and antithrombin deficiency, the calculations based on a total of 12 studies showed that a strategy of thrombophilia testing followed by avoidance of COCs in antithrombin-deficient women would lead to 19.39 fewer VTE events (ranging from 15.30-23.90) per 1000 women per year compared with a no-testing strategy. Because women with a second-degree family history have a 25% prevalence of the thrombophilia known in the proband, the number of VTE episodes prevented is half of that estimated in women with a first-degree family history.
For women with a first-degree family history of VTE and protein C deficiency, the calculations based on a total of 12 studies showed that a strategy of thrombophilia testing followed by avoidance of COCs in protein C–deficient women would lead to 13.84 fewer VTE events (ranging from 11.34-15.45) per 1000 women per year compared with a no-testing strategy. Because women with a second-degree family history have a 25% prevalence of the thrombophilia known in the proband, the number of VTE episodes prevented is half of that estimated in women with a first-degree family history.
For women with a first-degree family history of VTE and protein S deficiency, the calculations based on a total of 13 studies showed that a strategy of thrombophilia testing followed by avoidance of COCs in protein S–deficient women would lead to 10.49 fewer VTE events (ranging from 8.71-11.48) per 1000 women per year compared with a no-testing strategy. Because women with a second-degree family history have a 25% prevalence of the thrombophilia known in the proband, the number of VTE episodes prevented is half of that estimated in women with a first-degree family history.
Harms and burden
Potential harms and burden of thrombophilia testing and avoidance of COCs in women with thrombophilia are intangible, as they fall into a wider scope than VTE. The ASH guideline panel considered unwanted pregnancies, labeling women as thrombophilia positive, and other potential consequences of testing without calculating the effects on VTE from these potential harms.
Certainty in the evidence of effects
We rated the overall certainty in the evidence of effects as very low because our estimates were based on calculations with serious indirectness and imprecision of the estimates.
Other EtD criteria and considerations
The panel determined that on balance, with small (for FVL and PGM) to moderate (for antithrombin, protein C, and protein S deficiency) desirable effects (preventing VTE) and intangible undesirable effects (including unwanted pregnancies and other consequences of avoiding COCs), a strategy of not testing for thrombophilia and using COCs in women with a family history of VTE would probably be favored for women with a family history of FVL and the PGM, whereas the testing strategy would probably be favored for women with a family history of antithrombin, protein C, or protein S deficiency. The panel considered that there is important variability because younger women may value a different trade-off between benefit and risk than older women who are candidates for COCs. The panel considered moderate costs of thrombophilia testing of women with a family history of VTE and thrombophilia intending to use COCs.
Conclusions and research needs for this recommendation
A family history of VTE increases the risk of VTE by twofold, regardless of the presence of thrombophilia.33 The ASH recommendations do not consider that women without thrombophilia but with a family history of VTE are at increased risk of VTE as compared with the general population. Hence, the family history of VTE and thrombophilia in itself may lead to cautious use of COCs in this population.
The guideline panel acknowledges the fact that our recommendation is based on calculations using RR estimates of thrombophilia for VTE.
For women with a family history of VTE and thrombophilia, should selective thrombophilia testing be performed to guide the use of HRT?
Recommendation 20
For women with a family history of VTE and known FVL or PGM in the family (low-risk thrombophilia), the ASH guideline panel suggests not testing for the known familial thrombophilia to guide the use of HRT (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
For women with a family history of VTE and known antithrombin, protein C, or protein S deficiency in the family (high-risk thrombophilia), the ASH guideline panel suggests testing for the known familial thrombophilia. The panel suggests avoidance of HRT for women with high-risk thrombophilia (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
Remarks:
A strategy with selective testing for the known familial thrombophilia would mean that women with thrombophilia would avoid HRT and women without thrombophilia would use HRT.
A positive family history is defined as having a first- or second-degree relative with VTE.
These recommendations do not address homozygous defects or combinations of thrombophilia types.
Summary of the evidence
We did not identify direct studies to answer this question. The effect of thrombophilia testing and a subsequent strategy to avoid HRT in women with thrombophilia was indirectly calculated using separate studies for overall risk, thrombophilia prevalence, RR of a first episode of VTE in women with thrombophilia vs women without thrombophilia, and effect of HRT on VTE risk. We calculated effects for specific hereditary thrombophilia defects separately. We did not address this strategy for homozygous defects or combinations of thrombophilia types. Given the autosomal dominant inheritance pattern, the prevalence of thrombophilia was set at 50% in women with a first-degree family history and 25% in women with a second-degree family history.
We identified 1 observational study for the overall risk of VTE, 2 observational studies for the RR of a first episode of VTE in women with thrombophilia vs women without thrombophilia, and 1 systematic review to assess the effect of estrogen-only HRT and combined HRT on the risk of VTE. See the online evidence profile for study references.
We used RRs for a first event in women with thrombophilia vs women without thrombophilia as follows: FVL 2.6 (95% CI, 1.3-5.2), PGM 0.8 (95% CI, 0.3-2.2), antithrombin deficiency 1.7 (95% CI, 0.9-3.2), protein C deficiency 1.8 (95% CI, 0.9-3.8), and protein S deficiency 1.9 (95% CI, 0.9-4.1). The effect of HRT use was estimated at 2.22 (95% CI, 1.12-4.39) for estrogen-only HRT and 4.28 (95% CI, 2.49-7.34) for combined HRT. The overall risk for a first VTE was estimated at 2.5 per 1000 for individuals with a first-degree family history of VTE in patients with FVL or the PGM, 8.4 per 1000 for antithrombin deficiency, 6.3 per 1000 for protein C deficiency, and 4.9 per 1000 for protein S deficiency.
The evidence profile and EtD framework are shown online at:
Benefits
We used a strategy of no thrombophilia testing and the use of HRT in all women with a family history of VTE and thrombophilia as the comparison. Therefore, the potential benefits of thrombophilia testing and avoiding HRT in women with thrombophilia would consist of fewer VTE.
For women with a first-degree family history of VTE and FVL, the calculations based on a total of 4 studies showed that a strategy of thrombophilia testing followed by avoidance of estrogen-only HRT in women with FVL would lead to 2.20 fewer VTE events (ranging from 0.25-4.79) per 1000 women per year compared with a no-testing strategy. A testing strategy followed by avoidance of combined HRT in women with FVL would lead to 5.92 fewer VTE events (ranging from 3.12-8.96) per 1000 women per year compared with a no-testing strategy. Because women with a second-degree family history have a 25% prevalence of the thrombophilia known in the proband, the number of VTE episodes prevented is half of that estimated in women with a first-degree family history.
For women with a first-degree family history of VTE and PGM, the calculations based on a total of 4 studies showed that a strategy of thrombophilia testing followed by avoidance of estrogen-only HRT in women with PGM would lead to 1.36 fewer VTE events (ranging from 0.21-1.96) per 1000 women per year compared with a no-testing strategy. A testing strategy followed by avoidance of combined HRT in women with PGM would lead to 3.64 fewer VTE events (ranging from 2.56-3.66) per 1000 women per year compared with a no-testing strategy.
For women with a first-degree family history of VTE and antithrombin deficiency, the calculations based on a total of 4 studies showed that a strategy of thrombophilia testing followed by avoidance of estrogen-only HRT in antithrombin-deficient women would lead to 6.45 fewer VTE events (ranging from 0.77-13.49) per 1000 women per year compared with a no-testing strategy. A testing strategy followed by avoidance of combined HRT in antithrombin-deficient women would lead to 17.35 fewer VTE events (ranging from 9.54-25.23) per 1000 women per year compared with a no-testing strategy.
For women with a first-degree family history of VTE and protein C deficiency, the calculations based on a total of 4 studies showed that a strategy of thrombophilia testing followed by avoidance of estrogen-only HRT in protein C–deficient women would lead to 4.94 fewer VTE events (ranging from 0.60-10.12) per 1000 women per year compared with a no-testing strategy. A testing strategy followed by avoidance of combined HRT in protein C–deficient women would lead to 13.28 fewer VTE events (ranging from 7.43-18.92) per 1000 women per year compared with a no-testing strategy.
For women with a first-degree family history of VTE and protein S deficiency, the calculations based on a total of 4 studies showed that a strategy of thrombophilia testing followed by avoidance of estrogen-only HRT in protein S–deficient women would lead to 3.92 fewer VTE events (ranging from 0.47-7.87) per 1000 women per year compared with a no-testing strategy. A testing strategy followed by avoidance of combined HRT in protein S–deficient women would lead to 10.53 fewer VTE events (ranging from 5.87-14.72) per 1000 women per year compared with a no-testing strategy.
Harms and burden
Potential harms and burden of thrombophilia testing and avoidance of HRT in women with thrombophilia are intangible because they fall into a wider scope than VTE. The ASH guideline panel considered not alleviating postmenopausal symptoms, labeling women as thrombophilia positive, and potential other consequences of testing.
Certainty in the evidence of effects
We rated the overall certainty in the evidence of effects as very low because our estimates were based on calculations with serious indirectness and imprecision of the estimates.
Other EtD criteria and considerations
The panel determined that on balance, with small (for FVL and PGM) to moderate (for antithrombin, protein C, and protein S deficiency) desirable effects (preventing VTE) and intangible undesirable effects (including not alleviating postmenopausal symptoms, and other consequences of avoiding HRT), a strategy of not testing for thrombophilia and using HRT in women with a family history of VTE would probably be favored for women from families with FVL and PGM, whereas the testing strategy would probably be favored for women from families with antithrombin, protein C, or protein S deficiency, particularly based on the estimated additional VTE with combined HRT. The panel considered the moderate costs of thrombophilia testing for women with a family history of VTE and thrombophilia in women intending to use HRT.
Conclusions and research needs for this recommendation
A family history of VTE increases the risk of VTE by twofold, regardless of the presence of thrombophilia.33 The ASH recommendations do not consider that women without thrombophilia but with a family history of VTE and thrombophilia are at increased risk of VTE as compared with the general population. Hence, the family history of VTE and thrombophilia in itself may lead to cautious use of HRT in this population.
The guideline panel acknowledges the fact that our recommendation is based on calculations using RR estimates of thrombophilia for VTE.
Thrombophilia testing for women with a family history of VTE and thrombophilia to prevent VTE associated with pregnancy and the postpartum period
The panel deliberated separately on thrombophilia testing and offering thromboprophylaxis to women found to have thrombophilia in the scenarios of antepartum (recommendation 21) and postpartum (recommendation 22) prophylaxis. The choice was the result of considering the different duration and risk of VTE during pregnancy and the puerperium, even though the panel is aware that antepartum prophylaxis is usually extended into the postpartum period of 6 weeks.
The panel considered selective thrombophilia testing for high-risk thrombophilia only, as the ASH guidelines on VTE in the context of pregnancy provided recommendations suggesting the use of antepartum thromboprophylaxis in women with a family history of VTE and antithrombin deficiency, homozygous FVL, or combined thrombophilias.27 Because the ASH guidelines on VTE in the context of pregnancy suggested not to use antepartum thromboprophylaxis in women with a family history of VTE and heterozygous FVL or heterozygous PGM, and test results would not affect treatment, we did not issue recommendations about selective testing for these women. Of note, the ASH pregnancy panel used the estimated absolute risk reduction of VTE by thrombosis prophylaxis as the main approach, which differs from the number needed to test, with a subsequent different prophylactic strategy in women with vs women without thrombophilia than was used by the thrombophilia panel. However, the ASH thrombophilia panel valued the consistency of the entire ASH guideline body and abstained from issuing recommendations where recommendations had already been made.
For women with a family history of VTE and thrombophilia, should selective thrombophilia testing be performed to guide the use of thromboprophylaxis during pregnancy?
Recommendation 21
For women with a family history of VTE and known homozygous FVL, a combination of FVL and PGM, or an antithrombin deficiency in the family, the ASH guideline panel suggests testing for the known familial thrombophilia. The panel suggests antepartum thromboprophylaxis for women with the same familial thrombophilia (ie, homozygous FVL, combination of FVL and PGM, or antithrombin deficiency) and no antepartum prophylaxis for women without the same familial thrombophilia (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
For women with a family history of VTE and a known protein C or protein S deficiency in the family, the ASH guideline panel suggests either testing for the known familial thrombophilia or not testing for thrombophilia to guide antepartum prophylaxis (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
Remarks:
Pharmacological thromboprophylaxis based on antepartum thrombophilia testing is generally continued postpartum.
Conditions can include the duration and burden of the treatment, which involves injections with low-molecular-weight heparin, and patient preference.
A strategy with selective testing for the known familial thrombophilia type would mean that relatives with a positive result would receive thromboprophylaxis and relatives with a negative result would not receive thromboprophylaxis.
A positive family history is defined as having a first- or second-degree relative with VTE; for homozygous FVL, these recommendations only concern siblings, not children, as these would most often be heterozygous for FVL; management of women with a second-degree family history was not addressed.
These recommendations do not address heterozygous FVL or PGM alone, as the ASH guidelines on the management of VTE in the context of pregnancy suggest not using thromboprophylaxis for these women.
For women with a family history of VTE and thrombophilia, should selective thrombophilia testing be performed to guide the use of thromboprophylaxis postpartum?
Recommendation 22
For women with a first-degree family history of VTE and known homozygous FVL, a combination of FVL and PGM, antithrombin deficiency, protein C deficiency, or protein S deficiency in the family, the ASH guideline panel suggests testing for the known familial thrombophilia. The panel suggests postpartum thromboprophylaxis for women with the same familial thrombophilia (ie, homozygous FVL, combination of FVL and PGM, or antithrombin deficiency) and no postpartum prophylaxis for women without the same familial thrombophilia (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
For women with a second-degree family history of VTE and a known combination of FVL and PGM, or antithrombin deficiency in the family, the ASH guideline panel suggests testing for the known familial thrombophilia. The panel suggests postpartum thromboprophylaxis for women with thrombophilia and no postpartum prophylaxis for women without thrombophilia (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
For women with a second-degree family history of VTE and a known protein C or protein S deficiency in the family, the ASH guideline panel suggests either testing for the known familial thrombophilia or not testing for thrombophilia to guide postpartum thromboprophylaxis (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
Remarks:
Thromboprophylaxis postpartum continues until 6 weeks after delivery.
Conditions can include the duration and burden of the treatment, which involves injections, and patient preference.
A strategy with selective testing for the known familial thrombophilia type would mean that women with thrombophilia would receive thromboprophylaxis, and women without thrombophilia would not receive thromboprophylaxis.
For homozygous FVL, these recommendations only concern siblings, not children, as these would most often be heterozygous for FVL; testing of women with a second-degree family history was not addressed.
These recommendations do not address heterozygous FVL or PT mutations alone, as the ASH guidelines on the management of VTE in the context of pregnancy suggest not prescribing thromboprophylaxis for these women.
Summary of the evidence
We did not identify direct studies to answer these questions. The effect of selective thrombophilia testing and a subsequent strategy of 8 months of antepartum thromboprophylaxis (recommendation 21) or 6 weeks postpartum thromboprophylaxis (recommendation 22) in women with thrombophilia and not in women without thrombophilia was indirectly calculated using the known thrombophilia prevalence depending on the relationship to the proband (ie, 50% in women with a first-degree family history, 25% in women with a second-degree family history, and 25% among those who have siblings with VTE and homozygous FVL or a combination of FVL and PGM), the overall risk for first-time VTE and RRs for a first event in women with thrombophilia vs women without thrombophilia from 3 to 6 observational studies (depending on the type of thrombophilia), and the effect of thromboprophylaxis on VTE from 1 systematic review. The overall risk for major bleeding and the effect of thromboprophylaxis on major bleeding were derived from 1 systematic review.
The following data regarding VTE were assumed to be the same for the antepartum (8 months) and postpartum (6 weeks) periods. The overall risk for a first VTE was estimated at 37.5 per 1000 for homozygous FVL, 18 per 1000 for antithrombin deficiency, 4 per 1000 for protein C deficiency, 8 per 1000 for protein S deficiency, and 20.25 per 1000 for the combination of FVL and the PGM. The RRs for a first VTE in women with thrombophilia vs women without thrombophilia was estimated to be as follows: 20.96 (95% CI, 7.17-53.34) for homozygous FVL, 10.51 (95% CI, 2.48-44.54) for antithrombin deficiency, 6.04 (95% CI, 0.81-45.19) for protein C deficiency, 5.03 (95% CI, 0.57-44.51) for protein S deficiency, and 9.36 (95% CI, 2.97-25.66) for the combination of FVL and the PGM. The effect of thromboprophylaxis on VTE was estimated to be 0.41 (95% CI, 0.32-0.54).
We estimated the overall risk of major bleeding at 6.34 per 1000 for the antepartum period and 8.46 per 1000 for the postpartum period. The effect of thromboprophylaxis on major bleeding was estimated to be 0.34 (95% CI, 0.04-3.21) antepartum and 0.75 (95% CI, 0.17-3.38) postpartum.
The evidence profiles and EtD frameworks are shown online at:
Benefits
We used a strategy of no thrombophilia testing and no thromboprophylaxis during pregnancy or the postpartum period as the comparison. Therefore, the potential benefits of thrombophilia testing and providing thromboprophylaxis to women with thrombophilia would be to reduce VTE. The calculations based on a total of 4 to 7 studies (depending on the type of thrombophilia) showed that a strategy of selective thrombophilia testing in women with a first-degree family history, followed by thromboprophylaxis in women with thrombophilia and not providing thromboprophylaxis in women without thrombophilia during pregnancy, that is, for about 8 months, would result in 19.35 fewer VTE events (ranging from 12.16-24.14) per 1000 women with a family history of VTE and homozygous FVL, 9.70 fewer VTE events (ranging from 5.90-11.97) per 1000 women for antithrombin deficiency, 2.02 fewer VTE events (ranging from 0.82-2.66) per 1000 women for protein C deficiency, 3.94 fewer VTE events (ranging from 1.34-5.32) per 1000 women for protein S deficiency, and 9.05 fewer VTE events (ranging from 4.63-12.33) per 1000 women for a combination of FVL and the PGM.
The estimated number of VTE events prevented by a strategy of selective thrombophilia testing in women with a first-degree family history of VTE and thrombophilia, followed by thromboprophylaxis in women with thrombophilia and not providing thromboprophylaxis in women without thrombophilia postpartum, that is, for 6 weeks, is similar to that for the 8-month antepartum period, as approximately half of all VTE episodes related to pregnancy occur during pregnancy and half in the 6 weeks postpartum.
Because women with a second-degree family history have a 25% prevalence of antithrombin, protein C, or protein S deficiency, or a combination of FVL and the PGM, the number of VTE episodes prevented is half of that estimated in women with a first-degree family history and these thrombophilic defects.
We did not address second-degree relatives for homozygous FVL because the ASH guidelines on VTE in the context of pregnancy suggest not using antepartum or postpartum thromboprophylaxis in women with a family history of VTE and a heterozygous FVL mutation.
Harms and burden
Potential harms and burden of thrombophilia testing and providing thromboprophylaxis to women with thrombophilia would be an increase in major bleeding. The calculations based on 1 systematic review showed that a strategy of thrombophilia testing followed by thromboprophylaxis during pregnancy in women with thrombophilia and not providing thromboprophylaxis in women without thrombophilia would result in 2.09 fewer (from 3.04 fewer to 7.01 more) antepartum major bleeds per 1000 pregnancies when testing for antithrombin, protein C, or protein S deficiency and 1.05 fewer (from 1.52 fewer to 3.50 more) antepartum major bleeds when testing for homozygous FVL or the combination of FVL and the PGM. A strategy of thrombophilia testing followed by thromboprophylaxis postpartum in women with thrombophilia and not providing thromboprophylaxis in women without thrombophilia would result in 1.06 fewer (from 3.51 fewer to 10.07 more) postpartum major bleeds per 1000 pregnancies when testing for antithrombin, protein C, or protein S deficiency and 0.53 fewer (from 1.76 fewer to 5.03 more) postpartum major bleeds when testing for homozygous FVL or the combination of FVL and the PGM.
Certainty in the evidence of effects
We rated the overall certainty in the evidence of effects as very low because our estimates were based on calculations with serious indirectness and imprecision of the estimates.
Other EtD criteria and considerations
The panel determined that on balance, with small effects (preventing VTE during pregnancy) and trivial undesirable effects (more major bleeding) for women with a sibling with homozygous FVL and women with a first-degree family history of a combination of FVL and PGM or antithrombin deficiency, a strategy of testing for thrombophilia and thromboprophylaxis during pregnancy in women with thrombophilia would probably be favored. For women with a family history of VTE and protein C or protein S deficiency, the panel determined that the balance between benefits and harms did not favor either selective testing or not testing. For women with a second-degree family history of VTE and antithrombin, protein C, or protein S deficiency, or a combination of FVL and PGM, the panel decided that the balance between benefits and harms did not favor either selective testing or not testing.
The panel determined that on balance, with small effects (preventing VTE in the postpartum period) and trivial undesirable effects (more major bleeding) for women with a sibling with homozygous FVL and women with a first-degree family history of a combination of FVL and PGM, or antithrombin, protein C, or protein S deficiency, a strategy of testing for thrombophilia and thromboprophylaxis postpartum in women with thrombophilia would probably be favored. For women with a second-degree family history of VTE and a combination of FVL and PGM or antithrombin deficiency, a strategy of selective testing for thrombophilia and thromboprophylaxis postpartum in women with thrombophilia would probably be favored. For women with a second-degree family history of protein C or protein S deficiency, the panel decided that the balance between benefits and harms did not favor either selective testing or no testing.
The panel did consider the potential moderate costs of the intervention by testing for thrombophilia and the subsequent costs of thromboprophylaxis. The intervention of thrombophilia testing was considered acceptable by patients and health care providers and probably feasible, although several studies have described inappropriate and inadequate thrombophilia testing. Finally, when a decision for thrombophilia testing is made based on the consequences of postpartum thromboprophylaxis but not antepartum prophylaxis, it would be recommended to perform thrombophilia testing preconceptionally to avoid spurious results, particularly of protein S.
Conclusions and research needs for this recommendation
The absolute risk of VTE during pregnancy and the postpartum period in women with a family history of VTE and high-risk thrombophilia in the absence of thromboprophylaxis is based on retrospective cohort studies with their inherent biases, and the panel used the best available evidence. The evidence used to estimate the effect of thromboprophylaxis in pregnant and postpartum women was based on a systematic review of relatively small trials that suggested a decrease in major bleeding antepartum and no increased risk postpartum. There is a need for high-quality evidence on the efficacy and safety of thromboprophylaxis in pregnant and postpartum women to better be able to balance the risks and benefits.
Thrombophilia testing for individuals with a family history of VTE and/or family history of thrombophilia to prevent cancer-associated VTE
For ambulatory patients with cancer receiving systemic therapy with a family history of VTE, should thrombophilia testing (using a panel of tests) be performed to guide the use of thromboprophylaxis?
Recommendation 23
For ambulatory patients with cancer receiving systemic therapy who have a family history of VTE and are otherwise determined to be at low or intermediate risk for VTE, the ASH guideline panel suggests testing for hereditary thrombophilia. The panel suggests ambulatory thromboprophylaxis for patients with thrombophilia and no thromboprophylaxis for patients without thrombophilia (conditional recommendation based on very low certainty in the evidence about effects ⊕○○○).
Remarks:
This question only addresses patients with cancer receiving systemic therapy, without a personal history of VTE who are at low or intermediate risk for VTE. The ASH VTE guidelines on prevention and treatment in patients with cancer suggest using DOAC prophylaxis for all ambulatory patients with cancer with high VTE risk as assessed by a validated risk assessment tool complemented by clinical judgment and experience.
Patient preference is an important factor to consider, as undergoing the thrombophilia test, knowing the positive test result, and receiving additional medication can be an added burden.
A strategy with testing for hereditary thrombophilia (using a panel of tests) would mean that ambulatory patients with cancer with thrombophilia would receive thromboprophylaxis, and ambulatory patients with cancer without thrombophilia would not receive thromboprophylaxis.
A positive family history is defined as having a first-degree relative with VTE.
This recommendation does not address homozygous defects or combinations of thrombophilia types.
Summary of the evidence
In the ASH VTE guidelines for prevention and treatment in patients with cancer,28 ambulatory patients with cancer receiving systemic therapy who are at high risk for VTE are suggested to use thromboprophylaxis with a DOAC (apixaban or rivaroxaban) for primary prevention of VTE.28 Classification of patients as being at low, intermediate, or high risk for VTE should be based on a validated risk assessment tool (ie, Khorana score) complemented by clinical judgment and experience. For patients at low risk, the guideline suggests not using thromboprophylaxis, and for patients at intermediate risk, the ASH guideline panel suggests thromboprophylaxis with a DOAC (apixaban or rivaroxaban) or no thromboprophylaxis. Given these recommendations in the cancer guideline,28 we assessed the risk of a first VTE in ambulatory patients with cancer who are at low or intermediate risk for VTE.
We did not identify direct studies to answer this question. The effect of thrombophilia testing and a subsequent strategy of thromboprophylaxis for patients with cancer with thrombophilia and not for patients with cancer without thrombophilia was indirectly calculated using the known thrombophilia prevalence in patients with VTE (Table 3) and the subsequent prevalence depending on the relationship to the proband (ie, 50% in individuals with a first-degree family history, and 25% in individuals with a second-degree family history), RRs for a first VTE event in thrombophilia-positive vs thrombophilia-negative relatives for FVL and PGM from 11 cancer-specific observational studies and for antithrombin, protein C, and protein S deficiency from Table 4, evidence for the effect of thromboprophylaxis with a DOAC for 6 months on VTE and on major bleeding from 3 RCTs, and the overall risk for VTE and major bleeding from 1 systematic review each.
The overall risk of a first episode of VTE during ambulatory cancer treatment in patients at low risk of VTE was estimated at 50 per 1000 per 6 months and in patients at intermediate risk of VTE at 66 per 1000 per 6 months. The RR for a first VTE event in heterozygous FVL vs negative individuals was estimated at 1.86 (95% CI, 1.20-2.90) and for heterozygous PGM vs negative individuals at 1.78 (95% CI, 1.40-2.27). The effect of thromboprophylaxis using a DOAC for 6 months on VTE was estimated at RR 0.61 (95% CI, 0.31-1.21) in both risk groups.
The overall risk of major bleeding in patients at low risk of bleeding was estimated to be 3.6 per 1000 per 6 months, and in patients at intermediate risk of bleeding, it was 8.0 per 1000 per 6 months. The effect of thromboprophylaxis using a DOAC during 6 months on major bleeding was estimated at RR 1.65 (95% CI, 0.72-3.80) in both risk groups.
The evidence profile and EtD framework are shown online at:
Benefits
We used a strategy of no thrombophilia testing and no thromboprophylaxis during systemic cancer treatment as the comparison. Therefore, potential benefits of thrombophilia testing and providing thromboprophylaxis to patients with cancer and a positive family history of VTE who have thrombophilia would be to reduce VTE. The calculations based on a total of 41 studies showed that a strategy of hereditary thrombophilia testing for patients with cancer with a first-degree family history of VTE who are at low risk for VTE according to a validated risk assessment tool, followed by thromboprophylaxis for patients with cancer with thrombophilia, and not providing thromboprophylaxis for patients with cancer without thrombophilia, would result in 6.85 fewer VTE events (ranging from 23.37 fewer to 0.16 more) per 1000 patients per 6 months. For patients with cancer and a positive family history of VTE who are at intermediate risk for VTE, such a strategy would result in 9.04 fewer VTE events (ranging from 30.85 fewer to 0.21 more) per 1000 patients per 6 months compared with a no-testing strategy.
Harms and burden
Potential harms and burden of thrombophilia testing and providing thromboprophylaxis to patients with cancer with thrombophilia would be an increase in major bleeding. The calculations based on a total of 24 studies showed that a strategy of thrombophilia testing followed by thromboprophylaxis for patients with cancer with thrombophilia and not providing thromboprophylaxis for patients with cancer without thrombophilia would result in 0.33 more major bleeds (ranging from 0.10 fewer to 2.02 more) per 1000 patients per 6 months in those at low risk for VTE, and in 0.74 more major bleeds (ranging from 0.22 fewer to 4.49 more) per 1000 patients per 6 months in those at intermediate risk for VTE, compared with a no-testing strategy.
Certainty in the evidence of effects
We rated the overall certainty in the evidence of effects as very low because our estimates were based on calculations with serious indirectness and imprecision of the estimates.
Other EtD criteria and considerations
The panel determined that on balance, with small desirable effects (preventing VTE during ambulatory cancer treatment) and trivial undesirable effects (more major bleeding) for patients with cancer with a first-degree family history of VTE, thrombophilia testing followed by thromboprophylaxis for patients with thrombophilia would probably be favored.
The panel did consider the potential moderate costs of the intervention by testing for thrombophilia and the subsequent costs of thromboprophylaxis. The intervention of thrombophilia testing was considered acceptable by patients and health care providers and probably feasible, although several studies have described inappropriate and inadequate thrombophilia testing.
Conclusions and research needs for this recommendation
The absolute risk of VTE during ambulatory cancer treatment in patients with a family history of VTE in the absence of thromboprophylaxis is based on estimates of the prevalence of thrombophilia in patients with VTE and the RRs of thrombophilia from observational studies with their inherent biases, and the panel used the best available evidence. Risk assessment tools to categorize patients with cancer into low-, intermediate-, and high-risk groups for VTE may be suboptimal.
The guideline panel acknowledges the fact that our recommendation is based on calculations with a prevalence of any type of hereditary thrombophilia in patients with VTE that may vary geographically.
What is new in these ASH guidelines?
The contribution of these guidelines in the broader space of treatment and prevention of VTE is to ensure that a patient-centered, individualized approach is adopted whenever appropriate. Although establishing unbiased estimates of the effect of specific antithrombotic treatments requires large RCTs, the same trials often do not provide sufficiently granular evidence to optimize the choice of whom to treat. Ultimately, the goal is to reduce the number needed to treat as much as possible, thus avoiding treatment of those patients who will not benefit from treatment or denying treatment to those who will.
To accomplish this overarching goal of finding out whether thrombophilia testing could lead to better individualized treatment, we believe the value of testing is to drive subsequent treatment decisions. We have devised an approach to appraise the value of thrombophilia testing built on combining prevalence data (how likely it is for an individual to have thrombophilia), risk association data (how likely it is for an individual with or without thrombophilia to have an event), and measures of treatment effect (how many fewer VTEs and how many more bleeding events will result from treating patients with thrombophilia and not treating patients without thrombophilia). Thus, what our panel decided upon was the number of events prevented (or provoked) by adopting a personalized treatment approach for the patients with a positive result for thrombophilia in several clinical scenarios. Of note, we have included cost, feasibility, acceptability, and equity considerations in the process. We argue that this is the best approach to making an evidence-based decision on the appropriateness of thrombophilia testing until robust prospective observations (and maybe RCTs) confirm or contradict the results of our simulations.
As a consequence of the rigorous and novel process described above, the panel found value in thrombophilia testing for a series of clinical conditions and issued conditional recommendations in favor of testing for thrombophilia in the following: patients with VTE associated with nonsurgical triggering conditions, including COCs and pregnancy; patients with CVT or splanchnic venous thrombosis in settings where short-term primary treatment is the standard of care; individuals with a family history of antithrombin, protein C, or protein S deficiency when considering VTE prophylaxis for minor VTE risk factors or avoidance of COCs/HRT; pregnant women with a sibling with homozygous FVL or a family history of a combination of FVL and PGM or antithrombin deficiency; patients with cancer who are otherwise at low or intermediate risk of thrombosis and who have a family history of VTE. For other considered conditions, the panel provided recommendations against testing for thrombophilia, including a strong recommendation against testing in the general population before starting COCs and a conditional recommendation against testing in the general population before starting HRT.
Some of these recommendations introduce the potential for change in clinical practice and therefore deserve some additional consideration. First, the recommendations are all conditional, based mostly on patient preferences and values attached to relevant outcomes. We do acknowledge that conditional recommendations might be less appealing than strong ones to be applied to most patients and that applying conditional recommendations will require the education of patients and physicians to effectively elicit those preferences and appropriately use them for shared decision making. However, risk stratification is necessary to accomplish individualized optimal treatment, which makes our panel stand behind the present deliberations. Second, some of the recommendations may appear counterintuitive: for example, one may feel that it is inappropriate to test young people with COC-related VTE or patients with VTE provoked by transient nonsurgical risk factors, as their risk is generally considered too low for them to be candidates for lifelong treatment if they have thrombophilia. Against this uneasiness, we invite the reader to consider how reluctant one can be to treat a young patient with unprovoked VTE for life. These patients are not the majority of people enrolled in clinical trials, so we have little direct evidence about the need to treat them for life, yet that is the recommended approach.1 Furthermore, one would realize that cases of VTE related to COCs or nonsurgical risk factors are relatively few compared with the many more exposed to the same risk factors, and it is likely that a fraction of them might have a relevant provoking cofactor represented by thrombophilia. Others might be worried by the cost associated with testing for thrombophilia; for them, we note that irrespective of whether thrombophilia testing results will be used to start or withhold treatment, the cost of testing is negligible compared with the cost of lifelong anticoagulation (which we considered in our process), likely even after including indirect costs stemming from the treatment of additional events in untreated patients (which we did not consider). Finally, someone might be confused by having “divergent” recommendations for the same condition (thrombosis at unusual sites) depending on the standard of care adopted in a specific setting, whereby testing for thrombophilia is recommended to prolong treatment where the standard of care is short-term treatment duration and not recommended when the standard of care is long-term treatment for everyone. Under the perspective of choosing the best management option for patients with thrombophilia, patients with thrombophilia will indeed receive indefinite treatment because of both recommendations (because of testing in 1 case and regardless of testing in the other). Again, this is the result of a robust, pragmatic, and logical process that assessed the value of testing within the context in which the results of testing will be used.
What are others saying?
Some of the recommendations in this guideline are consistent with those from others, whereas some recommendations differ from other guidelines. Over the past 10 years, several guidelines or guidance statements on thrombophilia testing have been issued: the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group34 (limited to FVL and the PGM), National Institute for Health and Clinical Excellence (2012, partially amended 2020, NICE, United Kingdom),35 Choosing Wisely Campaign (2013),36 the Anticoagulation Forum (2016, AC Forum, United States),37 and the Thrombosis and Haemostasis Society of Australia and New Zealand (2019, THANZ, Australia and New Zealand).38
Furthermore, some evidence-based guidelines on the treatment or prevention of VTE have implicitly or explicitly mentioned the relevance, or lack thereof, of thrombophilia testing for patient management.
For patients with unprovoked VTE, the recommendation in this ASH guideline not to test for thrombophilia is consistent with those of EGAPP, NICE, the Anticoagulation Forum, and THANZ. The recommendation in this ASH guideline is based on the comparison (standard of care) with indefinite anticoagulation in all patients with unprovoked VTE, that is, regardless of the presence of thrombophilia. In line with the ASH recommendation, the NICE guideline explicitly states “not to offer testing for hereditary thrombophilia to people who are continuing anticoagulation treatment, and to consider testing for APLA in people who have had unprovoked DVT or PE and for hereditary thrombophilia in people who have had unprovoked DVT or PE and who have a first-degree relative who has had DVT or PE, if it is planned to stop anticoagulation treatment.” Likewise, the THANZ guidelines state that “young patients (<45 years) with unprovoked proximal DVT and PE may be tested for antithrombin and protein C and S deficiency if it influences treatment duration,” and “patients with unprovoked proximal DVT and PE should be tested for antiphospholipid syndrome.”
For patients with VTE provoked by surgery, the recommendation in this ASH guideline not to test for thrombophilia is consistent with those of others. Interestingly, the ASH Choosing Wisely guidance that aims to reduce inappropriate thrombophilia testing also states: “patients who experience VTE in the setting of a major transient risk factor but who have additional risk factors such as a positive family history or concurrent exposure to hormonal therapy, ASH recommends that such patients seek guidance from an expert in VTE,”36 highlighting the need for the current guidelines.
For patients with VTE provoked by a nonsurgical major transient risk factor or VTE associated with COCs, HRT, pregnancy, or postpartum, the ASH guideline suggests testing patients for thrombophilia. These recommendations are new and may cause considerable discussion, as many currently view these VTE episodes as provoked and are generally inclined to use short-term anticoagulation for such patients. It is important to note, however, that most guidelines or guidance statements on thrombophilia testing do not distinguish between major and minor provoking risk factors, which current science suggests is appropriate. For example, the ASH VTE treatment guideline,1 which the thrombophilia panel has used to define clinical scenarios and standards of care, distinguishes between major and minor provoking risk factors for VTE. The role of thrombophilia in decisions to guide treatment duration was not discussed by that panel, as it was assigned to the ASH thrombophilia panel. The European Society of Cardiology guidelines for the diagnosis and management of acute PE developed in collaboration with the European Respiratory Society (ERS) (2019) also distinguish major and minor provoking risk factors for VTE to assess VTE recurrence risk and suggest “to test for high-risk thrombophilia (but not heterozygous FVL or PGM) in patients in whom VTE occurs at a young age (eg, aged <50 years) and in the absence of an otherwise identifiable risk factor, especially when this occurs against the background of a strong family history of VTE, as these are often candidates for indefinite anticoagulant treatment after a first episode of PE occurring in the absence of a major reversible risk factor.”39 In summary, the suggestions to consider thrombophilia testing in deciding on the duration of VTE treatment after a nonsurgical risk factor may appear counterintuitive to some, but in fact are in line with considerations mentioned in other guidelines or guidance statements. It has to be noted that, to our knowledge, the ASH recommendations are the first to have formally used a rigorous modeling approach to assess the effect of thrombophilia testing for patients with VTE provoked by a nonsurgical major transient risk factor, supporting with quantitative and comprehensive considerations the suggestion of testing and the consequent indefinite duration of anticoagulation for the patients found to be positive and therefore at higher risk.
For patients with CVT, this thrombophilia guideline has issued 2 separate recommendations, depending on whether the standard of care is to discontinue anticoagulant treatment after 3 to 6 months (suggesting to test) or to continue indefinitely (suggesting not to test). This is in part consistent with guidelines of the European Stroke Organization, which on the one hand suggest not testing for thrombophilia to prevent recurrent venous thrombosis but on the other hand suggest testing patients who have a high probability of carrying severe thrombophilia (ie, a personal and/or family history of venous thrombosis, a young age at CVT, CVT without a transient or permanent risk factor) to prevent recurrent VTE.32 Likewise, for patients with splanchnic venous thrombosis, the 2 recommendations, dependent on the standard of care, are partially consistent with the implicit guidance statement from the ISTH on the duration of anticoagulant treatment, where patients with high-risk thrombophilia are mentioned to likely benefit from indefinite anticoagulation.31 Once more, the novelty of our statements is not in the recommendations themselves but in the objective way we have used to examine the role of thrombophilia in light of the best available evidence.
For individuals with a family history of VTE and/or thrombophilia, several recommendations in the current ASH thrombophilia guideline suggest testing to guide the use of thromboprophylaxis during minor transient VTE risk factors, during pregnancy or postpartum, or to avoid hormone use in women with thrombophilia, depending on the clinical setting and type of thrombophilia. The NICE guideline suggests not to routinely offer thrombophilia testing to first-degree relatives of people with a history of DVT or PE and thrombophilia, because “it does not alter the decision of whether to give these people thromboprophylaxis as it is routinely given to all first-degree relatives of those who have had thromboembolic disease.”35 Similar reasoning is provided with regard to avoiding COCs or HRT. It is, however, discussed that “there are rare circumstances where this test could be of benefit, particularly in issues related to pregnancy (which is not within the scope of the guideline).” Although at first glance, the ASH recommendations differ from those from the Anticoagulation Forum, where family testing is generally mentioned to be of limited value, an exception is made for female relatives of patients with VTE and known hereditary thrombophilia, provided thrombophilia testing changes decisions regarding hormone use or thromboprophylaxis around pregnancy. The ASH recommendations regarding testing pregnant women with a family history of VTE, inherited thrombophilia, or both for high-risk thrombophilia are consistent with the recommendations to provide thromboprophylaxis to these women from the ASH guideline on VTE in pregnancy.27 Reflecting on our process, we have to acknowledge that among the strongest drivers in considering whether to suggest testing was the very clear and consistent view of our patient representatives, who were very supportive of ensuring individualized treatment and testing whenever supported by evidence.
For ambulatory patients with cancer who are at low or intermediate risk for VTE as determined by a validated risk assessment tool and who have a family history of VTE, this thrombophilia guideline suggests testing for hereditary thrombophilia to guide the use of thromboprophylaxis during systemic treatment. This recommendation is novel and not discussed in previously published guidance documents, including the ASH VTE guideline on treatment and prevention of VTE for patients with cancer.28 However, the ASH guideline on VTE in cancer28 suggests providing thromboprophylaxis to patients at high risk for other considerations, and therefore, considering the additional risk associated with thrombophilia, this new recommendation should be seen as a new application of an established risk stratification approach.
Limitations of these guidelines
Direct evidence to answer our questions would have come from randomized or well-designed observational studies comparing management strategies embedding or not embedding thrombophilia testing strategies. Similar studies exist for the use of D-dimer and other risk stratification strategies, but none are focused specifically on the role of thrombophilia. Because of the lack of direct evidence, we used a modeling approach, using the best available evidence from observational studies and applying relative treatment effects from other ASH guidelines. Hence, most of our evidence was graded low to very low quality for risk of bias and precision and often downgraded for indirectness when we had to borrow prevalence and risk association from the most to the least common scenarios. Besides the quality of the underlying evidence, other considerations were considered to grade our confidence in the body of evidence, which was very low in most cases. In particular, we adopted a simplified modeling approach without the use of formal forecasting techniques such as Bayesian approaches or Monte Carlo–type simulations; we also did not discount risk and benefits over time and used a 1-year horizon to estimate the risk of recurrent VTE, whereas it is known that recurrence risk decreases over time. Finally, we modeled the variability in prevalence and association, but we did not consider diagnostic test characteristics and clinical pitfalls in laboratory testing of thrombophilia. Specifically, we did not account for the impact of false-positive test results. Of note, in all strategies where the comparator is “treat all,” there is no material impact of false-positive test results, but there is when the comparator is “treat none.”
Implications for practice and research of these guidelines
Our work has several implications for practice and research. First, for the practicing clinician and for patients, our guideline suggests that shared decision making, covering the pros and cons and the practical implications of thrombophilia testing and the adoption of the associated VTE prevention strategies, may improve the quality of care for individuals with increased risk of clotting events, particularly so if they are at high risk of bleeding or have an indication or preference for hormonal therapy. Implementation of the guideline from this perspective will require educational tools and opportunities, which we strongly recommend being provided by scientific societies and patient organizations. Second, as pointed out many times in the guideline and for each recommendation, it is very critical that the proper thrombophilia tests are performed by high-quality clinical laboratories. Too often, thrombophilia testing includes tests with no supportive evidence, and too often the laboratory results are reported with insufficient details or interpretation. Training of physicians and laboratory medicine clinicians will be required for a positive impact of the proposed recommendations. Third, as the guidelines suggest against thrombophilia testing in many clinical scenarios, overdiagnosis may be decreased. Finally, more research is urgently needed. In particular, large implementation studies comparing the impact, in terms of outcome rates, among management strategies involving or not involving thrombophilia testing. This is a typical field where academically initiated guideline implementation studies might be warranted, as it is unlikely that this research will be sponsored by drug manufacturers because personalized medicine approaches often restrict the indication to pharmacological treatment. However, large organizations such as ASH might facilitate networks of independent researchers accessing public research funds to answer these burning questions.
Revision or adaptation of the guidelines
Plans for updating these guidelines
After publication of these guidelines, ASH will maintain them through surveillance for new evidence, ongoing review by experts, and regular revisions.
Updating or adapting recommendations locally
Adaptation of these guidelines will be necessary in many circumstances. These adaptations should be based on the associated EtD framework.
Acknowledgments
The authors acknowledge Trevor Baglin, Mark Crowther, and Beth Waldron for participation on the panel during the initial stages of the guideline development process, including prioritizing questions and health outcomes, and Danielle Barrett for participation on the panel during a later stage, including to review evidence and agree on recommendations. The authors acknowledge Farid Foroutan for checking the calculation methods. Holger Schünemann prepared a template of these guidelines for all panels that was critically reviewed by Adam Cuker, Rob Kunkle, the ASH Guideline Oversight Subcommittee, the Methods Group supporting the guidelines, and editors of Blood Advances.40 The authors also thank the people who provided input into the manuscript during the public comment phase.
Authorship
Contribution: S. Middeldorp and A.I. wrote the first draft of the manuscript and revised the manuscript based on the authors’ suggestions; L.B.K., M.C., D.H., A.J., E.L., S. Moll, and T.M. (guideline panel members) critically reviewed the manuscript and provided suggestions for improvement; R.N., C.C.-A., M.B., L.E.C.-L., S.G.K., and Y.Z. (members of the knowledge synthesis team) contributed evidence summaries to the guidelines and critically reviewed the manuscript; R.N., W.W., and H.J.S. (coordinators of the knowledge synthesis team) provided support for the creation of evidence summaries and critically reviewed the manuscript; S. Middeldorp and A.I. were the chair and vice-chair, respectively, of the panel and led the panel meetings; and all authors approved of the content of the manuscript.
Conflict-of-interest disclosure: All authors were members of either the guideline panel or of the systematic review team. Consequently, they completed a disclosure-of-interest form, which was reviewed by ASH and is available as Supplements 2 and 3.
Correspondence: Saskia Middeldorp, Department of Internal Medicine, Radboud University Medical Center, Geert Grooteplein 10, 6525 GA Nijmegen, The Netherlands; email: saskia.middeldorp@radboudumc.nl.
References
Author notes
Data are available through ASH/McMaster University GRADE Centre.
The full-text version of this article contains a data supplement.