Key Points
PROs were associated with a proinflammatory gene expression pattern in allogeneic HCT–treated patients.
Social well-being was the most important predictor of the proinflammatory transcriptome.
Abstract
Patient-reported outcomes (PROs) capture subjective social determinants of health (SDOHs), which can affect health outcomes through the stress response pathway. The conserved transcriptional response to adversity (CTRA) is a stress-mediated proinflammatory transcriptomic pattern that has been linked to adverse hematopoietic cell transplant (HCT) outcomes. This study examined the association of pretransplant CTRA with patient-reported SDOHs in allogeneic HCT recipients. In this cross-sectional study, pre-HCT SDOH-related PROs included the 36-Item Short Form Health Survey and the Functional Assessment of Cancer Therapy–Bone Marrow Transplant (FACT-BMT). CTRA was assessed by RNA sequencing of whole blood specimens, with mixed effects linear regression models relating CTRA expression to PRO scores while controlling for age, sex, race, disease, and performance status. Among 121 patients, the median age was 54 years, 42% were female, and 91% were White. CTRA was elevated in participants reporting lower scores on the FACT-BMT (P = .003), including the general (P = .003) and BMT-specific (P = .014) components. Effects were driven by the social well-being domain (P = .0001). This corresponded to an 8% to 15% difference in CTRA RNA expression across a 4 standard deviation range in patient-reported SDOHs. Ancillary bioinformatics analyses confirmed the association of well-being with reduced proinflammatory transcription pathway activity [cyclic AMP response element-binding protein, (CREB), NF-κB, and activating protein-1 (AP-1)]. In conclusion, HCT-treated patients who experience unfavorable social conditions show elevated CTRA expression in pretransplant blood samples. These data highlight the biologic sequelae of social well-being and community context and suggest a potential molecular mechanism for the impact of social gradients in HCT outcomes. Targeting this pathway could optimize outcomes in this high-risk population.
Introduction
Social determinants of health (SDOHs) are the nonmedical factors in patients’ life experience and social context that influence their health outcomes and include the multidimensional individual, societal, environmental, political, cultural, and psychosocial forces that shape daily life.1 For example, inadequate health insurance,2 poverty,3,4 experienced discrimination,5 and housing instability6 have all been linked to inferior medical outcomes, including for individuals with cancer.7,8 Within this framework, social and community support have consistently been identified as critical factors.9 Patients with cancer undergoing hematopoietic cell transplantation (HCT) may experience even more pronounced challenges from adverse SDOH, given their prolonged isolation and heightened risks of morbidity, mortality, and late effects.10,11
Significant disparities in HCT outcomes based on race, insurance status, education, and socioeconomic disadvantage have been widely and repeatedly demonstrated.12-15 However, these assessments often rely on external, objective metrics and do not account for a patients’ perception of their social condition. Patient-reported SDOH measures augment our understanding of these complex phenomena and may improve precision in estimating relationships between SDOHs and medical outcomes.16 Such internalized or “experienced” measures of socioenvironmental, economic, and psychosocial factors influence outcomes in the HCT-treated population.17,18 Indeed, a recent analysis of Center for International Blood and Marrow Transplant Research (CIBMTR) data found a significant relationship between baseline patient-reported well-being and overall survival after allogeneic HCT.19 However, there is a gap in our understanding of the mechanisms responsible for these biobehavioral relationships.
The stress response, activated through internal or external triggers, is a potential pathway linking patient-reported SDOHs and medical outcomes in HCT.17,20 A stressor can act through neuroimmune and endocrine pathways to alter molecular signaling profiles that result in dysregulated immune and nervous system functions.21,22 One such profile, termed the conserved transcriptional response to adversity (CTRA), is a gene-regulatory program involving an increased expression of proinflammatory genes and decreased expression of type 1 interferon response genes after β-adrenergic signaling from the sympathetic nervous system.23 Previous studies have shown that circulating immune cells show a systematic increase in basal CTRA expression profiles during extended periods of stress, threat, or uncertainty, consistent with the physiology of stress-associated illness24 and favoring a tumor-promoting milieu.25,26 Knight et al have demonstrated that HCT recipients of lower socioeconomic status (SES), a condition associated with experienced psychological distress,27 display significantly increased expression of the CTRA gene profile, which was associated with increased relapse and decreased disease-free survival.28 However, this study assessed objective measures of SES rather than patients’ subjective experience of their social conditions; inclusion of patient-reported data is important, given the key role of subjective perceptions in controlling the biologic stress response.22
A more comprehensive understanding of the socio-biological mechanisms of SDOHs through incorporation of the subjective patient experience would have substantial impact on efforts to interrupt social disparities in HCT outcomes. Here, to the best of our knowledge, we leverage the largest cohort to date of allogeneic HCT recipients with centralized, standardized patient-reported outcome (PRO) measures and parallel biospecimen data to test the hypothesis that CTRA gene expression will vary inversely with patients’ subjectively experienced level of well-being (general, physical, emotional, social, and functional) and that such relationships will be most pronounced for perceived social well-being.
Methods
This was a cross-sectional analysis from a subset of a larger study published by Shaw et al; the primary aim of the larger study was to test the feasibility of collecting centralized PROs.19 This cohort was chosen based on results demonstrating differential survival at 1 year based on a PRO score. Transplant centers were invited to participate in the larger study based on participation in CIBMTR data collection, their interest in survivorship, and their willingness to enroll patients for centralized PRO collection.
CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry, Autologous Blood and Marrow Transplant Registry, and the National Marrow Donor Program Office of Research. The CIBMTR Research Sample Repository maintains research biospecimens at the Medical College of Wisconsin. CIBMTR data submission and quality compliance are monitored by a rigorous audit process. Data are collected and reported at 2 levels: Transplant Essential Data (TED) and Comprehensive Report Form. TED data include disease type, age, sex, pretransplant disease stage, and chemotherapy responsiveness, date of diagnosis, graft type, conditioning regimen, posttransplant disease progression and survival, development of a new malignancy, and cause of death. All CIBMTR centers contribute TED data. More detailed clinical information was collected via the Comprehensive Report Form mechanism for a subset of randomly selected patients.
The study was approved by the National Marrow Donor Program institutional review board. All patients provided written informed consent for the research use of their biospecimens and clinical data.
Patient eligibility
Eligible patients for this analysis were those aged ≥18 years undergoing allogeneic HCT who completed at least baseline PRO measures and had available pretransplant whole blood biospecimens. To qualify for the larger parent PRO study, patients were also required to be English-speaking and have access to a telephone and a valid mailing address in the United States.
PRO SDOH measures
Baseline PRO measures were filled out with paper and pencil by patients within 30 days before HCT and were returned to CIBMTR. In addition to a sociodemographic survey, patients completed the 36-Item Short Form Health Survey (SF-36) and Functional Assessment of Cancer Therapy–Bone Marrow Transplant (FACT-BMT), measures commonly used in HCT studies.29 The SF-36 is a 36-item survey measure that broadly assesses health and functioning with 2 subscales: the Physical Component Summary and Mental Component Summary (MCS). The normal population mean for the Physical Component Summary and MCS is 50, with a standard deviation (SD) of 10.30 The FACT-BMT is a 50-item measure composed of the Functional Assessment of Cancer Therapy–General (FACT-G) plus a transplant-specific subscale. The FACT-G is composed of 4 domains: physical (eg, “I have a lack of energy”), social (eg, “I feel close to my friends”), emotional (eg, “I feel sad”), and functional well-being (eg, “I am able to work”); transplant-specific items include questions regarding appetite, appearance, mobility, and fatigue. The minimal clinically important difference for the FACT-BMT is 2 to 3 points on the transplant-specific domains and 3 to 7 on the general domains.31 For all measures, higher scores indicate better functioning.
CTRA assay
Pre-HCT CTRA expression was quantified in genome-wide transcriptional profiles obtained from RNA sequencing as previously described,32 with total RNA extracted from buffy coat blood samples, tested for suitable mass and integrity, converted to complementary DNA using a high-efficiency messenger RNA–targeted enzyme system (Lexogen QuantSeq 3′ FWD), and sequenced on an Illumina NextSeq instrument using the manufacturer’s standard protocols. Sequencing targeted 4 million reads per sample (achieved median, 4.6 million), each of which was mapped to the GRCh38 reference human transcriptome (achieved median, 99.0% mapping rate) and quantified as gene transcripts per million mapped reads using the Spliced Transcripts Alignment to a Reference (STAR) aligner. Transcript-per-million values were log2 transformed and floored at 0 (1 transcript per million) for statistical analysis as described later in the article.
Data analysis
Descriptive analyses of patient-, disease-, and transplant-related factors were prepared, listing means and SDs for continuous variables and counts with percentages for categorical variables. Sample PRO scores were compared with means of the general US population using 1-sample t tests. Association of CTRA expression with patient-reported SDOH measures were quantified by mixed effect linear model analyses as previously described.28,32 Briefly, primary analyses analyzed an a priori–defined composite score representing the CTRA profile of upregulated proinflammatory gene transcripts (18 indicator transcripts positively weighted: IL1A, IL1B, IL8/CXCL8, TNF, PTGS1, PTGS2, FOS, FOSB, FOSL1, FOSL2, JUN, JUNB, JUND, NFKB1, NFKB2, REL, RELA, and RELB) and downregulated expression of genes involved in type 1 interferon and antibody responses (29 indicator transcripts negatively weighted: GBP1, IFI27, IFI27L1-2, IFI30, IFI35, IFI44, IFI44L, IFI6, IFIH1, IFIT1-3, IFIT5, IFIT1B, IFITM1-3, IRF2, IRF7-8, MX1-2, OAS1-3, OASL, JCHAIN, and IGLL1). Data were analyzed by mixed effect linear models relating average CTRA indicator gene expression to PRO scores while controlling for systematic effects of indicator gene and patient age, sex, race, malignant vs nonmalignant indication for HCT, and Karnofsky performance status, with a fully saturated (unstructured) residual variance-covariance matrix accounting for within-patient correlation of gene transcript residuals. Data analysis was conducted using SAS software (SAS Institute Inc, Cary, NC).
For specific PRO domains that showed significant CTRA association in primary analyses, we conducted secondary confirmatory analyses using a complementary measure of CTRA biology involving the activities of transcription factors known to mediate CTRA gene expression.28,32 These analyses used the Transcription Element Listening System promoter-based bioinformatic analysis of empirical genome-wide transcriptome differences to quantify the activities of 2 key proinflammatory transcription factor families involved in CTRA proinflammatory signaling (NF-κB and AP-1) as well as the CREB transcription factor family that mediates β-adrenergic signaling from sympathetic nervous system stress responses. As specificity controls, we also examined the glucocorticoid receptor (GR) transcription control pathway that is activated by hypothalamus-pituitary-adrenal axis stress signaling, in contrast to sympathetic nervous system β-adrenergic signaling. Transcription Element Listening System analyses took as input all gene transcripts (genome-wide) that showed more than twofold difference in average expression over the range of continuously varying patient-reported SDOH measures from 2 SDs below the average value to 2 SDs above the average value (ie, the general 4-SD range of variation). Analyses compared the prevalence of transcription factor–binding motifs (TFBMs) in the core promoter sequences of genes that were upregulated in association with favorable patient-reported SDOH status (more well-being) in comparison with those upregulated in association with unfavorable patient-reported SDOH status (less well-being). TFBMs were identified using position-specific weight matrixes from the JASPAR library: CREB1, NF-κB1, and FOS::JUN; and the TRANSFAC library: V$GR_Q6. To confirm involvement of myeloid lineage cells as would be expected in the CTRA profile,23 we also assessed JASPAR MEIS1-3 and MSX2 TFBMs. These analyses were repeated over 9 parametric variations of TRANSFAC MatInspector scan stringency and core promoter length, with average (log2) TFBM ratios tested for statistical significance using standard errors derived from bootstrap resampling of linear model residual vectors from primary linear statistical models described earlier. This study was not designed or powered for de novo discovery of statistically significant associations between patient-reported SDOH measures and individual gene transcripts, and we did not conduct any analysis of statistical significance at the level of individual genes.
Results
Population characteristics
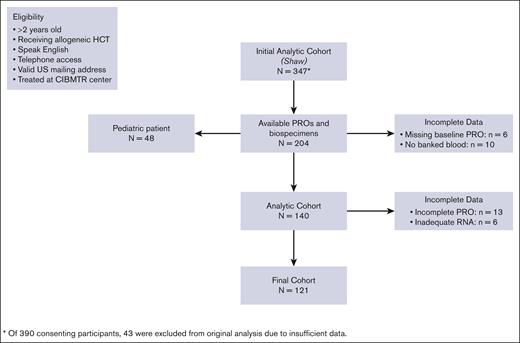
A total of 121 patients representing 8 transplant centers in the United States met the criteria for inclusion in the analyses (Figure 1). Patient characteristics are depicted in Table 1. The median age was 54 years (range, 18-74 years), 42% were female, and 91% identified as White. The majority of the patients (95%) had a hematologic malignancy, with acute myelogenous leukemia being the most common (35%), followed by myelodysplastic syndrome (15%) and chronic myeloid leukemia (10%). Most patients (63%) went on to receive a myeloablative preparative conditioning regimen, and 44% received total body irradiation before transplant. According to patient-reported sociodemographic surveys, 70% of patients were married or living with a partner. Only more than half of patients (53%) had private insurance, compared with 10% with Medicare and 10% with Medicaid. Patient-reported income data were available for approximately half of the participants, of whom nearly two-thirds reported a household gross annual income of ≥$60 000 compared with one-third in the <$60 000 category. There were no unexplained sociodemographic differences between excluded patients and the analytic cohort (supplemental Table 1).
Patient characteristics
| Characteristic . | . |
|---|---|
| No. of patients | 121 |
| Age at HCT, y, n (%) | |
| Median (min-max) | 54 (18-74) |
| 18-29 | 20 (17) |
| 30-39 | 13 (11) |
| 40-49 | 16 (13) |
| 50-59 | 30 (25) |
| 60-69 | 37 (31) |
| ≥70 | 5 (4) |
| Recipient sex, n (%) | |
| Male | 70 (58) |
| Female | 51 (42) |
| Race, n (%) | |
| White | 110 (91) |
| Black or African American | 8 (7) |
| Asian | 3 (2) |
| Performance score at HCT, n (%) | |
| ≥90 | 77 (64) |
| <90 | 44 (36) |
| Indication for transplant, n (%) | |
| Malignant | |
| Acute Myeloid leukemia | 42 (35) |
| Acute lymphoblastic leukemia | 10 (8) |
| Other leukemia | 8 (7) |
| Chronic myeloid leukemia | 12 (10) |
| Myelodysplastic syndrome | 18 (15) |
| Other acute leukemia | 3 (2) |
| Non-Hodgkin lymphoma | 12 (10) |
| Hodgkin disease | 4 (3) |
| Plasma cell disorder/Multiple myeloma | 3 (2) |
| Myeloproliferative neoplasm | 3 (2) |
| Nonmalignant | |
| Severe aplastic anemia | 3 (2) |
| SCID and other immune system disorders | 2 (2) |
| Histiocytic disorders | 1 (1) |
| Prior autologous HCT, n (%) | |
| No | 107 (88) |
| Yes | 14 (12) |
| Conditioning intensity, n (%) | |
| Myeloablative conditioning | 76 (63) |
| Reduced intensity conditioning | 42 (35) |
| Undecided at the time of data collection | 3 (2) |
| TBI use, n (%) | |
| No | 68 (56) |
| Yes | 53 (44) |
| Marital status, n (%) | |
| Married or living with partner | 85 (70) |
| Single/separated/divorced/widowed | 28 (23) |
| Missing | 8 (7) |
| Work status, n (%) | |
| Full time | 60 (50) |
| Other | 49 (40) |
| Missing | 12 (10) |
| Household gross annual income, n (%) | |
| <$60 000 | 21 (17) |
| ≥$60 000 | 35 (29) |
| Declines/unknown | 65 (54) |
| Health insurance, n (%) | |
| Employer | 7 (6) |
| Medicaid | 12 (10) |
| Medicare | 12 (10) |
| Private | 64 (53) |
| Medicaid + private | 3 (2) |
| Medicare + private | 9 (7) |
| Self-pay | 1 (1) |
| Other | 8 (7) |
| Unknown | 5 (4) |
| Y of transplant, n (%) | |
| 2011 | 13 (11) |
| 2012 | 83 (69) |
| 2013 | 25 (21) |
| Follow-up in mo, median (range) | 73 (13-98) |
| Characteristic . | . |
|---|---|
| No. of patients | 121 |
| Age at HCT, y, n (%) | |
| Median (min-max) | 54 (18-74) |
| 18-29 | 20 (17) |
| 30-39 | 13 (11) |
| 40-49 | 16 (13) |
| 50-59 | 30 (25) |
| 60-69 | 37 (31) |
| ≥70 | 5 (4) |
| Recipient sex, n (%) | |
| Male | 70 (58) |
| Female | 51 (42) |
| Race, n (%) | |
| White | 110 (91) |
| Black or African American | 8 (7) |
| Asian | 3 (2) |
| Performance score at HCT, n (%) | |
| ≥90 | 77 (64) |
| <90 | 44 (36) |
| Indication for transplant, n (%) | |
| Malignant | |
| Acute Myeloid leukemia | 42 (35) |
| Acute lymphoblastic leukemia | 10 (8) |
| Other leukemia | 8 (7) |
| Chronic myeloid leukemia | 12 (10) |
| Myelodysplastic syndrome | 18 (15) |
| Other acute leukemia | 3 (2) |
| Non-Hodgkin lymphoma | 12 (10) |
| Hodgkin disease | 4 (3) |
| Plasma cell disorder/Multiple myeloma | 3 (2) |
| Myeloproliferative neoplasm | 3 (2) |
| Nonmalignant | |
| Severe aplastic anemia | 3 (2) |
| SCID and other immune system disorders | 2 (2) |
| Histiocytic disorders | 1 (1) |
| Prior autologous HCT, n (%) | |
| No | 107 (88) |
| Yes | 14 (12) |
| Conditioning intensity, n (%) | |
| Myeloablative conditioning | 76 (63) |
| Reduced intensity conditioning | 42 (35) |
| Undecided at the time of data collection | 3 (2) |
| TBI use, n (%) | |
| No | 68 (56) |
| Yes | 53 (44) |
| Marital status, n (%) | |
| Married or living with partner | 85 (70) |
| Single/separated/divorced/widowed | 28 (23) |
| Missing | 8 (7) |
| Work status, n (%) | |
| Full time | 60 (50) |
| Other | 49 (40) |
| Missing | 12 (10) |
| Household gross annual income, n (%) | |
| <$60 000 | 21 (17) |
| ≥$60 000 | 35 (29) |
| Declines/unknown | 65 (54) |
| Health insurance, n (%) | |
| Employer | 7 (6) |
| Medicaid | 12 (10) |
| Medicare | 12 (10) |
| Private | 64 (53) |
| Medicaid + private | 3 (2) |
| Medicare + private | 9 (7) |
| Self-pay | 1 (1) |
| Other | 8 (7) |
| Unknown | 5 (4) |
| Y of transplant, n (%) | |
| 2011 | 13 (11) |
| 2012 | 83 (69) |
| 2013 | 25 (21) |
| Follow-up in mo, median (range) | 73 (13-98) |
CMV, cytomegalovirus; GVHD, graft-versus-host disease; SCID, severe combined immunodeficiency; TBI, total body irradiation.
Patient-reported SDOH outcomes
Baseline (pre-HCT) PRO scores are listed in Table 2 along with published normative values for the general US population for reference.30,31 With the exception of the SF-36 MCS and FACT social well-being subdomains, all PRO scores were statistically significantly lower in the HCT cohort than in the general US population, indicating lower self-reported well-being.
PRO measures compared with those of the general US population
| Outcome measure . | Mean (SD) . | US population mean (SD) . | P∗ . |
|---|---|---|---|
| SF-36 | |||
| SF-36 MCS | 50.0 (10.3) | 50 (10) | .97 |
| SF-36 PCS | 40.8 (9.3) | 50 (10) | <.001 |
| FACT-BMT | |||
| FACT-G | 76.8 (13.4) | 86.5 (15.2) | <.001 |
| Social well-being | 20.9 (4.0) | 20.2 (5.8) | .07 |
| Emotional well-being | 17.6 (3.7) | 19.5 (4.5) | <.001 |
| Physical well-being | 21.1 (5.4) | 24.9 (4.1) | <.001 |
| Functional well-being | 17.2 (5.3) | 21.4 (5.5) | <.001 |
| BMT subdomain | 26.7 (5.9) | — | — |
| FACT-BMT total | 103.5 (17.8) | — | — |
| Outcome measure . | Mean (SD) . | US population mean (SD) . | P∗ . |
|---|---|---|---|
| SF-36 | |||
| SF-36 MCS | 50.0 (10.3) | 50 (10) | .97 |
| SF-36 PCS | 40.8 (9.3) | 50 (10) | <.001 |
| FACT-BMT | |||
| FACT-G | 76.8 (13.4) | 86.5 (15.2) | <.001 |
| Social well-being | 20.9 (4.0) | 20.2 (5.8) | .07 |
| Emotional well-being | 17.6 (3.7) | 19.5 (4.5) | <.001 |
| Physical well-being | 21.1 (5.4) | 24.9 (4.1) | <.001 |
| Functional well-being | 17.2 (5.3) | 21.4 (5.5) | <.001 |
| BMT subdomain | 26.7 (5.9) | — | — |
| FACT-BMT total | 103.5 (17.8) | — | — |
PCS, physical component summary.
P values were calculated using 1-sample t test.
CTRA associations with patient-reported SDOH outcomes
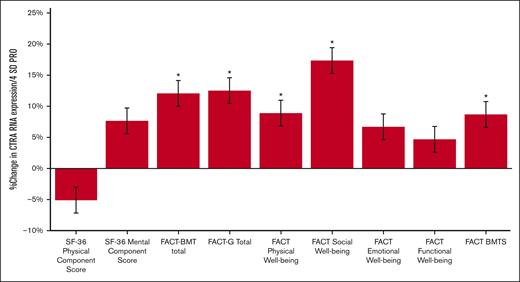
Of the 121 patients with leukocyte RNA profiles, analyses showed an inverse relationship between multiple measures of patient-reported SDOH and CTRA gene expression (Figure 2). Elevated CTRA expression was associated with lower FACT-BMT total score (10.7% increase in average CTRA expression across the 4-SD range in scores from from high to low FACT-BMT well-being; P = .003) as well as the FACT-G component score (11.1% CTRA increase; P = .003) and its BMT-specific subcomponent (8.7% CTRA increase; P = .014). When both the BMT and general components of the FACT were analyzed simultaneously in a single multivariate model (ie, each controlled for the other), only FACT-G continued to show a significant inverse association with CTRA gene expression (12.7% CTRA increase; P = .006; BMT-specific component: 0.6% decrease; P = .90). Within the FACT-G, CTRA gene expression showed the strongest association with its social well-being subdomain (14.9% CTRA increase; P = .0001) and a more modest but still significant association with lower patient-reported physical well-being (8.2% CTRA increase; P = .031). The SF-36 MCS also showed a trend toward a significant inverse association with CTRA (P = .052), corresponding to a 7.2% increase in CTRA expression across the 4-SD range in SF-36 MCS scores from more to less well-being.
CTRA expression based on PROs. Percent change in the average CTRA expression across the 4-SD range of variation from an advantaged status 2 SD above the mean to a disadvantaged status 2 SD below the mean score for the sample.; FACT BMTS, FACT-BMT–specific subdomain. ∗P < .05.
CTRA expression based on PROs. Percent change in the average CTRA expression across the 4-SD range of variation from an advantaged status 2 SD above the mean to a disadvantaged status 2 SD below the mean score for the sample.; FACT BMTS, FACT-BMT–specific subdomain. ∗P < .05.
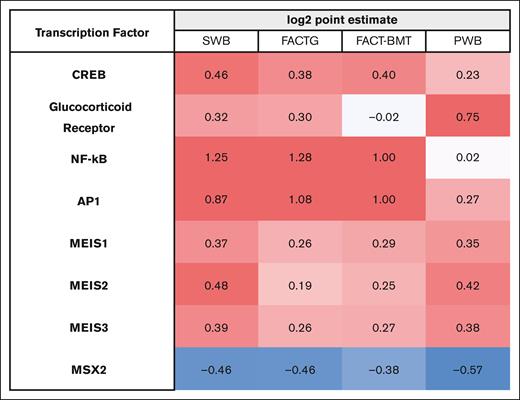
To confirm significant results from the primary analyses of the prespecified CTRA indicator gene sets, we conducted secondary bioinformatics analyses to assess the activity of CTRA-mediating upstream transcription factors in unbiased empirical differences in genome-wide transcriptional profiles. The ratio of upregulated compared with downregulated TFBMs were quantified as log2 point estimates, which can be conceptualized similar to effect sizes, with respect to specific PRO domains. These analyses confirmed the expected association of reduced patient-reported well-being with increased activity of multiple risk-related transcription control pathways, including the CREB transcription factors (involved in mediating β-adrenergic signaling from the sympathetic nervous system), the proinflammatory NF-κB and AP-1 transcription control pathways, and the myeloid lineage factors MEIS1-3 (Figure 3). These effects were specific to adrenergic sympathetic nervous system CTRA-related transcription control pathways, because no significant associations emerged for the GR transcription factor involved in cortisol signaling from the hypothalamus-pituitary-adrenal axis. As in the analyses of the prespecified CTRA indicator gene sets described earlier, these effects appeared most pronounced for the social well-being subscale of the FACT-G measure. Although not hypothesized a priori, we noted a consistent bioinformatic indication of increased MSX2 transcription factor activity associated with the patient-reported SDOH disadvantage measures (Figure 3).
TFBM analyses. Bioinformatic analyses quantified ratio of upregulated vs downregulated TFBMs within these 4 PRO domains. There was a pattern of increased activation of inflammation-related TFBMs (NF-κB and AP-1) with decreased patient-reported well-being, with the most pronounced difference in the social well-being domain. Differences in myeloid lineage TFBMs (MEIS1-3) were observed, consistent with CTRA immunobiology as previously described. PWB, physical well-being; SWB, social well-being.
TFBM analyses. Bioinformatic analyses quantified ratio of upregulated vs downregulated TFBMs within these 4 PRO domains. There was a pattern of increased activation of inflammation-related TFBMs (NF-κB and AP-1) with decreased patient-reported well-being, with the most pronounced difference in the social well-being domain. Differences in myeloid lineage TFBMs (MEIS1-3) were observed, consistent with CTRA immunobiology as previously described. PWB, physical well-being; SWB, social well-being.
Discussion
Among this cohort of allogeneic HCT recipients, patient-reported measures of SDOH risk were associated with higher pretransplant CTRA expression, with less social well-being identified as the most prominent association. These findings support the importance of perceived social support and community context within the wider paradigm of SDOH. Identifying modifiable biomarkers predictive of patient-reported SDOHs and medical outcomes is important; capturing a downstream biologic signature associated with subjective experience can enhance the clinical predictive utility of standard PROs and facilitate the identification of mechanistic drivers of pathology. This could ultimately assist in more comprehensive biopsychosocial risk stratification and subsequent risk-adapted care for the HCT population.20
Social isolation and the resulting subjective experience (loneliness) have long been tied to negative medical outcomes, including cardiovascular disease, infections, and even all-cause mortality.33 Conversely, higher perceived social support has been linked to better quality of life and lower psychological distress after HCT.18 We lack a complete understanding of the underlying mechanisms linking social support and biology, but it is plausible that a dysregulated immune response plays an important role in inflammatory pathology.33,34 Expression of the CTRA immunoregulatory pattern results in a global proinflammatory leukocyte phenotype, and it has appeared across a spectrum of adverse psychosocial conditions, including loneliness, poverty, bereavement, and trauma.24,28 Additionally, the CTRA pattern is consistently observed in various disease phenotypes, including cardiovascular disease, viral infections, chronic fatigue, and depression.23 Thus, the CTRA has emerged as a molecular framework to help understand the connection between socioenvironmental exposure and human health and disease.
The role of the CTRA in HCT biology is an area of active translational investigation, given the pronounced overlap between social strain and immune dysregulation in malignant processes. Prior studies have established a relationship between CTRA expression and clinical cancer and HCT outcomes. Allogeneic HCT recipients with low SES have consistently demonstrated elevated CTRA expression compared with patients of high SES, and this has been, in turn, associated with inferior clinical outcomes.15,28 Importantly, the CTRA profile can be modified through pharmacologic or behavioral intervention. For example, both β-adrenergic antagonism with propranolol and cognitive-behavioral stress reduction training can reduce CTRA gene expression and related transcriptome dynamics.32,35,36 Furthermore, in a phase 2 biomarker trial among autologous HCT patients, those participants randomized to the β-antagonist (propranolol) arm demonstrated upregulation of CD34-associated gene transcripts and a trend toward accelerated engraftment and reduced posttransplant infections.32 Immune cell–mediated inflammation and infections drive a significant proportion of short- and long-term morbidity and mortality in the HCT population37; identifying modifiable predictors of these outcomes could significantly impact patient outcomes.
Results from our study further support a potential role for CTRA in mediating socioenvironmental influences on HCT outcomes. Individuals with lower levels of perceived well-being and lower social well-being, in particular, demonstrated higher CTRA gene expression and increased activation of upstream adrenergic stress-mediated transcription control pathways. Targeting the psychosocial-CTRA pathway through behavioral (ie, social support, stress reduction, or positive psychology interventions) and/or pharmacologic interruption of the adrenergic stress response (ie, beta-blocker therapy) may be a promising strategy to improve multidimensional outcomes in the HCT population. Although this study was not designed or powered to detect changes in clinical outcomes, the larger parent cohort did report differences in overall survival based on certain PRO quartiles; this suggests that CTRA may play a role in the PRO-outcomes relationship, though definitive conclusions will require additional investigation.19 Future, larger studies should seek to further clarify potential risk groups for CTRA activation over time using patient-reported SDOH measures, which could serve as a helpful clinical adjunct in our effort to provide optimal whole-person care.
There are important limitations to consider when interpreting this study’s results. This cohort was quite homogenous with respect to race, with 91% of participants identifying as White, although this is comparable with the general US HCT recipient population (ranging from 83% to 86% White from 2010 to 2020).38 Additionally, there were missing data in relevant SDOH variables (income, work status, and marital status), limiting our ability to include these variables in our primary analyses. However, although our sample size was modest, it was similar to that of other studies evaluating high-resolution genomic data in a biopsychosocial context.15,28,36,39 There are distinct advantages to using a large database repository for robust and rigorous data capture, but there were likely unmeasured confounders in the relationship between CTRA and patient-reported SDOH outcomes. For example, PROs at initial cancer diagnosis, lines of prior therapy, and some health behavior data were not captured in this analysis and may also influence CTRA expression.23 Data were analyzed at a single time point pre-HCT, restricting our ability to infer causality and quantify changes over time. Of note, self-reported social well-being did not differ statistically between our study cohort and the general US population (Table 2). This is consistent with data showing perceived social support declines after HCT and then likely increases further out from transplant.31 Finally, there is no established clinically significant change in CTRA RNA expression, limiting our ability to comment on the overall health significance of the absolute changes in this biomarker seen in our study. These data warrant further exploration in a larger, prospective, more sociodemographically diverse HCT population.
In summary, this study sought to quantify the association between patient-reported measures of social and behavioral determinants of health and a specific pretransplant social genomic profile among allogeneic HCT recipients. We identified multiple domains of experienced SDOH risk linked to CTRA expression, with low social well-being emerging as the most significant factor. These results align with prior studies of the relationship between CTRA and adverse socioenvironmental factors and suggest the potential for clinical impacts in the HCT setting. The CTRA molecular pattern is easily measured and is modifiable, making it an appealing candidate as a targetable biopsychosocial biomarker. Ultimately, applying a social genomics lens to biomarker discovery will help identify patients at risk of poor outcomes and facilitate the development of targeted interventions to improve comprehensive outcomes in the HCT population.
Acknowledgments
This study was supported by a Supportive Care research grant (712551) from the St. Baldrick's Foundation and a Career Development Award from the Seattle Children's Research Institute. The CIBMTR is supported primarily by the Public Health Service U24CA076518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases; 75R60222C00011 from the Health Resources and Services Administration; N00014-21-1-2954 and N00014-23-1-2057 from the Office of Naval Research; support was also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, Gateway for Cancer Research, Pediatric Transplantation and Cellular Therapy Consortium, and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc; Adaptimmune; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Allogene; Allovir, Inc; Amgen, Inc; Angiocrine; Astellas Pharma US; Atara Biotherapeutics; BeiGene; bluebird bio, Inc; Bristol Myers Squibb Co; CareDx Inc; CSL Behring; CytoSen Therapeutics, Inc; Elevance Health; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida-Cell, Ltd; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc; Karius; Kiadis Pharma; Kite, a Gilead company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt Pharmaceuticals; Merck & Co; Mesoblast; Millennium, the Takeda Oncology Co; Miltenyi Biotec, Inc; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc; Ossium Health, Inc; Pfizer, Inc; Pharmacyclics, LLC, an AbbVie company; PPD Development, LP; Regimmune; Sanofi; Sarah Cannon; Sobi, Inc; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc; and Xenikos BV. Visual Abstract art credit: Katie Vicari.
Authorship
Contribution: M.R.T., J.M.K., S.W.C., K.S.B., R.P., D.B., B.H., and H.S. conceived, designed, and refined the study and wrote the draft manuscript; J.S., R.B., and S.W.C. performed data analysis and interpretation; and all authors reviewed, revised, and approved the final manuscript.
Conflict-of-interest disclosure: B.H. reports compensation for participation in Nkarta ad hoc advisory board, Angiocrine data safety monitoring committee, Kadmon/Sanofi ad hoc advisory board, and Equilium ad hoc advisory board and receives Incyte consultancy and Therakos/Mallinkrodt speaker fees. H.S. reports having received personal fees from Incyte, Janssen, Novartis, Sanofi, and the Belgian Hematological Society (BHS), as well as research grants from Novartis and the BHS paid to her institution; has received nonfinancial support from Gilead, Pfizer, the European Society for Blood and Marrow transplantation, and the CIBMTR; and also serving as a volunteer for European Society for Blood and Marrow transplantation, CIBMTR, and EUPATI. A.S. reports having received a consultant fee from Spotlight Therapeutics, Medexus Inc, Vertex Pharmaceuticals, and Sangamo Therapeutics; research funding from CRISPR Therapeutics and honoraria from Vindico Medical Education; is the St. Jude Children’s Research Hospital site principal investigator of clinical trials for genome editing of sickle cell disease sponsored by Vertex Pharmaceuticals/CRISPR Therapeutics, Novartis Pharmaceuticals Corp, and Beam Therapeutics; the industry sponsors provide funding for the clinical trial, which includes salary support paid to his institution; and has no direct financial interest in these therapies. R.P. reports advisory board participation with bluebird bio and research funding from Amgen. The remaining authors declare no conflicting financial interests.
Correspondence: Mallory R. Taylor, Ben Towne Center for Childhood Cancer Research, Seattle Children's Research Institute, 1920 Terry Ave, MS Cure 4, Seattle, WA 98101; e-mail: molly.taylor@seattlechildrens.org.
References
Author notes
Presented in oral abstract form at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 10 December 2022.
CIBMTR supports accessibility of research in accord with the National Institutes of Health Data Sharing Policy and the National Cancer Institute Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases deidentified data sets that comply with all relevant global regulations regarding privacy and confidentiality.
The final analysis data set will be deposited in the CIBMTR website at https://cibmtr.org/CIBMTR/Resources/Publicly-Available-Datasets1#.
The full-text version of this article contains a data supplement.