TO THE EDITOR:
Axicabtagene ciloleucel (axi-cel), an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell product approved for patients with relapsed and/or refractory large B-cell lymphoma (LBCL), results in cytokine release syndrome (CRS) in 90% to 95% of patients and immune effector cell–associated neurotoxicity syndrome (ICANS) in 60% to 64% of patients.1,2 Corticosteroids represent the only current treatment option for ICANS, and although their negative impact on outcomes remains controversial, high doses and prolonged duration of corticosteroid treatment may hamper CAR T-cell function, in addition to potentially increasing the risk of infectious complications.3-5 We have previously shown that the presence of myeloid-like ICANS-associated cells (IACs) expressing high levels of interlukin-1β (IL-1β) within axi-cel infusion products is associated with the development of high-grade ICANS.6 In line with this, serum IL-1β levels peak within the first 7 days after axi-cel infusion, and higher serum IL-1β levels are also associated with an increased risk of ICANS.1 Preclinical studies have shown that anakinra, an IL-1 receptor antagonist currently approved by the Food and Drug Administration for the treatment of patients with refractory rheumatoid arthritis at a dose of 100 mg to 200 mg subcutaneous (SC) daily, may abrogate CRS and ICANS without negatively affecting CAR T-cell function.7-9 The use of anakinra to treat CRS or ICANS after axi-cel has previously been reported and shown to be safe, without any increase in infectious complications.10,11 In this study, we conducted a phase 1 study to determine whether prophylactic anakinra can be safely used to mitigate CRS and/or ICANS in patients with LBCL treated with axi-cel (NCT04432506). The trial was approved by the University of Texas MD Anderson Cancer Center and was conducted in accordance with the Declaration of Helsinki.
This is a single-center phase 1 study, conducted at the University of Texas MD Anderson Cancer Center from November 2020 through May 2022. Detailed eligibility criteria are provided in the supplemental Material. All patients received standard axi-cel. Starting 6 hours before axi-cel infusion on day 0, anakinra was administered subcutaneously at a dose of 100 mg daily (n = 10, dose level 1) or 100 mg every 12 hours (n = 10, dose level 2) for 7 days.
The primary end point was the safety and tolerability of anakinra after axi-cel infusion in patients with LBCL. CRS and ICANS were graded according to the criteria set by the American Society for Transplantation and Cellular Therapy, and all other adverse events were graded according to the Common Toxicity Criteria for Adverse Events version 5.12 Response status was assessed by the Lugano 2014 classification.13 IACs in the infusion products were quantified using single-cell RNA-sequencing with capture–based cell identification and unsupervised clustering; CAR T-cell amplification in peripheral blood was quantified by polymerase chain reaction using vector-specific primers6; cytokine levels were measured using immunoassay of plasma samples using the V-PLEX Human Cytokine 30-Plex Kit (Meso Scale Discovery, Rockville, MD). A 3 + 3 design was used for a safety run-in phase within each dose level cohort, and dose-limiting toxicity was evaluated during the first 30 days after axi-cel infusion (details are provided in supplemental Material). Additional patients were treated during the expansion phase, for a total of 10 patients at each dose level. CAR T-cell amplification and cytokine production in the study cohort were compared with that of a contemporaneous matched cohort of patients with LBCL, treated with axi-cel without anakinra (statistical details are provided in supplemental Material and baseline characteristics in supplemental Table 1).
All 20 patients treated at dose levels 1 and 2 completed the full course of anakinra and were evaluable for safety and efficacy. Baseline characteristics are shown in Table 1. No dose-limiting toxicity was observed at either of the dose levels, and 100 mg SC every 12 hours is the recommended phase 2 dose. Incidence and grade for all adverse events are detailed in supplemental Table 2. Adverse events, possibly because of anakinra, included grade 2 nausea (1 patient) and grade 3 diarrhea (1 patient). Five patients (25%) had ongoing grade 3 to 4 cytopenia at day 30, including 3 of 10 (30%) patients at dose level 1 and 2 of 10 (20%) at dose level 2.
Baseline characteristics
| Characteristic . | Number (%), median (range) . | ||
|---|---|---|---|
| Dose level 1, 100 mg daily (n = 10) . | Dose level 2, 100 mg twice daily (n = 10) . | Total (n = 20) . | |
| DLBCL | 8 (80) | 7 (70) | 15 (75) |
| HGBCL | 1 (10) | 0 (0) | 1 (5) |
| PMBCL | 0 (0) | 1 (10) | 1 (5) |
| TFL | 1 (10) | 2 (20) | 3 (15) |
| Age (y) | 60 (39-81) | 58 (26-81) | 58 (26-81) |
| Age > 60 y | 5 (50) | 5 (50) | 10 (50) |
| Male | 6 (60) | 10 (100) | 16 (80) |
| ECOG performance status ≥ 1 | 3 (30) | 5 (50) | 8 (40) |
| Ann Arbor stage III-IV | 10 (100) | 9 (90) | 19 (95) |
| Extranodal sites > 1 | 3 (30) | 6 (60) | 9 (45) |
| International Prognostic Index score ≥ 3 | 7 (70) | 7 (70) | 14 (70) |
| Absolute neutrophil count, 109/L | 3 (1.4-9.7) | 3.7 (1.3-8.9) | 3.3 (1.3-9.7) |
| Absolute lymphocyte count, 109/L | 0.7 (0.3-1.1) | 0.4 (0.2-2.3) | 0.6 (0.2-2.3) |
| Absolute monocyte count, 109/L | 0.6 (0.2-2) | 0.6 (0.3-1.2) | 0.6 (0.2-2) |
| Hemoglobin, g/dL | 11.6 (8.3-13.8) | 11.6 (6.3-14.7) | 11.6 (6.3-14.7) |
| Platelet count, 109/L | 149 (90-231) | 115 (71-362) | 144 (71-362) |
| C-reactive protein, mg/L | 8.2 (0.7-63) | 10.4 (0.4-79) | 8.6 (0.4-79) |
| Ferritin, mg/L | 277 (20-3190) | 300 (58-1520) | 300 (20-3190) |
| Lactate dehydrogenase, U/L | 250 (200-348) | 302 (144-756) | 268 (144-756) |
| Lactate dehydrogenase > UNL | 7 (70) | 8 (80) | 15 (75) |
| Creatinine clearance, mL/min | 79 (64-105) | 80 (40-127) | 80 (40-127) |
| Previous therapies | 4 (2-8) | 3 (2-5) | 3 (2-8) |
| Bridging therapy use | 5 (50) | 7 (70) | 12 (60) |
| Bridging | |||
| Chemotherapy | 2 (20) | 2 (20) | 4 (20) |
| Radiation therapy | 1 (10) | 4 (40) | 5 (25) |
| Biological therapy | 2 (10) | 1 (10) | 3 (15) |
| Refractory disease | 10 (100) | 10 (100) | 20 (100) |
| Previous autologous SCT | 0 (0) | 1 (10) | 1 (5) |
| Previous allogeneic SCT | 0 (0) | 0 (0) | 0 (0) |
| Total metabolic tumor volume, mL | 25 (6-85) | 38 (3-226) | 31 (3-226) |
| Characteristic . | Number (%), median (range) . | ||
|---|---|---|---|
| Dose level 1, 100 mg daily (n = 10) . | Dose level 2, 100 mg twice daily (n = 10) . | Total (n = 20) . | |
| DLBCL | 8 (80) | 7 (70) | 15 (75) |
| HGBCL | 1 (10) | 0 (0) | 1 (5) |
| PMBCL | 0 (0) | 1 (10) | 1 (5) |
| TFL | 1 (10) | 2 (20) | 3 (15) |
| Age (y) | 60 (39-81) | 58 (26-81) | 58 (26-81) |
| Age > 60 y | 5 (50) | 5 (50) | 10 (50) |
| Male | 6 (60) | 10 (100) | 16 (80) |
| ECOG performance status ≥ 1 | 3 (30) | 5 (50) | 8 (40) |
| Ann Arbor stage III-IV | 10 (100) | 9 (90) | 19 (95) |
| Extranodal sites > 1 | 3 (30) | 6 (60) | 9 (45) |
| International Prognostic Index score ≥ 3 | 7 (70) | 7 (70) | 14 (70) |
| Absolute neutrophil count, 109/L | 3 (1.4-9.7) | 3.7 (1.3-8.9) | 3.3 (1.3-9.7) |
| Absolute lymphocyte count, 109/L | 0.7 (0.3-1.1) | 0.4 (0.2-2.3) | 0.6 (0.2-2.3) |
| Absolute monocyte count, 109/L | 0.6 (0.2-2) | 0.6 (0.3-1.2) | 0.6 (0.2-2) |
| Hemoglobin, g/dL | 11.6 (8.3-13.8) | 11.6 (6.3-14.7) | 11.6 (6.3-14.7) |
| Platelet count, 109/L | 149 (90-231) | 115 (71-362) | 144 (71-362) |
| C-reactive protein, mg/L | 8.2 (0.7-63) | 10.4 (0.4-79) | 8.6 (0.4-79) |
| Ferritin, mg/L | 277 (20-3190) | 300 (58-1520) | 300 (20-3190) |
| Lactate dehydrogenase, U/L | 250 (200-348) | 302 (144-756) | 268 (144-756) |
| Lactate dehydrogenase > UNL | 7 (70) | 8 (80) | 15 (75) |
| Creatinine clearance, mL/min | 79 (64-105) | 80 (40-127) | 80 (40-127) |
| Previous therapies | 4 (2-8) | 3 (2-5) | 3 (2-8) |
| Bridging therapy use | 5 (50) | 7 (70) | 12 (60) |
| Bridging | |||
| Chemotherapy | 2 (20) | 2 (20) | 4 (20) |
| Radiation therapy | 1 (10) | 4 (40) | 5 (25) |
| Biological therapy | 2 (10) | 1 (10) | 3 (15) |
| Refractory disease | 10 (100) | 10 (100) | 20 (100) |
| Previous autologous SCT | 0 (0) | 1 (10) | 1 (5) |
| Previous allogeneic SCT | 0 (0) | 0 (0) | 0 (0) |
| Total metabolic tumor volume, mL | 25 (6-85) | 38 (3-226) | 31 (3-226) |
DLBCL, diffuse LBCL; ECOG, European Cooperative Oncology Group; HGBCL, high-grade BCL; PMBCL, primary mediastinal BCL; SCT, stem cell transplant; TFL, transformed follicular lymphoma; UNL, upper normal limit.
Across all 20 patients, CRS was observed in 95% of patients and was grade 3 to 4 in 5% of patients. ICANS occurred in 35% of patients and was grade 3 to 4 in 20% of patients. There were no grade 5 CRS or ICANS events. The median times to onset of CRS and ICANS were 3 days (range, 1-8) and 7 days (range, 3-9), respectively, after axi-cel infusion. The median CRS duration was 5 days (range, 2-9), and the median ICANS duration was 2 days (range, 2-17). Seven patients (35%) required dexamethasone, with a median cumulative dose of 100 mg (range, 10-1728). As compared to a contemporaneous matched cohort, patients treated with anakinra experienced less frequent ICANS of any grade (35% vs 60%) and lower use (35% vs 55%) and duration of corticosteroid treatment (4 days vs 6 days) for management of CRS or ICANS. Remaining toxicity outcomes, including CRS incidence and grade and use of tocilizumab remained numerically similar between the 2 cohorts. Further details regarding toxicities and their management according to each dose level and the matched cohort are provided in supplemental Table 3.
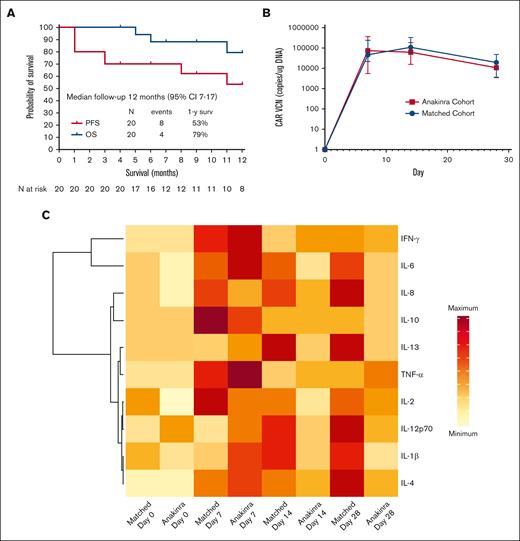
On day 30 after axi-cel infusion, the overall and complete response rates were found to be 85% and 65%, respectively, and at day 90, 60% of patients were in complete response. After a median follow-up of 12 months (95% confidence interval, 7-17), the response rate was 65%, the 1-year progression-free survival rate was 53%, and the 1-year overall survival rate was 79% (Figure 1A). All 5 deaths observed during follow-up were due to cancer progression.
Survival and biological effects. (A) Progression-free survival and overall survival in the 20 patients treated with anakinra with axi-cel. (B) CAR T-cell amplification in peripheral blood compared with a contemporaneous matched cohort as measured by quantitative polymerase chain reaction; VCN, vector copy number. (C) Heatmap of inflammatory markers in the plasma of patients treated with anakinra compared with a contemporaneous matched cohort receiving axi-cel only. For CAR T-cell amplification, comparisons between cohorts was performed using Mann-Whitney U test: ∗∗ P < .01; ∗∗∗ P < .001. Median values are displayed with error bars showing interquartile range. In the heatmap, data for median plasma levels of each cytokine are represented by the same scale for both cohorts.
Survival and biological effects. (A) Progression-free survival and overall survival in the 20 patients treated with anakinra with axi-cel. (B) CAR T-cell amplification in peripheral blood compared with a contemporaneous matched cohort as measured by quantitative polymerase chain reaction; VCN, vector copy number. (C) Heatmap of inflammatory markers in the plasma of patients treated with anakinra compared with a contemporaneous matched cohort receiving axi-cel only. For CAR T-cell amplification, comparisons between cohorts was performed using Mann-Whitney U test: ∗∗ P < .01; ∗∗∗ P < .001. Median values are displayed with error bars showing interquartile range. In the heatmap, data for median plasma levels of each cytokine are represented by the same scale for both cohorts.
IACs were measured in 15 patients using single-cell RNA-sequencing of the infusion products. Nine of 15 patients (60%) had detectable IACs, comprising a median of 0.25% of cells (range, 0.03-3.09) in patients in whom they were detected. Of these 9 patients, 3 (33%) had grade 3 to 4 ICANS. CAR T-cell amplification in peripheral blood was similar between the study cohort and the contemporaneous matched cohort at all time points (Figure 1B). Inflammatory cytokine levels in the peripheral blood were lower beyond day 7 in patients treated with anakinra than with the contemporaneous matched cohort (Figure 1C).
Our results, to our knowledge, demonstrated for the first time that prophylactic anakinra can be safely administered in patients with LBCL treated with axi-cel. With the limitations of an interstudy comparison, no apparent difference in the incidence, grade, and duration of CRS was observed in our study cohort compared with patients treated with axi-cel in the ZUMA-1 trial, despite previously published preclinical data suggesting that anakinra mitigates CRS associated with CAR T-cell therapy.1,8,9 This similarity in CRS may be because of the fact that multiple cytokines are involved in the pathophysiology of CRS, and therefore, the combination of anakinra with other agents or a higher dose of anakinra may be needed to prevent CRS.1 However, compared with patients treated in the ZUMA-1 trial, a lower incidence of all grades of ICANS was observed in patients treated in this study, independently from the presence of IACs. It remains unknown whether a dose-dependent decrease in the ICANS rate could be observed with higher doses of anakinra. High doses of anakinra (up to 8 mg/kg) for the management of CAR T-cell therapy–associated toxicities have been shown to be safe and effective.14 However, as the intent of this trial was prophylaxis (rather than treatment), and as anakinra is currently available as a prefilled syringe containing 100 mg of active agent, requiring multiple SC injections when high doses are required, no higher dose levels were explored. Intravenous administration of anakinra has been advocated as a more favorable route that may improve blood-brain barrier penetration.15 Besides alternative doses, routes of administration, and schedules of anakinra, other novel agents may need to be investigated for the prevention and/or management of CAR T-cell therapy–related toxicities, including more potent IL-1 receptor antagonists, such as isunakinra, and monoclonal antibodies targeting interferon-γ, such as emapalumab.16,17
Our study also demonstrated that prophylactic anakinra does not appear to affect CAR T-cell expansion kinetics but may lower levels of proinflammatory cytokines, along with lowering the incidence of ICANS (but with similar rates of high-grade ICANS). Thus, despite the small sample size and single-center nature of the study, anakinra showed favorable pharmacokinetic and pharmacodynamic effects and an excellent safety profile while maintaining the efficacy of axi-cel at a level similar to that seen in ZUMA-1, indicating that randomized trials are warranted to confirm the ability of this agent to mitigate ICANS either alone or in combination with other agents to block additional proinflammatory molecules including early or prophylactic corticosteroids.4,5,17
Acknowledgments: This research is supported in part by The University of Texas MD Anderson Cancer Center support grant from National Institutes of Health (P30 CA016672) and by a National Institutes of Health R21 grant (1R21CA259694-01). Salary allocated to P.S. is supported by the Lymphoma Research Foundation Career Development Award, the Leukemia Lymphoma Society Scholar in Clinical Research Career Development Program, the Kite Gilead Scholar in Clinical Research Award, and the Sabin Family Fellowship Award. M.R.G. is supported by a Leukemia and Lymphoma Society scholar award. Anakinra (Kineret) was provided free of charge by Swedish Orphan Biovitrum AB (publication) (also known as Sobi) under an Investigator-Initiated Study Agreement between Sobi and The University of Texas MD Anderson Cancer Center. Sobi was not involved in the design or conduct of the study, data collection and analysis, or preparation of the manuscript. The manuscript was edited by Sarah Bronson, ELS, of the Research Medical Library at MD Anderson.
Contribution: P.S., A.J., Q.D., S.S.N., and M.R.G. designed the study, analyzed the data, and wrote the manuscript; A.H. and T.E.D. analyzed imaging data on all patients and coauthored the manuscript; L.F. and R.S. provided statistical support and coauthored the manuscript; P.S., S. Ahmed, T.C., R.E.S., S. Adkins, S.J., J.R.W., D.S.H., D.C., L.E.F., S.P.I., S.H., L.J.N., R.N., M.H., M.A.R., E.J.S., J.L.R., P.K., and S.S.N. provided clinical care to patients and coauthored the manuscript; S. Adkins, S.J., S.H., and M.H. collected clinical data; A.J., Q.D., X.L., and M.R.G. performed correlative analyses.
Conflict-of-interest disclosure: P.S. is a consultant for Roche-Genentech, Kite/Gilead, Hutchison MediPharma, AstraZeneca-Acerta, ADC Therapeutics, Sobi, and TG Therapeutics; he has received research funds from AstraZeneca-Acerta, ALX Oncology, and ADC Therapeutics. S. Ahmed reports research support to the institution for clinical trials from Seattle Genetics, Merck, Xencor, Chimagen, and Tessa Therapeutics; has membership on Tessa Therapeutic’s and Chimagen’s scientific advisory committees; serves on the data safety monitoring board for Myeloid Therapeutics; and is a consultant for ADC Therapeutics and Kite/Gilead. M.R.G. reports research funding from Sanofi, Kite/Gilead, AbbVie, and Allogene; consulting for AbbVie, Allogene, and Bristol Myers Squibb; honoraria from Tessa Therapeutics, Monte Rosa Therapeutics, and Daiichi Sankyo; and stock ownership of KDAc Therapeutics. E.J.S. is a consultant for Adaptimmune, Axio, Navan, Fibroblasts and FibroBiologics, New York Blood Center, and Celaid Therapeutics; and holds patents with Takeda and Affimed. S.S.N. received research support from Kite/Gilead, BMS, Allogene, Precision Biosciences, Adicet Bio, and Sana Biotechnology; served as advisory board member/consultant for Kite/Gilead, Merck, Sellas Life Sciences, Athenex, Allogene, Incyte, Adicet Bio, BMS, bluebird bio, Fosun Kite, Sana Biotechnology, Caribou, Astellas Pharma, MorphoSys, Janssen, Chimagen, ImmunoACT, Orna Therapeutics, Takeda, and Synthekine; has stock options from Longbow Immunotherapy, Inc; and has intellectual property related to cell therapy. The remaining authors declare no competing financial interests.
Correspondence: Paolo Strati, Department of Lymphoma and Myeloma, Department of Translational Molecular Pathology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 429, Houston, TX 77030; e-mail: pstrati@mdanderson.org.
References
Author notes
∗P.S., A.J., and Q.D. contributed equally to this study.
†S.S.N. and M.R.G. contributed equally to this study.
Data are available on request from the corresponding author, Paolo Strati (pstrati@mdanderson.org).
The full-text version of this article contains a data supplement.